Influence of Seed Coat Integrity on the Response of Pepper Seeds to Dielectric Barrier Discharge Plasma Treatment
Abstract
1. Introduction
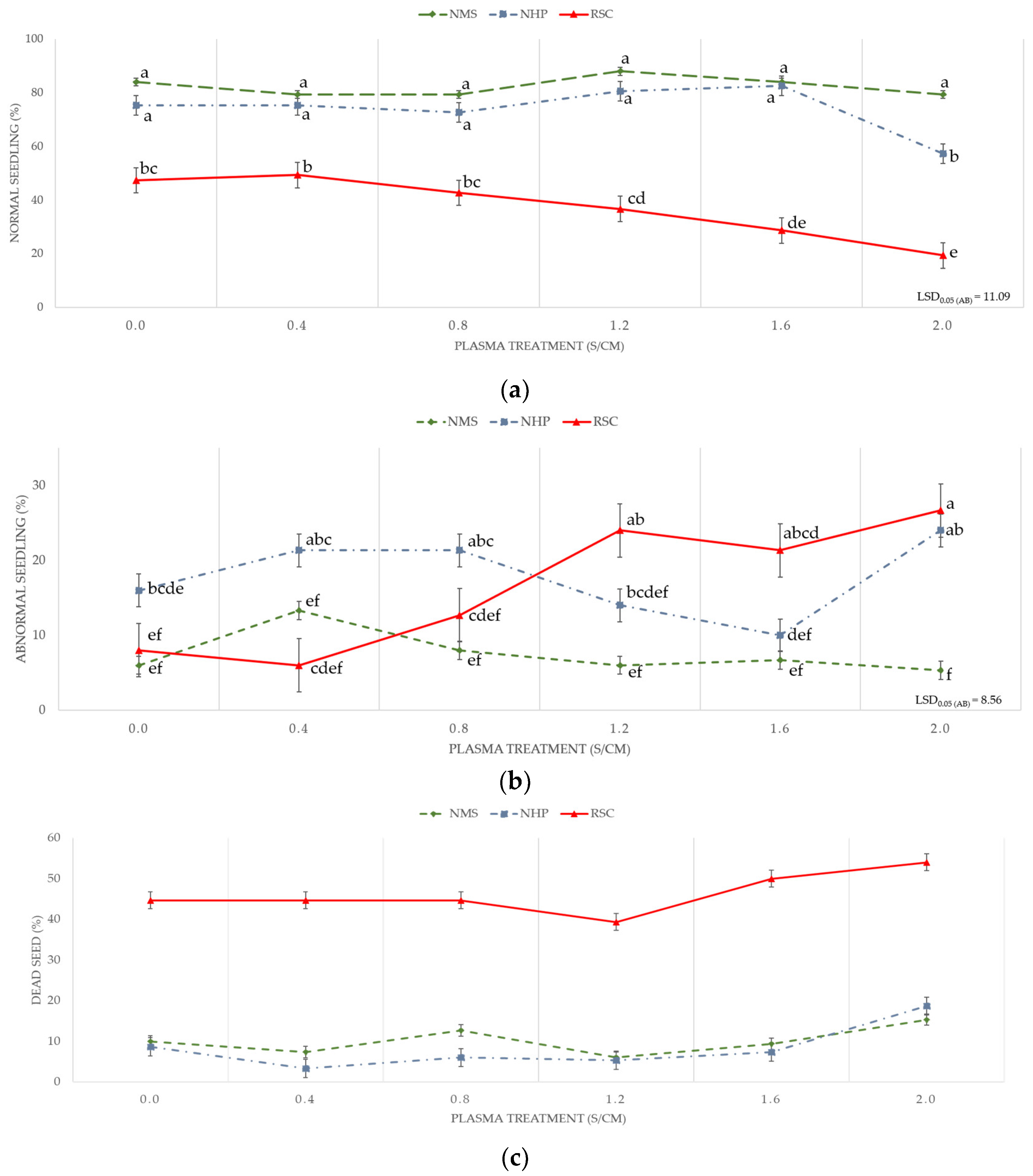
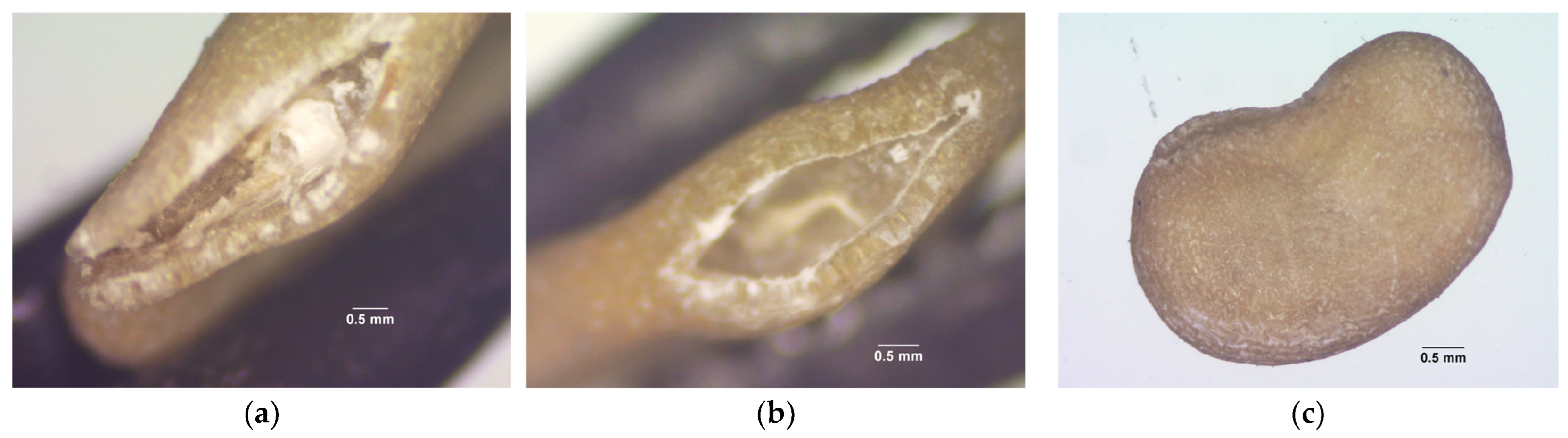
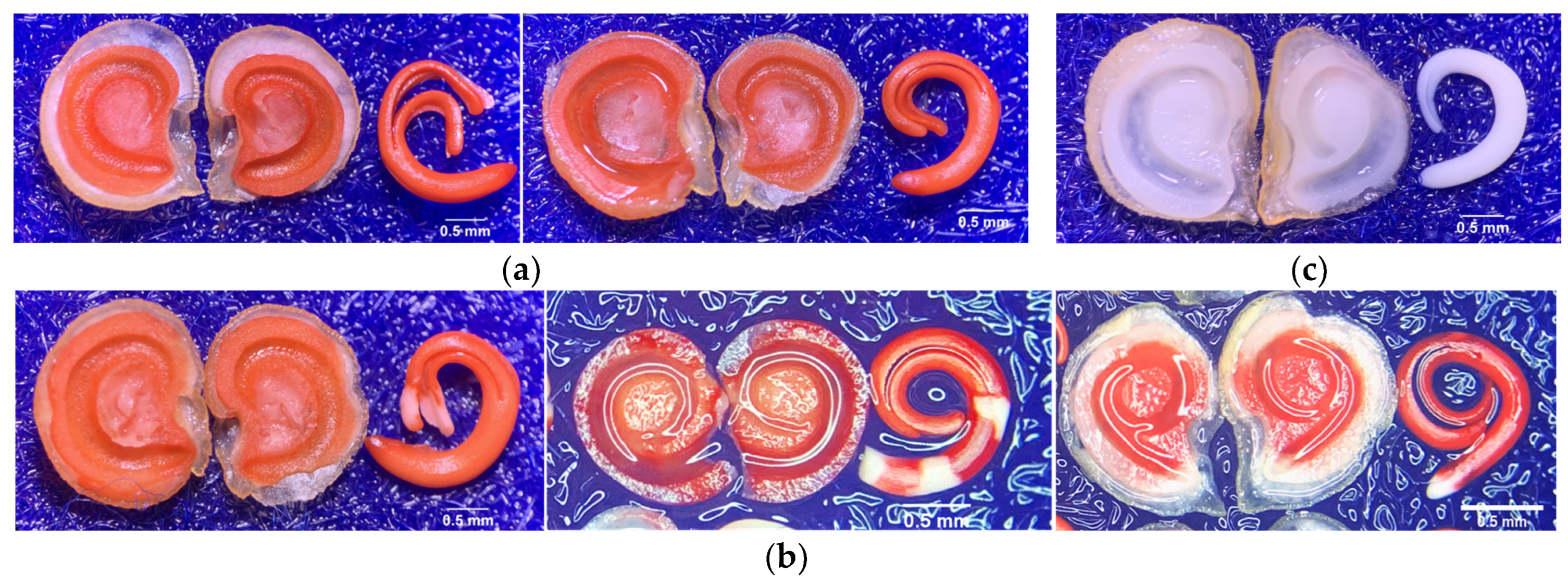
2. Results
3. Discussion
4. Materials and Methods
4.1. Seed Material and Preparation
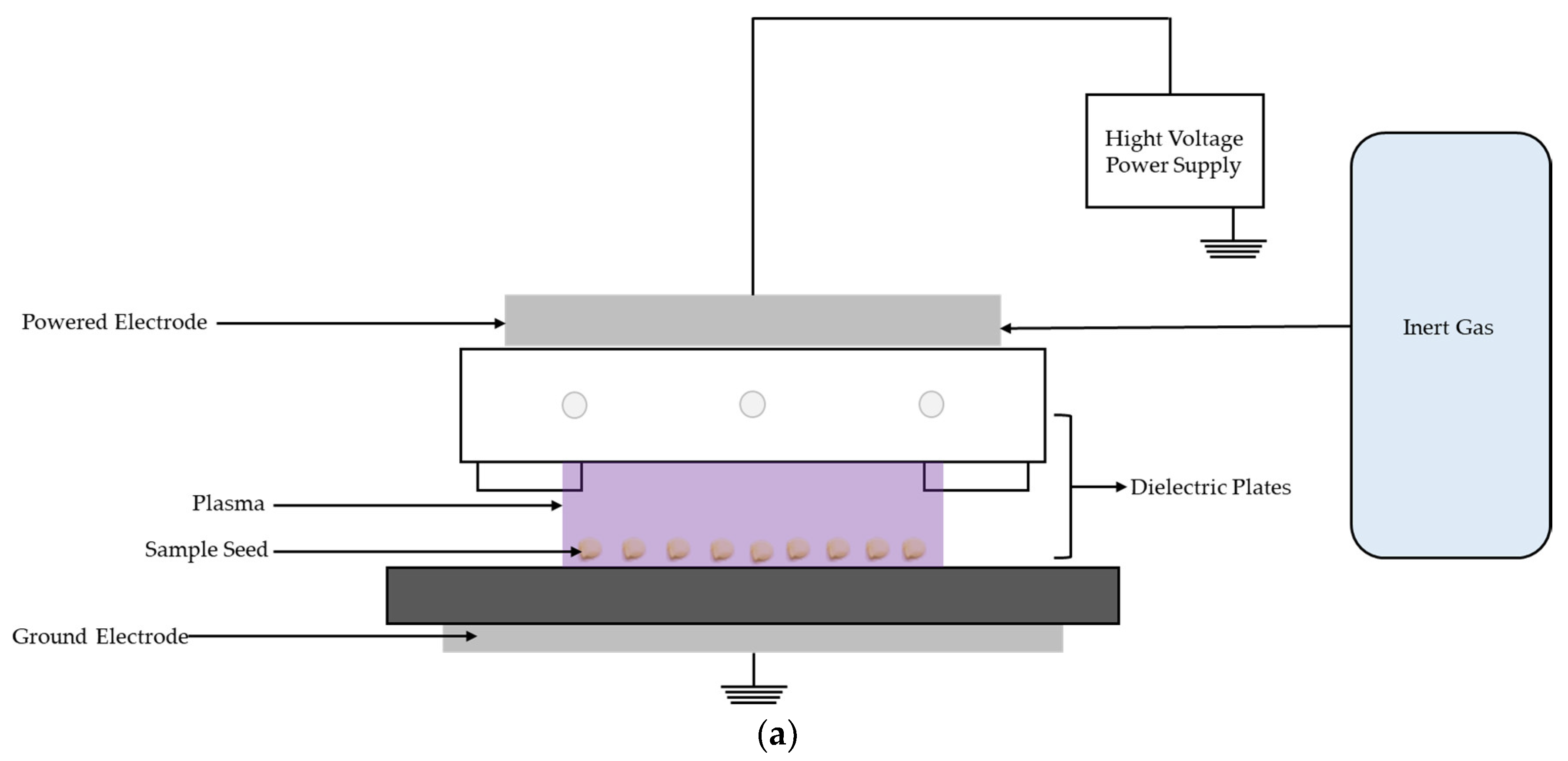
4.2. Characteristics of the DBD Plasma Device
4.3. Experimental Setup
4.4. PT
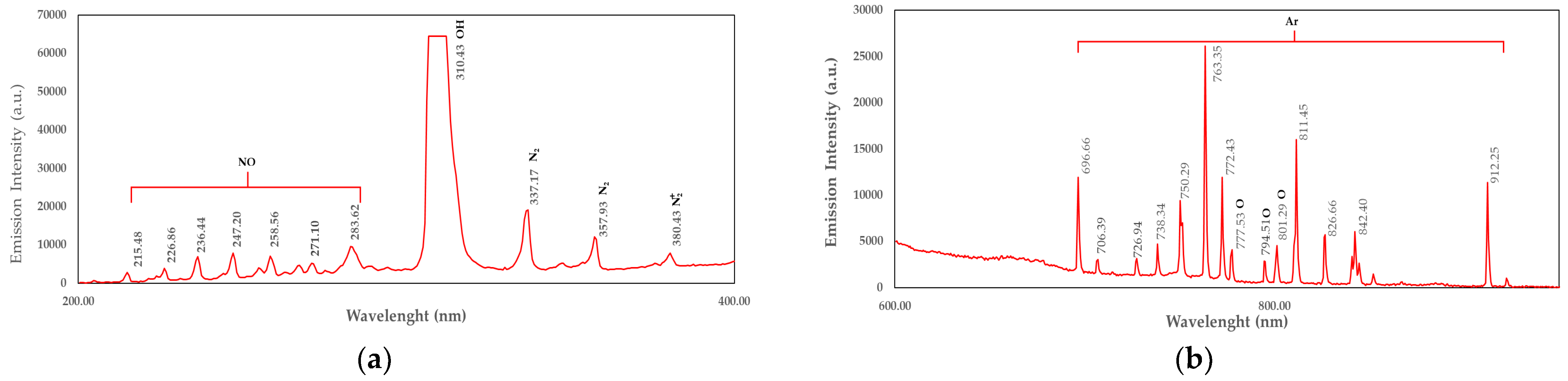
4.5. OES Measurement
4.6. Effect of Plasma Treatment to Pepper Seed Qualities
4.6.1. Biochemical Test for Viability
4.6.2. Germination Tests (Gs)
4.6.3. EC Test
4.6.4. RE Test
4.6.5. GI Test
4.6.6. SGT Test
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Modern Agriculture Program Group. 2022. Available online: https://www.nstda.or.th/home/mission_post/rdim/seed/ (accessed on 6 February 2025).
- Germains Seed Technology. Benefits of Seed Priming. Germains. Available online: https://germains.com/us/benefits-seed-priming/ (accessed on 13 March 2025).
- Marthandan, V.; Geetha, R.; Kumutha, K.; Renganathan, V.G.; Karthikeyan, A.; Ramalingam, J. Seed priming: A feasible strategy to enhance drought tolerance in crop plants. Int. J. Mol. Sci. 2020, 21, 8258. [Google Scholar] [CrossRef] [PubMed]
- Pyle, A.R. Techniques to Improve Seed Germination. J. W. Jung Seed Company. Available online: https://blog.jungseed.com/wp-content/uploads/2016/02/Techniques-to-Improve-Success-with-Seed-Germination.pdf (accessed on 13 March 2025).
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; CABI: Wallingford, UK, 2015; p. 761. [Google Scholar]
- Ardiarini, N.; Lase, J.A.; Hidayat, Y.; Habeahan, K.B. The effect of seed scarification on the germination process and the growth of long bean (Vigna sinensis) sprout. E3S Web Conf. 2021, 306, 01002. [Google Scholar] [CrossRef]
- Kermanshah, H.; Saeedi, R.; Ahmadi, E.; Ranjbar Omrani, L. Efficacy of cavity liners with/without atmospheric cold helium plasma jet for dentin remineralization. Biomater. Investig. Dent. 2020, 7, 120–125. [Google Scholar] [CrossRef]
- Abeysingha, D.N.; Dhaliwal, H.K.; Du, L.; De Silva, C.; Szczyglowski, K.; Roopesh, M.S.; Thilakarathna, M.S. The potential of cold plasma-based seed treatments in legume–rhizobia symbiotic nitrogen fixation: A review. Crops 2024, 4, 95–114. [Google Scholar] [CrossRef]
- Ling, L.; Jiafeng, J.; Jiangang, L.; Minchong, S.; Xin, H.; Hanliang, S.; Yuanhua, D. Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci. Rep. 2014, 4, 5859. [Google Scholar] [CrossRef]
- Varilla, C.; Marcone, M.; Annor, G.A. Potential of cold plasma technology in ensuring the safety of foods and agricultural produce: A review. Foods 2020, 9, 1435. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Kindel, E.; von Woedtke, T.; Hähnel, M.; Stieber, M.; Brandenburg, R. Atmospheric-pressure plasma sources: Prospective tools for plasma medicine. Pure Appl. Chem. 2010, 82, 1223–1237. [Google Scholar] [CrossRef]
- Tong, J.; He, R.; Zhang, X.; Zhan, R.; Chen, W.; Yang, S. Effects of atmospheric pressure air plasma pretreatment on the seed germination and early growth of Andrographis paniculata. Plasma Sci. Technol. 2014, 16, 260–266. [Google Scholar] [CrossRef]
- Waskow, A.; Howling, A.; Furno, I. Mechanisms of plasma-seed treatments as a potential seed processing technology. Front. Phys. 2021, 9, 617345. [Google Scholar] [CrossRef]
- Rajan, A.; Boopathy, B.; Radhakrishnan, M.; Rao, L.; Schlüter, O.K.; Tiwari, B.K. Plasma processing: A sustainable technology in agri-food processing. Sustain. Food Technol. 2023, 1, 9–49. [Google Scholar] [CrossRef]
- Konchekov, E.M.; Gusein-zade, N.; Burmistrov, D.E.; Kolik, L.V.; Dorokhov, A.S.; Izmailov, A.Y.; Shokri, B.; Gudkov, S.V. Advancements in plasma agriculture: A review of recent studies. Int. J. Mol. Sci. 2023, 24, 15093. [Google Scholar] [CrossRef] [PubMed]
- Pal, U.N.; Kumar, M.; Khatun, H.; Sharma, A.K. Discharge characteristics of dielectric barrier discharge (DBD) based VUV/UV sources. J. Phys. Conf. Ser. 2008, 114, 012065. [Google Scholar] [CrossRef]
- Ollegott, K.; Wirth, P.; Oberste-Beulmann, C.; Awakowicz, P.; Muhler, M. Fundamental properties and applications of dielectric barrier discharges in plasma-catalytic processes at atmospheric pressure. Chem. Ing. Tech. 2020, 92, 1542–1558. [Google Scholar] [CrossRef]
- Ongrak, P.; Poolyarat, N.; Suksaengpanomrung, S.; Saidarasamoot, K.; Jirakiattikul, Y.; Rithichai, P. Germination, physicochemical properties, and antioxidant enzyme activities in Kangkong (Ipomoea aquatica Forssk.) seeds as affected by dielectric barrier discharge plasma. Horticulturae 2023, 9, 1269. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.; Meng, Y.; Qu, G.; Sun, Q.; Liang, D.; Hu, S. Air atmospheric dielectric barrier discharge plasma induced germination and growth enhancement of wheat seed. Plasma Chem. Plasma Process. 2017, 37, 1621–1634. [Google Scholar] [CrossRef]
- Iranbakhsh, A.; Ghoranneviss, M.; Oraghi Ardebili, Z.; Oraghi Ardebili, N.; Hesami Tackallou, S.; Nikmaram, H. Non-thermal plasma modified growth and physiology in Triticum aestivum via generated signaling molecules and UV radiation. Biol. Plant. 2017, 61, 702–708. [Google Scholar] [CrossRef]
- El Shaer, M.; Abdel-azim, M.; El-welily, H.; Hussein, Y.; Abdelghani, A.; Zaki, A.; Mobasher, M. Effects of DBD direct air plasma and gliding arc indirect plasma activated mist on germination, and physiological parameters of rice seed. Plasma Chem. Plasma Process. 2023, 43, 1169–1193. [Google Scholar] [CrossRef]
- Rongsangchaicharean, T.; Srisonphan, S.; Onwimol, D. Responses of rice seed quality to large-scale atmospheric nonthermal plasmas. Plasma Chem. Plasma Process. 2022, 42, 1127–1141. [Google Scholar] [CrossRef]
- Gómez-Ramírez, A.; López-Santos, C.; Cantos, M.; García, J.L.; Molina, R.; Cotrino, J.; Espinós, J.P.; González-Elipe, A.R. Surface chemistry and germination improvement of Quinoa seeds subjected to plasma activation. Sci. Rep. 2017, 7, 5924. [Google Scholar] [CrossRef]
- Rithichai, P.; Jirakaittikul, Y.; Singhawiboon, M.; Poolyarat, N. Effect of dielectric barrier discharge plasma on growth and secondary metabolite contents of lettuce sprout. Thai Sci. Technol. J. (TSTJ) 2020, 28, 2001–2012. [Google Scholar]
- Rithichai, P.; Jirakaittikul, Y.; Chansri, S.; Suksaengpanomrung, S.; Poolyarat, N. Effect of dielectric barrier discharge plasma on seed sermination and seedling growth of chinese kale. Thai Sci. Technol. J. (TSTJ) 2023, 31, 40–49. [Google Scholar]
- Billah, M.; Sajib, S.A.; Roy, N.C.; Rashid, M.M.; Reza, M.A.; Hasan, M.M.; Talukder, M.R. Effects of DBD air plasma treatment on the enhancement of black gram (Vigna mungo L.) seed germination and growth. Arch. Biochem. Biophys. 2020, 681, 108253. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.H.; Choi, K.H.; Pengkit, A.; Im, J.S.; Kim, J.S.; Kim, Y.H.; Park, Y.; Hong, E.J.; Jung, S.K.; Choi, E.-H.; et al. Effects of high voltage nanosecond pulsed plasma and micro DBD plasma on seed germination, growth development and physiological activities in spinach. Arch. Biochem. Biophys. 2016, 605, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Le, T.Q.X.; Nguyen, L.N.; Nguyen, T.T.; Choi, E.H.; Nguyen, Q.L.; Kaushik, N.K.; Dao, N.T. Effects of cold plasma treatment on physical modification and endogenous hormone regulation in enhancing seed germination and radicle growth of mung bean. Appl. Sci. 2022, 12, 10308. [Google Scholar] [CrossRef]
- Ahmed, N.; Siow, K.S.; Wee, M.; Patra, A. A study to examine the ageing behaviour of cold plasma-treated agricultural seeds. Sci. Rep. 2023, 13, 1675. [Google Scholar] [CrossRef]
- Souza, F.H.; Marcos-Filho, J. The seed coat as a modulator of seed-environment relationships in Fabaceae. Brazil. J. Bot. 2001, 24, 365–375. [Google Scholar] [CrossRef]
- Byju’s. Difference Between Hilum and Micropyle. BYJU’S. Available online: https://byjus.com/neet/difference-between-hilum-and-micropyle/ (accessed on 29 March 2025).
- Maekawa, S.; Carpenter, W.J. Verbena seed hilum morphology contributes to irregular germination. HortScience 1991, 26, 129–132. [Google Scholar] [CrossRef]
- Ruggiero, A.; Landi, S.; Punzo, P.; Possenti, M.; Van Oosten, M.J.; Costa, A.; Morelli, G.; Maggio, A.; Grillo, S.; Batelli, G. Salinity and ABA seed responses in pepper: Expression and interaction of ABA core signaling components. Front. Plant Sci. 2019, 10, 304. [Google Scholar] [CrossRef]
- Zhao, Q.; Shi, X.; Yan, L.; Yang, C.; Liu, C.; Feng, Y.; Zhang, M.; Yang, Y.; Liao, H. Characterization of the common genetic basis underlying seed hilum size, yield, and quality traits in soybean. Front. Plant Sci. 2021, 12, 610214. [Google Scholar] [CrossRef]
- Asghar, A.H.; Galaly, A.R. The effect of oxygen admixture with argon discharges on the impact parameters of atmospheric pressure plasma jet characteristics. Appl. Sci. 2021, 11, 6870. [Google Scholar] [CrossRef]
- Jangra, S.; Mishra, A.; Mishra, R.; Pandey, S.; Prakash, R. Transformative impact of atmospheric cold plasma on mung bean seeds: Unveiling surface characteristics, physicochemical alterations, and enhanced germination potential. AIP Adv. 2024, 14, 075215. [Google Scholar] [CrossRef]
- Wojtyla, L.; Lechowska, K.; Kubala, S.; Garnczarska, M. Different modes of hydrogen peroxide action during seed germination. Front. Plant Sci. 2016, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Commey, L.; Tengey, T.K.; Cobos, C.J.; Dampanaboina, L.; Dhillon, K.K.; Pandey, M.K.; Sudini, H.K.; Falalou, H.; Varshney, R.K.; Burow, M.D.; et al. Peanut seed coat acts as a physical and biochemical barrier against Aspergillus flavus infection. J. Fungi 2021, 7, 1000. [Google Scholar] [CrossRef]
- Jing, D.; Zhang, Q.; Li, J. Plasma treatment enhances seed germination and vigor via modulation of aquaporin activity. Plant Physiol. Biochem. 2021, 160, 112–120. [Google Scholar]
- Montechiarini, N.H.; Morandi, E.N.; Gosparini, C.O. Developing soybean seed germination: Low ABA and high EXP1 gene expression promote embryonic axis growth whereas the seed coat delays radicle protrusion. Seed Sci. Res. 2022, 32, 23–33. [Google Scholar] [CrossRef]
- Favoretto, M.D.M.G.; Krzyzanowski, F.C.; Emrich, P.P.; Zucareli, C. Primary root emission and electrical conductivity test for wheat seed vigor evaluation. J. Seed Sci. 2024, 46, e202446032. [Google Scholar]
- Randeniya, L.K.; de Groot, G.J.J.B. Non-thermal plasma treatment of agricultural seeds for stimulation of germination, removal of surface contamination and other benefits: A review. Plasma Process. Polym. 2015, 12, 608–623. [Google Scholar] [CrossRef]
- Bormashenko, E.; Grynyov, R.; Bormashenko, Y.; Drori, E. Cold radiofrequency plasma treatment modifies wettability and germination speed of plant seeds. Sci. Rep. 2012, 2, 741. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Shen, M.; Zhang, C.; Dong, Y. Cold plasma treatment enhances oilseed rape seed germination under drought stress. Sci. Rep. 2015, 5, 13033. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, Y.; Li, J.; Li, L.; He, X.; Shao, H.; Dong, Y. Effect of seed treatment by cold plasma on the resistance of tomato to Ralstonia solanacearum (Bacterial Wilt). PLoS ONE 2014, 9, e97753. [Google Scholar] [CrossRef]
- França-Neto, J.d.B.; Krzyzanowski, F.C. Use of the tetrazolium test for estimating the physiological quality of seeds. Seed Sci. Technol. 2022, 50, 31–44. [Google Scholar] [CrossRef]
- Sivachandiran, L.; Khacef, A. Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: Combined effect of seed and water treatment. RSC Adv. 2017, 7, 1822–1832. [Google Scholar] [CrossRef]
- Priatama, R.A.; Pervitasari, A.N.; Park, S.; Park, S.J.; Lee, Y.K. Current advancements in the molecular mechanism of plasma treatment for seed germination and plant growth. Int. J. Mol. Sci. 2022, 23, 4609. [Google Scholar] [CrossRef] [PubMed]
- Song, J.S.; Kim, S.B.; Ryu, S.; Oh, J.; Kim, D.S. Emerging plasma technology that alleviates crop stress during the early growth stages of plants: A review. Front. Plant Sci. 2020, 11, 988. [Google Scholar] [CrossRef]
- Starič, P.; Vogel-Mikuš, K.; Mozetič, M.; Junkar, I. Effects of nonthermal plasma on morphology, genetics and physiology of seeds: A review. Plants 2020, 9, 1736. [Google Scholar] [CrossRef]
- Selcuk, M.; Oksuz, L.; Basaran, P. Decontamination of grains and legumes infected with Aspergillus spp. and Penicillum spp. by cold plasma treatment. Bioresour. Technol. 2008, 99, 5104–5109. [Google Scholar] [CrossRef]
- Thakur, N.; Vasudevan, S.N. Plasma treatment and seed quality advancement: A review. Agric. Rev. 2021, 42, 197–202. [Google Scholar] [CrossRef]
- Mohajer, M.H.; Khademi, A.; Rahmani, M.; Monfaredi, M.; Hamidi, A.; Mirjalili, M.H.; Ghomi, H. Optimizing beet seed germination via dielectric barrier discharge plasma parameters. Heliyon 2024, 10, e40020. [Google Scholar] [CrossRef]
- Johnson, A.J.; Geary, B.; Hulet, A.; Madsen, M.D. Fungicide Seed Coating Increases Emergence of Bluebunch Wheatgrass (Pseudoroegneria spicata) Under High-Fungal-Biomass Conditions. Plants 2025, 14, 679. [Google Scholar] [CrossRef]
- Kusumawardana, A.; Pujiasmanto, B.; Pardono, P. Short communication: Tetrazolium test for evaluating viability of Capsicum annum seeds. Nusant. Biosci. 2018, 10, 142–145. [Google Scholar] [CrossRef]
- Vidigal, D.D.S.; Dias, D.C.F.D.S.; Dias, L.A.D.S.; Finger, F.L. Changes in seed quality during fruit maturation of sweet pepper. Sci. Agric. 2011, 68, 535–539. [Google Scholar] [CrossRef]
- Don, R. ISTA Handbook on Seedling Evaluation, 4th ed.; The International Seed Testing Association: Bassersdorf, Switzerland, 2018. [Google Scholar]
- Gagliardi, B.; Marcos Filho, J. Assessment of the physiological potential of bell pepper seeds and relationship with seedling emergence. Rev. Bras. Sementes 2011, 33, 162–170. [Google Scholar] [CrossRef]
- International Seed Testing Association (ISTA). International Rules for Seed Testing, 2024th ed.; The International Seed Testing Association: Wallisellen, Switzerland, 2024. [Google Scholar]



| Factor | Quality Testing | ||||
|---|---|---|---|---|---|
| TZ (%) | |||||
| Viable | Non-Viable | ||||
| VG | NVG | ||||
| CDC | HDC | RDC | |||
| SC (A) | |||||
| NMS | 97 a | 0.22 b | 0.00 b | 0.22 b | 2 c |
| NHP | 95 b | 0.00 b | 0.00 b | 0.44 ab | 4 a |
| RSC | 94 c | 0.66 a | 1.44 a | 0.56 a | 3 b |
| F-test | ** | ** | ** | * | ** |
| PT (B) | |||||
| control | 99 a | 0.00 b | 0.00 c | 0.00 c | 1 d |
| 0.4 s/cm | 96 bc | 0.56 a | 0.00 c | 0.89 ab | 2 c |
| 0.8 s/cm | 97 b | 0.00 b | 0.67 b | 0.44 bc | 2 c |
| 1.2 s/cm | 97 b | 0.00 b | 0.00 c | 0.00 c | 3 b |
| 1.6 s/cm | 95 c | 0.00 b | 1.11 a | 0.00 c | 4 b |
| 2.0 s/cm | 88 d | 0.33 b | 1.11 a | 1.11 a | 9 a |
| F-test | ** | ** | ** | ** | ** |
| A × B | ** | ** | ** | ** | ** |
| CV (%) | 1.18 | 91.86 | 71.50 | 136.91 | 29.34 |
| LSD0.05(A) | 0.90 | 0.25 | 0.50 | 0.25 | 0.33 |
| LSD0.05(B) | 1.08 | 0.26 | 0.33 | 0.54 | 0.48 |
| Factor | Quality Testing | |||||||
|---|---|---|---|---|---|---|---|---|
| Germination (G) (%) | Seed Vigor | |||||||
| G1st | GFinal | EC (μS cm−1 g−1) | RE (%) | GI | SGT (cm) | |||
| 7th | 14th | Root | Shoot | |||||
| SC (A) | ||||||||
| NMS | 55 a | 82 a | 673.88 a | 55.56 a | 5.45 b | 28.14 a | 2.09 b | 3.67 a |
| NHP | 55 a | 74 a | 741.48 a | 55.33 a | 7.56 a | 29.42 a | 2.31 a | 3.59 a |
| RSC | 15 b | 37 b | 256.77 b | 23.00 b | 1.70 c | 11.84 b | 2.09 b | 2.71 b |
| F-test | ** | ** | ** | ** | ** | ** | * | ** |
| PT (B) | ||||||||
| control | 40 bc | 69 a | 518.13 bc | 42.44 c | 4.93 ab | 23.67 b | 2.81 a | 3.39 ab |
| 0.4 s/cm | 41 b | 68 a | 465.18 c | 41.78 c | 5.27 ab | 23.49 b | 2.42 b | 3.51 a |
| 0.8 s/cm | 42 b | 65 a | 536.54 bc | 41.78 c | 5.14 ab | 23.53 b | 2.19 b | 3.53 a |
| 1.2 s/cm | 50 a | 68 a | 622.12 a | 54.00 a | 5.78 a | 26.03 a | 1.83 c | 3.16 bc |
| 1.6 s/cm | 44 b | 65 a | 619.34 a | 48.00 b | 4.72 b | 23.46 b | 2.17 b | 3.32 abc |
| 2.0 s/cm | 35 c | 52 b | 582.96 ab | 39.78 c | 3.58 c | 18.65 c | 1.58 c | 3.02 c |
| F-test | ** | ** | ** | ** | ** | ** | ** | * |
| A × B | ** | ** | ns | ** | * | ** | ** | ** |
| CV (%) | 12.58 | 10.30 | 14.67 | 10.95 | 21.11 | 7.94 | 12.92 | 9.36 |
| LSD0.05(A) | 6.10 | 12.52 | 100.78 | 7.48 | 1.20 | 4.00 | 0.17 | 0.39 |
| LSD0.05(B) | 5.07 | 6.40 | 78.72 | 4.71 | 1.00 | 1.77 | 0.27 | 0.30 |
| Factor | Total AS | Abnormal Seedlings Type (AS) | Dead | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Seedling as a Whole | Root System | Shoot System | |||||||||
| SD | SDPI | SDSI | SR | PSS | PTS | PM | CD | HD | |||
| SC (A) | |||||||||||
| NMS | 8 | 0.11 | 0.00 | 0.00 b | 0.00 b | 6.33 | 1.00 b | 0.11 | 0.00 | 0.00 | 10 b |
| NHP | 18 | 0.67 | 0.33 | 0.44 b | 1.22 a | 5.56 | 7.44 a | 2.00 | 0.00 | 0.11 | 8 b |
| RSC | 16 | 1.00 | 0.00 | 2.56 a | 0.11 b | 8.00 | 3.00 ab | 0.78 | 0.33 | 0.67 | 46 a |
| F-test | ns | ns | ns | ** | ** | ns | * | ns | ns | ns | ** |
| PT (B) | |||||||||||
| control | 10 c | 0.00 | 0.00 | 1.11 | 0.00 b | 3.33 | 5.33 | 0.00 b | 0.22 | 0.00 | 21 b |
| 0.4 s/cm | 14 b | 0.44 | 0.00 | 0.00 | 0.00 b | 7.56 | 5.33 | 0.00 b | 0.00 | 0.22 | 18 b |
| 0.8 s/cm | 14 ab | 0.44 | 0.22 | 0.67 | 0.22 b | 7.33 | 4.22 | 0.67 b | 0.22 | 0.00 | 21 b |
| 1.2 s/cm | 15 ab | 0.44 | 0.44 | 1.56 | 0.00 b | 8.67 | 3.33 | 0.00 b | 0.00 | 0.22 | 17 b |
| 1.6 s/cm | 13 b | 1.56 | 0.00 | 0.44 | 0.22 b | 5.56 | 2.22 | 2.00 a | 0.22 | 0.44 | 22 b |
| 2.0 s/cm | 19 a | 0.67 | 0.00 | 0.74 | 2.22 a | 7.33 | 2.44 | 3.11 a | 0.00 | 0.67 | 29 a |
| F-test | * | ns | ns | ns | ** | ns | ns | ** | ns | ns | ** |
| A × B | ** | ns | ns | ns | ** | ** | ns | ** | ns | ns | ns |
| CV (%) | 36.86 | 235.09 | 502.00 | 240.68 | 109.54 | 55.41 | 82.65 | 133.77 | 424.26 | 369.67 | 30.93 |
| LSD0.05(A) | - | - | - | 1.15 | 0.31 | - | 5.17 | - | - | - | 8.32 |
| LSD0.05(B) | 4.94 | - | - | - | 0.47 | - | - | 1.24 | - | - | 6.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sriruksa, C.; Sawangrat, C.; Sansongsiri, S.; Boonyawan, D.; Thanapornpoonpong, S.-n. Influence of Seed Coat Integrity on the Response of Pepper Seeds to Dielectric Barrier Discharge Plasma Treatment. Plants 2025, 14, 1938. https://doi.org/10.3390/plants14131938
Sriruksa C, Sawangrat C, Sansongsiri S, Boonyawan D, Thanapornpoonpong S-n. Influence of Seed Coat Integrity on the Response of Pepper Seeds to Dielectric Barrier Discharge Plasma Treatment. Plants. 2025; 14(13):1938. https://doi.org/10.3390/plants14131938
Chicago/Turabian StyleSriruksa, Chanyanuch, Choncharoen Sawangrat, Sakon Sansongsiri, Dheerawan Boonyawan, and Sa-nguansak Thanapornpoonpong. 2025. "Influence of Seed Coat Integrity on the Response of Pepper Seeds to Dielectric Barrier Discharge Plasma Treatment" Plants 14, no. 13: 1938. https://doi.org/10.3390/plants14131938
APA StyleSriruksa, C., Sawangrat, C., Sansongsiri, S., Boonyawan, D., & Thanapornpoonpong, S.-n. (2025). Influence of Seed Coat Integrity on the Response of Pepper Seeds to Dielectric Barrier Discharge Plasma Treatment. Plants, 14(13), 1938. https://doi.org/10.3390/plants14131938






