Non-Invasive Micro-Test Technology in Plant Physiology Under Abiotic Stress: From Mechanism to Application
Abstract
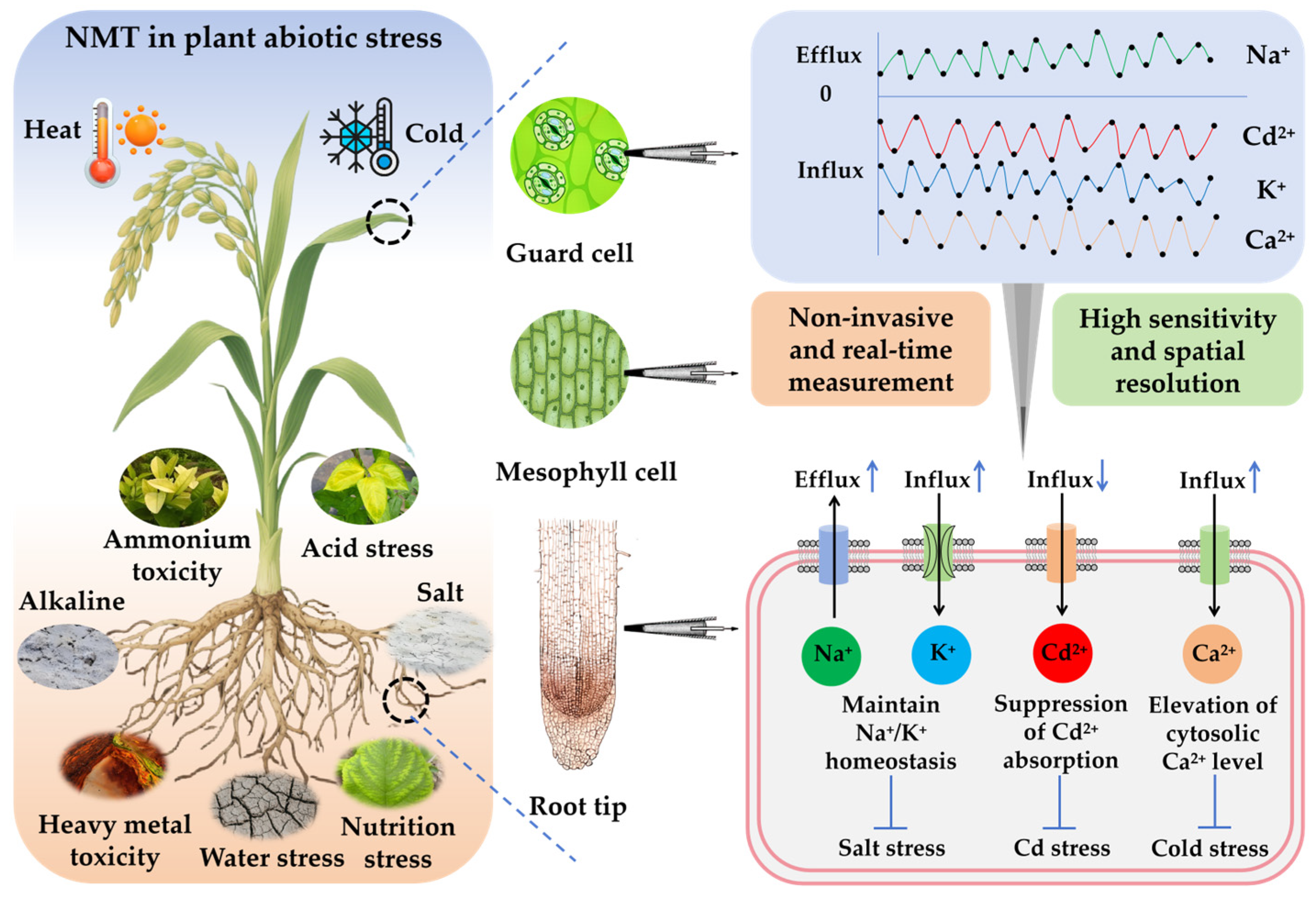
1. Introduction
2. An Overview of NMT
2.1. Technical Principles
2.2. Features, Advantages, and Application Fields
3. NMT in Plant Physiology Under Abiotic Stress
3.1. Salt Stress
3.2. Alkali Stress
3.3. Water Stress
3.4. Low- and High-Temperature Stress
3.5. Nutrition Stress
3.6. Ammonium Toxicity and Acid Stress
3.7. Heavy Metal Toxicity
4. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.Y.; Li, J.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M. Molecular mechanisms of plant abiotic stress tolerance. Int. J. Mol. Sci. 2025, 26, 2731. [Google Scholar] [CrossRef] [PubMed]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Salehin, M. Emerging roles of auxin in plant abiotic stress tolerance. Physiol. Plant. 2024, 176, e14601. [Google Scholar] [CrossRef]
- Michard, E.; Lima, P.T.; Borges, F.; Silva, A.C.; Portes, M.T.; Carvalho, J.E.; Gilliham, M.; Liu, L.H.; Obermeyer, G.; Feijó, J.A. Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 2011, 332, 434–437. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D.; et al. COLD1 confers chilling tolerance in rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef]
- Li, L.; Verstraeten, I.; Roosjen, M.; Takahashi, K.; Rodriguez, L.; Merrin, J.; Chen, J.; Shabala, L.; Smet, W.; Ren, H.; et al. Cell surface and intracellular auxin signalling for H+ fluxes in root growth. Nature 2021, 599, 273–277. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Ye, N. Noninvasive micro-test technology: Monitoring ion and molecular flow in plants. Trends Plant Sci. 2023, 28, 123–124. [Google Scholar] [CrossRef]
- Thor, K.; Jiang, S.; Michard, E.; George, J.; Scherzer, S.; Huang, S.; Dindas, J.; Derbyshire, P.; Leitão, N.; DeFalco, T.A.; et al. The calcium-permeable channel OSCA1.3 regulates plant stomatal immunity. Nature 2020, 585, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Wang, Y.; Li, Y.; Zhu, R.; Gu, Y.; Li, J.; Guo, H.; Ye, W.; Nabi, H.G.; Yang, T.; et al. The OsNAC41-RoLe1-OsAGAP module promotes root development and drought resistance in upland rice. Mol. Plant 2024, 17, 1573–1593. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Xu, Y.; Cao, J.; Guo, X.; Han, J.; Zhang, Y.; Niu, Y.; Zhang, M.; Wang, Y.; Liang, G.; et al. COLD6-OSM1 module senses chilling for cold tolerance via 2’,3’-cAMP signaling in rice. Mol. Cell 2024, 84, 4224–4238.e9. [Google Scholar] [CrossRef]
- Kunkel, J.G.; Cordeiro, S.; Xu, Y.; Shipley, A.M.; Feijo, J.A. The use of non-invasive ion-selective microelectrode techniques for the study of plant development. In Plant Electrophysiology-Theory and Methods; Volkov, A.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Chapter V; pp. 109–137. [Google Scholar] [CrossRef]
- Shabala, S. Non-invasive microelectrode ion flux measurements in plant stress physiology. In Plant Electrophysiology; Volkov, A.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; Chapter III; pp. 35–71. [Google Scholar] [CrossRef]
- Newman, I.A.; Kochian, L.V.; Grusak, M.A.; Lucas, W.J. Fluxes of H+ and K+ in corn roots: Characterization and stoichiometries using ion-selective microelectrodes. Plant Physiol. 1987, 84, 1177–1184. [Google Scholar] [CrossRef]
- Kühtreiber, W.M.; Jaffe, L.F. Detection of extracellular calcium gradients with a calcium-specific vibrating electrode. J. Cell Biol. 1990, 110, 1565–1573. [Google Scholar] [CrossRef]
- Jaffe, L.F. Fast calcium waves. Cell Calcium 2010, 48, 102–113. [Google Scholar] [CrossRef]
- Smith, P.J. Non-invasive ion probes-tools for measuring transmembrane ion flux. Nature 1995, 378, 645–646. [Google Scholar] [CrossRef]
- ISO 9001:2015; Quality Management Systems–Requirements. International Organization for Standardization: Geneva, Switzerland, 2015.
- Sun, K.; Liu, Y.; Pan, Y.; Di, D.; Li, J.; Xu, F.; Li, L.; Mimata, Y.; Chen, Y.; Xie, L.; et al. Non-invasive micro-test technology and applications. Biophys. Rep. 2025, 11, 96–111. [Google Scholar] [CrossRef]
- Han, M.; Yang, H.; Yu, G.; Jiang, P.; You, S.; Zhang, L.; Lin, H.; Liu, J.; Shu, Y. Application of Non-invasive Micro-test Technology (NMT) in environmental fields: A comprehensive review. Ecotoxicol. Environ. Saf. 2022, 240, 113706. [Google Scholar] [CrossRef]
- Jiang, W.; You, S.; Shi, Y.; Jiang, P.; Chen, M.; Yang, X. Application progress of Non-invasive Micro-test Technology in environmental bioremediation and stress physiology research. J. Plant Interact. 2023, 18, 2268131. [Google Scholar] [CrossRef]
- Li, L.Z.; Yu, S.Y.; Peijnenburg, W.J.G.M.; Luo, Y.M. Determining the fluxes of ions (Pb2+, Cu2+ and Cd2+) at the root surface of wetland plants using the scanning ion-selective electrode technique. Plant Soil 2017, 414, 1–12. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, T.; Yin, L.P. Application of non-invasive microsensing system to simultaneously measure both H+ and O2 fluxes around the pollen tube. J. Integr. Plant Biol. 2006, 48, 823–831. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, H.; Shabala, S.; Li, H.; Yang, X.; Zhang, H. Tissue tolerance mechanisms conferring salinity tolerance in a halophytic perennial species Nitraria sibirica Pall. Tree Physiol. 2021, 41, 1264–1277. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yao, L.; Li, B.; Ma, X.; Si, E.; Yang, K.; Zhang, H.; Meng, Y.; Wang, J.; Wang, H. HgS2, a novel salt-responsive gene from the Halophyte Halogeton glomeratus, confers salt tolerance in transgenic Arabidopsis. Physiol. Plant. 2024, 176, e14356. [Google Scholar] [CrossRef]
- Stéger, A.; Hayashi, M.; Lauritzen, E.W.; Herburger, K.; Shabala, L.; Wang, C.; Bendtsen, A.K.; Nørrevang, A.F.; Madriz-Ordeñana, K.; Ren, S.; et al. The evolution of plant proton pump regulation via the R domain may have facilitated plant terrestrialization. Commun. Biol. 2022, 5, 1312. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, D.; Gao, M.; Wu, Y.; Zhai, L.; Sun, S.; Wu, T.; Zhang, X.; Xu, X.; Han, Z.; et al. MxMPK6-2-mediated phosphorylation enhances the response of apple rootstocks to Fe deficiency by activating PM H+-ATPase MxHA2. Plant J. 2023, 116, 69–86. [Google Scholar] [CrossRef]
- Newman, I.A. Ion transport in roots: Measurement of fluxes using ion-selective microelectrodes to characterize transporter function. Plant Cell Environ. 2001, 24, 1–14. [Google Scholar] [CrossRef]
- Jing, H.; Wilkinson, E.G.; Sageman-Furnas, K.; Strader, L.C. Auxin and abiotic stress responses. J. Exp. Bot. 2023, 74, 7000–7014. [Google Scholar] [CrossRef]
- Xu, H.; Chen, H.; Halford, N.G.; Xu, R.; He, T.; Yang, B.; Zhou, L.; Guo, H.; Liu, C. Ion homeostasis and coordinated salt tolerance mechanisms in a barley (Hordeum vulgare L.) doubled haploid line. BMC Plant Biol. 2025, 25, 52. [Google Scholar] [CrossRef]
- Kou, Y.; Su, B.; Yang, S.; Gong, W.; Zhang, X.; Shan, X. Phosphorylation of Arabidopsis NRT1.1 regulates plant stomatal aperture and drought resistance in low nitrate condition. BMC Plant Biol. 2025, 25, 95. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Xie, T.; Shen, J.; Liang, T.; Yin, L.; Liu, K.; He, Y.; Chen, M.; Tang, H.; Chen, S.; et al. Potassium transporter OsHAK9 regulates seed germination under salt stress by preventing gibberellin degradation through mediating OsGA2ox7 in rice. J. Integr. Plant Biol. 2024, 66, 731–748. [Google Scholar] [CrossRef]
- Bazihizina, N.; Vita, F.; Balestrini, R.; Kiferle, C.; Caparrotta, S.; Ghignone, S.; Atzori, G.; Mancuso, S.; Shabala, S. Early signalling processes in roots play a crucial role in the differential salt tolerance in contrasting Chenopodium quinoa accessions. J. Exp. Bot. 2022, 73, 292–306. [Google Scholar] [CrossRef]
- Tanveer, M.; Wang, L.; Huang, L.; Zhou, M.; Chen, Z.H.; Shabala, S. Understanding mechanisms for differential salinity tissue tolerance between quinoa and spinach: Zooming on ros-inducible ion channels. Crop J. 2024, 12, 1357–1368. [Google Scholar] [CrossRef]
- Rasouli, F.; Yun, P.; Kiani-Pouya, A.; Movahedi, A.; Rasouli, M.; Salehi, M.; Shabala, S. One size does not fit all: Different strategies employed by triticale and barley plants to deal with soil salinity. Environ. Exp. Bot. 2024, 218, 105585. [Google Scholar] [CrossRef]
- Wei, S.; Chen, M.; Wang, F.; Tu, Y.; Xu, Y.; Fu, L.; Zeng, F.; Zhang, G.; Wu, D.; Shen, Q. OsCaM1-1 is responsible for salt tolerance by regulating Na+/K+ homoeostasis in rice. Plant Cell Environ. 2025, 48, 1393–1408. [Google Scholar] [CrossRef]
- Lou, L.; Yu, F.; Tian, M.; Liu, G.; Wu, Y.; Wu, Y.; Xia, R.; Pardo, J.M.; Guo, Y.; Xie, Q. ESCRT-I component VPS23A sustains salt tolerance by strengthening the SOS module in Arabidopsis. Mol. Plant 2020, 13, 1134–1148. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, C.; Xue, Y.; Liu, X.; Chen, S.; Song, C.; Yang, Y.; Guo, Y. Calcium-activated 14-3-3 proteins as a molecular switch in salt stress tolerance. Nat. Commun. 2019, 10, 1199. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, K.; Yao, J.; Zhao, Z.; Liu, Z.; Yan, C.; Zhang, Y.; Liu, J.; Li, J.; Zhao, N.; et al. Populus euphratica GLABRA3 binds PLDδ promoters to enhance salt tolerance. Int. J. Mol. Sci. 2023, 24, 8208. [Google Scholar] [CrossRef]
- Li, J.; Zhao, R.; Liu, J.; Yao, J.; Ma, S.; Yin, K.; Zhang, Y.; Liu, Z.; Yan, C.; Zhao, N.; et al. Populus euphratica GRP2 interacts with target mRNAs to negatively regulate salt tolerance by interfering with photosynthesis, Na+, and ROS homeostasis. Int. J. Mol. Sci. 2024, 25, 2046. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liu, X.; Meng, F.; Jiang, A.; Zhou, Y.; Yuan, F.; Chen, M. LbHKT1;1 negatively regulates salt tolerance of Limonium bicolor by decreasing salt secretion rate of salt glands. Plant Cell Environ. 2025, 48, 3544–3558. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Gao, S.; Li, P.; Zhang, Y.; Ma, B.; Wang, Y. Exogenous methyl jasmonate promotes salt stress-induced growth inhibition and prioritizes defense response of Nitraria tangutorum Bobr. Physiol. Plant. 2021, 172, 162–175. [Google Scholar] [CrossRef]
- Dong, X.; Sun, L.; Guo, J.; Liu, L.; Wang, B. Exogenous boron alleviates growth inhibition by NaCl stress by reducing Cl uptake in sugar beet (Beta vulgaris). Plant Soil 2021, 464, 423–439. [Google Scholar] [CrossRef]
- Qu, M.; Havshøi, N.W.; Huang, X.; Shabala, L.; Yu, M.; Fuglsang, A.T.; Shabala, S. Understanding the mechanistic basis of ameliorative effects of boron on salinity in barley (Hordeum vulgare). Environ. Exp. Bot. 2024, 220, 105690. [Google Scholar] [CrossRef]
- Huang, H.; Zhuang, L.; Tang, H.; Guo, Z.; Li, Q.; Lin, Z.; Dai, M.; Wang, X.; Wang, Y.; Zheng, H.; et al. Biosynthesis-based spatial metabolome of condensed tannin reveals its role in salt tolerance of non-salt-secretor mangrove Kandelia obovata. Plant Cell Environ. 2025, 48, 1874–1889. [Google Scholar] [CrossRef]
- Wu, X.; Li, J.; Song, L.Y.; Zeng, L.L.; Guo, Z., J.; Ma, D.N.; Wei, M.Y.; Zhang, L.D.; Wang, X.X.; Zheng, H.L. NADPH oxidase-dependent H2O2 production mediates salicylic acid-induced salt tolerance in mangrove plant Kandelia obovata by regulating Na+/K+ and redox homeostasis. Plant J. 2024, 118, 1119–1135. [Google Scholar] [CrossRef]
- Zeng, L.L.; Song, L.Y.; Wu, X.; Ma, D.N.; Song, S.W.; Wang, X.X.; Zheng, H.L. Brassinosteroid enhances salt tolerance via S-nitrosoglutathione reductase and nitric oxide signaling pathway in mangrove Kandelia obovata. Plant Cell Environ. 2024, 47, 511–526. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, M.; Liang, X.; Li, F.; Shi, Y.; Yang, X.; Jiang, C. Natural variation of an EF-hand Ca2+-binding-protein coding gene confers saline-alkaline tolerance in maize. Nat. Commun. 2020, 11, 186. [Google Scholar] [CrossRef]
- Xie, Q.; Yang, Y.; Wang, Y.; Pan, C.; Hong, S.; Wu, Z.; Song, J.; Zhou, Y.; Jiang, X. The calcium sensor CBL10 negatively regulates plasma membrane H+-ATPase activity and alkaline stress response in Arabidopsis. Environ. Exp. Bot. 2022, 194, 104752. [Google Scholar] [CrossRef]
- Cui, M.; Li, Y.; Li, J.; Yin, F.; Chen, X.; Qin, L.; Wei, L.; Xia, G.; Liu, S. Ca2+-dependent TaCCD1 cooperates with TaSAUR215 to enhance plasma membrane H+-ATPase activity and alkali stress tolerance by inhibiting PP2C-mediated dephosphorylation of TaHA2 in wheat. Mol. Plant 2023, 16, 571–587. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Zhan, Y.; Mu, Y.; Zhang, J.; Xu, W. MNSs-mediated N-glycan processing is essential for auxin homeostasis in Arabidopsis roots during alkaline response. iScience 2022, 25, 104298. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Sun, L.; Wang, W.; Zhang, F. Exogenous calcium application mediates K+ and Na+ homeostasis of different salt-tolerant rapeseed varieties under NaHCO3 stress. Plant Growth Regul. 2024, 102, 367–378. [Google Scholar] [CrossRef]
- Xu, W.; Jia, L.; Shi, W.; Liang, J.; Zhou, F.; Li, Q.; Zhang, J. Abscisic acid accumulation modulates auxin transport in the root tip to enhance proton secretion for maintaining root growth under moderate water stress. New Phytol. 2013, 197, 139–150. [Google Scholar] [CrossRef]
- Mak, M.; Babla, M.; Xu, S.C.; O’Carrigan, A.; Liu, X.H.; Gong, Y.M.; Holford, P.; Chen, Z.H. Leaf mesophyll K+, H+ and Ca2+ fluxes are involved in drought-induced decrease in photosynthesis and stomatal closure in soybean. Environ. Exp. Bot. 2014, 98, 1–12. [Google Scholar] [CrossRef]
- Feng, X.; Liu, W.; Qiu, C.W.; Zeng, F.; Wang, Y.; Zhang, G.; Chen, Z.H.; Wu, F. HvAKT2 and HvHAK1 confer drought tolerance in barley through enhanced leaf mesophyll H+ homoeostasis. Plant Biotechnol. J. 2020, 18, 1683–1696. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, H.; Chen, J.; Chen, L.; Chang, N.; Ge, G.; Wang, X. Higher ROS scavenging ability and plasma membrane H+-ATPase activity are associated with potassium retention in drought tolerant tea plants. J. Plant Nutr. Soil Sci. 2020, 183, 406–415. [Google Scholar] [CrossRef]
- Sun, W.; Xia, L.; Deng, J.; Sun, S.; Yue, D.; You, J.; Wang, M.; Jin, S.; Zhu, L.; Lindsey, K.; et al. Evolution and subfunctionalization of CIPK6 homologous genes in regulating cotton drought resistance. Nat. Commun. 2024, 15, 5733. [Google Scholar] [CrossRef]
- Huang, X.; Shabala, L.; Zhang, X.; Zhou, M.; Voesenek, L.A.C.J.; Hartman, S.; Yu, M.; Shabala, S. Cation transporters in cell fate determination and plant adaptive responses to a low-oxygen environment. J. Exp. Bot. 2022, 73, 636–645. [Google Scholar] [CrossRef]
- Liu, C.Y.; Zhang, F.; Zhang, D.J.; Srivastava, A.K.; Wu, Q.S.; Zou, Y.N. Mycorrhiza stimulates root-hair growth and IAA synthesis and transport in trifoliate orange under drought stress. Sci. Rep. 2018, 8, 1978. [Google Scholar] [CrossRef]
- Li, B.; Zhang, M.; Sun, W.; Yue, D.; Ma, Y.; Zhang, B.; Duan, L.; Wang, M.; Lindsey, K.; Nie, X.; et al. N6-methyladenosine RNA modification regulates cotton drought response in a Ca2+ and ABA-dependent manner. Plant Biotechnol. J. 2023, 21, 1270–1285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, L.; Zhang, L.; Kong, X.; Zhang, J.; Wang, X.; Pei, Y.; Jin, Z. H2S-mediated balance regulation of stomatal and non-stomatal factors responding to drought stress in Chinese cabbage. Hortic. Res. 2022, 10, uhac284. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Guo, Y.; Li, J.; Liu, L.; Liu, J.; Yuan, L.; Wei, C.; Ma, J.; Zhang, Y.; Ahammed, G.J.; et al. Cyclic nucleotide-gated ion channel 20 regulates melatonin-induced calcium signaling and cold tolerance in watermelon. Plant Physiol. 2024, 26, kiae630. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, X.; Xu, Y.; Li, H.; Ma, L.; Yao, X.; Weng, Y.; Guo, Y.; Liu, C.M.; Chong, K. OsCIPK7 point-mutation leads to conformation and kinase-activity change for sensing cold response. J. Integr. Plant Biol. 2019, 61, 1194–1200. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Y.; Liu, X.; Luo, S.; Zhang, X.; Liu, X.; Lin, Q.; Zhu, S.; Wan, H.; Yang, Y.; et al. Transcriptional activation and phosphorylation of OsCNGC9 confer enhanced chilling tolerance in rice. Mol. Plant 2021, 14, 315–329. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, M.; Ma, S.; Feng, Q.; Wang, Y.; Di, Q.; Zhou, M.; He, C.; Li, Y.; Gao, L.; et al. Mechanism of CsGPA1 in regulating cold tolerance of cucumber. Hortic. Res. 2022, 9, uhac109. [Google Scholar] [CrossRef]
- Zimmermann, M.J.; Bose, J.; Kramer, E.M.; Atkin, O.K.; Tyerman, S.D.; Baskin, T.I. Oxygen uptake rates have contrasting responses to temperature in the root meristem and elongation zone. Physiol. Plant. 2022, 174, e13682. [Google Scholar] [CrossRef]
- Guo, Z.; Zuo, Y.; Wang, S.; Zhang, X.; Wang, Z.; Liu, Y.; Shen, Y. Early signaling enhance heat tolerance in Arabidopsis through modulating jasmonic acid synthesis mediated by HSFA2. Int. J. Biol. Macromol. 2024, 267, 131256. [Google Scholar] [CrossRef]
- Chen, F.; Dong, G.; Wang, F.; Shi, Y.; Zhu, J.; Zhang, Y.; Ruan, B.; Wu, Y.; Feng, X.; Zhao, C.; et al. A β-ketoacyl carrier protein reductase confers heat tolerance via the regulation of fatty acid biosynthesis and stress signaling in rice. New Phytol. 2021, 232, 655–672. [Google Scholar] [CrossRef]
- Huang, H.; Han, Y.; Hao, C.F.S. Exogenous spermidine modulates osmoregulatory substances and leaf stomata to alleviate the damage to lettuce seedlings caused by high temperature stress. J. Plant Growth Regul. 2023, 42, 1236–1255. [Google Scholar] [CrossRef]
- Kan, Y.; Mu, X.R.; Zhang, H.; Gao, J.; Shan, J.X.; Ye, W.W.; Lin, H.X. TT2 controls rice thermotolerance through SCT1-dependent alteration of wax biosynthesis. Nat. Plants 2022, 8, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chen, P.; Liu, P.; Bu, C.; Zhang, D. High-temperature-responsive poplar lncRNAs modulate target gene expression via RNA interference and act as RNA scaffolds to enhance heat tolerance. Int. J. Mol. Sci. 2020, 21, 6808. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Chen, Y.; Zhang, Y.; An, X.; Li, X.; Yang, A.; Kang, G.; Zhou, J.; Cheng, C. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of apple dwarfing rootstock root morphogenesis under nitrogen and/or phosphorus deficient conditions. Front. Plant Sci. 2023, 14, 1120777. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Zhang, Y.; Hu, L.; Zhang, Y.; Kong, L. Low-nitrogen stress stimulates lateral root initiation and nitrogen assimilation in wheat: Roles of phytohormone signaling. J. Plant Growth Regul. 2021, 40, 436–450. [Google Scholar] [CrossRef]
- Sun, H.; Guo, X.; Zhu, X.; Gu, P.; Zhang, W.; Tao, W.; Wang, D.; Wu, Y.; Zhao, Q.; Xu, G.; et al. Strigolactone and gibberellin signaling coordinately regulate metabolic adaptations to changes in nitrogen availability in rice. Mol. Plant 2023, 16, 588–598. [Google Scholar] [CrossRef]
- Alber, A.; Ehlting, B.; Ehlting, J.; Hawkins, B.J.; Rennenberg, H. Net NH4+ and NO3- flux, and expression of NH4+ and NO3- transporters in roots of Picea glauca. Trees 2012, 26, 1403–1411. [Google Scholar] [CrossRef]
- Hawkins, B.J.; Robbins, S. Comparison of ammonium, nitrate, and proton fluxes in mycorrhizal and nonmycorrhizal roots of lodgepole pine in contrasting nitrogen treatments. Can. J. For. Res. 2022, 52, 1245–1253. [Google Scholar] [CrossRef]
- Sun, Q.; Zhai, L.; Zhao, D.; Gao, M.; Wu, Y.; Wu, T.; Zhang, X.; Xu, X.; Han, Z.; Wang, Y. Kinase MxMPK4-1 and calmodulin-binding protein MxIQM3 enhance apple root acidification during Fe deficiency. Plant Physiol. 2023, 191, 1968–1984. [Google Scholar] [CrossRef]
- Kong, L.; Sun, M.; Wang, F.; Liu, J.; Feng, B.; Si, J.; Zhang, B.; Li, S.; Li, H. Effects of high NH4+ on K+ uptake, culm mechanical strength and grain filling in wheat. Front. Plant Sci. 2014, 5, 703. [Google Scholar] [CrossRef]
- Jian, S.; Liao, Q.; Song, H.; Liu, Q.; Lepo, J.E.; Guan, C.; Zhang, J.; Ismail, A.M.; Zhang, Z. NRT1.1-related NH4+ toxicity is associated with a disturbed balance between NH4+ uptake and assimilation. Plant Physiol. 2018, 178, 1473–1488. [Google Scholar] [CrossRef]
- Xiao, C.; Sun, D.; Liu, B.; Fang, X.; Li, P.; Jiang, Y.; He, M.; Li, J.; Luan, S.; He, K. Nitrate transporter NRT1.1 and anion channel SLAH3 form a functional unit to regulate nitrate-dependent alleviation of ammonium toxicity. J. Integr. Plant Biol. 2022, 64, 942–957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, H.; Chen, L.; Li, Y.; Wan, X. Efficient iron plaque formation on tea (Camellia sinensis) roots contributes to acidic stress tolerance. J. Integr. Plant Biol. 2019, 61, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.Y.; Tian, W.H.; Zhou, M.; Zhu, Q.Y.; Du, W.X.; Zhu, Y.X.; Liu, X.X.; Lin, X.Y.; Zheng, S.J.; Jin, C.W. STOP1 activates NRT1.1-mediated nitrate uptake to create a favorable rhizospheric pH for plant adaptation to acidity. Plant Cell 2021, 33, 3658–3674. [Google Scholar] [CrossRef]
- Lehmann, J.; Jørgensen, M.E.; Fratz, S.; Müller, H.M.; Kusch, J.; Scherzer, S.; Navarro-Retamal, C.; Mayer, D.; Böhm, J.; Konrad, K.R.; et al. Acidosis-induced activation of anion channel SLAH3 in the flooding-related stress response of Arabidopsis. Curr. Biol. 2021, 31, 3575–3585.e9. [Google Scholar] [CrossRef]
- Wang, C.; Bian, C.; Li, J.; Han, L.; Guo, D.; Wang, T.; Sun, Z.; Ma, C.; Liu, X.; Tian, Y.; et al. Melatonin promotes Al3+ compartmentalization via H+ transport and ion gradients in Malus hupehensis. Plant Physiol. 2023, 193, 821–839. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, D.; Meng, H.; Li, S.; Li, N. Magnesium alleviates aluminum toxicity by promoting polar auxin transport and distribution and root alkalization in the root apex in populus. Plant Soil 2020, 448, 565–585. [Google Scholar] [CrossRef]
- Lu, Z.; Yu, M.; Han, X.; Qiao, G.; Xu, J.; Wu, L.; Qiu, W.; Zhuo, R. SpbZIP60 confers cadmium tolerance by strengthening the root cell wall compartmentalization in Sedum plumbizincicola. J. Hazard. Mater. 2024, 480, 135936. [Google Scholar] [CrossRef]
- Liu, M.; Liu, X.; Kang, J.; Korpelainen, H.; Li, C. Are males and females of Populus cathayana differentially sensitive to Cd stress? J. Hazard. Mater. 2020, 393, 122411. [Google Scholar] [CrossRef]
- Yin, K.; Liu, Y.; Liu, Z.; Zhao, R.; Zhang, Y.; Yan, C.; Zhao, Z.; Feng, B.; Zhang, X.; An, K.; et al. Populus euphratica CPK21 interacts with NF-YC3 to enhance cadmium tolerance in Arabidopsis. Int. J. Mol. Sci. 2024, 25, 7214. [Google Scholar] [CrossRef]
- Liu, L.; Gui, H.; Zou, D.; Jiao, W.; Wang, S.; Wan, X. Long-term adaptation of water hyacinth to low cadmium involves antioxidant enzyme and metallothionein transcriptional regulation. Chemosphere 2024, 365, 143346. [Google Scholar] [CrossRef]
- Su, N.; Niu, M.; Liu, Z.; Wang, L.; Zhu, Z.; Zou, J.; Chen, Y.; Cui, J. Hemin-decreased cadmium uptake in pak choi (Brassica chinensis L.) seedlings is heme oxygenase-1 dependent and relies on its by-products ferrous iron and carbon monoxide. Environ. Pollut. 2021, 274, 115882. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhou, B.; Tan, Q.; Zhang, L.; Pan, K. The roles of silicon in combating cadmium challenge in the Marine diatom Phaeodactylum tricornutum. J. Hazard. Mater. 2020, 389, 121903. [Google Scholar] [CrossRef] [PubMed]
- Mak, M.; Zhang, M.; Randall, D.; Holford, P.; Chen, Z.H. Chloride transport at plant-soil interface modulates barley cd tolerance. Plant Soil 2019, 441, 409. [Google Scholar] [CrossRef]
- Zhang, Y.; Sa, G.; Zhang, Y.; Hou, S.; Wu, X.; Zhao, N.; Zhang, Y.; Deng, S.; Deng, C.; Deng, J.; et al. Populus euphratica annexin1 facilitates cadmium enrichment in transgenic Arabidopsis. J. Hazard. Mater. 2021, 405, 124063. [Google Scholar] [CrossRef]
- He, J.; Zhuang, X.; Zhou, J.; Sun, L.; Wan, H.; Li, H.; Lyu, D. Exogenous melatonin alleviates cadmium uptake and toxicity in apple rootstocks. Tree Physiol. 2020, 40, 746–761. [Google Scholar] [CrossRef]
- Kuromori, T.; Fujita, M.; Takahashi, F.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Inter-tissue and inter-organ signaling in drought stress response and phenotyping of drought tolerance. Plant J. 2022, 109, 342–358. [Google Scholar] [CrossRef]
- Liu, H.; Able, A.J.; Able, J.A. Priming crops for the future: Rewiring stress memory. Trends Plant Sci. 2022, 2, 699–716. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Kim, J.S.; Kidokoro, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulatory networks in plant responses to drought and cold stress. Plant Physiol. 2024, 195, 170–189. [Google Scholar] [CrossRef]
- McLamore, E.S.; Diggs, A.; Calvo Marzal, P.; Shi, J.; Blakeslee, J.J.; Peer, W.A.; Murphy, A.S.; Porterfield, D.M. Non-invasive quantification of endogenous root auxin transport using an integrated flux microsensor technique. Plant J. 2010, 63, 1004–1016. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Fu, Y.; Cui, Y.; Wu, N.; Li, Y.; Yang, Z.; Zhang, C.; Song, H.; He, G.; et al. HTT1, a Stearoyl-Acyl Carrier Protein Desaturase involved unsaturated fatty acid biosynthesis, affects rice heat tolerance. Plant Cell Environ. 2025, 48, 3391–3405. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Feng, Y.; Luo, S.; Cheng, L.; Tong, W.; Lu, X.; Li, Y.; Zhang, P. The aquaporin MePIP2;7 improves MeMGT9-mediated Mg2+ acquisition in cassava. J. Integr. Plant Biol. 2023, 65, 2349–2367. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Zhang, H.H.; Zhu, Q.Y.; Ye, J.Y.; Zhu, Y.X.; Jing, X.T.; Du, W.X.; Zhou, M.; Lin, X.Y.; Zheng, S.J.; et al. Phloem iron remodels root development in response to ammonium as the major nitrogen source. Nat. Commun. 2022, 13, 561. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Devi, L.L.; Gupta, S.; Prasad, P.; Agrwal, K.; Asif, M.H.; Pandey, A.K.; Bandyopadhyay, K.; Singh, A.P. Jasmonate signaling modulates root growth by suppressing iron accumulation during ammonium stress. Plant Physiol. 2024, 196, 2213–2231. [Google Scholar] [CrossRef]
- Bolan, N.; Sarmah, A.K.; Bordoloi, S.; Bolan, S.; Padhye, L.P.; Van Zwieten, L.; Sooriyakumar, P.; Khan, B.A.; Ahmad, M.; Solaiman, Z.M.; et al. Soil acidification and the liming potential of biochar. Environ. Pollut. 2023, 317, 120632. [Google Scholar] [CrossRef]
- Dhandapani, S.; Sng, Y.H.; Agisha, V.N.; Suraby, E.J.; Park, B.S. Mitigating aluminum toxicity and promoting plant resilience in acidic soil with Penicillium olsonii TLL1. Front. Plant Sci. 2024, 15, 1423617. [Google Scholar] [CrossRef]
- Feng, Z.; Ji, S.; Ping, J.; Cui, D. Recent advances in metabolomics for studying heavy metal stress in plants. TrAC-Trend Anal. Chem. 2021, 143, 116402. [Google Scholar] [CrossRef]

| Stress | Samples | Detection Site | Ions/Molecules | References |
|---|---|---|---|---|
| Rice | Seed embryo | K+, Na+ | [36] | |
| Quinoa | Root elongation and mature zone | K+ | [37] | |
| Quinoa | Leaf mesophyll cells | Ca2+, K+, H+ | [38] | |
| Barley and triticale | Root elongation and mature zone | Ca2+, K+, H+ | [39] | |
| Rice | Root mature zone | K+, Ca2+ | [40] | |
| Arabidopsis | Root meristematic zone | Na+ | [41] | |
| Arabidopsis | Roots | Na+, H+ | [42] | |
| Populus euphratica | Roots | Na+ | [43] | |
| Populus euphratica | Root meristematic zone | Na+ | [44] | |
| Limonium bicolor | Salt glands | Na+ | [45] | |
| Nitraria tangutorum | Root tip | Na+, K+ | [46] | |
| Sugar beet | Roots | Cl- | [47] | |
| Barley | Roots | K+, H+ | [48] | |
| Kandelia obovata | Leaves | Na+ | [49] | |
| Kandelia obovata | Roots | Na+, K+, H+, Ca2+ | [50] | |
| Kandelia obovata | Roots | Na+, K+ | [51] | |
| Alkali stress | Maize | Root meristematic zone | Na+, H+ | [52] |
| Arabidopsis | Leaf mesophyll cells | H+ | [53] | |
| Wheat | Roots | H+ | [54] | |
| Arabidopsis | Root elongation zone | H+ | [55] | |
| Rapeseed | Leaf mesophyll cells | Na+, K+, Ca2+ | [56] | |
| Water stress | Upland rice | Root tip | IAA | [12] |
| Rice, Arabidopsis | Root tip | H+ | [57] | |
| Soybean | Leaf mesophyll cells | K+, H+, Ca2+ | [58] | |
| Barley | Leaf mesophyll cells, roots | K+, H+, Ca2+ | [59] | |
| Tea | Roots | K+ | [60] | |
| Cotton | Guard cell | K+ | [61] | |
| Barley | Roots | K+ | [62] | |
| Trifoliate orange | Root hair zone | IAA | [63] | |
| Cotton | Leaf mesophyll cells | Ca2+ | [64] | |
| Chinese cabbage | Guard cell | Cl−, K+, H+ | [65] | |
| Low-temperature stress | Rice | Root meristematic zone | Ca2+ | [8] |
| Watermelon | Intracellular | Ca2+ | [66] | |
| Rice | Roots | Ca2+ | [67] | |
| Rice | Roots | Ca2+ | [68] | |
| Cucumber | Roots | Ca2+ | [69] | |
| Arabidopsis | Roots | O2 | [70] | |
| High-temperature stress | Arabidopsis | Leaf mesophyll cells | H+, K+, Ca2+ | [71] |
| Rice | Leaf mesophyll cells | Ca2+ | [72] | |
| Lettuce | Guard cell | K+, Ca2+ | [73] | |
| Rice | Root and aboveground parts | Ca2+ | [74] | |
| Poplar | Roots | Ca2+ | [75] | |
| Nutrition stress | Apple | Stock root elongation zone | H+ | [29] |
| Apple | Stock roots | H+, NO3− | [76] | |
| Wheat | Roots | IAA, H+ | [77] | |
| Rice | Root meristem zone | NH4+ | [78] | |
| White spruce | Roots | H+, NH4+, NO3− | [79] | |
| Lodgepole pine | Root and aboveground parts | H+, NH4+, NO3− | [80] | |
| Apple rootstock | Root mature zone | H+ | [81] | |
| Ammonium toxicity | Wheat | Roots | K+ | [82] |
| Arabidopsis | Roots | NH4+, NO3− | [83] | |
| Arabidopsis | Roots | NO3− | [84] | |
| Acid stress | Tea | Root mature area | H+ | [85] |
| Arabidopsis | Root meristem zone, elongation zone and mature zone | H+ | [86] | |
| Arabidopsis | Roots | Cl−, NO3− | [87] | |
| Malus hupehensis | Roots | H+ | [88] | |
| Populus | Roots | Mg2+, IAA | [89] | |
| Heavy metal toxicity | Sedum plumbizincicola | Roots | Cd2+ | [90] |
| Cathay poplar | Roots | Cd2+ | [91] | |
| Populus euphratica | Root tip | Cd2+ | [92] | |
| Eichhornia crassipes | Roots, stem, leaves | H2O2, O2 | [93] | |
| Pak choi | Roots | Cd2+ | [94] | |
| Diatom | Frustule | Cd2+ | [95] | |
| Barley | Roots | Cd2+, K+, H+, Cl−, Ca2+ | [96] | |
| Populus euphratica | Roots | Cd2+ | [97] | |
| Apple rootstocks | Roots | Cd2+ | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Yin, P.; Yang, X.; Liu, Y.; Xu, R. Non-Invasive Micro-Test Technology in Plant Physiology Under Abiotic Stress: From Mechanism to Application. Plants 2025, 14, 1932. https://doi.org/10.3390/plants14131932
Zhang T, Yin P, Yang X, Liu Y, Xu R. Non-Invasive Micro-Test Technology in Plant Physiology Under Abiotic Stress: From Mechanism to Application. Plants. 2025; 14(13):1932. https://doi.org/10.3390/plants14131932
Chicago/Turabian StyleZhang, Tianpeng, Peipei Yin, Xinghong Yang, Yunqi Liu, and Ruirui Xu. 2025. "Non-Invasive Micro-Test Technology in Plant Physiology Under Abiotic Stress: From Mechanism to Application" Plants 14, no. 13: 1932. https://doi.org/10.3390/plants14131932
APA StyleZhang, T., Yin, P., Yang, X., Liu, Y., & Xu, R. (2025). Non-Invasive Micro-Test Technology in Plant Physiology Under Abiotic Stress: From Mechanism to Application. Plants, 14(13), 1932. https://doi.org/10.3390/plants14131932







