OsXTH19 Overexpression Improves Aluminum Tolerance via Xyloglucan Reduction in Rice Root Cell Wall
Abstract
1. Introduction
2. Results
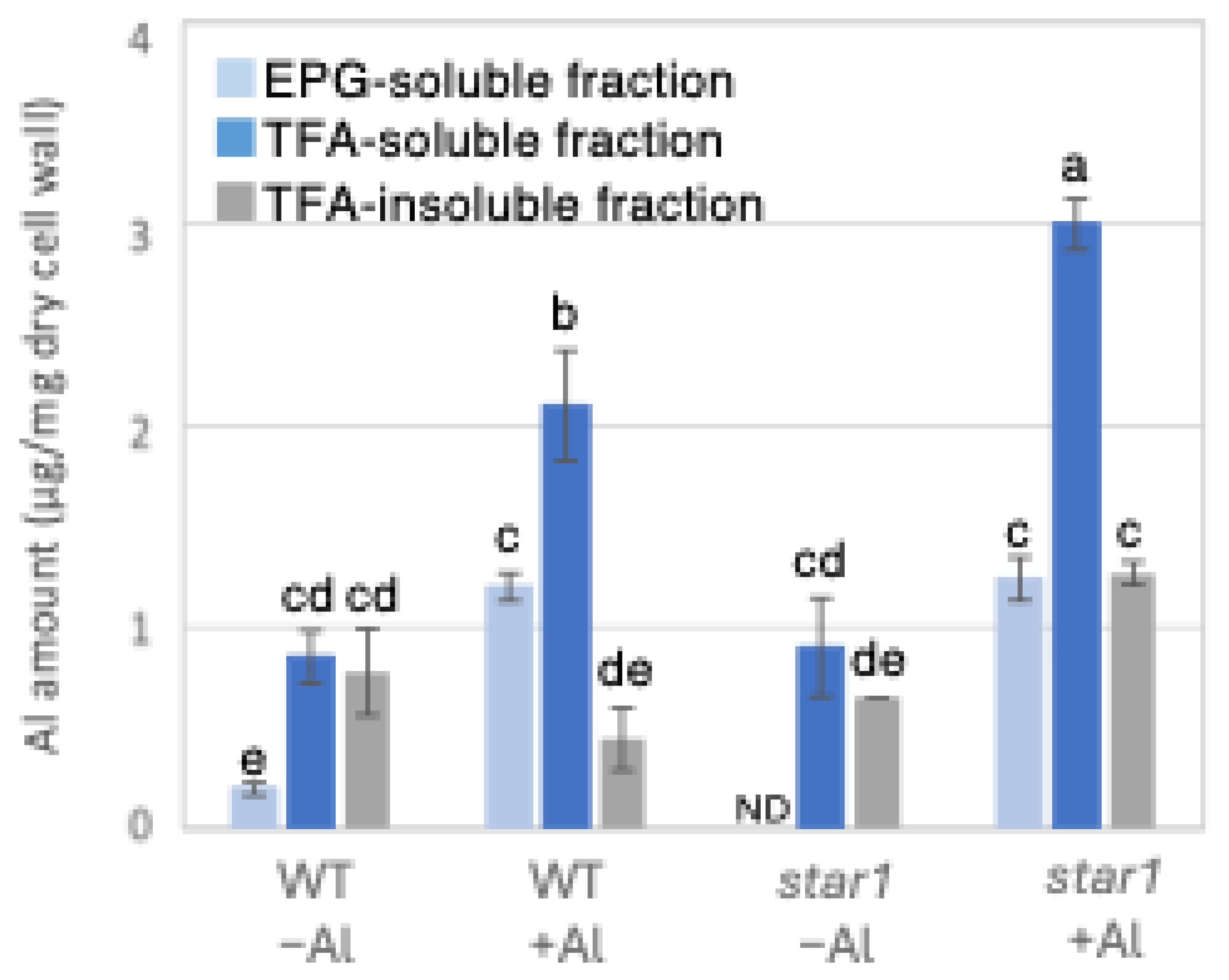
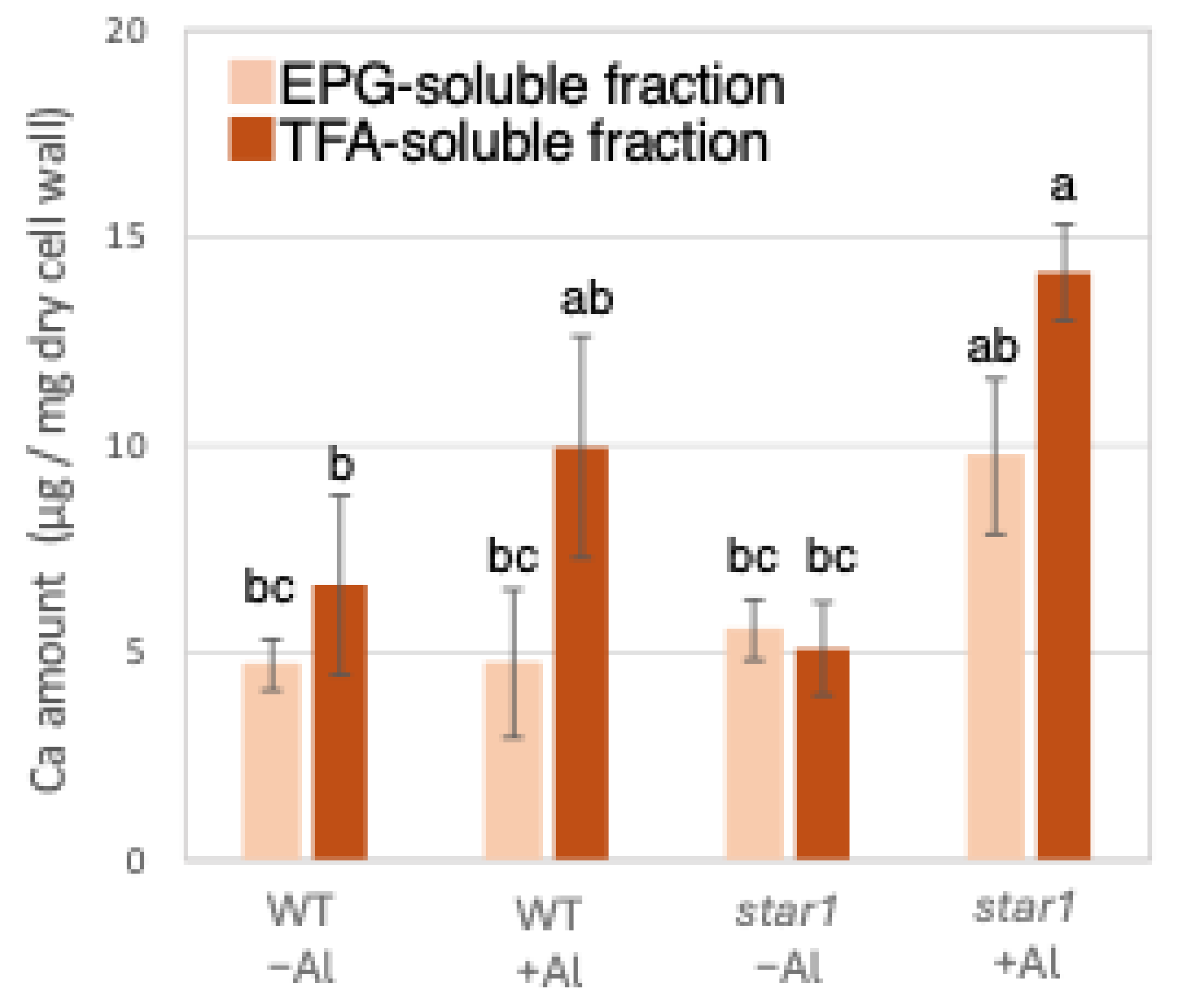
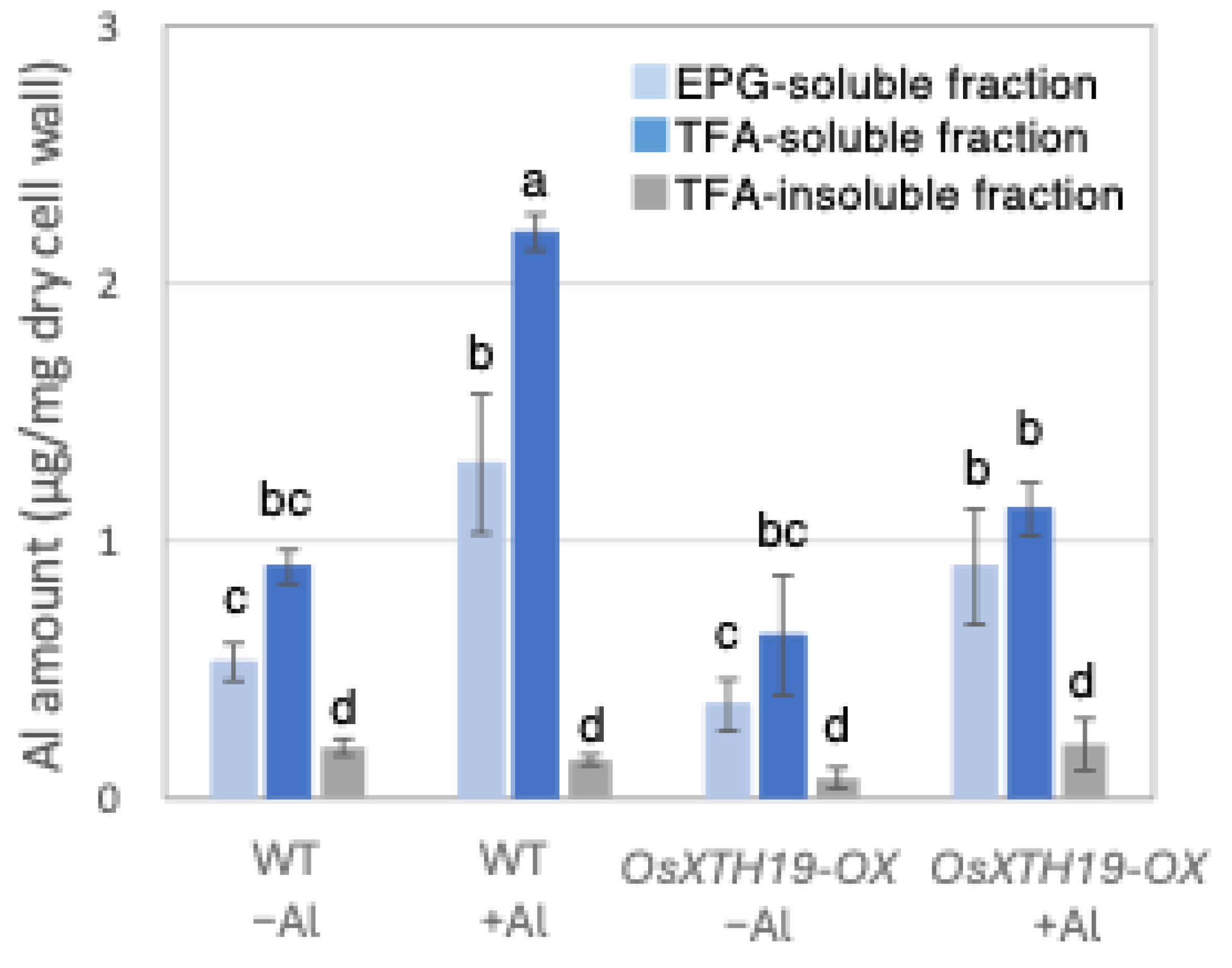
2.1. The Quantification of the Amount of Al and Ca Present in Each Fraction of the Cell Wall
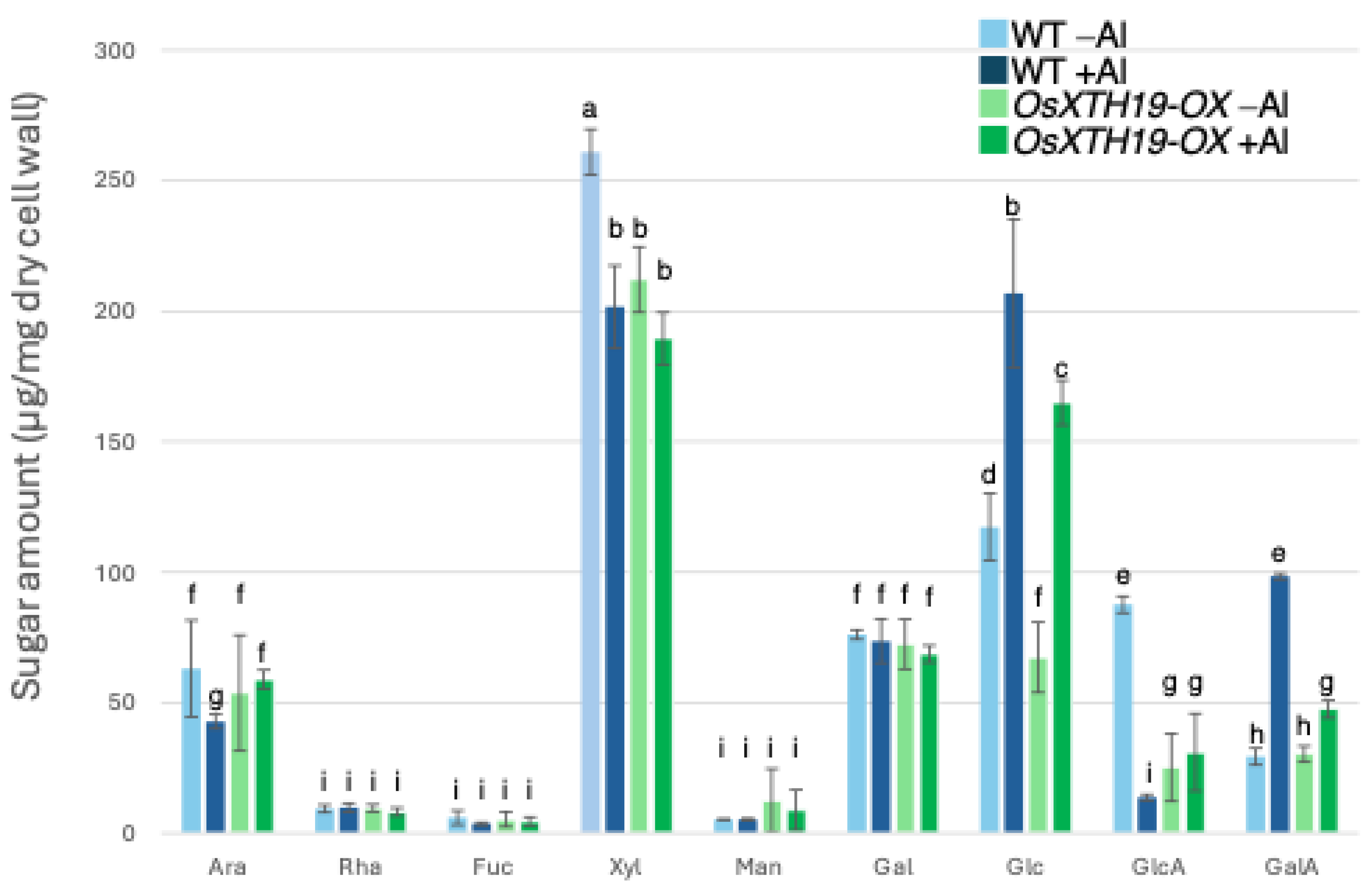
2.2. Analysis of Cell Wall Sugars in WT and Star1
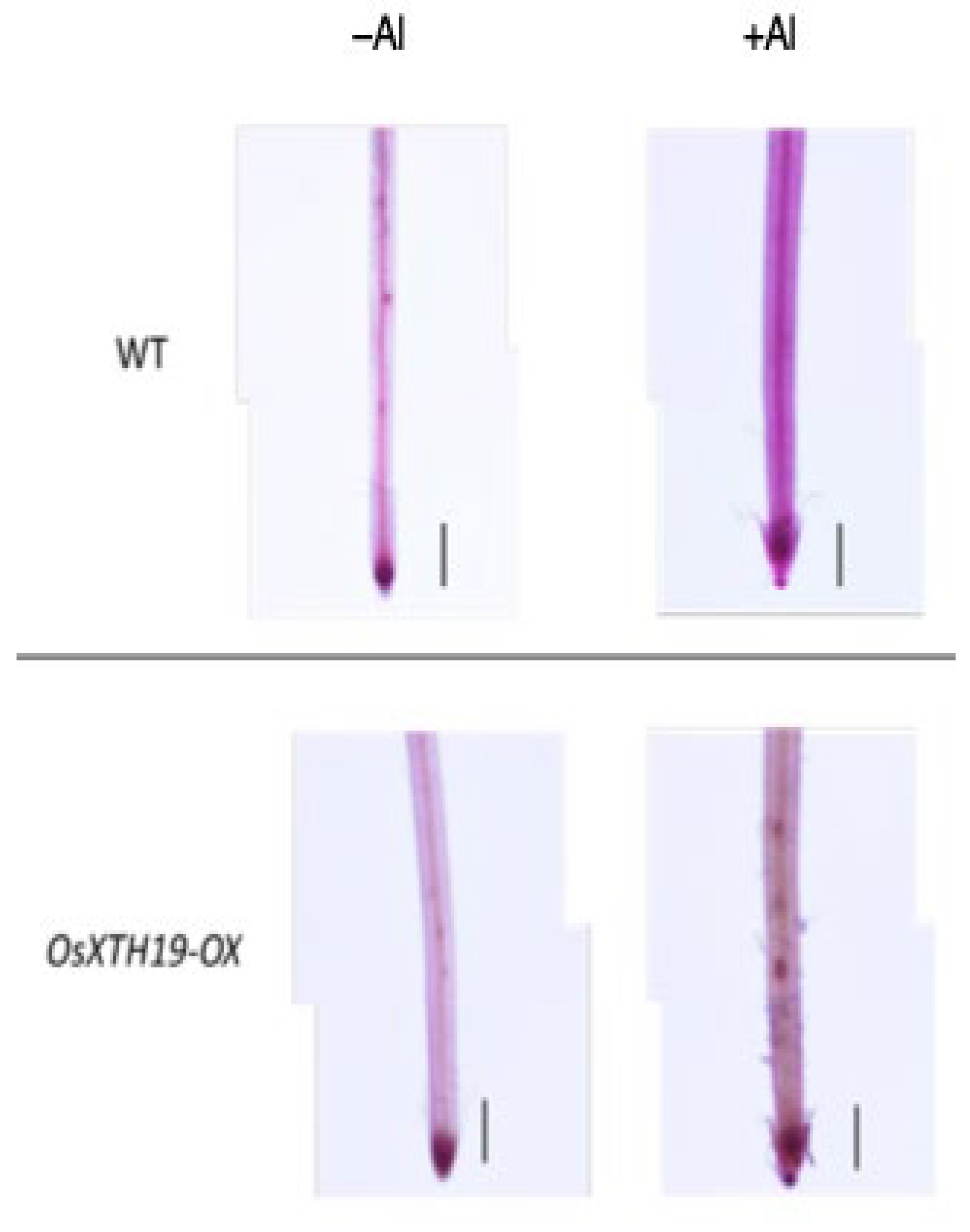
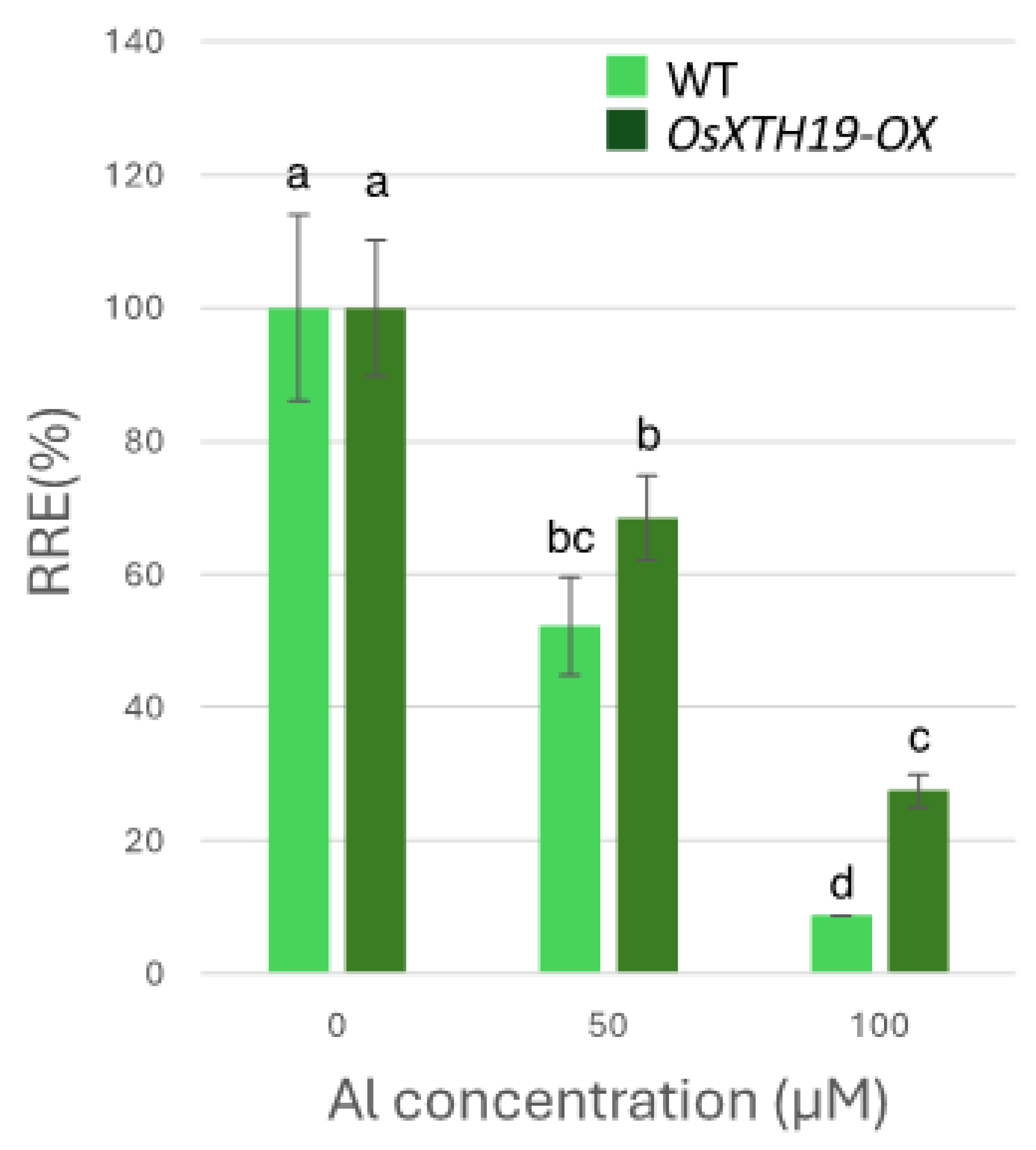
2.3. Measurement of Al Tolerance and Changes in Cell Wall Components in OsXTH19-OX
3. Discussion
3.1. Reevaluating the Primary Target of Aluminum Toxicity and the Role of Hemicellulose in Rice Cell Walls
3.2. Accumulation of Aluminum and Calcium in Cell Wall and Their Roles in Aluminum Tolerance
3.3. Xyloglucan Accumulation and Its Role in Aluminum Binding and Cell Wall Modification in Rice
3.4. Reduced Xyloglucan Content Enhances Aluminum Tolerance Despite Lower Pectin Levels
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Cell Wall Analysis
4.3. Quantification of Al and Ca
4.4. Staining of Total Pectin with Ruthenium Red
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICP-AES | inductively coupled plasma atomic emission spectroscopy |
| XTH | xyloglucan endotransglucosylase/hydrolase |
| EPG | endo-polygalacturonase |
| TFA | trifluoroacetic acid |
| GC | gas chromatography |
References
- Foy, C.D. Plant Adaptation to Acid, Aluminum-toxic Soils. Commun. Soil Sci. Plant Anal. 1988, 19, 959–987. [Google Scholar] [CrossRef]
- von Uexküll, H.R.; Mutert, E. Global Extent, Development and Economic Impact of Acid Soils. Plant Soil 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Duan, J.; Gregory, J. Coagulation by Hydrolysing Metal Salts. Adv. Colloid. Interface Sci. 2003, 100, 475–502. [Google Scholar] [CrossRef]
- Famoso, A.N.; Clark, R.T.; Shaff, J.E.; Craft, E.; McCouch, S.R.; Kochian, L.V. Development of a Novel Aluminum Tolerance Phenotyping Platform Used for Comparisons of Cereal Aluminum Tolerance and Investigations into Rice Aluminum Tolerance Mechanisms. Plant Physiol. 2010, 153, 1678–1691. [Google Scholar] [CrossRef]
- Kochian, L.V. Cellular Mechanisms of Aluminum Toxicity and Resistance in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995, 46, 237–260. [Google Scholar] [CrossRef]
- Kochian, L.V.; Piñeros, M.A.; Hoekenga, O.A. The Physiology, Genetics and Molecular Biology of Plant Aluminum Resistance and Toxicity. Plant Soil 2005, 274, 175–195. [Google Scholar] [CrossRef]
- Lu, H.; Dong, G.; Hua, H.; Zhao, W.; Li, J.; Xu, R. Method for Initially Selecting Al-Tolerant Rice Varieties Based on the Charge Characteristics of Their Roots. Ecotoxicol. Environ. Saf. 2020, 187, 109813. [Google Scholar] [CrossRef]
- Jones, D.L.; Blancaflor, E.B.; Kochian, L.V.; Gilroy, S. Spatial Coordination of Aluminium Uptake, Production of Reactive Oxygen Species, Callose Production and Wall Rigidification in Maize Roots. Plant Cell Environ. 2006, 29, 1309–1318. [Google Scholar] [CrossRef]
- Van, H.L.; Kuraishi, S.; Sakurai, N. Aluminum-Induced Rapid Root Inhibition and Changes in Cell-Wall Components of Squash Seedlings. Plant Physiol. 1994, 106, 971–976. [Google Scholar] [CrossRef]
- Clarkson, D.T. Interactions between Aluminium and Phosphorus on Root Surfaces and Cell Wall Material. Plant Soil 1967, 27, 347–356. [Google Scholar] [CrossRef]
- Schmohl, N.; Horst, W.J. Cell Wall Pectin Content Modulates Aluminium Sensitivity of Zea mays (L.) Cells Grown in Suspension Culture. Plant Cell Environ. 2000, 23, 735–742. [Google Scholar] [CrossRef]
- Yang, J.L.; Zhu, X.F.; Peng, Y.X.; Zheng, C.; Li, G.X.; Liu, Y.; Shi, Y.Z.; Zheng, S.J. Cell Wall Hemicellulose Contributes Significantly to Aluminum Adsorption and Root Growth in Arabidopsis. Plant Physiol. 2011, 155, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, T.; Nakamura, A.; Yamaji, N.; Satoh, S.; Furukawa, J.; Iwai, H. Changes in the Distribution of Pectin in Root Border Cells Under Aluminum Stress. Front. Plant Sci. 2019, 10, 1216. [Google Scholar] [CrossRef]
- Nagayama, T.; Tatsumi, A.; Nakamura, A.; Yamaji, N.; Satoh, S.; Furukawa, J.; Iwai, H. Effects of Polygalacturonase Overexpression on Pectin Distribution in the Elongation Zones of Roots under Aluminium Stress. AoB Plants 2022, 14, plac003. [Google Scholar] [CrossRef]
- Huang, C.F.; Yamaji, N.; Mitani, N.; Yano, M.; Nagamura, Y.; Ma, J.F. A Bacterial-Type ABC Transporter Is Involved in Aluminum Tolerance in Rice. Plant Cell 2009, 21, 655–667. [Google Scholar] [CrossRef]
- Delhaize, E.; Ryan, P.R. Aluminum Toxicity and Tolerance in Plants. Plant Physiol. 1995, 107, 315–321. [Google Scholar] [CrossRef]
- Hara, Y.; Yokoyama, R.; Osakabe, K.; Toki, S.; Nishitani, K. Function of Xyloglucan Endotransglucosylase/Hydrolases in Rice. Ann. Bot. 2014, 114, 1309–1318. [Google Scholar] [CrossRef]
- Horst, W.J.; Wang, Y.; Eticha, D. The Role of the Root Apoplast in Aluminium-Induced Inhibition of Root Elongation and in Aluminium Resistance of Plants: A Review. Ann. Bot. 2010, 106, 185–197. [Google Scholar] [CrossRef]
- Tabuchi, A.; Matsumoto, H. Changes in Cell-Wall Properties of Wheat (Triticum aestivum) Roots during Aluminum-Induced Growth Inhibition. Physiol. Plant. 2001, 112, 353–358. [Google Scholar] [CrossRef]
- Ma, J.F.; Nagao, S.; Huang, C.F.; Nishimura, M. Isolation and Characterization of a Rice Mutant Hypersensitive to Al. Plant Cell Physiol. 2005, 46, 1054–1061. [Google Scholar] [CrossRef]
- Dronnet, V.M.; Renard, C.M.G.C.; Axelos, M.A.V.; Thibault, J.-F. Characterisation and Selectivity of Divalent Metal Ions Binding by Citrus and Sugar-Beet Pectins. Carbohydr. Polym. 1996, 30, 253–263. [Google Scholar] [CrossRef]
- Iwai, H. Virtual Issue: Cell Wall Functions in Plant Growth and Environmental Responses. J. Plant Res. 2021, 134, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Calcium in Plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, D.T.; Sanderson, J. Inhibition of the Uptake and Long-Distance Transport of Calcium by Aluminium and Other Polyvalent Cations. J. Exp. Bot. 1971, 22, 837–851. [Google Scholar] [CrossRef]
- Blumwald, E.; Aharon, G.S.; Lam, B.C.-H. Early Signal Transduction Pathways in Plant–Pathogen Interactions. Trends Plant Sci. 1998, 3, 342–346. [Google Scholar] [CrossRef]
- Hatfield, R.D.; Rancour, D.M.; Marita, J.M. Grass Cell Walls: A Story of Cross-Linking. Front. Plant Sci. 2017, 7, 2056. [Google Scholar] [CrossRef]
- Vogel, J. Unique Aspects of the Grass Cell Wall. Curr. Opin. Plant Biol. 2008, 11, 301–307. [Google Scholar] [CrossRef]
- Li, X.F.; Ma, J.F.; Matsumoto, H. Pattern of Aluminum-Induced Secretion of Organic Acids Differs between Rye and Wheat. Plant Physiol. 2000, 123, 1537–1544. [Google Scholar] [CrossRef]
- Nishitani, K.; Vissenberg, K. Roles of the XTH Protein Family in the Expanding Cell. In The Expanding Cell; Plant Cell Monographs; Verbelen, J.-P., Vissenberg, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 6, ISBN 978-3-540-39114-2. [Google Scholar]
- Zhu, X.F.; Wan, J.X.; Wu, Q.; Zhao, X.S.; Zheng, S.J.; Shen, R.F. PARVUS Affects Aluminium Sensitivity by Modulating the Structure of Glucuronoxylan in Arabidopsis thaliana. Plant Cell Environ. 2017, 40, 1916–1925. [Google Scholar] [CrossRef]
- Carpita, N.C.; Gibeaut, D.M. Structural Models of Primary Cell Walls in Flowering Plants: Consistency of Molecular Structure with the Physical Properties of the Walls during Growth. Plant J. Cell Mol. Biol. 1993, 3, 1–30. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Relaxation in a High-Stress Environment: The Molecular Bases of Extensible Cell Walls and Cell Enlargement. Plant Cell 1997, 9, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Re-Constructing Our Models of Cellulose and Primary Cell Wall Assembly. Curr. Opin. Plant Biol. 2014, 22, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, R.; Rose, J.K.C.; Nishitani, K. A Surprising Diversity and Abundance of Xyloglucan Endotransglucosylase/Hydrolases in Rice. Classification and Expression Analysis. Plant Physiol. 2004, 134, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.B.; Cosgrove, D.J. A Revised Architecture of Primary Cell Walls Based on Biomechanical Changes Induced by Substrate-Specific Endoglucanases. Plant Physiol. 2012, 158, 1933–1943. [Google Scholar] [CrossRef]
- Zhu, X.F.; Shi, Y.Z.; Lei, G.J.; Fry, S.C.; Zhang, B.C.; Zhou, Y.H.; Braam, J.; Jiang, T.; Xu, X.Y.; Mao, C.Z.; et al. XTH31, Encoding an in Vitro XEH/XET-Active Enzyme, Regulates Aluminum Sensitivity by Modulating in Vivo XET Action, Cell Wall Xyloglucan Content, and Aluminum Binding Capacity in Arabidopsis. Plant Cell 2012, 24, 4731–4747. [Google Scholar] [CrossRef]
- Rengel, Z.; Zhang, W.-H. Role of Dynamics of Intracellular Calcium in Aluminium-Toxicity Syndrome. New Phytol. 2003, 159, 295–314. [Google Scholar] [CrossRef]
- Maejima, E.; Watanabe, T.; Osaki, M.; Wagatsuma, T. Phosphorus Deficiency Enhances Aluminum Tolerance of Rice (Oryza sativa) by Changing the Physicochemical Characteristics of Root Plasma Membranes and Cell Walls. J. Plant Physiol. 2014, 171, 9–15. [Google Scholar] [CrossRef]
- Shaff, J.E.; Schultz, B.A.; Craft, E.J.; Clark, R.T.; Kochian, L.V. GEOCHEM-EZ: A Chemical Speciation Program with Greater Power and Flexibility. Plant Soil 2010, 330, 207–214. [Google Scholar] [CrossRef]
- Sumiyoshi, M.; Nakamura, A.; Nakamura, H.; Hakata, M.; Ichikawa, H.; Hirochika, H.; Ishii, T.; Satoh, S.; Iwai, H. Increase in Cellulose Accumulation and Improvement of Saccharification by Overexpression of Arabinofuranosidase in Rice. PLoS ONE 2013, 8, e78269. [Google Scholar] [CrossRef]
- Ishii, T.; Matsuoka, K.; Ono, H.; Ohnishi-Kameyama, M.; Yaoi, K.; Nakano, Y.; Ohtani, M.; Demura, T.; Iwai, H.; Satoh, S. Characterization of Xylan in the Early Stages of Secondary Cell Wall Formation in Tobacco Bright Yellow-2 Cells. Carbohydr. Polym. 2017, 176, 381–391. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatsumi, A.; Nagayama, T.; Teramoto, A.; Nakamura, A.; Yokoyama, R.; Furukawa, J.; Iwai, H. OsXTH19 Overexpression Improves Aluminum Tolerance via Xyloglucan Reduction in Rice Root Cell Wall. Plants 2025, 14, 1912. https://doi.org/10.3390/plants14131912
Tatsumi A, Nagayama T, Teramoto A, Nakamura A, Yokoyama R, Furukawa J, Iwai H. OsXTH19 Overexpression Improves Aluminum Tolerance via Xyloglucan Reduction in Rice Root Cell Wall. Plants. 2025; 14(13):1912. https://doi.org/10.3390/plants14131912
Chicago/Turabian StyleTatsumi, Akane, Teruki Nagayama, Ayumi Teramoto, Atsuko Nakamura, Ryusuke Yokoyama, Jun Furukawa, and Hiroaki Iwai. 2025. "OsXTH19 Overexpression Improves Aluminum Tolerance via Xyloglucan Reduction in Rice Root Cell Wall" Plants 14, no. 13: 1912. https://doi.org/10.3390/plants14131912
APA StyleTatsumi, A., Nagayama, T., Teramoto, A., Nakamura, A., Yokoyama, R., Furukawa, J., & Iwai, H. (2025). OsXTH19 Overexpression Improves Aluminum Tolerance via Xyloglucan Reduction in Rice Root Cell Wall. Plants, 14(13), 1912. https://doi.org/10.3390/plants14131912







