The Fusion Gene BPI-LY, Encoding Human Bactericidal/Permeability-Increasing Protein Core Fragments and Lysozyme, Enhanced the Resistance of Transgenic Tomato Plants to Bacterial Wilt
Abstract
1. Introduction
2. Materials and Methods
2.1. Vectors, Bacterial Strains, and Preparation of Pathogenic Inoculum
2.2. Plant Materials and Inoculation Methods
2.3. Obtaining the Supernatant of Recombinant Protein Expressed in Yeast
2.4. Antimicrobial Activity Test Statistics
2.5. Determination of the Bacterial Growth Curves and the Bactericidal Activities of the Antibacterial Proteins
2.6. Determination of Conductivity
2.7. Construction of Plant Expression Vector
2.8. Acquisition of Disease-Resistant Transgenic Tomato Lines
2.9. Determination of the Tomato Plant Disease Index
2.10. Detection of the Number of R. solanacearum in Tomato Stems
2.11. Anatomical Observation of Tomato Stems
2.12. DAB and NBT Staining of Tomato Leaves
2.13. Quantitative Analysis of Gene Expression
2.14. Transcriptome Analysis
2.15. Statistical Analysis
3. Results
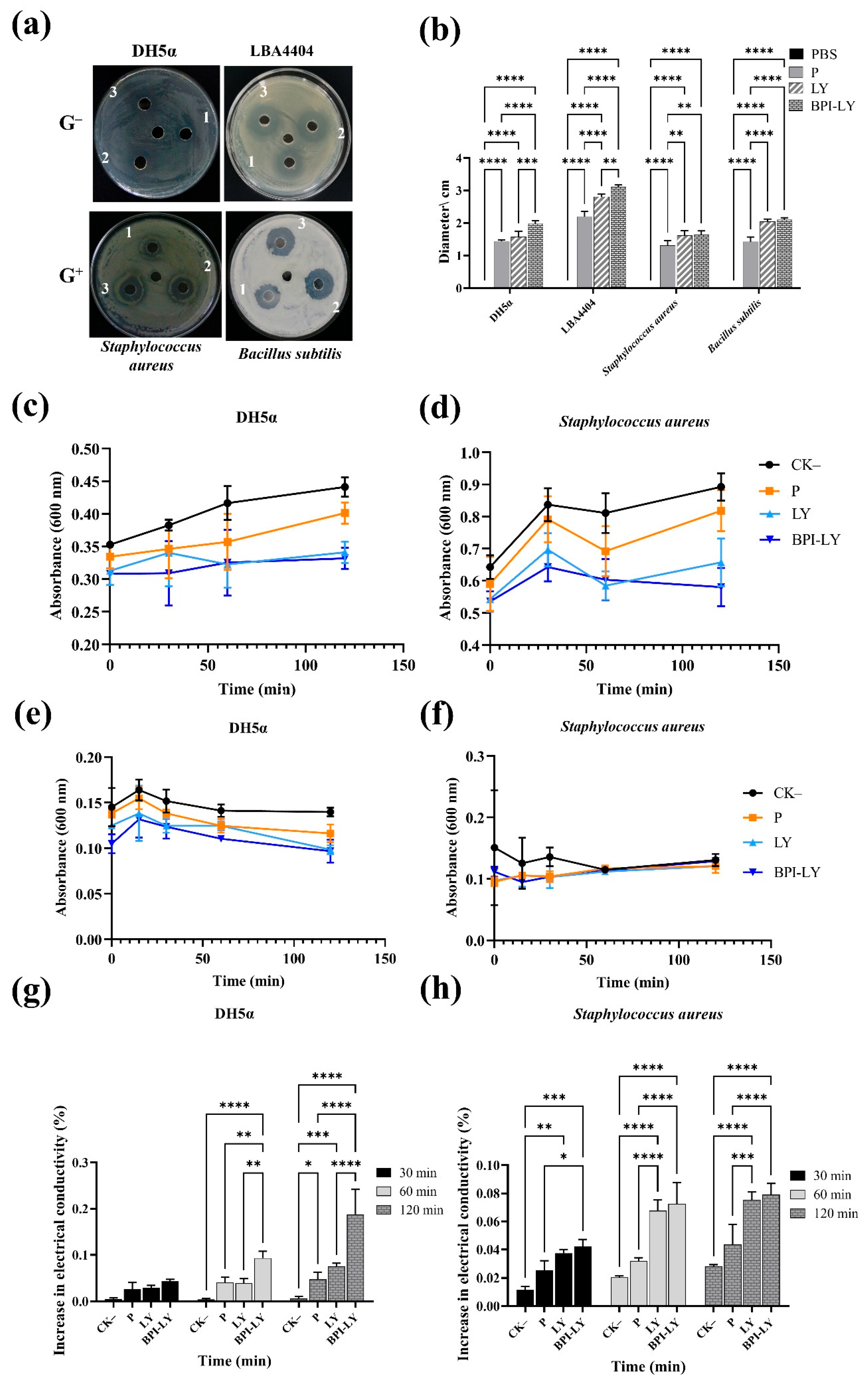
3.1. Recombinant BPI-LY Protein Increased the Bactericidal Activity Against G− Bacteria
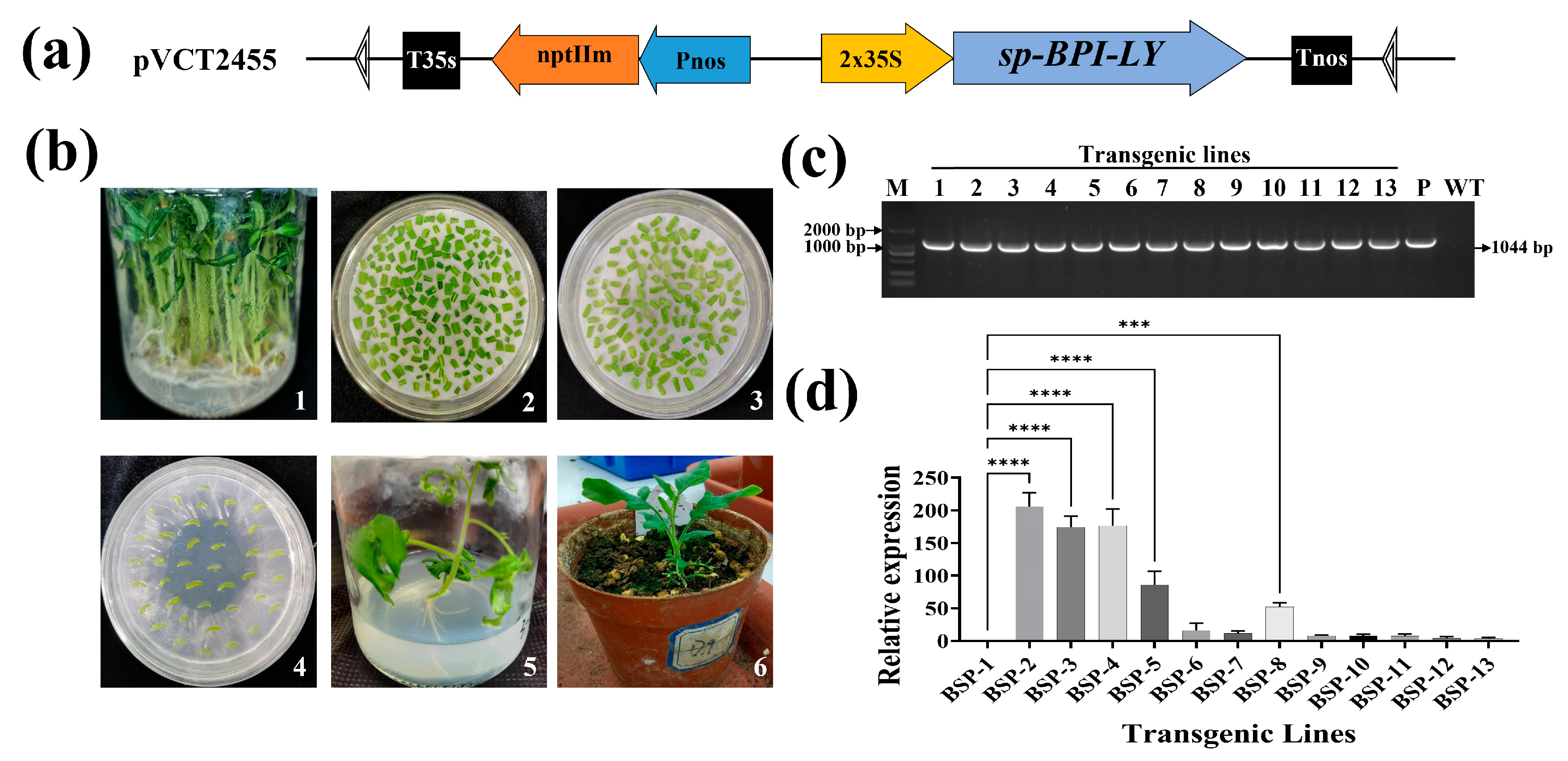
3.2. Creation of High-Expression sp-BPI-LY Transgenic Tomato Lines
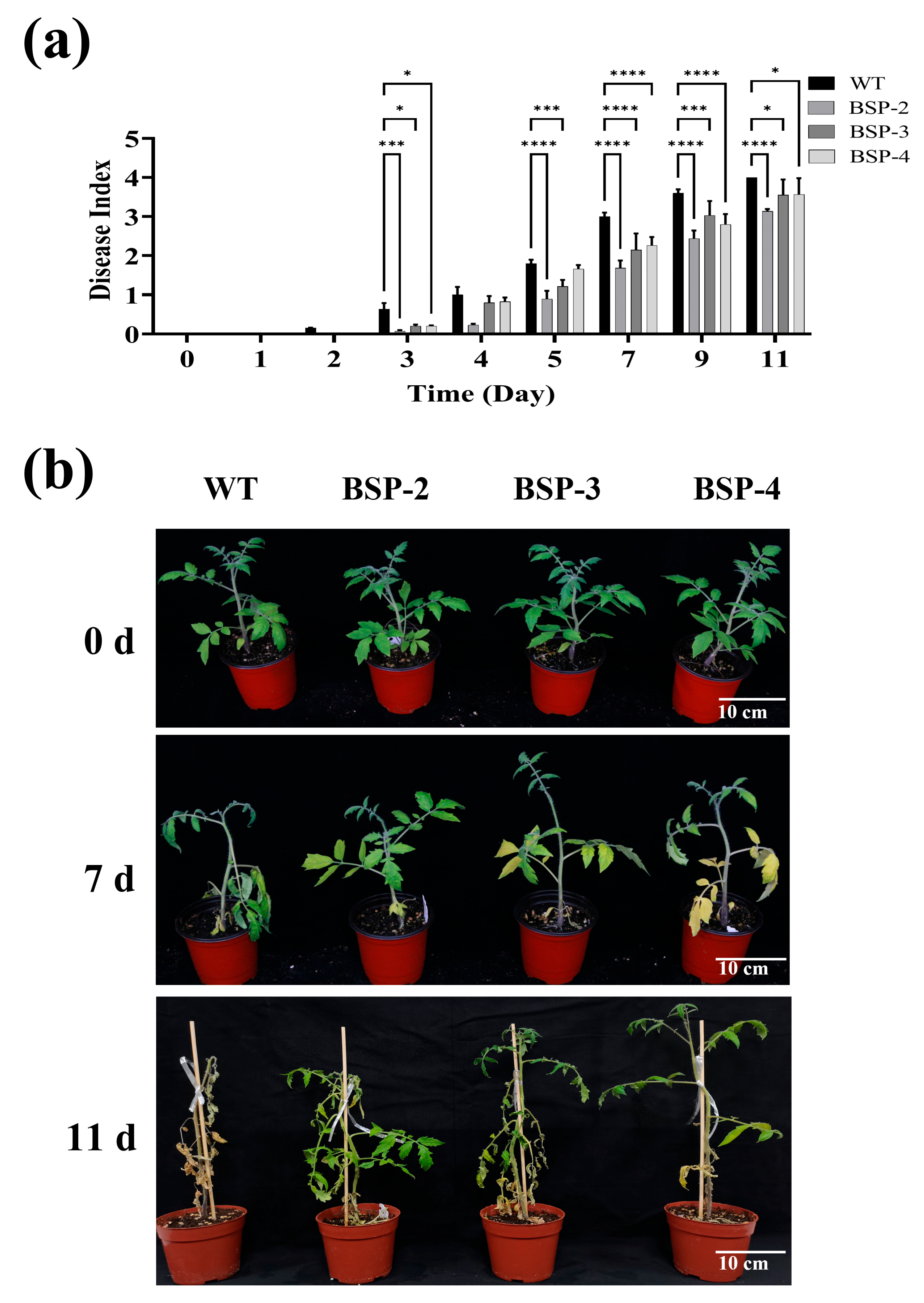
3.3. Overexpression of sp-BPI-LY Enhanced the Resistance to Bacterial Wilt Disease in the Transgenic Lines
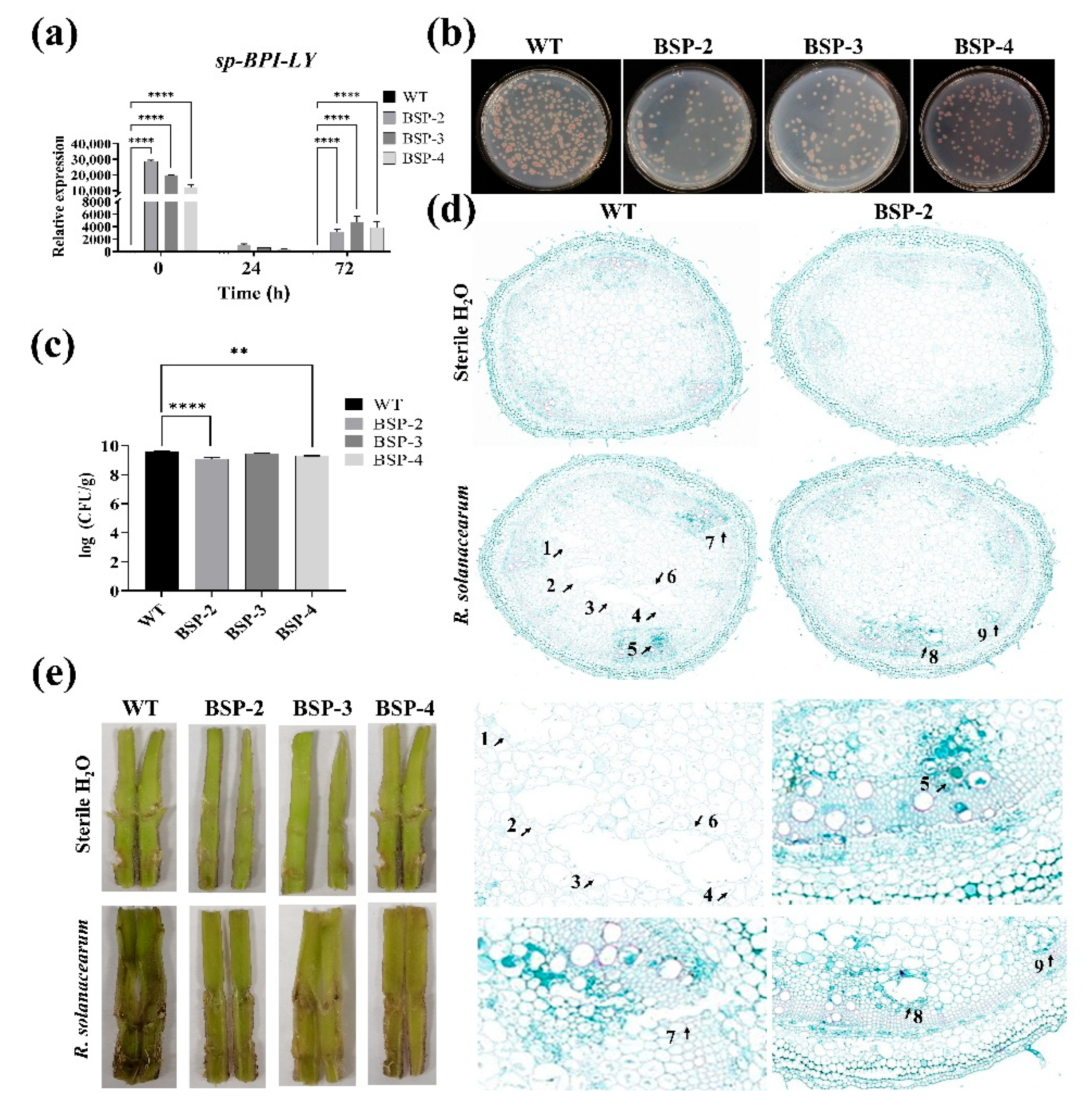
3.4. Effect of sp-BPI-LY Overexpression on Disease Resistance in Transgenic Tomato Stems
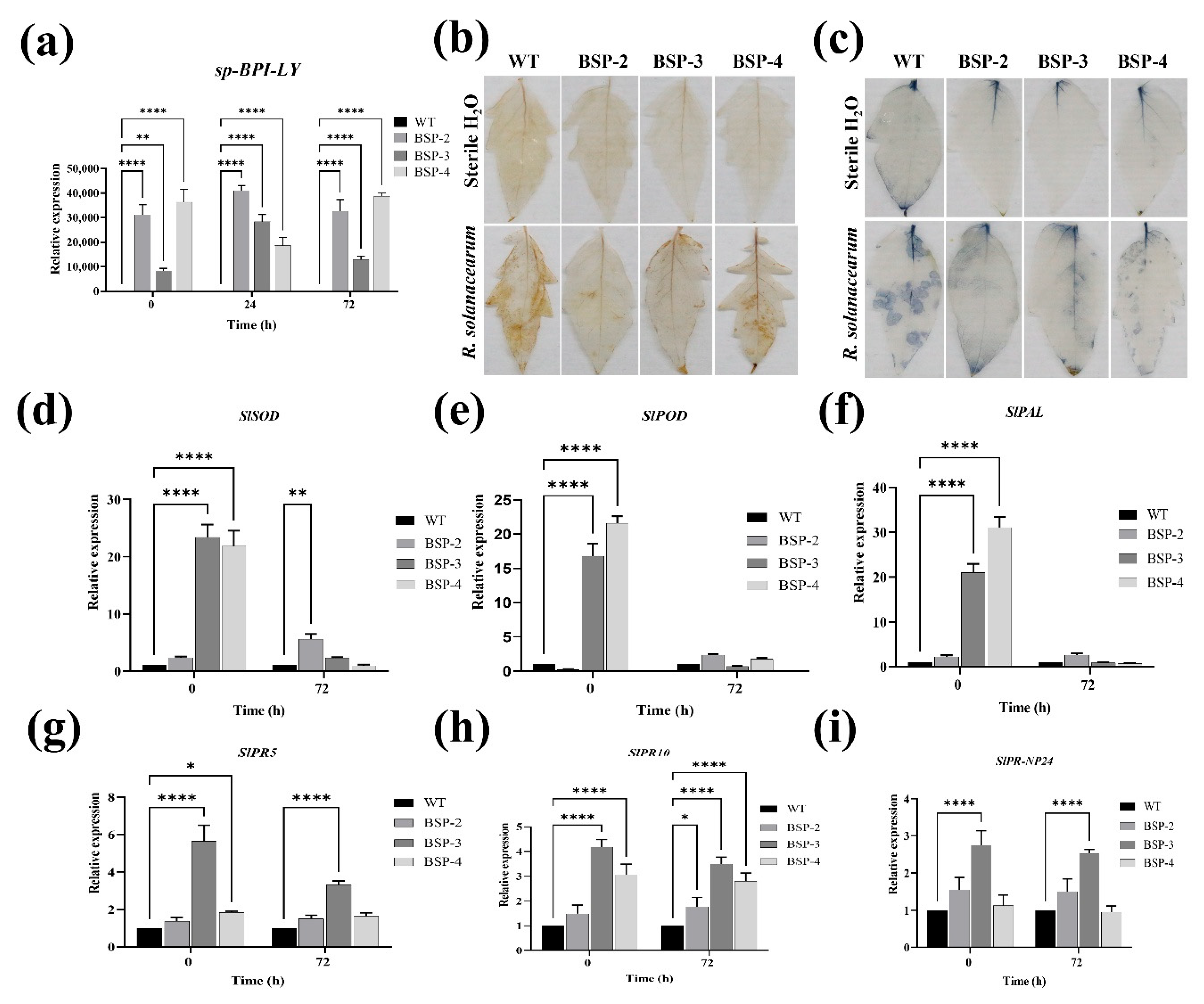
3.5. Effect of sp-BPI-LY Overexpression on ROS Accumulation and the Expression of Defense-Enzyme-Related Genes and Pathogenesis-Related Genes in Transgenic Tomato Plants
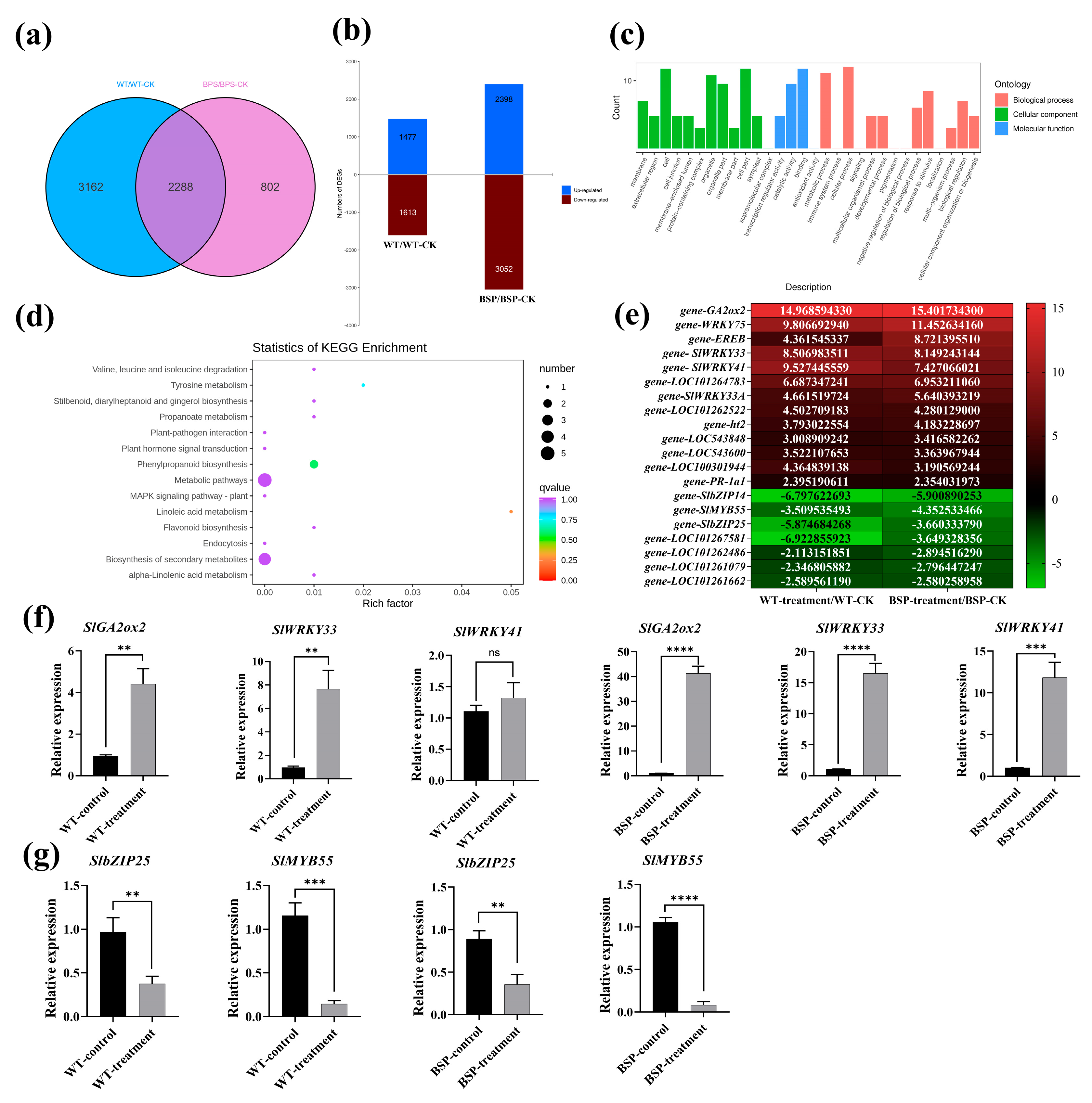
3.6. Effect of sp-BPI-LY Overexpression on the Gene Expression of Tomato Infected with R. solanacearum
4. Discussion
4.1. Recombinant Protein BPI-LY Could Enhance the Antibacterial Activity Against G− Bacteria
4.2. Overexpression of the sp-BPI-LY Gene Reduced ROS Damage in Transgenic Tomato and Enhanced the Expression of Defense Enzyme-Related Genes in Transgenic Tomato
4.3. Overexpression of the sp-BPI-LY Gene Enhanced the Expression of the Pathogenesis-Related Genes in Transgenic Tomato
4.4. Transcriptome Analysis of Tomato Overexpressing sp-BPI-LY
4.5. Biological Safety of sp-BPI-LY Transgenic Tomato
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salanoubat, M.; Genin, S.; Artiguenave, F.; Gouzy, J.; Mangenot, S.; Arlat, M.; Billault, A.; Brottier, P.; Camus, J.C.; Cattolico, L.; et al. Genome sequence of the plant pathogen Ralstonia solanacearum. Nature 2002, 415, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Lozano-Durán, R.; Macho, A.P. Insights into the root invasion by the plant pathogenic bacterium Ralstonia solanacearum. Plants 2020, 9, 516. [Google Scholar] [CrossRef]
- Wang, Y.; Xian, L.; Yu, G.; Macho, A.P. Tomato stem injection for the precise assessment of Ralstonia solanacearum fitness in planta. Bio. Protoc. 2021, 11, e4134. [Google Scholar] [CrossRef] [PubMed]
- Peeters, N.; Guidot, A.; Vailleau, F.; Valls, M. Ralstonia solanacearum, a widespread bacterial plant pathogen in the post-genomic era. Mol. Plant Pathol. 2013, 14, 651–662. [Google Scholar] [CrossRef]
- Genin, S. Molecular traits controlling host range and adaptation to plants in Ralstonia solanacearum. New Phytol. 2010, 187, 920–928. [Google Scholar] [CrossRef]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A. On a remarkable bacteriolytic element found in tissues and secretions. Proc. R. Soc. B 1922, 93, 306–317. [Google Scholar]
- Deckers, D.; Vanlint, D.; Callewaert, L.; Aertsen, A.; Michiels, C.W. Role of the lysozyme inhibitor Ivy in growth or survival of Escherichia coli and Pseudomonas aeruginosa bacteria in hen egg white and in human saliva and breast milk. Appl. Environ. Microbiol. 2008, 74, 4434–4439. [Google Scholar] [CrossRef]
- Caballero, M.; Ruiz, R.; Márquez de Prado, M.; Seco, M.; Borque, L.; Escanero, J.F. Development of a microparticle-enhanced nephelometric immunoassay for quantitation of human lysozyme in pleural effusion and plasma. J. Clin. Lab. Anal. 1999, 13, 301–307. [Google Scholar] [CrossRef]
- He, H.; Wu, S.; Mei, M.; Ning, J.; Li, C.; Ma, L.; Zhang, G.; Yi, L. A combinational strategy for effective heterologous production of functional human lysozyme in Pichia pastoris. Front. Bioeng. Biotechnol. 2020, 8, 118. [Google Scholar] [CrossRef]
- Nakajima, H.; Muranaka, T.; Ishige, F.; Akutsu, K.; Oeda, K. Fungal and bacterial disease resistance in transgenic plants expressing human lysozyme. Plant Cell Rep. 1997, 16, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.D.; Vollmer, W.; Foster, S.J. Different walls for rods and balls: The diversity of peptidoglycan. Mol. Microbiol. 2014, 91, 862–874. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Y.; Wang, L.; Guo, J.; Liu, W.; Meng, G.; Zhang, L.; Li, M.; Cong, L.; Sun, M. Recent Insights Into the Prognostic and Therapeutic Applications of Lysozymes. Front. Pharmacol. 2021, 12, 767642. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Yamada, M.; Kobayashi, K.; Kato, A. Bactericidal action of lysozyme against gram-negative bacteria due to insertion of a hydrophobic pentapeptide into its C-terminus. Biosci. Biotechnol. Biochem. 1992, 56, 1361–1363. [Google Scholar] [CrossRef]
- Weiss, J.; Elsbach, P.; Olsson, I.; Odeberg, H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J. Biol. Chem. 1978, 253, 2664–2672. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Sisson, R.B.; Kenyon, J.; Eichenwald, E.; Macone, A.B.; Goldmann, D. Enhancement of neonatal innate defense: Effects of adding an N-terminal recombinant fragment of bactericidal/permeability-increasing protein on growth and tumor necrosis factor-inducing activity of gram-negative bacteria tested in neonatal cord blood ex vivo. Infect. Immun. 2000, 68, 5120–5125. [Google Scholar] [PubMed]
- Abrahamson, S.L.; Wu, H.; Williams, R.E.; Der, K.; Ottah, N.; Little, R.; Gazzano-Santoro, H.; Theofan, G.; Bauer, R.; Leigh, S.; et al. Biochemical characterization of recombinant fusions of lipopolysaccharide binding protein and bactericidal/permeability-increasing protein. Implications in biological activity. J. Biol. Chem. 1997, 272, 2149–2155. [Google Scholar] [CrossRef]
- Lamping, N.; Hoess, A.; Yu, B.; Park, T.C.; Kirschning, C.J.; Pfeil, D.; Reuter, D.; Wright, S.D.; Herrmann, F.; Schumann, R.R. Effects of site-directed mutagenesis of basic residues (Arg 94, Lys 95, Lys 99) of lipopolysaccharide (LPS)-binding protein on binding and transfer of LPS and subsequent immune cell activation. J. Immunol. 1996, 157, 4648–4656. [Google Scholar] [CrossRef]
- Baron, O.L.; Deleury, E.; Reichhart, J.M.; Coustau, C. The LBP/BPI multigenic family in invertebrates: Evolutionary history and evidences of specialization in mollusks. Dev. Comp. Immunol. 2016, 57, 20–30. [Google Scholar] [CrossRef]
- Alexander, C.; Rietschel, E.T. Bacterial lipopolysaccharides and innate immunity. J. Endotoxin Res. 2001, 7, 167–202. [Google Scholar] [CrossRef]
- Chockalingam, A.; McKinney, C.E.; Rinaldi, M.; Zarlenga, D.S.; Bannerman, D.D. A peptide derived from human bactericidal/permeability-increasing protein (BPI) exerts bactericidal activity against Gram-negative bacterial isolates obtained from clinical cases of bovine mastitis. Vet. Microbiol. 2007, 125, 80–90. [Google Scholar] [CrossRef]
- Kim, H.; Jang, J.H.; Kim, S.C.; Cho, J.H. Enhancement of the antimicrobial activity and selectivity of GNU7 against Gram-negative bacteria by fusion with LPS-targeting peptide. Peptides 2016, 82, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Iizasa, S.; Iizasa, E.; Matsuzaki, S.; Tanaka, H.; Kodama, Y.; Watanabe, K.; Nagano, Y. Arabidopsis LBP/BPI related-1 and -2 bind to LPS directly and regulate PR1 expression. Sci. Rep. 2016, 6, 27527. [Google Scholar] [CrossRef] [PubMed]
- Rogy, M.A.; Oldenburg, H.S.; Calvano, S.E.; Montegut, W.J.; Stackpole, S.A.; Van Zee, K.J.; Marra, M.N.; Scott, R.W.; Seilhammer, J.J.; Moldawer, L.L.; et al. The role of bactericidal/permeability-increasing protein in the treatment of primate bacteremia and septic shock. J. Clin. Immunol. 1994, 14, 120–133. [Google Scholar] [CrossRef]
- Zhao, H.; Tang, J.; Cao, L.; Jia, G.; Long, D.; Liu, G.; Chen, X.; Cai, J.; Shang, H. Characterization of bioactive recombinant antimicrobial peptide parasin I fused with human lysozyme expressed in the yeast Pichia pastoris system. Enzym. Microb. Technol. 2015, 77, 61–67. [Google Scholar] [CrossRef]
- Shao, Y.; Li, C.; Che, Z.; Zhang, P.; Zhang, W.; Duan, X.; Li, Y. Cloning and characterization of two lipopolysaccharide-binding protein/bactericidal permeability-increasing protein (LBP/BPI) genes from the sea cucumber Apostichopus japonicus with diversified function in modulating ROS production. Dev. Comp. Immunol. 2015, 52, 88–97. [Google Scholar] [CrossRef]
- Hamilton, C.D.; Zaricor, B.; Dye, C.J.; Dresserl, E.; Michaels, R.; Caitilyn Allen, C. Ralstonia solanacearum pandemic lineage strain UW551 overcomes inhibitory xylem chemistry to break tomato bacterial wilt resistance. Mol. Plant Pathol. 2024, 25, e13395. [Google Scholar] [CrossRef] [PubMed]
- Jan, P.S.; Huang, H.Y.; Chen, H.M. Expression of a synthesized gene encoding cationic peptide cecropin B in transgenic tomato plants protects against bacterial diseases. Appl. Environ. Microbiol. 2010, 76, 769–775. [Google Scholar] [CrossRef]
- Morais, T.P.; Zaini, P.A.; Chakraborty, S.; Gouran, H.; Carvalho, C.P.; Almeida-Souza, H.O.; Souza, J.B.; Santos, P.S.; Goulart, L.R.; Luz, J.M.Q.; et al. The plant-based chimeric antimicrobial protein SlP14a-PPC20 protects tomato against bacterial wilt disease caused by Ralstonia solanacearum. Plant Sci. 2019, 280, 197–205. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Gao, Y.; Nakanishi, H.; Gao, X.; Li, Z. Highly efficient expression and secretion of human lysozyme using multiple strategies in Pichia pastoris. Biotechnol. J. 2023, 18, e2300259. [Google Scholar] [CrossRef]
- Xu, K.; Ma, C.; Wu, C.; Wu, D. The molecular modification, expression, and the antibacterial effects studies of human lysozyme. Biosci. Biotechnol. Biochem. 2024, 88, 546–554. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Q.; Liu, Y.; Zhang, L.; Ding, W. Overexpression of NtPR-Q. up-regulates multiple defense-related genes in Nicotiana tabacum and enhances plant resistance to Ralstonia solanacearum. Front. Plant Sci. 2017, 8, 1963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ni, L.; Chen, S.; Qin, Y.; Ding, X.; Li, J.; Pan, Y.; Zhang, X. Pterostilbene production of tomato transformed with resveratrol synthase and resveratrol O-methyltransferase genes. Plant sci. 2022, 322, 111343. [Google Scholar] [CrossRef]
- Qiu, J.; Ni, L.; Xia, X.; Chen, S.; Zhang, Y.; Lang, M.; Li, M.; Liu, B.; Pan, Y.; Li, J.; et al. Genome-wide analysis of the protein phosphatase 2C genes in tomato. Genes 2022, 13, 604. [Google Scholar] [CrossRef]
- Shi, P.; Wei, J.; You, H.; Chen, S.; Tan, F.; Lu, Z. Cloning, characterization, and heterologous expression of a candidate Hirudin gene from the salivary gland transcriptome of Hirudo nipponia. Sci. Rep. 2023, 13, 4943. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Xu, L.; Lu, W.; Li, M.; Zhang, R.; Sun, Y.; Liu, J.; Chu, X. Antibacterial peptide PMAP-37(F34-R), expressed in Pichia pastoris, is effective against pathogenic bacteria and preserves plums. Microb. Cell Fact. 2023, 22, 164. [Google Scholar] [CrossRef]
- Liu, X.L. Structural Analysis of Microbial Community in Fruit Juice and Study of Antibacterial Preservative. Master’s Thesis, Wuhan Institute of Technology, Wuhan, China, 2023. [Google Scholar]
- Liu, Q.; Liu, Y.; Tang, Y.; Chen, J.; Ding, W. Overexpression of NtWRKY50 Increases Resistance to Ralstonia solanacearum and Alters Salicylic Acid and Jasmonic Acid Production in Tobacco. Front. Plant Sci. 2017, 8, 1710. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.M.; Jin, X.; Zhu, J.; Mei, H.; Ma, Y.; Chu, F.; Wang, Y.; Li, X. Expression of the antimicrobial peptide cecropin fused with human lysozyme in Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 87, 2169–2176. [Google Scholar] [CrossRef]
- Meng, D.; Li, W.; Shi, L.; Lv, Y.; Sun, X.; Hu, J.; Fan, Z. Expression, purification and characterization of a recombinant antimicrobial peptide Hispidalin in Pichia pastoris. Protein Expr. Purif. 2019, 160, 19–27. [Google Scholar] [CrossRef]
- Ma, Y. Expression of Bactericidal Permeability-Increasing Protein BPI23 and Human Acidic Fibroblast Growth Factor haFGF Fusion Gene in Pichia pastoris and Study of its Biological Activity. Master’s Thesis, Guangdong Pharmaceutical University, Guangzhou, China, 2007. [Google Scholar]
- Meng, D.; Dai, H.; Gao, X.; Zhao, J.; Guo, Y.; Ling, X.; Dong, B.; Zhang, Z.; Fan, Z. Expression, purification and initial characterization of a novel recombinant antimicrobial peptide Mytichitin-A in Pichia pastoris. Protein Expr. Purif. 2016, 127, 35–43. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Lamb, C.; Dixon, R.A. The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef]
- De Gara, L.; de Pinto, M.C.; Tommasi, F. The antioxidant systems vis-à-vis reactive oxygen species during plant–pathogen interaction. Plant Physiol. Biochem. 2003, 41, 863–870. [Google Scholar] [CrossRef]
- Patel, S.J.; Subramanian, R.B.; Jha, Y.S. Biochemical and molecular studies of early blight disease in tomato. Phytoparasitica 2011, 39, 269–283. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Feng, K.; Yu, J.; Cheng, Y.; Ruan, M.; Wang, R.; Ye, Q.; Zhou, G.; Li, Z.; Yao, Z.; Yang, Y.; et al. The SOD gene family in tomato: Identification, Phylogenetic Relationships, and Expression Patterns. Front. Plant Sci. 2016, 7, 1279. [Google Scholar] [CrossRef]
- Su, W.; Raza, A.; Gao, A.; Jia, Z.; Zhang, Y.; Hussain, M.A.; Mehmood, S.S.; Cheng, Y.; Lv, Y.; Zou, X. Genome-wide analysis and expression profile of superoxide dsmutase (SOD) gene family in rapeseed (Brassica napus L.) under different hormones and abiotic stress conditions. Antioxidants 2021, 10, 1182. [Google Scholar] [CrossRef]
- Zhang, Z.; Dang, J.; Yuan, L.; Zhang, Y.; Zhou, F.; Li, T.; Hu, X. Exogenous 5-Aminolevulinic acid improved low-temperature tolerance tomato seedling by regulating starch content and phenylalanine metabolism. Plant Physiol. Biochem. 2024, 210, 108083. [Google Scholar] [CrossRef]
- van Loon, L.C.; Rep, M.; Pieterse, C.M. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, R.K.; Lemaux, P.G.; Buchanan, B.B.; Singh, J. Redox-dependent interaction between thaumatin-like protein and β-glucan influences malting quality of barley. Proc. Natl. Acad. Sci. USA 2017, 114, 7725–7730. [Google Scholar] [CrossRef]
- Cao, J.; Lv, Y.; Hou, Z.; Li, X.; Ding, L. Expansion and evolution of thaumatin-like protein (TLP) gene family in six plants. Plant Growth Regul. 2016, 79, 299–307. [Google Scholar] [CrossRef]
- King, G.J.; Hussey, C.E., Jr.; Turner, V.A. A protein induced by NaCl in suspension cultures of Nicotiana tabacum accumulates in whole plant roots. Plant Mol. Biol. 1986, 7, 441–449. [Google Scholar] [CrossRef]
- Grenier, J.; Potvin, C.; Trudel, J.; Asselin, A. Some thaumatin-like proteins hydrolyse polymeric beta-1,3-glucans. Plant J. 1999, 19, 473–480. [Google Scholar] [CrossRef]
- Park, C.J.; Kim, K.J.; Shin, R.; Park, J.M.; Shin, Y.C.; Paek, K.H. Pathogenesis-related protein 10 isolated from hot pepper functions as a ribonuclease in an antiviral pathway. Plant J. 2004, 37, 186–198. [Google Scholar] [CrossRef]
- Gómez-Gómez, L.; Rubio-Moraga, A.; Ahrazem, O. Molecular cloning and characterisation of a pathogenesis-related protein CsPR10 from Crocus sativus. Plant Biol. 2011, 13, 297–303. [Google Scholar] [CrossRef]
- Bahramnejad, B.; Goodwin, P.H.; Zhang, J.; Atnaseo, C.; Erickson, L.R. A comparison of two class 10 pathogenesis-related genes from alfalfa and their activation by multiple stresses and stress-related signaling molecules. Plant Cell Rep. 2010, 29, 1235–1250. [Google Scholar] [CrossRef]
- Wang, J.; Pan, C.; Wang, Y.; Ye, L.; Wu, J.; Chen, L.; Zou, T.; Lu, G. Genome-wide identification of MAPK, MAPKK, and MAPKKK gene families and transcriptional profiling analysis during development and stress response in cucumber. BMC Genom. 2015, 16, 386. [Google Scholar] [CrossRef]
- Pan, X.; Chen, J.; Yang, A.; Yuan, Q.; Zhao, W.; Xu, T.; Chen, B.; Ren, M.; Geng, R.; Zong, Z.; et al. Comparative transcriptome profiling reveals defense-related genes against Ralstonia solanacearum infection in tobacco. Front. Plant Sci. 2021, 12, 767882. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Tang, Y.; Ding, W. NtPR1a regulates resistance to Ralstonia solanacearum in Nicotiana tabacum via activating the defense-related genes. Biochem. Biophys. Res. Commun. 2019, 508, 940–945. [Google Scholar] [CrossRef]
- Chen, N.; Shao, Q.; Lu, Q.; Li, X.; Gao, Y. Transcriptome analysis reveals differential transcription in tomato (Solanum lycopersicum) following inoculation with Ralstonia solanacearum. Sci. Rep. 2022, 12, 22137. [Google Scholar] [CrossRef]
- Yan, L.; Jin, H.; Raza, A.; Huang, Y.; Gu, D.; Zou, X. WRKY genes provide novel insights into their role against Ralstonia solanacearum infection in cultivated peanut (Arachis hypogaea L.). Front. Plant Sci. 2022, 13, 986673. [Google Scholar] [CrossRef]
- Alves, M.S.; Dadalto, S.P.; Gonçalves, A.B.; De Souza, G.B.; Barros, V.A.; Fietto, L.G. Plant bZIP transcription factors responsive to pathogens: A review. Int. J. Mol. Sci. 2013, 14, 7815–7828. [Google Scholar] [CrossRef] [PubMed]
- Kishi-Kaboshi, M.; Seo, S.; Takahashi, A.; Hirochika, H. The MAMP-Responsive MYB Transcription Factors MYB30, MYB55 and MYB110 Activate the HCAA Synthesis Pathway and Enhance Immunity in Rice. Plant Cell Physiol. 2018, 59, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Ma, F.; Li, R.; Zhou, Q.; Yao, W.; Jiao, Y.; Zhang, C.; Zhang, J.; Wang, X.; Xu, Y.; et al. VpSTS29/STS2 enhances fungal tolerance in grapevine through a positive feedback loop. Plant Cell Environ. 2019, 42, 2979–2998. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y. Empirical analysis on the impact of trust in government, purposes of GM crops, and farmers’ expectations on the delayed commercialization of GM crops in China---Based on Xinjiang and Guangdong survey samples. GM Crops Food 2023, 14, 1–18. [Google Scholar] [CrossRef]
- Jin, Y.; Drabik, D.; Heerink, N.; Wesseler, J. Getting an Imported GM Crop Approved in China. Trends Biotechnol. 2019, 37, 566–569. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, L.; Zhang, Y.; Qin, Y.; Wang, M.; Tang, D.; Chen, L.; Ding, X.; Zheng, Y.; Pan, Y.; Li, J.; et al. The Fusion Gene BPI-LY, Encoding Human Bactericidal/Permeability-Increasing Protein Core Fragments and Lysozyme, Enhanced the Resistance of Transgenic Tomato Plants to Bacterial Wilt. Plants 2025, 14, 1897. https://doi.org/10.3390/plants14131897
Ni L, Zhang Y, Qin Y, Wang M, Tang D, Chen L, Ding X, Zheng Y, Pan Y, Li J, et al. The Fusion Gene BPI-LY, Encoding Human Bactericidal/Permeability-Increasing Protein Core Fragments and Lysozyme, Enhanced the Resistance of Transgenic Tomato Plants to Bacterial Wilt. Plants. 2025; 14(13):1897. https://doi.org/10.3390/plants14131897
Chicago/Turabian StyleNi, Lei, Yue Zhang, Yafei Qin, Mei Wang, Daodao Tang, Liantian Chen, Xing Ding, Yilin Zheng, Yu Pan, Jinhua Li, and et al. 2025. "The Fusion Gene BPI-LY, Encoding Human Bactericidal/Permeability-Increasing Protein Core Fragments and Lysozyme, Enhanced the Resistance of Transgenic Tomato Plants to Bacterial Wilt" Plants 14, no. 13: 1897. https://doi.org/10.3390/plants14131897
APA StyleNi, L., Zhang, Y., Qin, Y., Wang, M., Tang, D., Chen, L., Ding, X., Zheng, Y., Pan, Y., Li, J., & Zhang, X. (2025). The Fusion Gene BPI-LY, Encoding Human Bactericidal/Permeability-Increasing Protein Core Fragments and Lysozyme, Enhanced the Resistance of Transgenic Tomato Plants to Bacterial Wilt. Plants, 14(13), 1897. https://doi.org/10.3390/plants14131897






