The Growth-Promoting Effects of Piriformospora indica on Banana Under Different Concentrations of Phosphorus and Potassium Treatments
Abstract
1. Introduction
2. Results
2.1. Effects of P. indica on Banana Phenotypes Under Varying P and K Concentrations
2.2. Impact of P. indica on Banana Growth-Related Parameters Under Varying P and K Concentrations
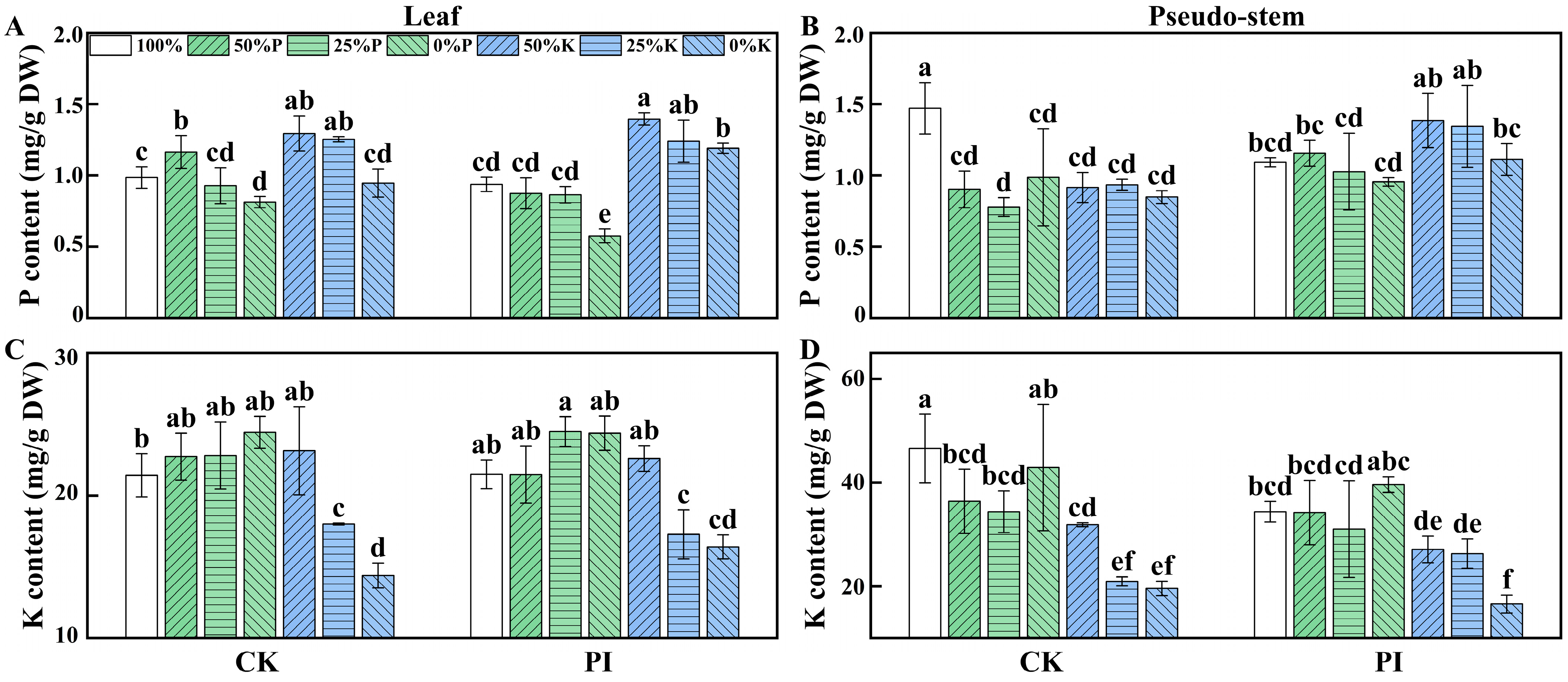
2.3. Effects of P. indica Colonization on the P and K Contents in Banana Leaves and Pseudo-Stems Under Varying P and K Concentrations
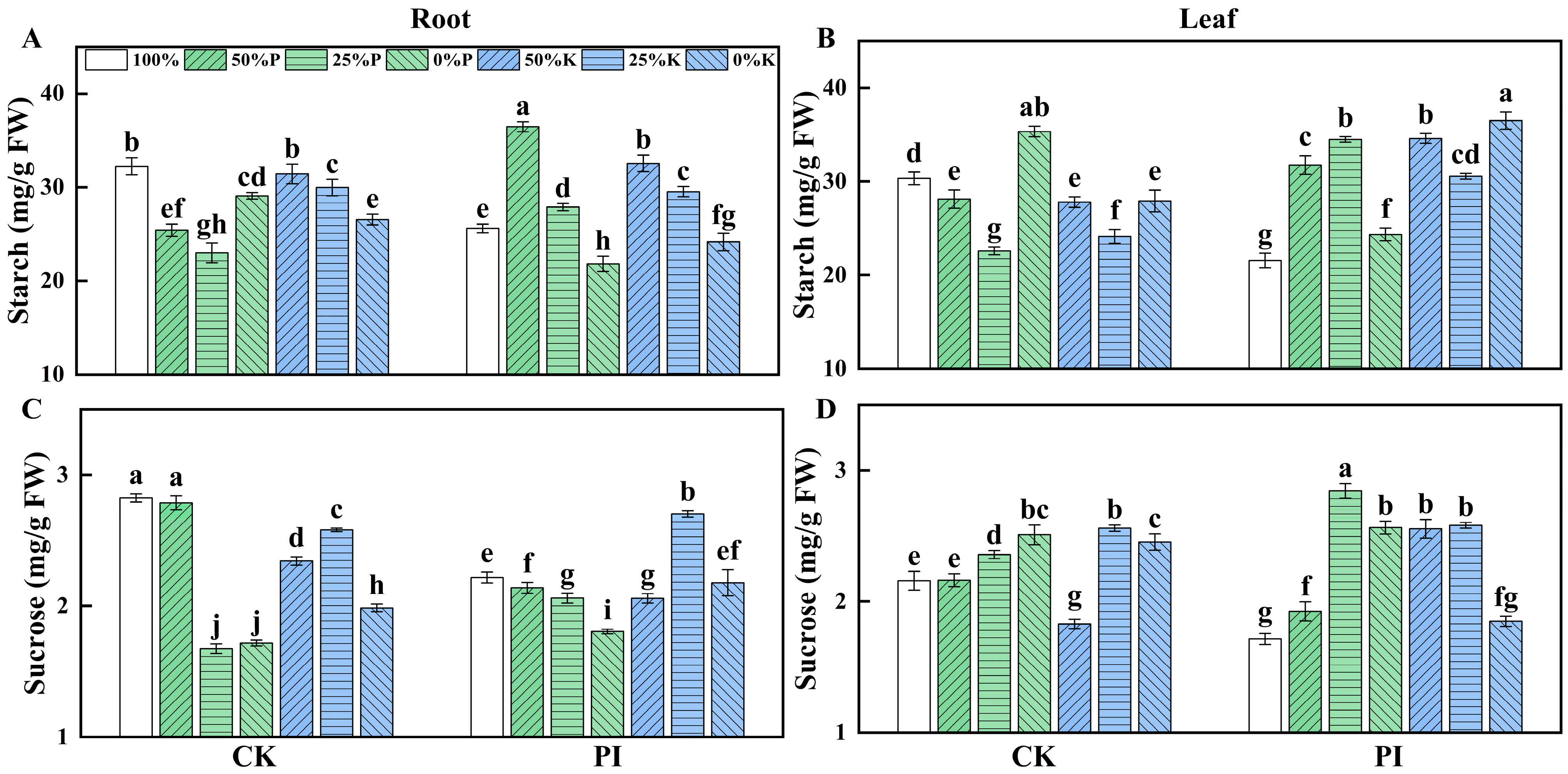
2.4. Effects of P. indica on Starch and Sucrose Accumulations in Banana Roots and Leaves Under Varying P and K Concentrations
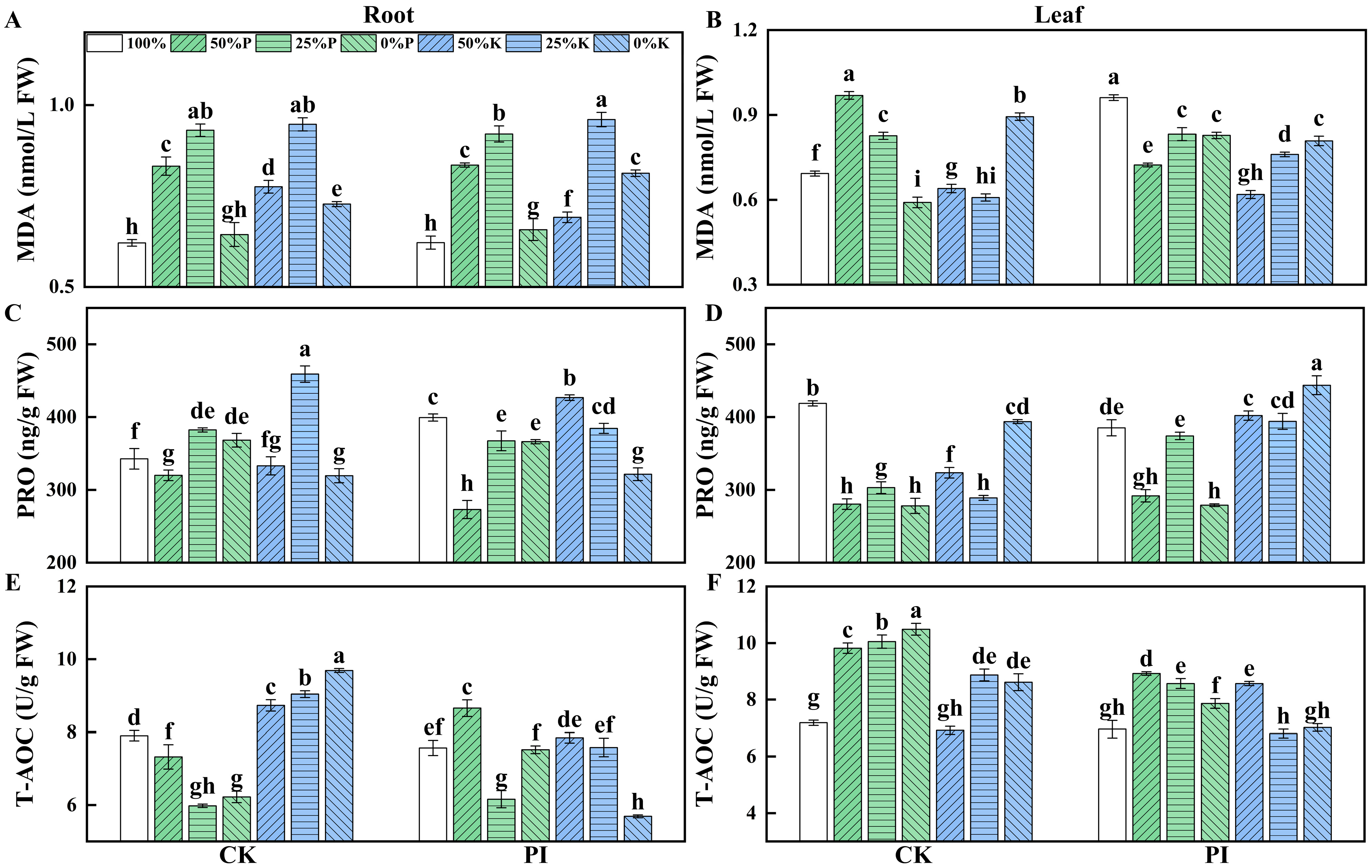
2.5. Effects of P. indica on Osmoprotectants Contents and Total Antioxidant Capacity in Banana Roots and Leaves Under Varying P and K Concentrations
2.6. Effects of P. indica on the Anthocyanins and Flavonoids Contents in Banana Roots and Leaves Under Varying P and K Concentrations
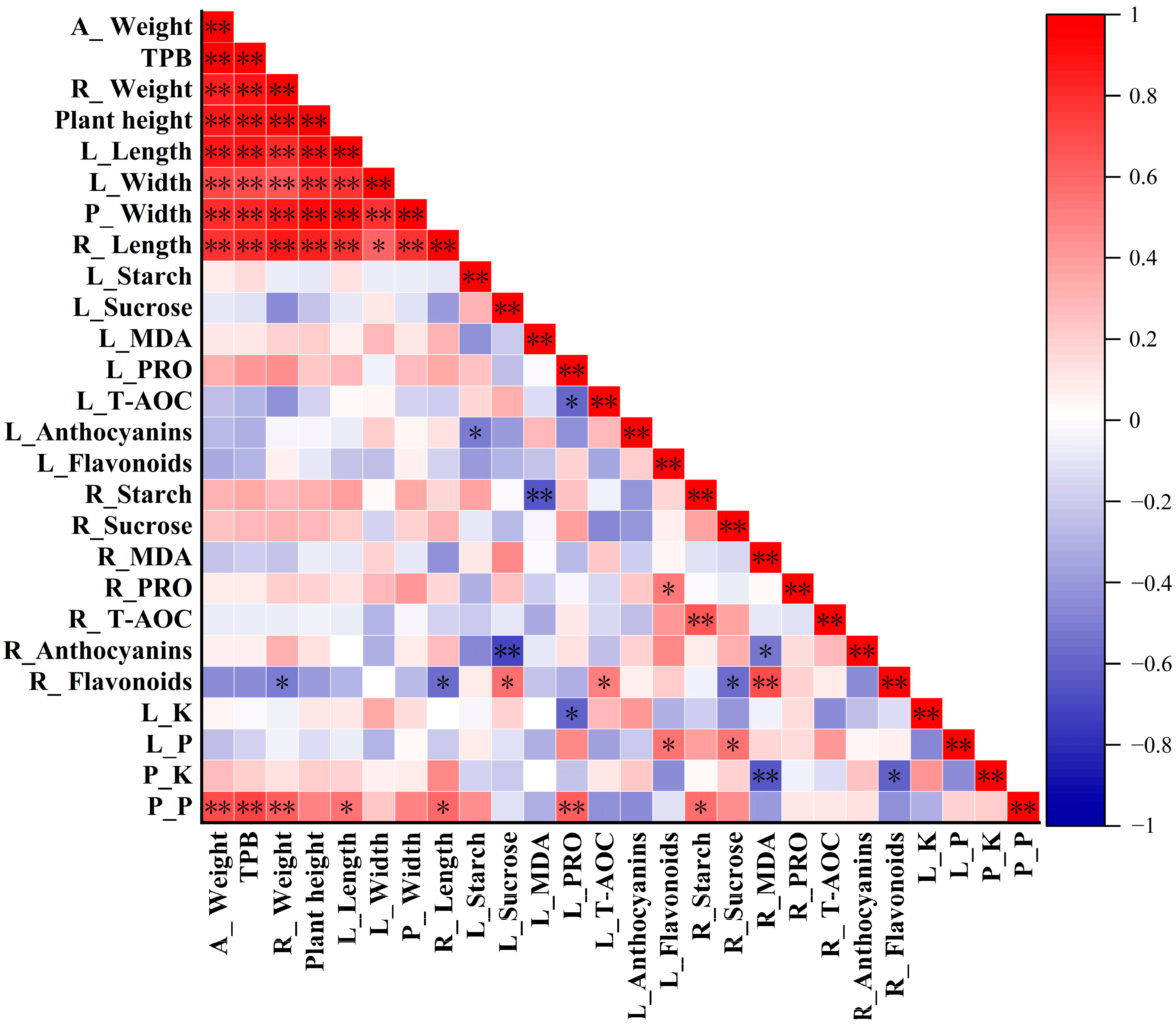
2.7. Correlation Analysis Results of Banana Growth-Related and Physio-Biochemical Parameters
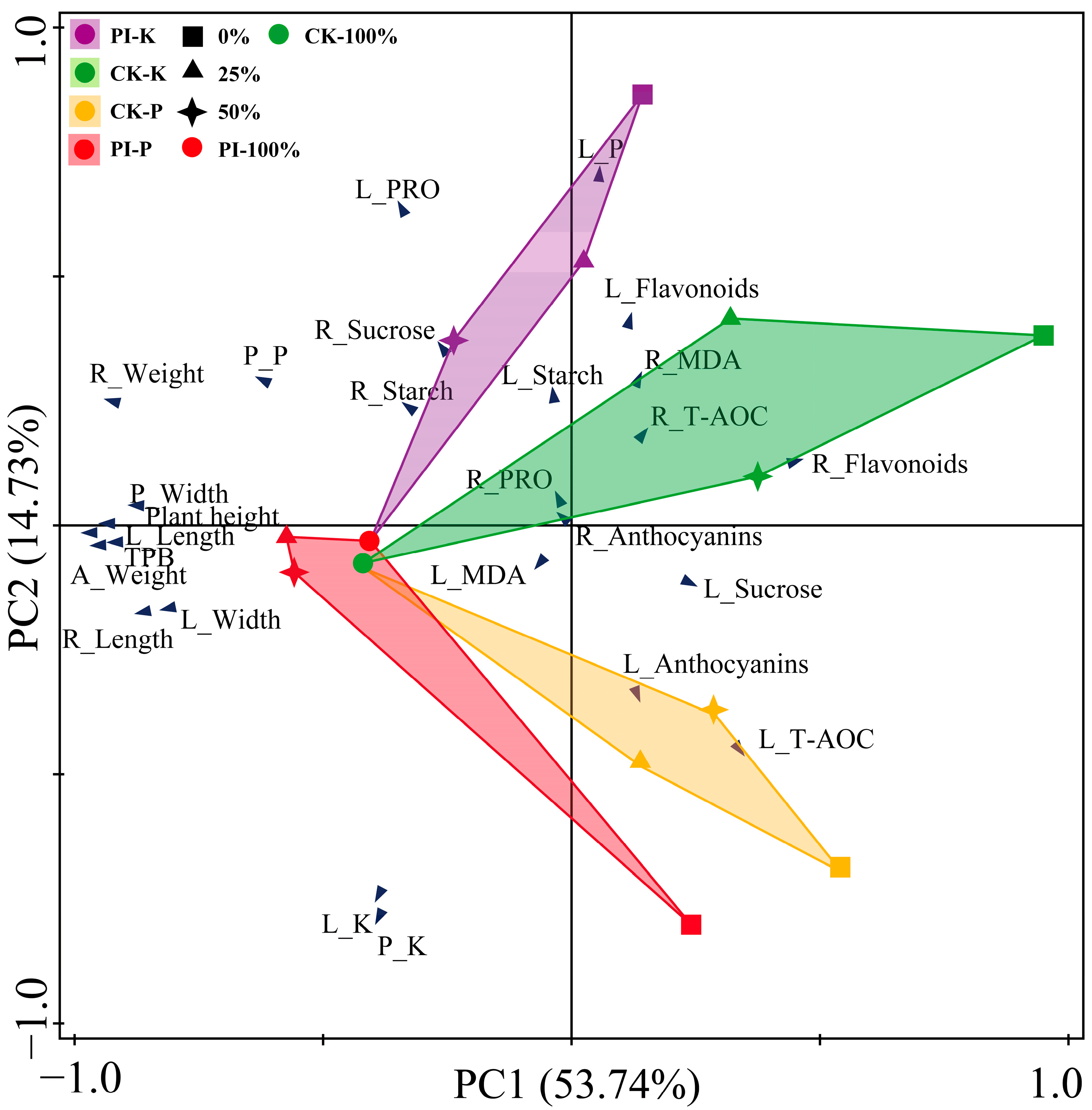
2.8. Principal Component Analysis (PCA) Results
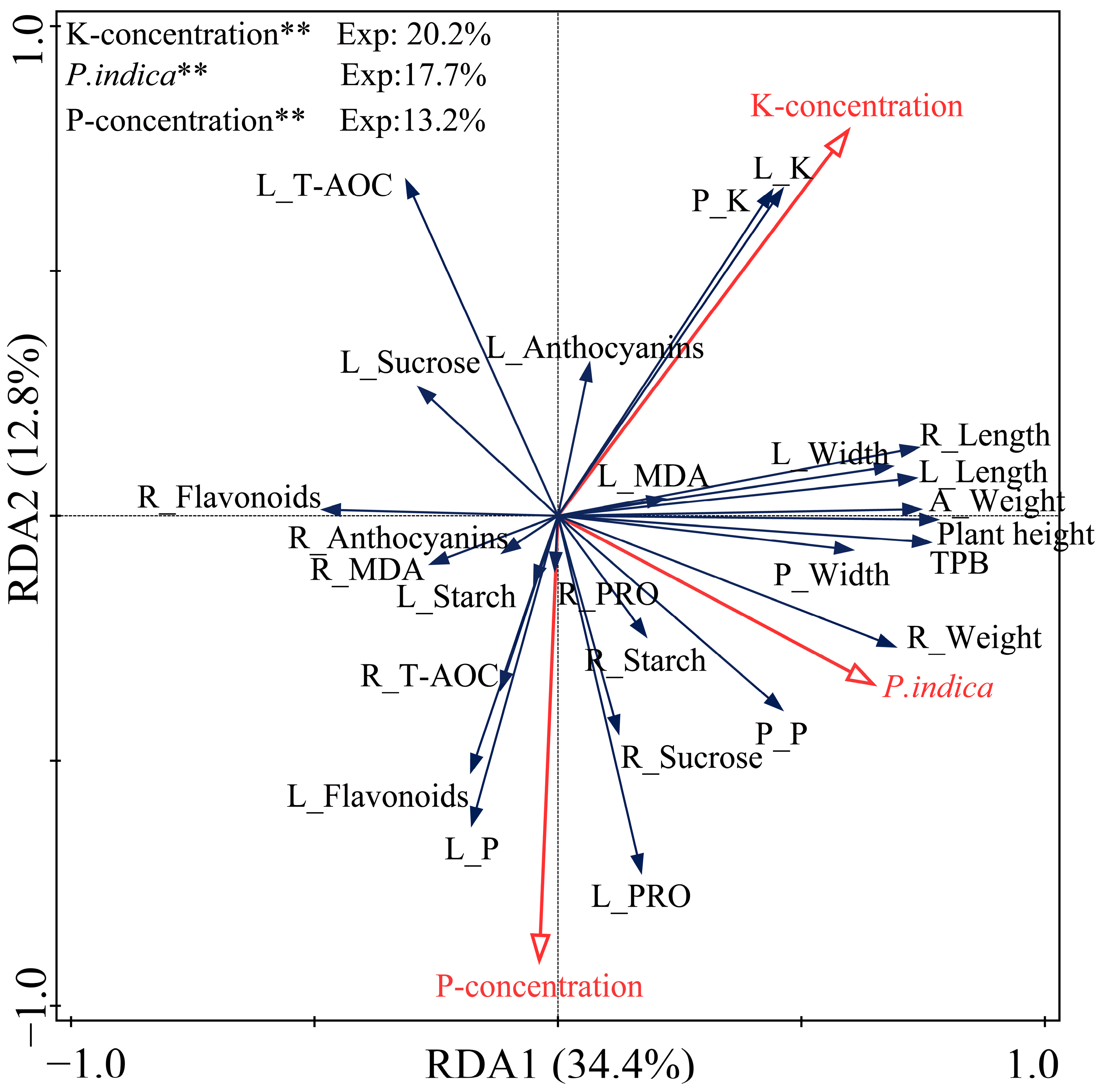
2.9. Redundancy Analysis (RDA) Results
3. Discussion
3.1. P. indica Promoted the Growth of Banana Plants Under Different Concentrations of P and K Treatments
3.2. P. indica Colonization Influences Starch and Sucrose Accumulation in Banana Leaves and Roots Across Different P and K Concentrations
3.3. P. indica Colonization Influenced the Accumulations of Osmoprotectants and Secondary Metabolites in Banana Under Low and Deficient P and K Conditions
4. Materials and Methods
4.1. Plant and Fungal Materials
4.2. P. indica Inoculation
4.3. Plant Treatments with Different Concentrations of P and K
4.4. Measurements of Growth-Related Parameters
4.5. Determinations of P and K Contents in Banana Leaf and Pseudo-Stem
4.6. Determinations of Starch and Sucrose Contents
4.7. Determinations of Malondiadehyde (MDA), Proline (PRO), Anthocyanin, and Flavonoid Contents and Total Antioxidant Capacity (T-AOC)
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CK | noncolonized control |
| DW | dry weight |
| FW | fresh weight |
| K | potassium |
| MDA | malondialdehyde |
| P | phosphorus |
| PCA | principal component analysis |
| PDA | potato dextrose agar |
| PDB | potato dextrose broth |
| PI | Piriformospora indica-colonized |
| PRO | proline |
| RDA | redundancy analysis |
| ROS | reactive oxygen species |
| T-AOC | total antioxidant capacity |
| TPB | total plant biomass |
References
- Verma, S.; Varma, A.; Rexer, K.-H.; Hassel, A.; Kost, G.; Sarbhoy, A.; Bisen, P.; Bütehorn, B.; Franken, P. Piriformospora indica, gen. et sp. nov., a new root-colonizing fungus. Mycologia 1998, 90, 896–903. [Google Scholar] [CrossRef]
- Mensah, R.A.; Li, D.; Liu, F.; Tian, N.; Sun, X.; Hao, X.; Lai, Z.; Cheng, C. Versatile Piriformospora indica and its potential applications in horticultural crops. Hortic. Plant J. 2020, 6, 111–121. [Google Scholar] [CrossRef]
- Sehar, S.; Feng, Q.; Adil, M.F.; Sahito, F.S.; Ibrahim, Z.; Baloch, D.M.; Ullah, N.; Ouyang, Y.; Guo, Y.; Shamsi, I.H. Tandem application of endophytic fungus Serendipita indica and phosphorus synergistically recuperate arsenic induced stress in rice. Front. Plant Sci. 2022, 13, 982668. [Google Scholar] [CrossRef]
- Saddique, M.A.B.; Ali, Z.; Khan, A.S.; Rana, I.A.; Shamsi, I.H. Inoculation with the endophyte Piriformospora indica significantly affects mechanisms involved in osmotic stress in rice. Rice 2018, 11, 34. [Google Scholar] [CrossRef]
- Jisha, S.; Sabu, K.K.; Manjula, S. Multifunctional aspects of Piriformospora indica in plant endosymbiosis. Mycology 2019, 10, 182–190. [Google Scholar]
- Rafique, M.; Naveed, M.; Mumtaz, M.Z.; Niaz, A.; Alamri, S.; ur Rehman, S.; Siddiqui, M.H.; Mustafa, A. Tripartite microbial augmentation of Bradyrhizobium diazoefficiens, Bacillus sp. MN54, and Piriformospora indica on growth, yield, and nutrient profiling of soybean (Glycine max L.). Front. Microbiol. 2025, 15, 1437489. [Google Scholar] [CrossRef]
- Yang, L.; Zou, Y.-N.; Tian, Z.-H.; Wu, Q.-S.; Kuča, K. Effects of beneficial endophytic fungal inoculants on plant growth and nutrient absorption of trifoliate orange seedlings. Sci. Hortic. 2021, 277, 109815. [Google Scholar] [CrossRef]
- Jahandideh Mahjen Abadi, V.A.; Sepehri, M.; Khatabi, B.; Rezaei, M. Alleviation of zinc deficiency in wheat inoculated with root endophytic fungus Piriformospora indica and rhizobacterium Pseudomonas putida. Rhizosphere 2021, 17, 100311. [Google Scholar] [CrossRef]
- Johri, A.K.; Oelmüller, R.; Dua, M.; Yadav, V.; Kumar, M.; Tuteja, N.; Varma, A.; Bonfante, P.; Persson, B.L.; Stroud, R.M. Fungal association and utilization of phosphate by plants: Success, limitations, and future prospects. Front. Microbiol. 2015, 6, 984. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Yadav, B.G.; Kumar, S.G.; Kumar, R.; Kogel, K.-H.; Kumar, S. Piriformospora indica and Azotobacter chroococcum consortium facilitates higher acquisition of N, P with improved carbon allocation and enhanced plant growth in Oryza sativa. J. Fungi 2022, 8, 453. [Google Scholar] [CrossRef]
- Rong, Z.-Y.; Lei, A.-Q.; Wu, Q.-S.; Srivastava, A.K.; Hashem, A.; Abd_Allah, E.F.; Kuča, K.; Yang, T. Serendipita indica promotes P acquisition and growth in tea seedlings under P deficit conditions by increasing cytokinins and indoleacetic acid and phosphate transporter gene expression. Front. Plant Sci. 2023, 14, 1146182. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wei, Q.; Xu, L.; Li, H.; Oelmüller, R.; Zhang, W. Piriformospora indica enhances phosphorus absorption by stimulating acid phosphatase activities and organic acid accumulation in Brassica napus. Plant Soil 2018, 432, 333–344. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, M.M.; Ghosh, T.K.; Kabir, A.H.; Abdelrahman, M.; Rahman Khan, M.A.; Mochida, K.; Tran, L.-S.P. Potassium in plant physiological adaptation to abiotic stresses. Plant Physiol. Bioch. 2022, 186, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Bioch. 2022, 172, 56–69. [Google Scholar] [CrossRef]
- Moreira, B.C.; Mendes, F.C.; Mendes, I.R.; Paula, T.A.; Prates Junior, P.; Salomão, L.C.C.; Stürmer, S.L.; Otoni, W.C.; Guarçoni, A.M.; Kasuya, M.C.M. The interaction between arbuscular mycorrhizal fungi and Piriformospora indica improves the growth and nutrient uptake in micropropagation-derived pineapple plantlets. Sci. Hortic. 2015, 197, 183–192. [Google Scholar] [CrossRef]
- Madaan, G.; Gosal, S.K.; Gosal, S.S.; Saroa, G.S.; Gill, M.I.S. Effect of microbial inoculants on the growth and yield of micropropagated banana (Musa indica) cv. Grand naine. J. Hortic. Sci. Biotechnol. 2013, 88, 643–649. [Google Scholar] [CrossRef]
- Li, D.; Mensah, R.A.; Liu, F.; Tian, N.; Qi, Q.; Yeh, K.; Xuhan, X.; Cheng, C.; Lai, Z. Effects of Piriformospora indica on rooting and growth of tissue-cultured banana (Musa acuminata cv. Tianbaojiao) seedlings. Sci. Hortic. 2019, 257, 108649. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, F.; Wang, B.; Qu, P.; Liu, J.; Zhang, Y.; Liu, W.; Tong, Z.; Deng, G. Influences of Serendipita indica and Dictyophorae echinovolvata on the growth and Fusarium wilt disease resistance of banana. Biology 2022, 11, 393. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, F.; Sun, X.; Wang, B.; Liu, J.; Ni, X.; Hu, C.; Deng, G.; Tong, Z.; Zhang, Y.; et al. Genome-wide identification of FAD gene family and their contributions to the temperature stresses and mutualistic and parasitic fungi colonization responses in banana. Int. J. Biol. Macromol. 2022, 204, 661–676. [Google Scholar] [CrossRef]
- Bodjrenou, M.D.; Sun, X.; Mensah, R.A.; Li, D.; Liu, F.; Tian, N.; Lai, Z.; Cheng, C. Physiological and biochemical mechanisms of Piriformospora indica-induced high-temperature resistance in banana. Chin. J. Appl. Environ. Biol. 2020, 6, 1466–1472. [Google Scholar]
- Bodjrenou, D.M.; Cheng, C.; Sun, X.; Chew, H.; Liu, Y.; Li, D.; Richard, Y.; Lai, Z. High temperature associated microRNAs and their potential roles in mediating heat tolerance in the leaf of banana inoculated with Serendipita indica. J. Hortic. Sci. Biotechnol. 2022, 97, 171–186. [Google Scholar] [CrossRef]
- Cheng, C.; Li, D.; Qi, Q.; Sun, X.; Anue, M.R.; David, B.M.; Zhang, Y.; Hao, X.; Zhang, Z.; Lai, Z. The root endophytic fungus Serendipita indica improves resistance of banana to Fusarium oxysporum f. sp. Cubense tropical race 4. Eur. J. Plant Pathol. 2020, 156, 87–100. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.; Huang, J.; Yang, S.; Mei, H.; Jiang, Y.; Hou, Y.; Peng, J.; Cheng, C.; Li, H.; et al. Integrated transcriptome and sRNAome analysis reveals the molecular mechanisms of Piriformospora indica-mediated resistance to Fusarium wilt in banana. Int. J. Mol. Sci. 2024, 25, 12446. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wu, S.; Deng, G.; Sheng, O.; Yi, G.; Yang, Q. Recent advances and future directions in banana molecular biology and breeding. Mol. Hortic. 2024, 4, 42. [Google Scholar] [CrossRef]
- Sathiamoorthy, S.; Jeyabaskaran, K.J. Potassium management of banana. In Proceedings of the IPI/NARCTT Regional Workshop: Potassium and Water Management in West Asia and North Africa, Amman (JOR), Amman, Jordan, 5–6 November 2001; pp. 499–516. [Google Scholar]
- Tian, N.; Wang, B.; Wu, H.; Wu, J.; Liu, J.; Liu, F.; Tian, Y.; Deng, G.; Cheng, C.; Huang, Y. Effects of Piriformospora indica on the growth and photosynthetic characteristics of bananas under short-term treatments with different concentrations of potassium, phosphorus, and calcium. Chin. J. Appl. Environ. Biol. 2022, 5, 1281–1287. [Google Scholar]
- Ma, R.; Tian, N.; Wang, B.; Liu, J.; Hu, M.; Liu, F.; Lv, P.; Cheng, C. Genome-wide identification and expression analysis of PHT1 gene family in banana. Chin. J. Appl. Environ. Biol. 2022, 28, 1510–1519. [Google Scholar]
- Chen, H. Study on Banana Nutrition Characteristics Under N, P, K, Ca, Mg, S Deficiency and Banana Nutrition Diagnosis. Ph.D. Dissertation, South China Agricultural University, Guangzhou, China, 2017. [Google Scholar]
- Jin, X.; Yang, Y.; Zhang, R.; Fu, C.; Deng, Y.; Huang, J.; Yin, L. Effects of phosphorus supply on nutrient absorption of “Baodao” and ‘Baxi’ banana seedling stage. Mol. Plant Breed. 2018, 21, 7209–7218. [Google Scholar]
- Ezawa, T.; Smith, S.E.; Smith, F.A. P Metabolism and transport in AM fungi. Plant Soil 2002, 244, 221–230. [Google Scholar] [CrossRef]
- Aslam, M.M.; Karanja, J.; Bello, S.K. Piriformospora indica colonization reprograms plants to improved P-uptake, enhanced crop performance, and biotic/abiotic stress tolerance. Physiol. Mol. Plant Pathol. 2019, 106, 232–237. [Google Scholar] [CrossRef]
- Cao, M.-A.; Liu, R.-C.; Xiao, Z.-Y.; Hashem, A.; Abd_Allah, E.F.; Alsayed, M.F.; Harsonowati, W.; Wu, Q.-S. Symbiotic fungi alter the acquisition of phosphorus in Camellia oleifera through regulating root architecture, plant phosphate transporter gene expressions and soil phosphatase activities. J. Fungi 2022, 8, 800. [Google Scholar] [CrossRef]
- Wei, Q.; Wu, M.; Zhang, W. Effects of Piriformospora indica on the growth and phosphorus absorption of lettuce under different phosphorus levels. J. Henan Agric. Sci. 2017, 1, 100–104. [Google Scholar]
- Li, L.; Feng, Y.; Qi, F.; Hao, R. Research progress of Piriformospora indica in improving plant growth and stress resistance to plant. J. Fungi 2023, 9, 965. [Google Scholar] [CrossRef] [PubMed]
- Boorboori, M.R.; Zhang, H.-Y. The role of Serendipita indica (Piriformospora indica) in improving plant resistance to drought and salinity stresses. Biology 2022, 11, 952. [Google Scholar] [CrossRef]
- Attia, H.; Rebah, F.; Ouhibi, C.; Saleh, M.A.; Althobaiti, A.T.; Alamer, K.H.; Ben Nasri, M.; Lachaâl, M. Effect of potassium deficiency on physiological responses and anatomical structure of Basil, Ocimum basilicum L. Biology 2022, 11, 1557. [Google Scholar] [CrossRef] [PubMed]
- Sakhai, F.S.; Movahedi, Z.; Ghabooli, M.; Fard, E.M. Positive effect of Serendipita indica on fenugreek and its tolerance against cadmium stress. Curr. Microbiol. 2025, 82, 182. [Google Scholar] [CrossRef]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef]
- Fang, S.; Wan, Z.; Shen, T.; Liang, G. Potassium attenuates drought damage by regulating sucrose metabolism and gene expression in sesame leaf. Plant Physiol. Biochem. 2024, 209, 108547. [Google Scholar] [CrossRef]
- Cui, J.; Lamade, E.; Tcherkez, G. Potassium deficiency reconfigures sugar export and induces catecholamine accumulation in oil palm leaves. Plant Sci. 2020, 300, 110628. [Google Scholar] [CrossRef]
- Thokchom, S.D.; Gupta, S.; Mewar, S.K.; Kumar, P.; Kalra, C.; Kapoor, R. Metabolome profiling of arbuscular mycorrhizal fungus treated Ocimum tenuiflorum L. provides insights into deviation in allocation of carbon compounds to secondary metabolism. Plant Physiol. Biochem. 2023, 203, 108039. [Google Scholar] [CrossRef]
- Zhu, S.; Shi, F.; Li, H.; Ding, Y.; Chang, W.; Ping, Y.; Song, F. Piriformospora indica alleviates soda saline-alkaline stress in Glycine max by modulating plant metabolism. Front. Plant Sci. 2024, 15, 1406542. [Google Scholar] [CrossRef]
- Farouk, S.; Al-Amri, S.M. Exogenous zinc forms counteract NaCl-induced damage by regulating the antioxidant system, osmotic adjustment substances, and ions in canola (Brassica napus L. cv. Pactol) plants. J. Soil Sci. Plant Nutr. 2019, 19, 887–899. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Zhang, L. AMF Inoculation and phosphorus supplementation alleviates drought induced growth and photosynthetic decline in Nicotiana tabacum by up-regulating antioxidant metabolism and osmolyte accumulation. Environ. Exp. Bot. 2020, 176, 104088. [Google Scholar] [CrossRef]
- Ran, Z.; Ding, W.; Cao, S.; Fang, L.; Zhou, J.; Zhang, Y. Arbuscular mycorrhizal fungi: Effects on secondary metabolite accumulation of traditional Chinese medicines. Plant Biol. 2022, 6, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yang, Y.; Wang, Y.; Zhang, W.; Sun, H. Colonization of root endophytic fungus Serendipita indica improves drought tolerance of Pinus taeda seedlings by regulating metabolome and proteome. Front. Microbiol. 2024, 15, 1294833. [Google Scholar] [CrossRef]
- Bajaj, R.; Huang, Y.; Gebrechristos, S.; Mikolajczyk, B.; Brown, H.; Prasad, R.; Varma, A.; Bushley, K.E. Transcriptional responses of soybean roots to colonization with the root endophytic fungus Piriformospora indica reveals altered phenylpropanoid and secondary metabolism. Sci. Rep. 2018, 8, 10227. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Muhammad Rizwan, H.; Liang, D.; Reichelt, M.; Mithöfer, A.; Scholz, S.S.; Oelmüller, R.; Chen, F. The effect of the root-colonizing Piriformospora indica on passion fruit (Passiflora edulis) development: Initial defense shifts to fitness benefits and higher fruit quality. Food Chem. 2021, 359, 129671. [Google Scholar] [CrossRef]
- Sehar, S.; Adil, M.F.; Ma, Z.; Karim, M.F.; Faizan, M.; Zaidi, S.S.A.; Siddiqui, M.H.; Alamri, S.; Zhou, F.; Shamsi, I.H. Phosphorus and Serendipita indica synergism augments arsenic stress tolerance in rice by regulating secondary metabolism related enzymatic activity and root metabolic patterns. Ecotoxicol. Environ. Saf. 2023, 256, 114866. [Google Scholar] [CrossRef]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Qu, P.; Zhang, Z.; Li, R.; Liu, R.; Zhang, Y.; Cheng, C. Insights into the rooting and growth-promoting effects of endophytic fungus Serendipita indica in blueberry (Vaccinium corymbosum). J. Plant Growth Regul. 2025, 44, 2235–2246. [Google Scholar] [CrossRef]
- Sitko, K.; Gieroń, Ż.; Szopiński, M.; Zieleźnik-Rusinowska, P.; Rusinowski, S.; Pogrzeba, M.; Daszkowska-Golec, A.; Kalaji, H.M.; Małkowski, E. Influence of short-term macronutrient deprivation in maize on photosynthetic characteristics, transpiration and pigment content. Sci. Rep. 2019, 9, 14181. [Google Scholar] [CrossRef]
- He, H.; Jin, X.; Ma, H.; Deng, Y.; Huang, J.; Yin, L. Changes of plant biomass partitioning, tissue nutrients and carbohydrates status in magnesium-deficient banana seedlings and remedy potential by foliar application of magnesium. Sci. Hortic. 2020, 268, 109377. [Google Scholar] [CrossRef]
- Wilschefski, S.; Baxter, M. Inductively coupled plasma mass spectrometry: Introduction to analytical aspects. Clin. Biochem. Rev. 2019, 40, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Qi, N.; Wang, C.; Li, C.; Huang, D.; Li, Y.; Wang, N.; Liao, W. Hydrogen-rich water promotes the formation of bulblets in Lilium davidii var. Unicolor through regulating sucrose and starch metabolism. Planta 2021, 254, 106. [Google Scholar] [CrossRef] [PubMed]
- Aguilar Diaz De Leon, J.; Borges, C.R. Evaluation of oxidative stress in biological samples using the thiobarbituric acid reactive substances assay. J. Vis. Exp. 2020, 159. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Qin, G.; Liu, Y.; Liu, J.; Bian, G.; Zhang, S.; Liu, Y.; Zuo, L.; Cheng, C. Physio-biochemical insights into the cold resistance variations among nectarine (Prunus persica (L.) Batsch var. Nectarina) cultivars. Biology 2024, 13, 222. [Google Scholar] [CrossRef]


| Groups | Plant Height (cm) | Leaf Length (cm) | Leaf Width (cm) | Pseudo-Stem Width (cm) | Root Length (cm) | Root Fresh Weight (g) | Aboveground Part Fresh Weight (g) | Total Plant Biomass (g) |
|---|---|---|---|---|---|---|---|---|
| CK-100% | 19.15 ± 1.02 b | 23.96 ± 1.09 bc | 9.25 ± 0.90 cd | 1.31 ± 0.05 abcd | 45.85 ± 8.84 a | 14.48 ± 1.23 abc | 53.07 ± 8.50 b | 67.55 ± 9.78 ab |
| PI-100% | 20.67 ± 1.55 b | 23.83 ± 1.55 bc | 9.78 ± 0.60 bc | 1.48 ± 0.28 ab | 46.20 ± 6.25 a | 18.30 ± 5.23 a | 49.04 ± 3.05 bc | 67.33 ± 5.49 ab |
| CK-50%P | 15.78 ± 1.21 de | 21.23 ± 1.41 de | 9.28 ± 0.66 cd | 1.23 ± 0.10 cd | 38.60 ± 1.54 abc | 7.55 ± 2.19 def | 31.81 ± 6.96 ef | 39.37 ± 7.31 de |
| PI-50%P | 23.43 ± 1.22 a | 25.87 ± 1.91 b | 10.71 ± 0.98 ab | 1.44 ± 0.04 abc | 43.38 ± 7.41 ab | 17.10 ± 3.48 ab | 65.98 ± 7.15 a | 83.07 ± 10.2 a |
| CK-25%P | 17.14 ± 0.80 cd | 22.98 ± 1.55 cd | 9.92 ± 1.35 bc | 1.31 ± 0.11 abcd | 38.12 ± 8.66 abc | 9.71 ± 2.79 cdef | 35.66 ± 8.40 cdef | 45.38 ± 8.45 cd |
| PI-25%P | 24.52 ± 1.57 a | 28.73 ± 2.62 a | 11.49 ± 1.01 a | 1.54 ± 0.14 a | 43.13 ± 2.73 ab | 16.39 ± 5.32 ab | 67.29 ± 8.90 a | 83.68 ± 14.21 a |
| CK-0%P | 12.41 ± 1.99 fg | 18.12 ± 1.31 fg | 6.81 ± 1.17 fg | 1.10 ± 0.14 de | 33.10 ± 1.50 cde | 6.08 ± 0.86 ef | 26.42 ± 9.84 ef | 32.50 ± 8.98 de |
| PI-0%P | 13.81 ± 1.54 ef | 20.40 ± 1.99 e | 9.72 ± 1.00 bc | 1.12 ± 0.25 de | 32.77 ± 1.05 cde | 6.80 ± 0.94 def | 39.09 ± 5.35 cde | 45.89 ± 6.21 cd |
| CK-50%K | 14.69 ± 1.78 e | 19.18 ± 1.48 ef | 7.72 ± 0.56 ef | 1.17 ± 0.14 de | 26.67 ± 2.31 e | 9.29 ± 2.41 cdef | 27.16 ± 8.69 ef | 36.45 ± 10.73 de |
| PI-50%K | 18.92 ± 1.57 bc | 24.76 ± 1.69 bc | 9.98 ± 0.68 bc | 1.52 ± 0.08 a | 40.27 ± 3.90 abc | 13.92 ± 2.20 abc | 46.57 ± 10.50 bcd | 60.49 ± 12.62 bc |
| CK-25%K | 15.65 ± 1.46 de | 21.01 ± 1.21 de | 8.27 ± 0.75 de | 1.26 ± 0.35 bcd | 27.35 ± 4.47 de | 9.25 ± 3.07 cdef | 33.19 ± 9.14 def | 42.44 ± 12.04 de |
| PI-25%K | 16.71 ± 1.51 d | 20.57 ± 1.68 e | 9.52 ± 0.95 bc | 1.25 ± 0.16 bcd | 36.27 ± 2.23 bcd | 11.69 ± 3.96 bcde | 36.77 ± 10.86 cde | 48.47 ± 14.82 cd |
| CK-0%K | 11.30 ± 1.62 g | 16.70 ± 2.21 g | 6.56 ± 0.85 g | 0.95 ± 0.10 e | 27.03 ± 2.89 e | 5.20 ± 1.73 f | 22.23 ± 2.62 f | 27.43 ± 4.27 e |
| PI-0%K | 14.16 ± 1.23 ef | 20.79 ± 2.02 de | 7.88 ± 0.53 ef | 1.16 ± 0.15 de | 34.65 ± 4.39 bcde | 12.10 ± 4.81 bcd | 36.15 ± 9.22 cdef | 48.26 ± 11.69 cd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.; Li, R.; Tian, N.; Li, Q.; Cheng, C.; Wang, M. The Growth-Promoting Effects of Piriformospora indica on Banana Under Different Concentrations of Phosphorus and Potassium Treatments. Plants 2025, 14, 1878. https://doi.org/10.3390/plants14121878
Zhao B, Li R, Tian N, Li Q, Cheng C, Wang M. The Growth-Promoting Effects of Piriformospora indica on Banana Under Different Concentrations of Phosphorus and Potassium Treatments. Plants. 2025; 14(12):1878. https://doi.org/10.3390/plants14121878
Chicago/Turabian StyleZhao, Boxiang, Ruide Li, Na Tian, Qian Li, Chunzhen Cheng, and Mingyuan Wang. 2025. "The Growth-Promoting Effects of Piriformospora indica on Banana Under Different Concentrations of Phosphorus and Potassium Treatments" Plants 14, no. 12: 1878. https://doi.org/10.3390/plants14121878
APA StyleZhao, B., Li, R., Tian, N., Li, Q., Cheng, C., & Wang, M. (2025). The Growth-Promoting Effects of Piriformospora indica on Banana Under Different Concentrations of Phosphorus and Potassium Treatments. Plants, 14(12), 1878. https://doi.org/10.3390/plants14121878







