Rotation Alleviated the Continuous Cropping Obstacle of Peanut (Arachis hypogaea L.) Cultivation and Optimized the Endophytic Bacterial Communities in Peanut Pods
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description and Experimental Design
2.2. Determination of Major Elements in Peanut Kernels
2.3. DNA Extraction, Amplification, and Sequencing of Pod Samples
2.4. Statistical Analysis and Bioinformatics Analysis
3. Results
3.1. Differences in the Macronutrient Content of Peanut Kernels and Peanut Yield Were Observed Under Various Planting Modes
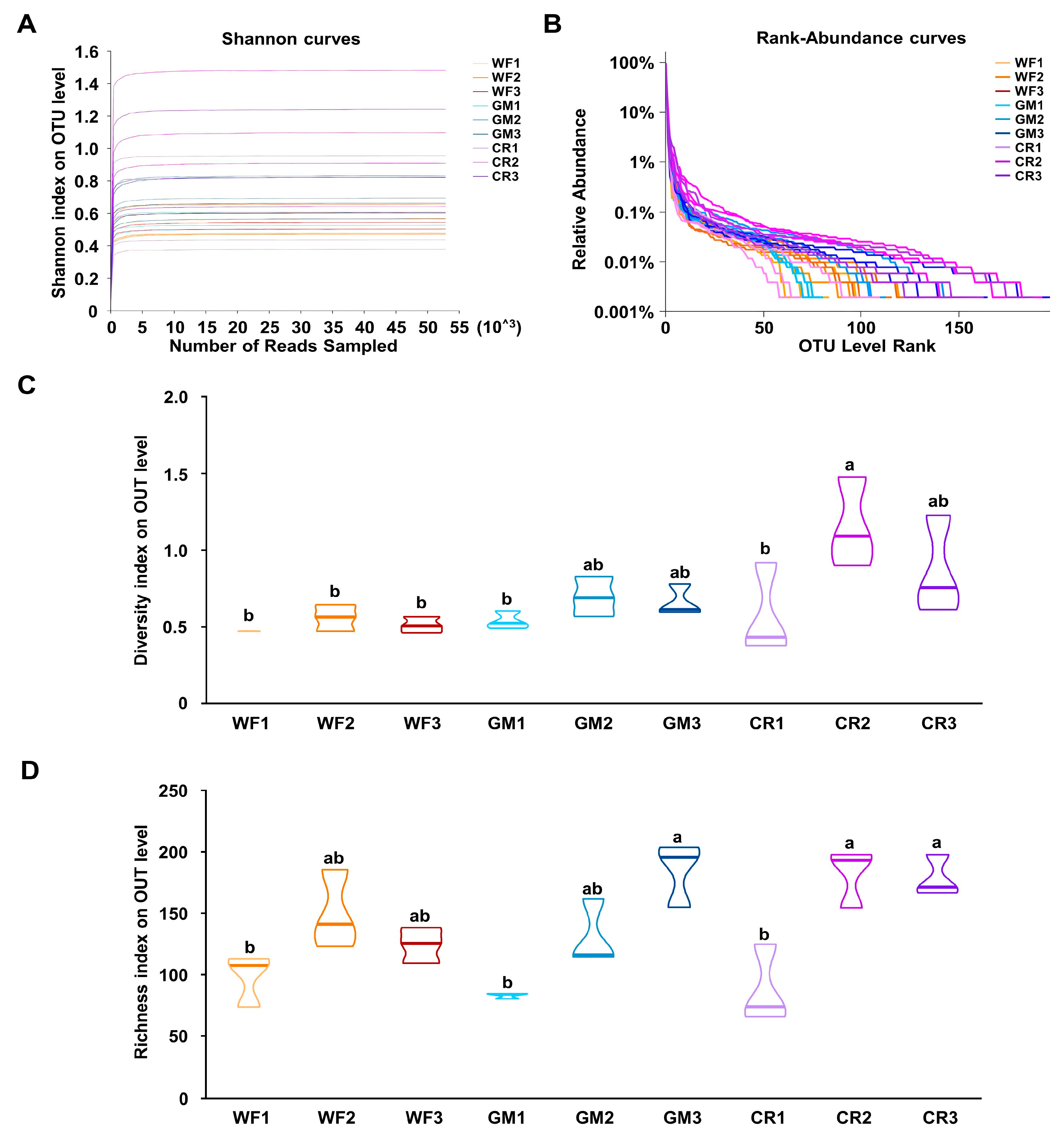
3.2. The α-Diversity Analysis of Endophytic Bacteria in Peanut Pods Under Different Planting Patterns
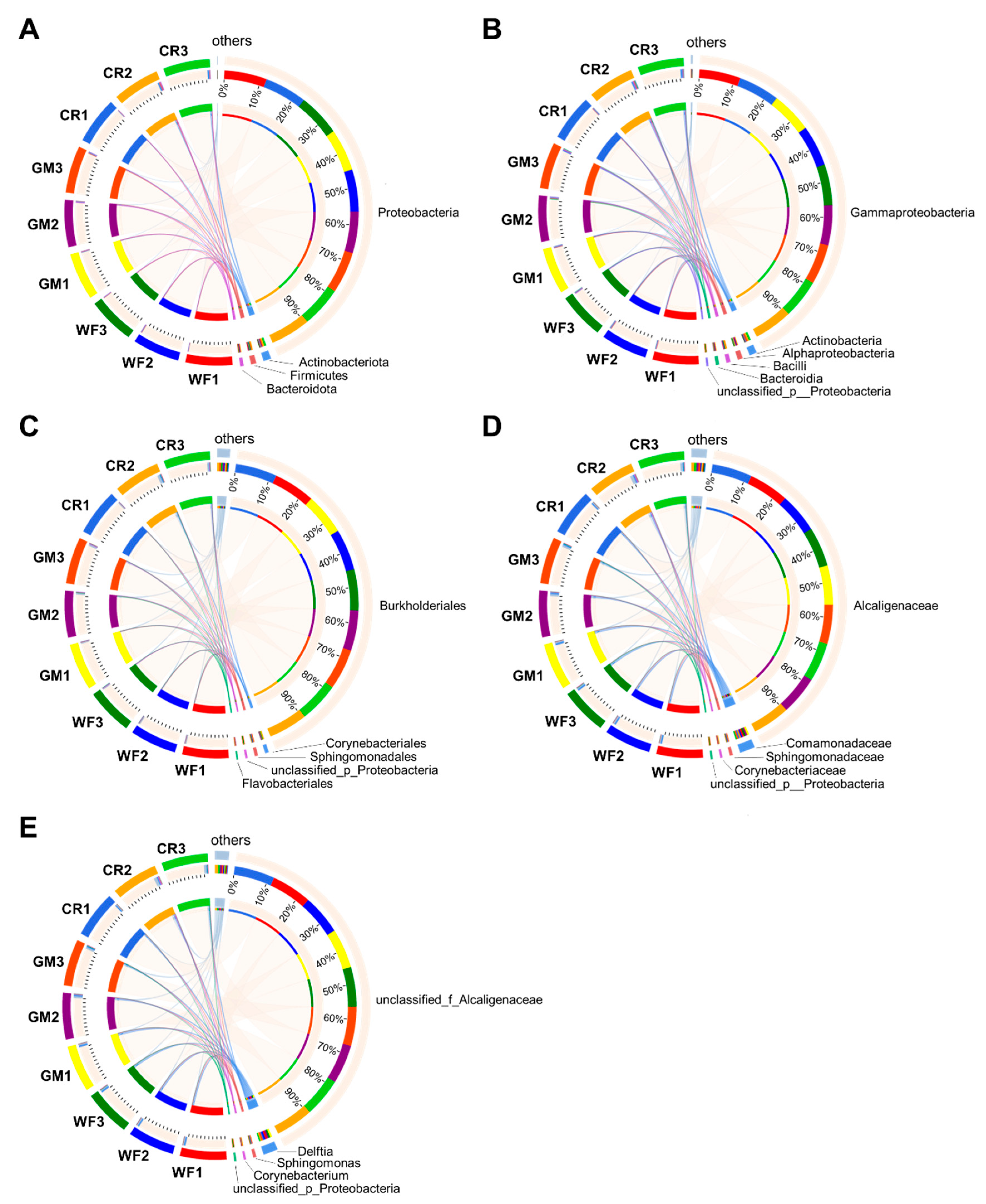
3.3. Circus Diagram Showing the Dominant Flora of Different Samples at Different Species Classification Levels
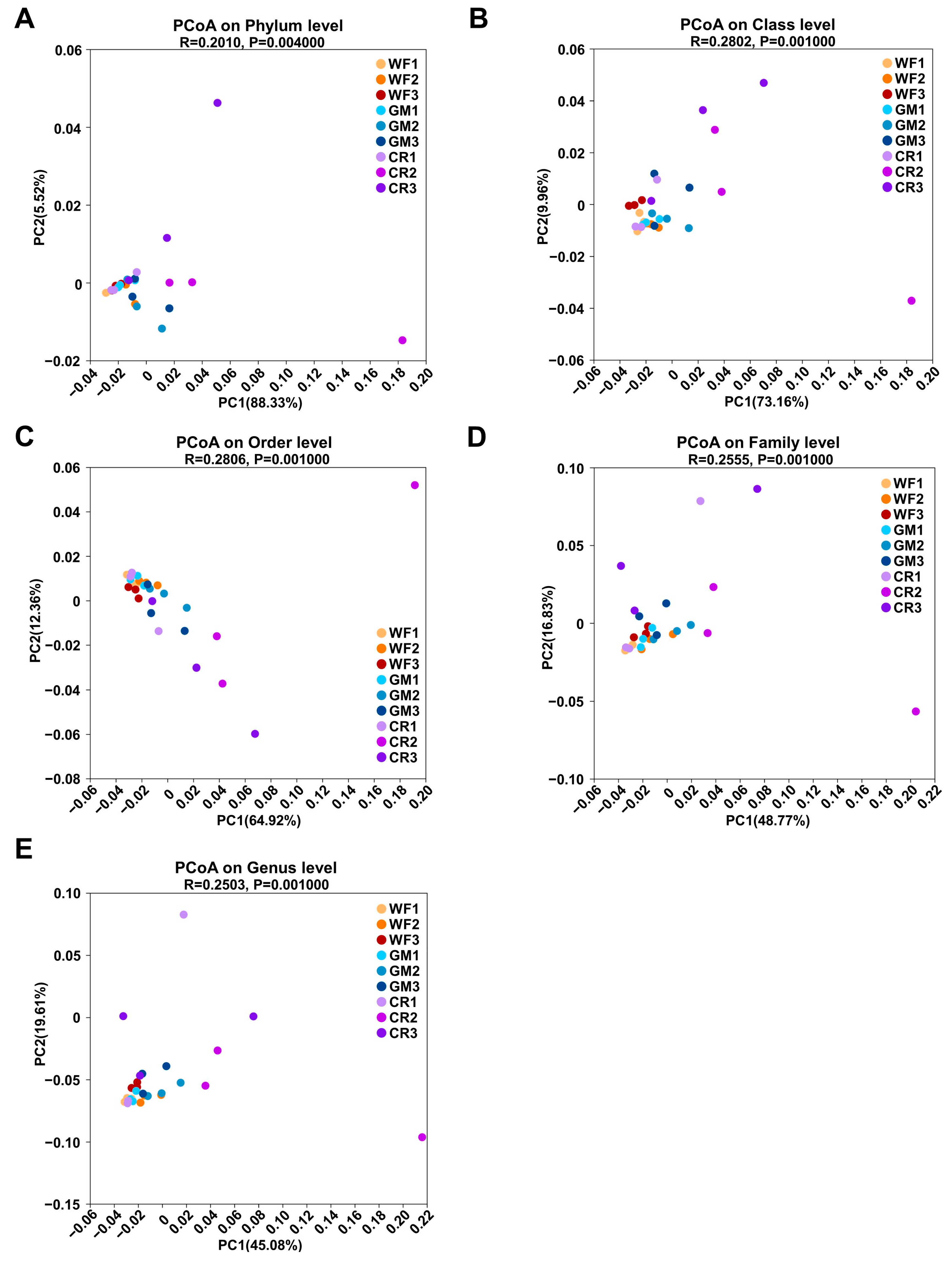
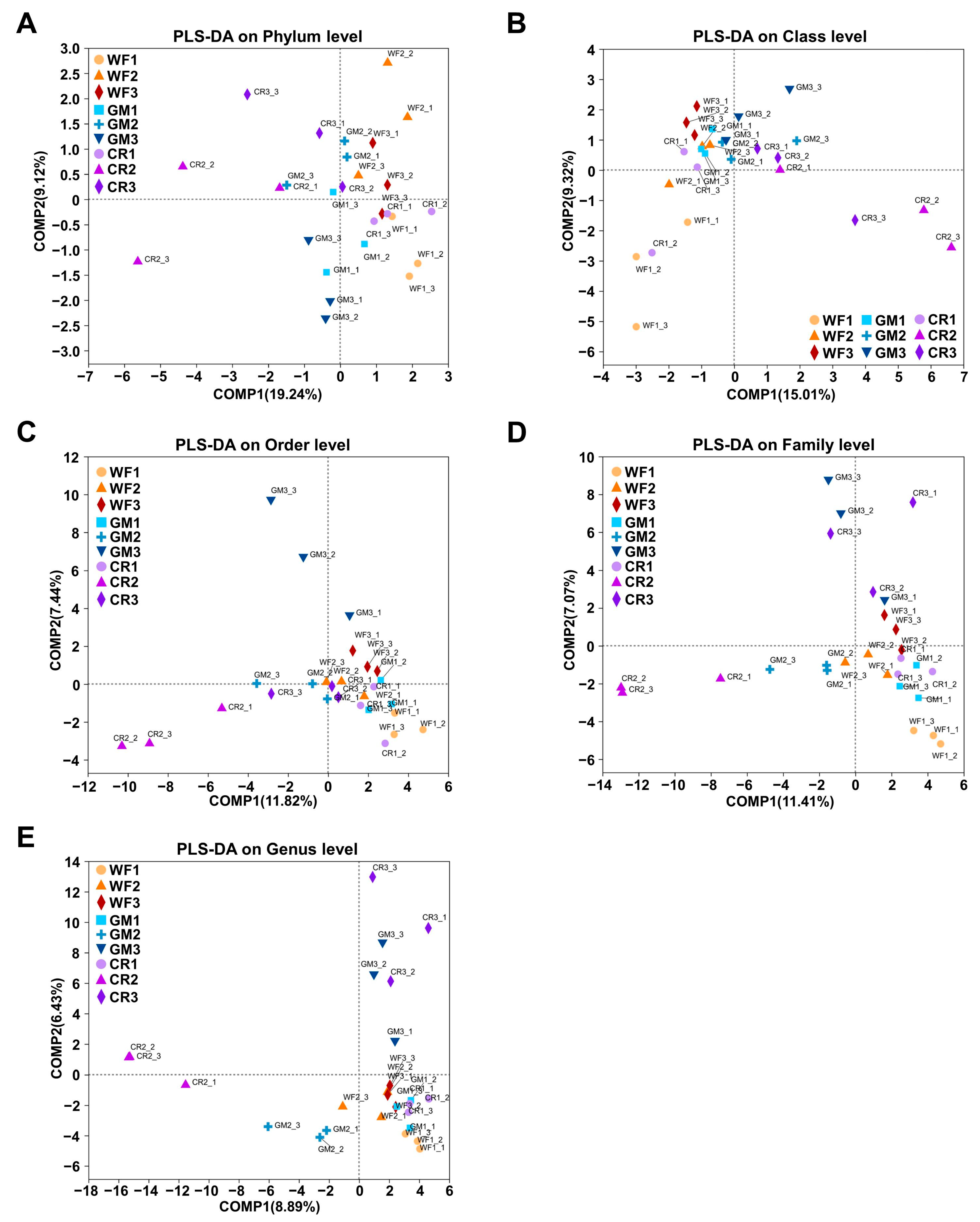
3.4. β-Diversity Analysis of Endophytic Bacteria in Peanut Pods Under Different Planting Patterns
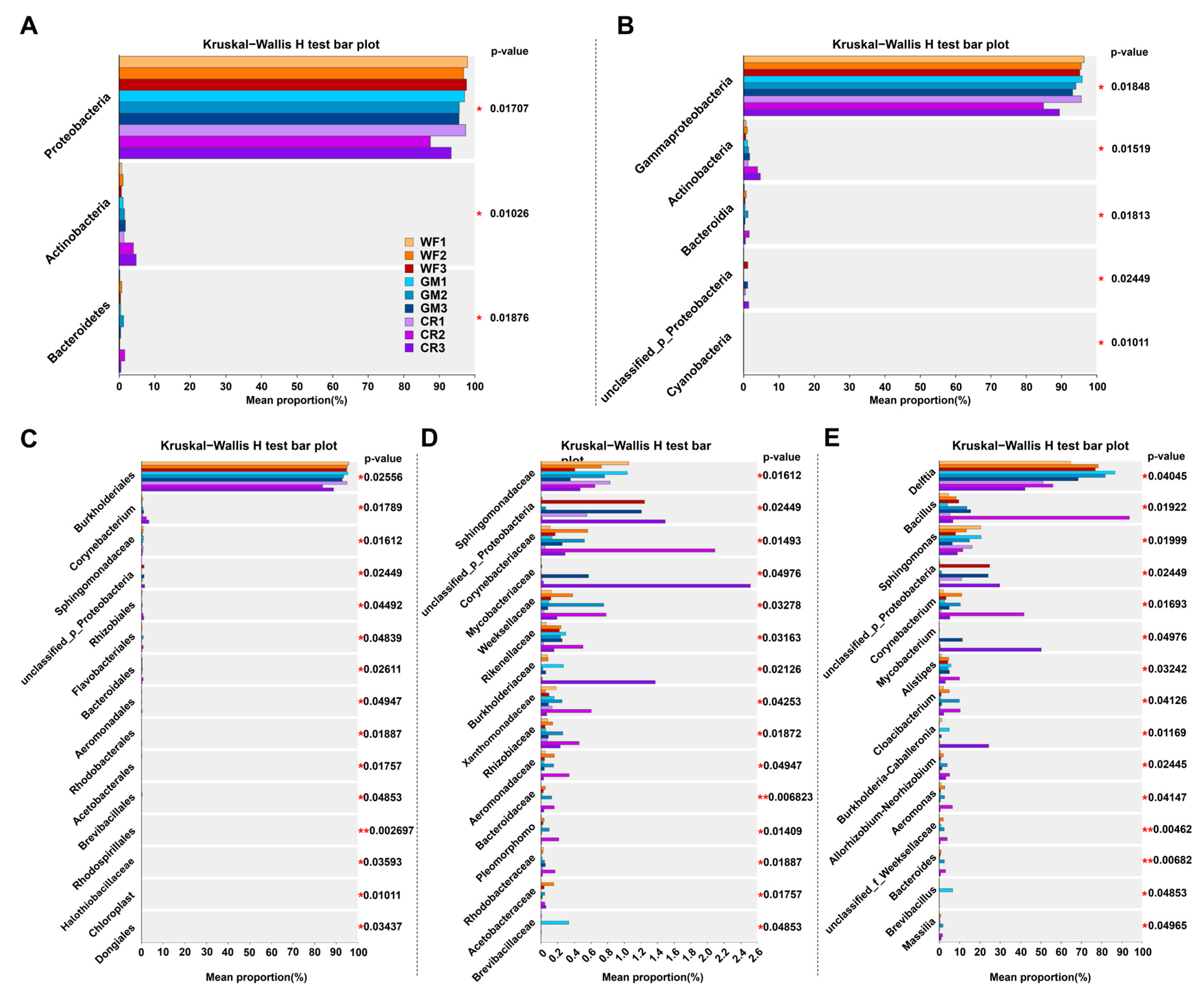
3.5. The LEfSe Multilevel Species Hierarchy Tree Diagram and Latent Dirichlet Assignment (LDA) Showed the Dominant Species Differences in Different Samples
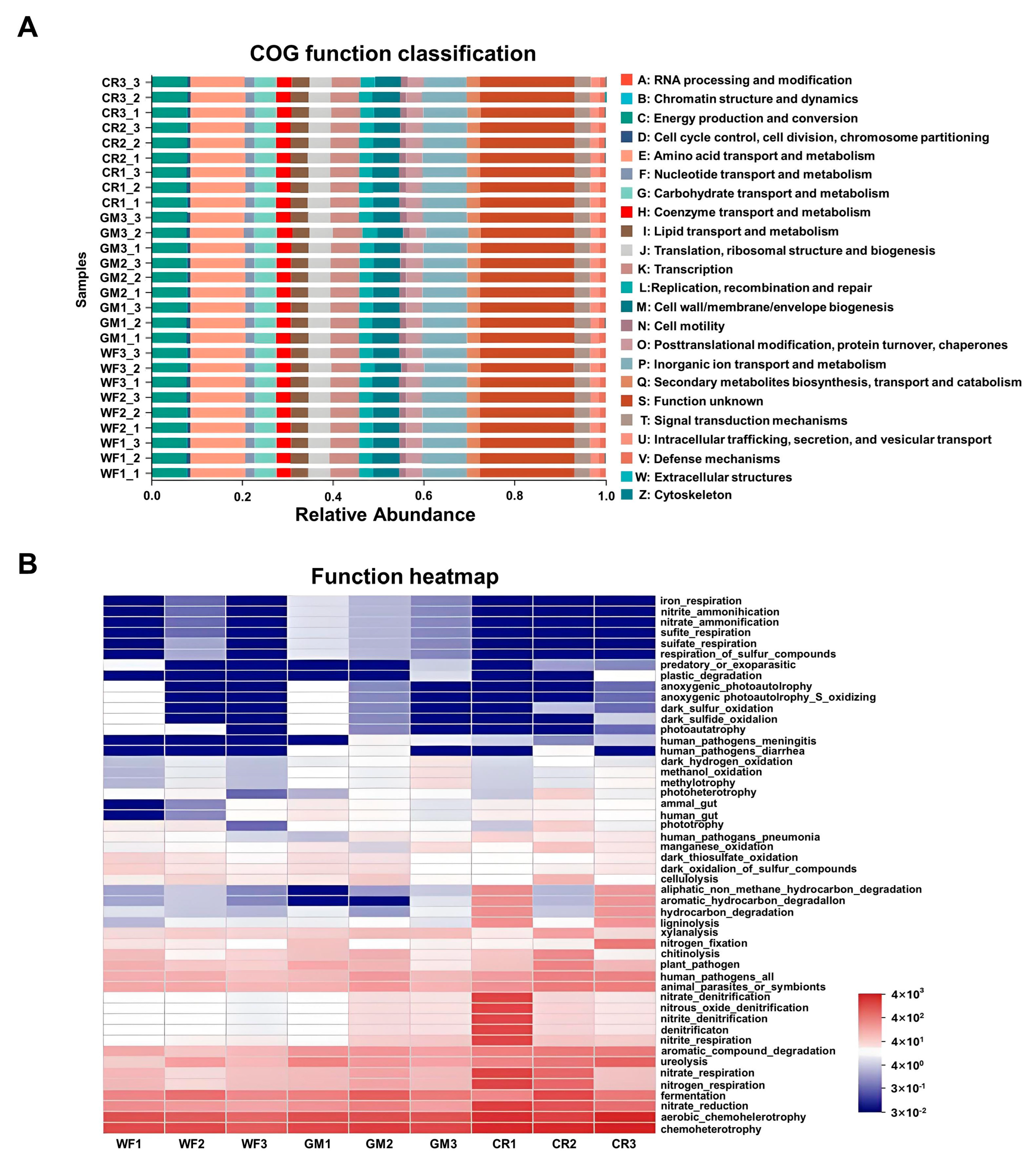
3.6. Microbial Function Prediction
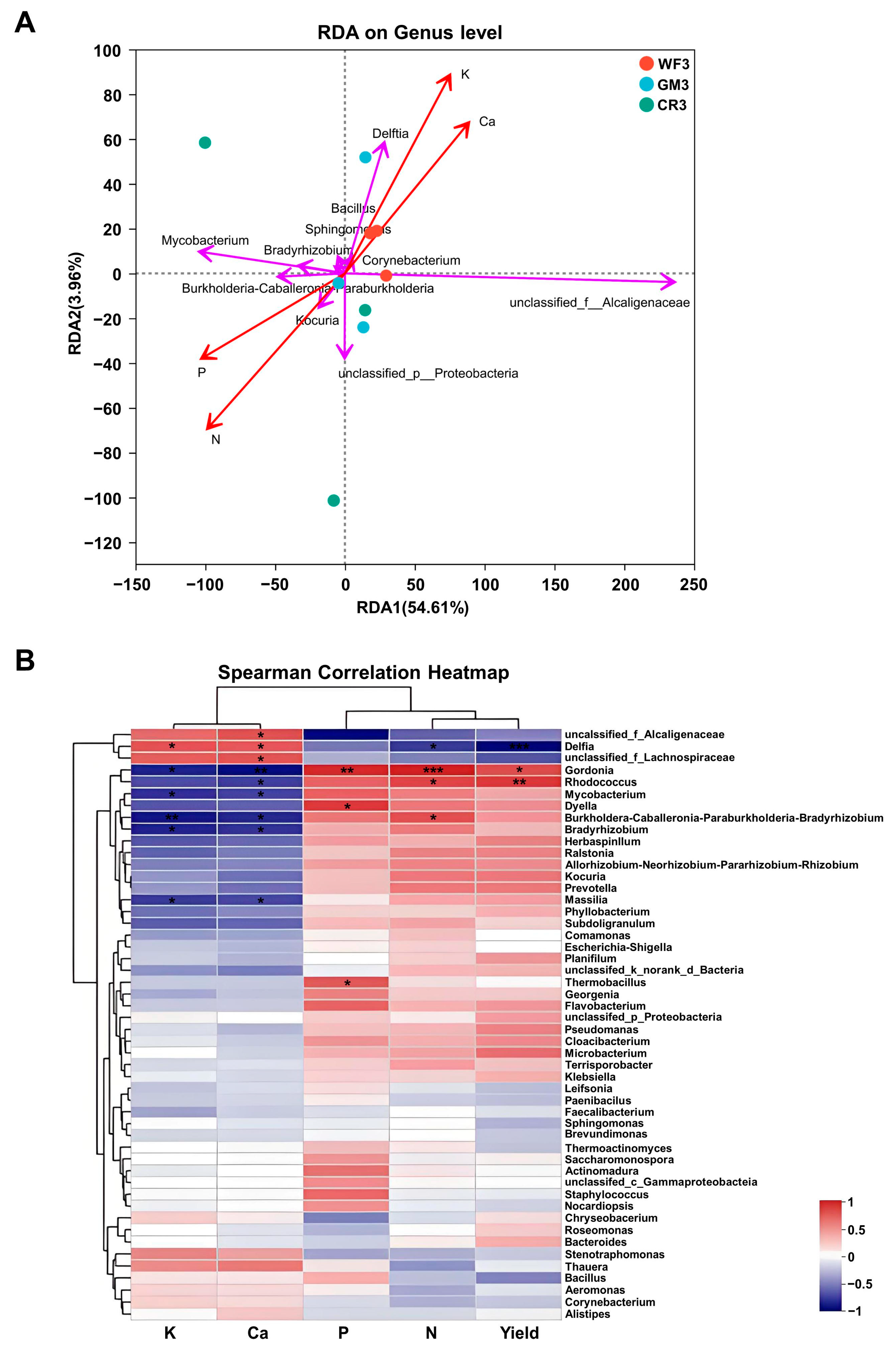
3.7. Correlation Analysis Between the Endophyte Community of Peanut Pods and the Macronutrient Contents of the Peanut Kernels Under Different Planting Methods
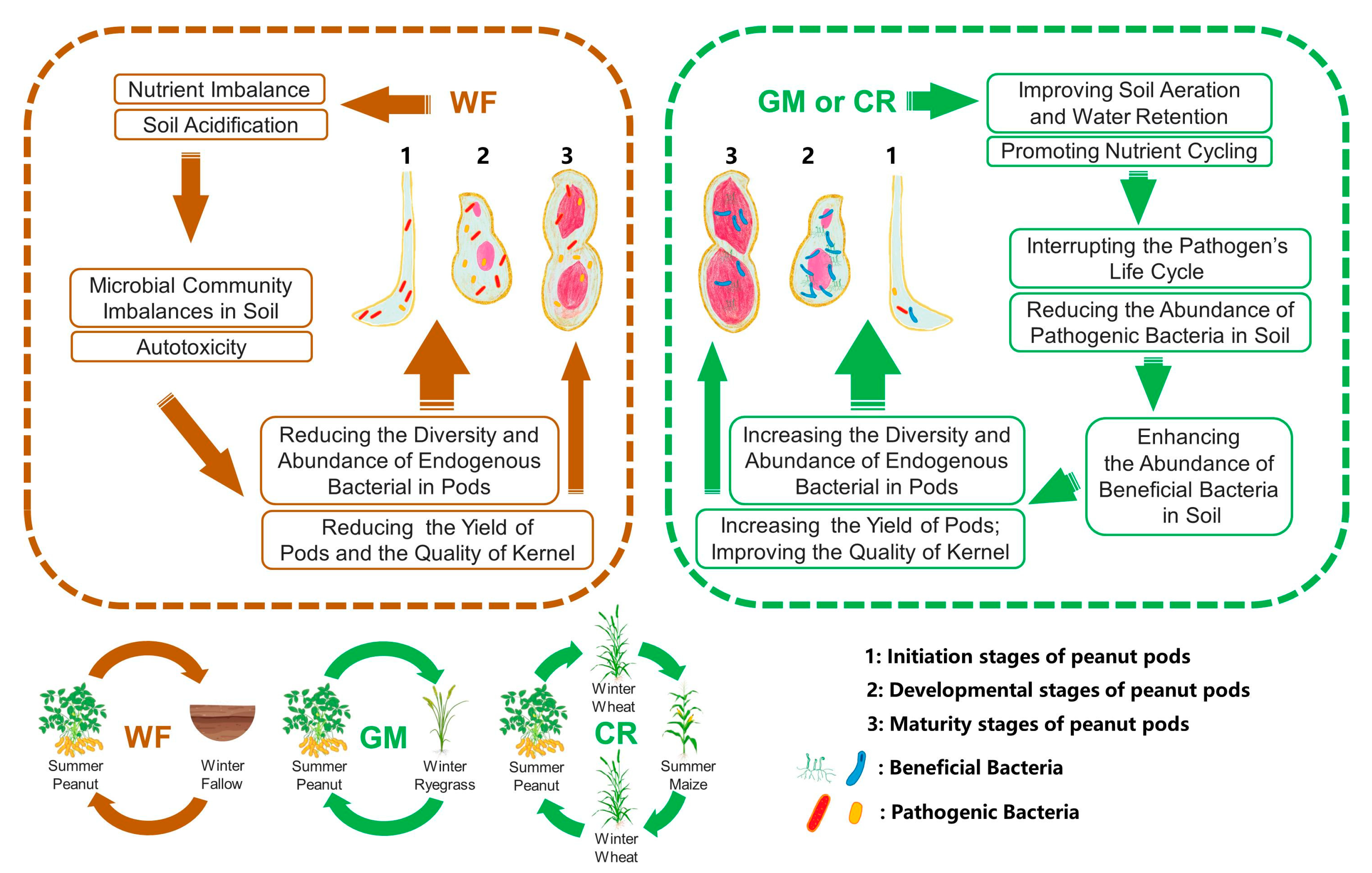
4. Discussion
4.1. Rotation Alleviates Continuous Cropping Obstacles and Increases the Accumulation of Nitrogen in Peanut Kernels and the Yield of Peanut Pods
4.2. Rotation Improves the Colony Structure of Endophytic Bacteria in Peanut Pods, Increasing the Diversity and Richness of Endophytic Bacteria in Peanut Pods
4.3. The Endophytic Bacteria in Peanut Pods Have Two Possible Sources: Vertical Transmission and the Colonization of Soil Rhizosphere Bacteria Within the Pods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Xia, Y.; Li, W.; Ma, X.; Chen, L.; Wang, D.; Qu, C. Recent processing of peanut protein in food industry: A molecular structure perspective. Int. J. Food Sci. Technol. 2024, 59, 2172–2185. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Song, X.; Liu, Y.; Gao, Q.; Zheng, R.; Li, J.; Zhang, P.; Liu, X. Impacts of continuous and rotational cropping practices on soil chemical properties and microbial communities during peanut cultivation. Sci. Rep. 2022, 12, 2758. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Song, Y.; Ge, L.; Pan, X.; Zhao, Y.; Cheng, L.; Li, Y.; Liu, X. Impact of various organic fertilizers on the growth, yield, and soil environment of peanuts subjected to continuous cropping obstacles. Pol. J. Environ. Stud. 2023, 32, 3683–3693. [Google Scholar] [CrossRef] [PubMed]
- Akram, N.; Shafiq, F.; Ashraf, M. Peanut (Arachis hypogaea L.): A prospective legume crop to offer multiple health benefits under changing climate. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1325–1338. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Yu, Z.; Yao, Q.; Li, Y.; Liang, A.; Zhang, W.; Mi, G.; Jin, J.; Liu, X.; et al. Long-term continuous cropping of soybean is comparable to crop rotation in mediating microbial abundance, diversity and community composition. Soil Tillage Res. 2020, 197, 104503. [Google Scholar] [CrossRef]
- Li, Y.; Fang, F.; Wei, J.; Wu, X.; Cui, R.; Li, G.; Zheng, F.; Tan, D. Humic acid fertilizer improved soil properties and soil microbial diversity of continuous cropping peanut: A three-year experiment. Sci. Rep. 2019, 9, 12014. [Google Scholar] [CrossRef]
- Dai, L.; Zhang, G.; Yu, Z.; Ding, H.; Xu, Y.; Zhang, Z. Effect of drought stress and developmental stages on microbial community structure and diversity in peanut rhizosphere soil. Int. J. Mol. Sci. 2019, 20, 2265. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, G.; Ding, H.; Ci, D.; Dai, L.; Zhang, Z. Influence of salt stress on the rhizosphere soil bacterial community structure and growth performance of groundnut (Arachis hypogaea L.). Int. Microbiol. 2020, 23, 453–465. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Q.; Wang, B.; Wang, X.; Franks, A.E.; Teng, Y.; Li, Z.; Luo, Y. Changes in the abundance and structure of bacterial communities under long-term fertilization treatments in a peanut monocropping system. Plant Soil 2015, 395, 415–427. [Google Scholar] [CrossRef]
- Brankatschk, G.; Finkbeiner, M. Modeling crop rotation in agricultural LCAs—Challenges and potential solutions. Agric. Syst. 2015, 138, 66–76. [Google Scholar] [CrossRef]
- Carrière, Y.; Brown, Z.; Aglasan, S.; Dutilleul, P.; Carroll, M.; Head, G.; Tabashnik, B.E.; Jørgensen, P.S.; Carroll, S.P. Crop rotation mitigates impacts of corn rootworm resistance to transgenic Bt corn. Proc. Natl. Acad. Sci. USA 2020, 117, 18385–18392. [Google Scholar] [CrossRef] [PubMed]
- He, H.M.; Liu, L.N.; Munir, S.; Bashir, N.H.; Wang, Y.; Yang, J.; Li, C.Y. Crop diversity and pest management in sustainable agriculture. J. Integr. Agric. 2019, 18, 1945–1952. [Google Scholar] [CrossRef]
- Triberti, L.; Nastri, A.; Baldoni, G. Long-term effects of crop rotation, manure and mineral fertilisation on carbon sequestration and soil fertility. Eur. J. Agron. 2016, 74, 47–55. [Google Scholar] [CrossRef]
- Zou, X.X.; Shi, P.X.; Zhang, C.J.; Si, T.; Wang, Y.F.; Zhang, X.J.; Yu, X.N.; Wang, H.X.; Wang, M.L. Rotational strip intercropping of maize and peanuts has multiple benefits for agricultural production in the northern agropastoral ecotone region of China. Eur. J. Agron. 2021, 129, 126304. [Google Scholar] [CrossRef]
- Qiao, M.; Sun, R.; Wang, Z.; Dumack, K.; Xie, X.; Dai, C.; Wang, E.; Zhou, J.; Sun, B.; Peng, X.; et al. Legume rhizodeposition promotes nitrogen fixation by soil microbiota under crop diversification. Nat. Commun. 2024, 15, 2924. [Google Scholar] [CrossRef]
- Rana, K.L.; Kour, D.; Kaur, T.; Devi, R.; Yadav, A.N.; Yadav, N.; Dhaliwal, H.S.; Saxena, A.K. Endophytic microbes: Biodiversity, plant growth-promoting mechanisms and potential applications for agricultural sustainability. Antonie Leeuwenhoek 2020, 113, 1075–1107. [Google Scholar] [CrossRef]
- Truyens, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Bacterial seed endophytes: Genera, vertical transmission and interaction with plants. Environ. Microbiol. Rep. 2015, 7, 40–50. [Google Scholar] [CrossRef]
- Lodewyckx, C.; Vangronsveld, J.; Porteous, F.; Moore, E.R.; Taghavi, S.; Mezgeay, M.; der Lelie, D.V. Endophytic bacteria and their potential applications. Crit. Rev. Plant Sci. 2002, 21, 583–606. [Google Scholar] [CrossRef]
- Lin, G.Y.; Lin, C.Y.; Chang, S.J.; Lin, W.Y. The dynamics of endophytic bacterial community structure in rice roots under different field management systems. Agronomy 2020, 10, 1623. [Google Scholar] [CrossRef]
- Wu, C.D.; Fan, Y.B.; Chen, X.; Cao, J.W.; Ye, J.Y.; Feng, M.L.; Liu, X.X.; Sun, W.J.; Liu, R.N.; Wang, A.Y. Analysis of endophytic bacterial diversity in seeds of different genotypes of cotton and the suppression of Verticillium wilt pathogen infection by a synthetic microbial community. BMC Plant Biol. 2024, 24, 263. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen-Total. In Methods of Soil Analysis: Part 3 Chemical Methods; SSSA Book Series 5; Soil Science Society of America, Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Isaac, R.A.; Johnson, W.C. Collaborative study of wet and dry ashing techniques for the elemental analysis of plant tissue by atomic absorption spectrophotometry. J. Assoc. Off. Anal. Chem. 1975, 58, 436–440. [Google Scholar] [CrossRef]
- Chen, W.; Teng, Y.; Li, Z.; Liu, W.; Ren, W.; Luo, Y.; Christie, P. Mechanisms by which organic fertilizer and effective microbes mitigate peanut continuous cropping yield constraints in a red soil of south China. Appl. Soil Ecol. 2018, 128, 23–34. [Google Scholar] [CrossRef]
- Yang, T.; Siddique, K.H.M.; Liu, K. Cropping systems in agriculture and their impact on soil health-A review. Glob. Ecol. Conserv. 2020, 23, e01118. [Google Scholar] [CrossRef]
- Chen, W.; Modi, D.; Picot, A. Soil and phytomicrobiome for plant disease suppression and management under climate change: A review. Plants 2023, 12, 2736. [Google Scholar] [CrossRef]
- Cui, J.; Bai, L.; Liu, X.; Jie, W.; Cai, B. Arbuscular mycorrhizal fungal communities in the rhizosphere of a continuous cropping soybean system at the seedling stage. Braz. J. Microbiol. 2018, 49, 240–247. [Google Scholar] [CrossRef]
- Smercina, D.N.; Evans, S.E.; Friesen, M.L.; Tiemann, L.K. To Fix or Not To Fix: Controls on Free-Living Nitrogen Fixation in the Rhizosphere. Appl. Environ. Microbiol. 2019, 85, e02546-18. [Google Scholar] [CrossRef]
- Crusciol, C.; Soratto, R. Nitrogen supply for cover crops and effects on peanut grown in succession under a no-till system. Agron. J. 2009, 101, 41–46. [Google Scholar] [CrossRef]
- Ajijah, N.; Fiodor, A.; Pandey, A.K.; Rana, A.; Pranaw, K. Plant growth-promoting bacteria (PGPB) with biofilm-forming ability: A multifaceted agent for sustainable agriculture. Diversity 2023, 15, 112. [Google Scholar] [CrossRef]
- Eberly, J.O.; Bourgault, M.; Dafo, J.M.; Yeoman, C.J.; Wyffels, S.A.; Lamb, P.F.; Boss, D.L. Soil bacterial community response to cover crop introduction in a wheat-based dryland cropping system. Front. Sustain. Food Syst. 2022, 6, 948220. [Google Scholar] [CrossRef]
- Eid, A.M.; Fouda, A.; Abdel-Rahman, M.A.; Salem, S.S.; Elsaied, A.; Oelmüller, R.; Hijri, M.; Bhowmik, A.; Elkelish, A.; Hassan, S.E.D. Harnessing bacterial endophytes for promotion of plant growth and biotechnological applications: An overview. Plants 2021, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Sahu, P.K.; Kumar, K.; Pal, G.; Gond, S.K.; Kharwar, R.N.; White, J.F. Endophyte roles in nutrient acquisition, root system architecture development and oxidative stress tolerance. J. Appl. Microbiol. 2021, 131, 2161–2177. [Google Scholar] [CrossRef] [PubMed]
- Reinhold-Hurek, B.; Hurek, T. Living inside plants: Bacterial endophytes. Curr. Opin. Plant Biol. 2011, 14, 435–443. [Google Scholar] [CrossRef]
- Ham, J.; Melanson, R.; Rush, M.C. Burkholderia glumae: Next major pathogen of rice? Mol. Plant Pathol. 2021, 12, 329–339. [Google Scholar] [CrossRef]
- Elshafie, H.; Camele, I. An overview of metabolic activity, beneficial and pathogenic aspects of Burkholderia Spp. Metabolites 2021, 11, 321. [Google Scholar] [CrossRef]
- El-Sayed, M.H.; Kobisi, A.E.N.A.; Elsehemy, I.A.; El-Sakhawy, M.A. Rhizospheric-derived Nocardiopsis alba BH35 as an effective biocontrol agent actinobacterium with antifungal and plant growth-promoting effects: In vitro studies. J. Microbiol. Biotechnol. 2023, 33, 607. [Google Scholar] [CrossRef] [PubMed]
- Blake, C.; Christensen, M.; Kovács, Á. Molecular aspects of plant growth promotion and protection by Bacillus subtilis. Mol. Plant Microbe Interact. 2021, 34, 15–25. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Kämpfer, P.; Glaeser, S.P.; McInroy, J.A.; Busse, H.J. Nocardioides zeicaulis sp. nov., an endophyte actinobacterium of maize. Int. J. Syst. Evol. Microbiol. 2016, 66, 1869–1874. [Google Scholar] [CrossRef]
- Samuel, S.O.; Suzuki, K.; Asiloglu, R.; Harada, N. Rice endophytic communities are strongly dependent on microbial communities specific to each soil. Biol. Fertil. Soils 2023, 59, 733–746. [Google Scholar] [CrossRef]

| Cropping Patterns | Nitrogen Content (g/kg) | Phosphorus Content (g/kg) | Potassium Content (g/kg) | Calcium Content (g/kg) | Peanut Pod Yield (t/ha) |
|---|---|---|---|---|---|
| WF | 40.39 ± 0.25 c | 2.17 ± 0.07 a | 6.70 ± 0.06 a | 7.68 ± 0.09 a | 2.71 ± 0.08 b |
| GM | 40.99 ± 0.07 b | 2.32 ± 0.09 a | 6.21 ± 0.15 b | 7.13 ± 0.19 b | 2.58 ± 0.50 b |
| CR | 43.14 ± 0.08 a | 2.32 ±0.11 a | 5.44 ± 0.25 c | 6.49 ± 0.13 c | 3.60 ± 0.20 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Shen, P.; Wu, Q.; Liang, H.; Chen, D.; Yang, L. Rotation Alleviated the Continuous Cropping Obstacle of Peanut (Arachis hypogaea L.) Cultivation and Optimized the Endophytic Bacterial Communities in Peanut Pods. Plants 2025, 14, 1799. https://doi.org/10.3390/plants14121799
Liu M, Shen P, Wu Q, Liang H, Chen D, Yang L. Rotation Alleviated the Continuous Cropping Obstacle of Peanut (Arachis hypogaea L.) Cultivation and Optimized the Endophytic Bacterial Communities in Peanut Pods. Plants. 2025; 14(12):1799. https://doi.org/10.3390/plants14121799
Chicago/Turabian StyleLiu, Miao, Pu Shen, Qi Wu, Haiyan Liang, Dianxu Chen, and Liyu Yang. 2025. "Rotation Alleviated the Continuous Cropping Obstacle of Peanut (Arachis hypogaea L.) Cultivation and Optimized the Endophytic Bacterial Communities in Peanut Pods" Plants 14, no. 12: 1799. https://doi.org/10.3390/plants14121799
APA StyleLiu, M., Shen, P., Wu, Q., Liang, H., Chen, D., & Yang, L. (2025). Rotation Alleviated the Continuous Cropping Obstacle of Peanut (Arachis hypogaea L.) Cultivation and Optimized the Endophytic Bacterial Communities in Peanut Pods. Plants, 14(12), 1799. https://doi.org/10.3390/plants14121799






