The Enhancement of a Saccharum spontaneum Population and a Genetic Impact Analysis of the Agronomic and Yield Traits of Its Progeny
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hybrid Crosses
2.3. Field Experiment
2.4. Statistical Analysis
3. Results
3.1. Analysis of Agronomic Traits and Screening of Excellent Offspring Based on Data from Experiment 1
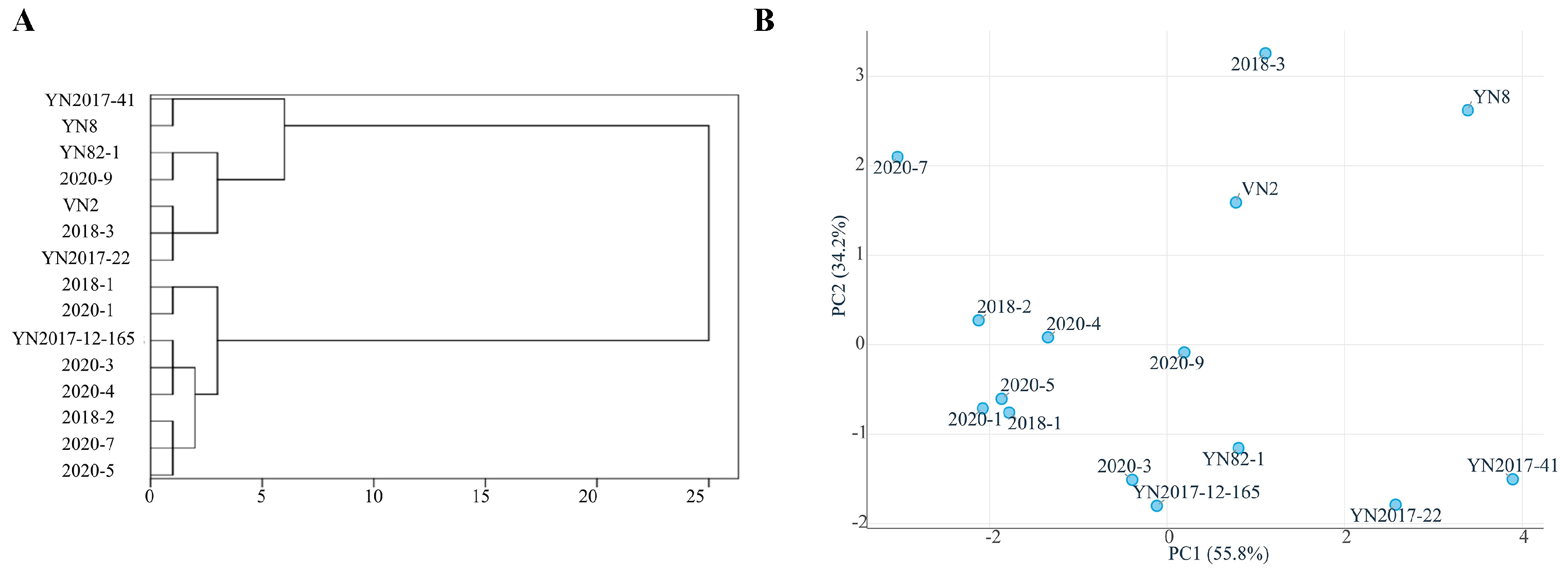
3.2. Genetic Progress and Comparative Analysis of Hybrids Based on Data from Experiment 2
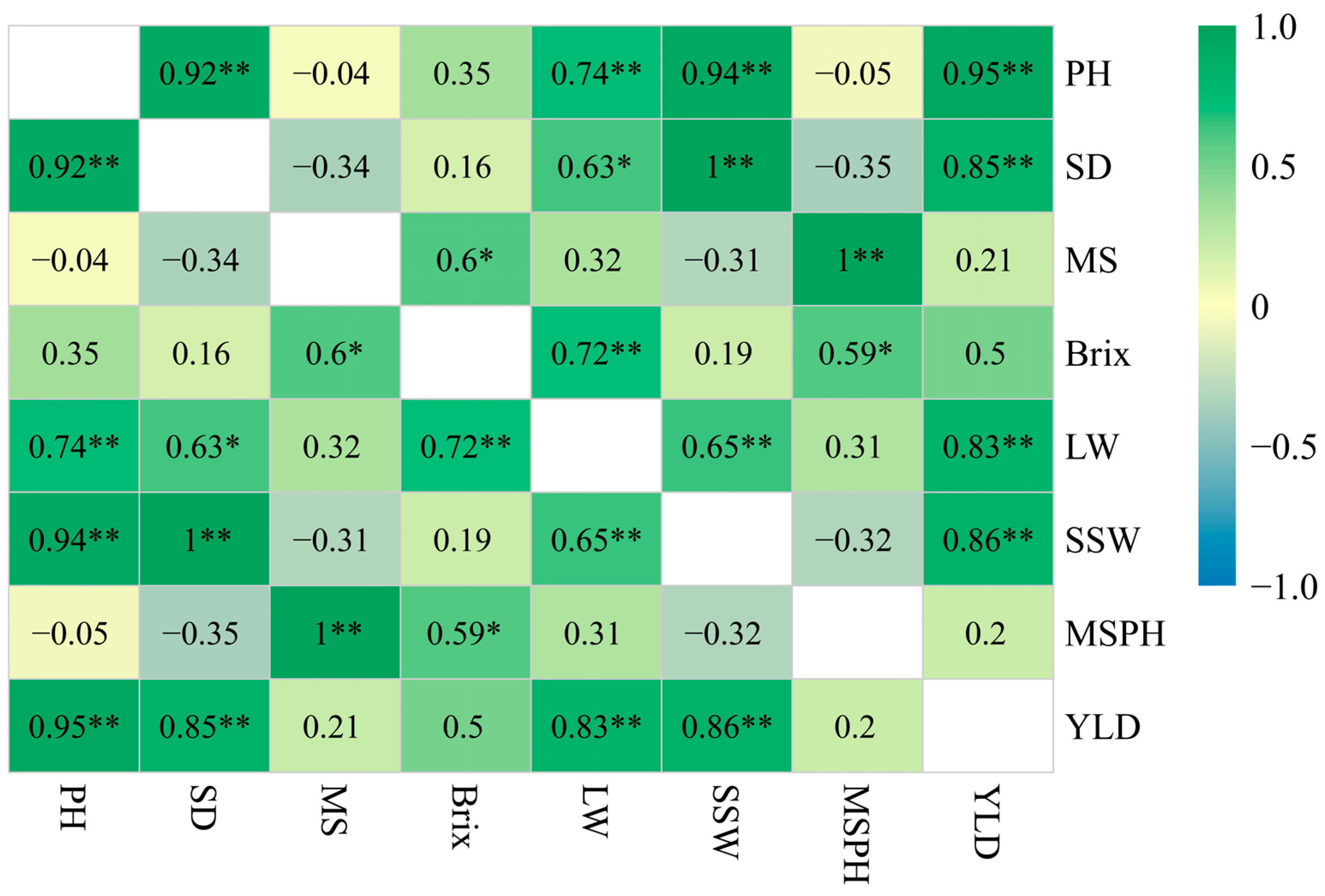
3.3. Correlation Analysis Between Yield and Agronomic Traits
4. Discussion
4.1. The Application Status and Improvement Methods of S. spontaneum
4.2. Correlation Effect
4.3. Application of Improved S. spontaneum Progeny in Sugarcane Breeding
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Garcia Tavares, R.; Lakshmanan, P.; Peiter, E.; O’Connell, A.; Caldana, C.; Vicentini, R.; Soares, J.S.; Menossi, M. ScGAI is a key regulator of culm development in sugarcane. J. Exp. Bot. 2018, 69, 3823–3837. [Google Scholar] [CrossRef] [PubMed]
- Dinesh Babu, K.S.; Janakiraman, V.; Palaniswamy, H.; Kasirajan, L.; Gomathi, R.; Ramkumar, T.R. A short review on sugarcane: Its domestication, molecular manipulations and future perspectives. Genet. Resour. Crop Evol. 2022, 69, 2623–2643. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Pan, Y.-B.; Wu, M.; Liu, J.; Yang, S.; Wu, Q.; Que, Y. Sugarcane genetics: Underlying theory and practical application. Crop J. 2024, 13, 328–338. [Google Scholar] [CrossRef]
- Li, Y.-R.; Zhang, B.-Q.; Song, X.-P.; Liang, Q.; Verma, K.K.; Li, D.-M. Development of sugar industry in China: R&D priorities for sustainable sugarcane production. Sugar Tech 2024, 26, 972–981. [Google Scholar]
- Jeswiet, J. The development of selection and breeding of the sugar cane in Java. Proc. Int. Soc. Sugarcane Technol. 1930, 3, 44–57. [Google Scholar]
- Ram, B.; Karuppaiyan, R.; Hemaprabha, G. Sugarcane breeding. In Fundamentals of Field Crop Breeding; Springer: Berlin/Heidelberg, Germany, 2022; pp. 499–570. [Google Scholar]
- Healey, A.; Garsmeur, O.; Lovell, J.; Shengquiang, S.; Sreedasyam, A.; Jenkins, J.; Plott, C.; Piperidis, N.; Pompidor, N.; Llaca, V. The complex polyploid genome architecture of sugarcane. Nature 2024, 628, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, A.; Zhao, P.; Xia, H.; Zhang, Y.; Que, Y. Theory to practice: A success in breeding sugarcane variety YZ08–1609 known as the King of Sugar. Front. Plant Sci. 2024, 15, 1413108. [Google Scholar] [CrossRef]
- Aitken, K.; Li, J.; Piperidis, G.; Qing, C.; Yuanhong, F.; Jackson, P. Worldwide genetic diversity of the wild species Saccharum spontaneum and level of diversity captured within sugarcane breeding programs. Crop Sci. 2018, 58, 218–229. [Google Scholar] [CrossRef]
- Liu, J.; Deng, Z.; Wu, C.; Tao, L.; Lu, X.; Zhao, P.; Zhang, Y. Progress and Discussion on Breeding and Utilization of Sugarcane Wild Species Saccharum spontaneum. J. Plant Genet. Resour. 2021, 22, 7. (In Chinese) [Google Scholar] [CrossRef]
- da Silva, J.A. The importance of the wild cane Saccharum spontaneum for bioenergy genetic breeding. Sugar Tech 2017, 19, 229–240. [Google Scholar] [CrossRef]
- Dhungana, S.R.; Braun, D.M. Genomic analyses of SUT and TST sugar transporter families in low and high sugar accumulating sugarcane species (Saccharum spontaneum and Saccharum officinarum). Trop. Plant Biol. 2022, 15, 181–196. [Google Scholar] [CrossRef]
- Hemaprabha, G.; Mohanraj, K.; Jackson, P.; Lakshmanan, P.; Ali, G.; Li, A.; Huang, D.; Ram, B. Sugarcane genetic diversity and major germplasm collections. Sugar Tech 2022, 24, 279–297. [Google Scholar] [CrossRef]
- Pan, Y.-B.; Burner, D.; Legendre, B.; Grisham, M.; White, W. An assessment of the genetic diversity within a collection of Saccharum spontaneum L. with RAPD-PCR. Genet. Resour. Crop Evol. 2005, 51, 895–903. [Google Scholar] [CrossRef]
- Labroo, M.R.; Studer, A.J.; Rutkoski, J.E. Heterosis and hybrid crop breeding: A multidisciplinary review. Front. Genet. 2021, 12, 643761. [Google Scholar] [CrossRef] [PubMed]
- Begna, T. Combining ability and heterosis in plant improvement. Open J. Plant Sci. 2021, 6, 108–117. [Google Scholar]
- Paril, J.; Reif, J.; Fournier-Level, A.; Pourkheirandish, M. Heterosis in crop improvement. Plant J. 2024, 117, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Gong, J.; Zhu, Z.; Li, Z.; Feng, Q.; Wang, C.; Zhao, Y.; Zhan, Q.; Zhou, C.; Wang, A. Structure and function of rice hybrid genomes reveal genetic basis and optimal performance of heterosis. Nat. Genet. 2023, 55, 1745–1756. [Google Scholar] [CrossRef]
- Andorf, C.; Beavis, W.D.; Hufford, M.; Smith, S.; Suza, W.P.; Wang, K.; Woodhouse, M.; Yu, J.; Lübberstedt, T. Technological advances in maize breeding: Past, present and future. Theor. Appl. Genet. 2019, 132, 817–849. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, X.; Han, B. Understanding the genetic basis of rice heterosis: Advances and prospects. Crop J. 2021, 9, 688–692. [Google Scholar] [CrossRef]
- Chang, H.; Guo, Y.; Zhou, F.; Fang, N.; Qiu, Y.; Chen, J.; Zhang, W.; Qin, Y.; Wu, J.; Zhang, C. Research on the Emasculation Technology by Hot Water about Sugarcane Tassels. Fujian J. Agric. Sci. 2020, 35, 591–597. [Google Scholar]
- Su, J.; Zhang, F.; Yang, X.; Feng, Y.; Yang, X.; Wu, Y.; Guan, Z.; Fang, W.; Chen, F. Combining ability, heterosis, genetic distance and their intercorrelations for waterlogging tolerance traits in chrysanthemum. Euphytica 2017, 213, 42. [Google Scholar] [CrossRef]
- Chai, J.; Wang, K.; Xie, Y.; Wang, A.; Zhong, H.; Yao, X.; Lin, P. Combining ability from complete diallel design of Camellia oleifera: Implications for the utility of GCA and SCA in oil-related traits breeding. Ind. Crops Prod. 2023, 205, 117434. [Google Scholar] [CrossRef]
- Manigben, K.A.; Beyene, Y.; Chaikam, V.; Tongoona, P.B.; Danquah, E.Y.; Ifie, B.E.; Aleri, I.; Chavangi, A.; Prasanna, B.M.; Gowda, M. Testcross performance and combining ability of intermediate maturing drought tolerant maize inbred lines in Sub-Saharan Africa. Front. Plant Sci. 2024, 15, 1471041. [Google Scholar] [CrossRef]
- Cursi, D.E.; Castillo, R.O.; Tarumoto, Y.; Umeda, M.; Tippayawat, A.; Ponragdee, W.; Racedo, J.; Perera, M.F.; Hoffmann, H.P.; Carneiro, M.S. Origin, genetic diversity, conservation, and traditional and molecular breeding approaches in sugarcane. In Cash Crops: Genetic Diversity, Erosion, Conservation and Utilization; Springer: Berlin/Heidelberg, Germany, 2022; pp. 83–116. [Google Scholar]
- Silva, J.A.G.d.; Costa, P.M.d.A.; Marconi, T.G.; Barreto, E.J.d.S.; Solís-Gracia, N.; Park, J.-W.; Glynn, N.C. Agronomic and molecular characterization of wild germplasm Saccharum spontaneum for sugarcane and energycane breeding purposes. Sci. Agric. 2018, 75, 329–338. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, Y.; Hua, X.; Wang, Y.; Wang, B.; Qi, Y.; Huang, Y.; Yu, Z.; Gao, R.; Zhang, Y. The highly allo-autopolyploid modern sugarcane genome and very recent allopolyploidization in Saccharum. Nat. Genet. 2025, 57, 242–253. [Google Scholar] [CrossRef]
- Piperidis, N.; D’Hont, A. Sugarcane genome architecture decrypted with chromosome-specific oligo probes. Plant J. 2020, 103, 2039–2051. [Google Scholar] [CrossRef]
- Glassop, D.; Perroux, J.; Rae, A. Differences in sugarcane stool branching within Saccharum spontaneum genotypes and compared to Saccharum officinarum and commercial varieties. Euphytica 2021, 217, 50. [Google Scholar] [CrossRef]
- Duanmeesuk, U.; Chutteang, C.; Somta, P.; Chatwachirawong, P. Estimation of genetic parameters, heritability, genetic advance and heterosis in sugarcane families. Thai Agric. Res. J. 2021, 39, 306–318. [Google Scholar]
- Engida, B.T.; Wegary, D.; Keno, T.; Mekonnen, T.W. Combining ability and genetic distance analysis of mid altitude sub-humid agroecology adapted maize inbred lines for high grain yield. Heliyon 2024, 10, e32267. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, X.; Yang, Z.; Li, F.; Ge, X. Synergistic optimization of crops by combining early maturation with other agronomic traits. Trends Plant Sci. 2023, 28, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, J.; Gao, Z.; Wang, H.; Liang, D.; Guo, Q.; Zhang, X.; Fan, X.; Wu, Y.; Liu, Q. Identification of heterosis and combining ability in the hybrids of male sterile and restorer sorghum [Sorghum bicolor (L.) Moench] lines. PLoS ONE 2024, 19, e0296416. [Google Scholar] [CrossRef] [PubMed]
- Yuqiang, G.; Cheng, F.; Huanying, X.; Hailong, C.; Jiantao, W.; Yongsheng, Q.; Qinnan, W. Construction of F1 Populations of S. spontaneum L. and Sugarcane and Molecular Detection of Genetic Diversity. Mol. Pathog. 2021, 12, 1–7. [Google Scholar]
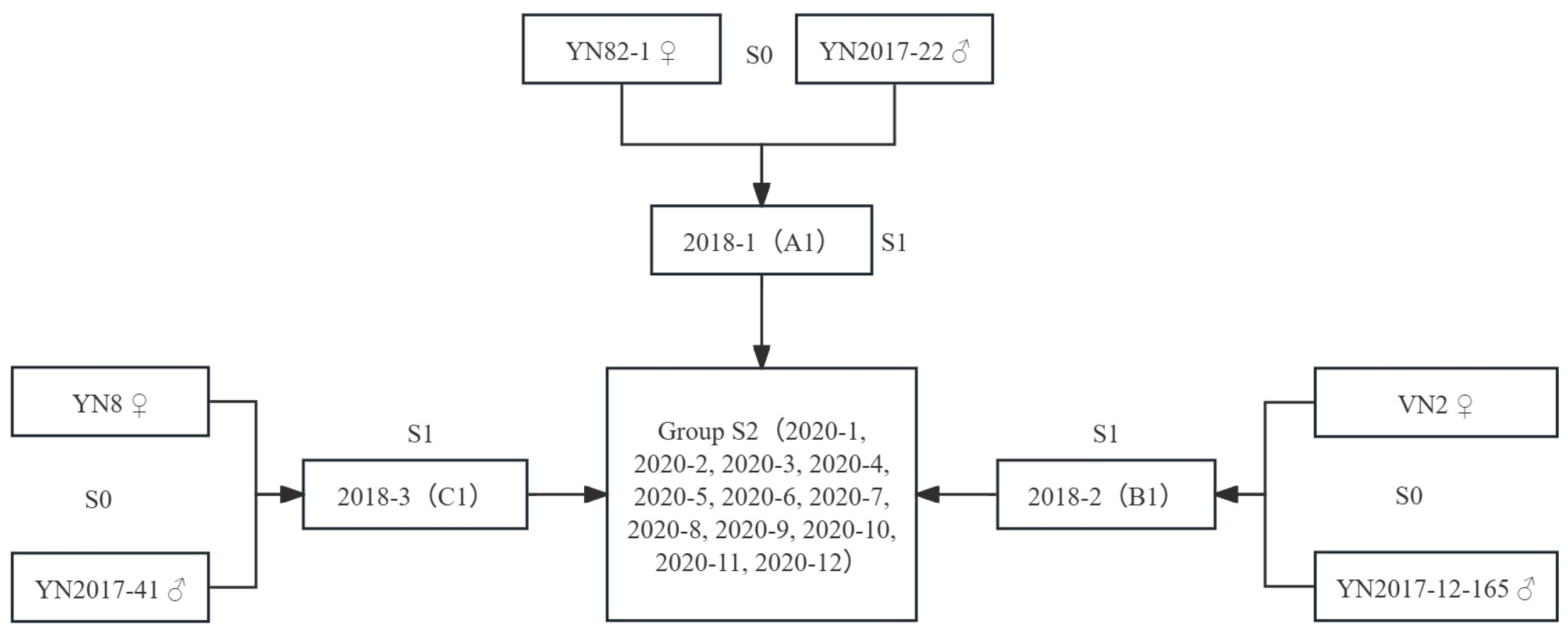
| Name of the Material | Classification | PH (cm) | SD (cm) | MS | LW (cm) | Brix (%) | CW (kg/Cluster) |
|---|---|---|---|---|---|---|---|
| YN82-1 | Male of A1 (S0) | 60 | 0.33 | 20 | 0.63 | 14.20 | 0.13 |
| YN2017-22 | Female of A1 (S0) | 80 | 0.67 | 31 | 0.85 | 13.00 | 1.10 |
| 2018-1 | A1 (S1) | 130 | 1.20 | 32 | 1.47 | 15.60 | 5.99 |
| VN2 | Male of B1 (S0) | 90 | 0.77 | 17 | 1.17 | 16.20 | 0.88 |
| YN2017-12-165 | Female of B1 (S0) | 100 | 0.90 | 16 | 0.83 | 13.20 | 1.32 |
| 2018-2 | B1 (S1) | 140 | 1.13 | 21 | 2.03 | 17.40 | 3.78 |
| YN8 | Male of C1 (S0) | 50 | 0.55 | 25 | 0.93 | 15.00 | 0.37 |
| YN2017-41 | Female of C1 (S0) | 60 | 0.50 | 21 | 0.67 | 14.20 | 0.31 |
| 2018-3 | C1 (S1) | 80 | 0.87 | 47 | 1.47 | 17.00 | 2.82 |
| Trait | Combined Mean | p | |||||
|---|---|---|---|---|---|---|---|
| A1 × B1 | A1 × C1 | B1 × A1 | B1 × C1 | C1 × A1 | C1 × B1 | ||
| PH (cm) | 168 | 172 | 148 | 170 | 171 | 159 | 0.047 |
| SD (cm) | 1.10 | 1.10 | 0.90 | 0.90 | 1.00 | 0.90 | 0.007 |
| MS (stalk/cluster) | 12 | 13 | 10 | 20 | 17 | 25 | 0.013 |
| Brix (%) | 7.70 | 7.70 | 6.80 | 7.00 | 7.30 | 5.70 | 0.010 |
| CW (kg) | 3.70 | 4.30 | 2.00 | 4.60 | 4.60 | 5.06 | 0.190 |
| LW (cm) | 1.70 | 1.80 | 1.80 | 1.80 | 1.90 | 1.58 | 0.350 |
| Name of the Material | Cross Source | PH (cm) | SD (cm) | MS (Stalk/Cluster) | Brix (%) | CW (kg) | LW (cm) | Type |
|---|---|---|---|---|---|---|---|---|
| 2020-1 | A1 × B1 | 202 | 1.2 | 17 | 8.2 | 3.97 | 1.6 | S2 |
| 2020-2 | A1 × B1 | 205 | 1.2 | 13 | 7.3 | 3.18 | 1.8 | S2 |
| 2020-3 | A1 × B1 | 197 | 0.9 | 24 | 9.3 | 3.23 | 1.6 | S2 |
| Mean of A1 and B1 | 211 | 1.1 | 11 | 8.3 | 2.20 | 2.1 | S1 | |
| Mean of parent of A1 and B1 | 176 | 0.8 | 14 | 8.3 | 1.24 | 1.5 | S0 | |
| 2020-4 | A1 × C1 | 207 | 1.1 | 13 | 9.4 | 2.60 | 1.7 | S2 |
| 2020-5 | A1 × C1 | 197 | 1.2 | 25 | 7.8 | 5.25 | 2.0 | S2 |
| Mean of A1 and C1 | 212 | 1.0 | 12 | 8.7 | 2.00 | 2.0 | S1 | |
| Mean of parent of A1amd C1 | 150 | 0.5 | 15 | 6.7 | 0.44 | 1.5 | S0 | |
| 2020-6 | B1 × A1 | 159 | 1.0 | 26 | 6.3 | 3.18 | 2.0 | S2 |
| Mean of B1 and A1 | 211 | 1.1 | 11 | 8.3 | 2.20 | 2.1 | S1 | |
| Mean of parent of B1 and A1 | 176 | 0.8 | 14 | 8.3 | 1.24 | 1.5 | S0 | |
| 2020-7 | B1 × C1 | 188 | 0.9 | 42 | 10.1 | 4.60 | 2.4 | S2 |
| 2020-8 | B1 × C1 | 157 | 0.9 | 30 | 8.6 | 3.17 | 1.4 | S2 |
| 2020-9 | B1 × C1 | 212 | 0.8 | 24 | 10.5 | 2.50 | 2.0 | S2 |
| Mean of B1 and C1 | 207 | 0.9 | 18 | 8.3 | 2.30 | 2.1 | S1 | |
| Mean of parent of B1 and C1 | 161 | 0.6 | 14 | 8.0 | 0.61 | 1.5 | S0 | |
| 2020-10 | C1 × A1 | 229 | 1.2 | 12 | 9.2 | 2.82 | 2.3 | S2 |
| 2020-11 | C1 × A1 | 161 | 0.9 | 29 | 12.9 | 2.92 | 1.4 | S2 |
| 2020-12 | C1 × A1 | 168 | 1.0 | 17 | 8.9 | 2.32 | 1.4 | S2 |
| Mean of C1 and A1 | 212 | 1.0 | 12 | 8.7 | 2.00 | 2.0 | S1 | |
| Mean of parent of C1 and A1 | 150 | 0.5 | 15 | 6.7 | 0.44 | 1.5 | S0 | |
| A1 | YN82-1 × YN2017-22 | 216 | 1.2 | 6 | 8.8 | 1.55 | 1.9 | S1 |
| B1 | VN2 × YN2017-12-165 | 206 | 1.0 | 17 | 7.9 | 2.85 | 2.3 | S1 |
| C1 | YN8 × YN2017-41 | 208 | 0.7 | 19 | 8.7 | 1.70 | 2.0 | S1 |
| YN2017-12-165 | 193 | 1.1 | 9 | 7.5 | 1.65 | 1.2 | S0 | |
| YN2017-22 | 138 | 0.6 | 23 | 6.0 | 1.00 | 1.5 | S0 | |
| YN2017-41 | 115 | 0.4 | 10 | 7.1 | 0.10 | 1.2 | S0 | |
| VN2 | 183 | 0.7 | 17 | 11.8 | 1.10 | 1.8 | S0 | |
| YN8 | 156 | 0.3 | 20 | 5.7 | 0.25 | 1.7 | S0 | |
| YN82-1 | 193 | 0.9 | 8 | 7.8 | 1.10 | 1.5 | S0 |
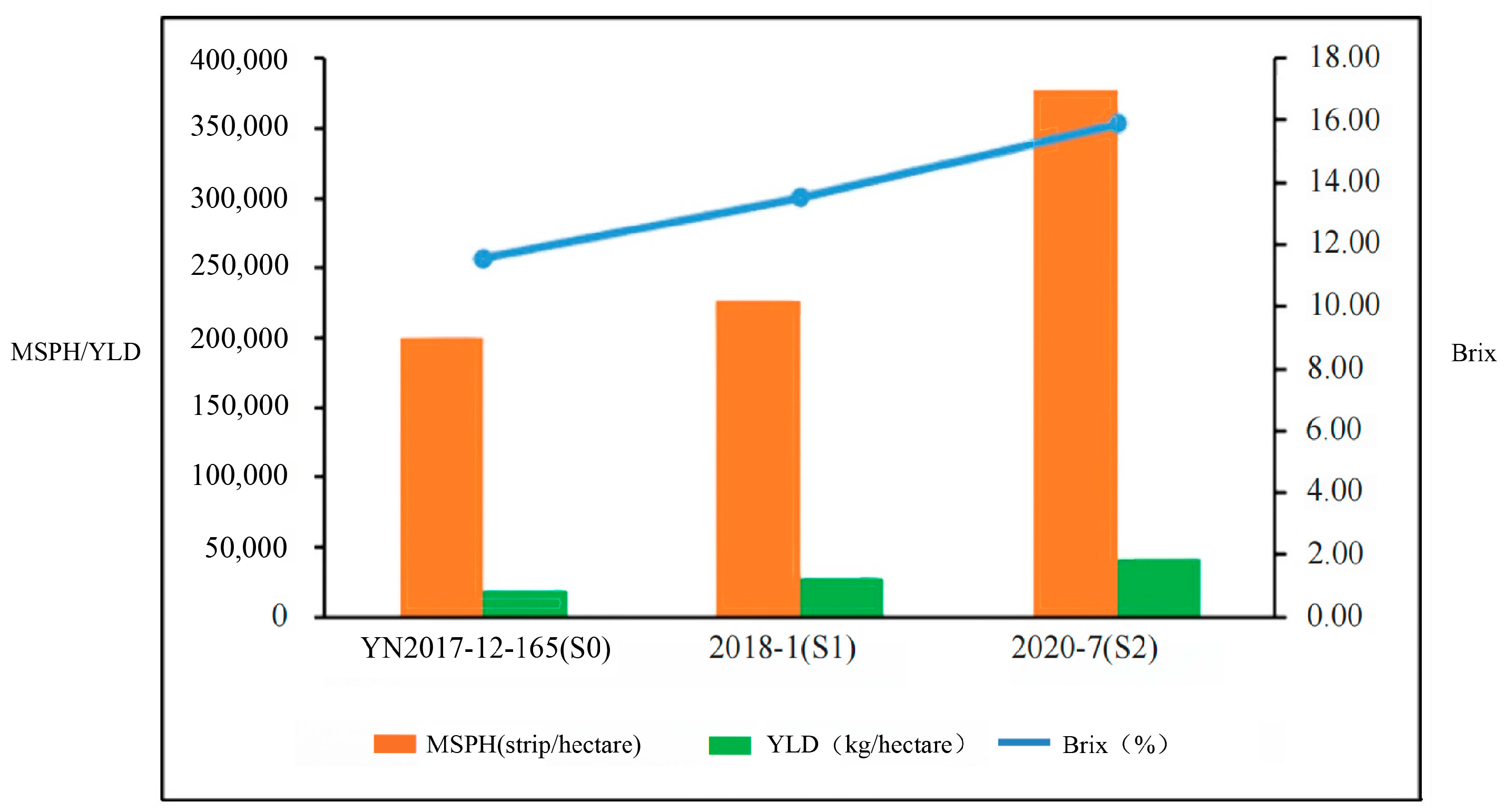
| Trait | S0 (CK) | S1 | Increase Compared with S0 % | S2 | Increase Compared with S0 % |
|---|---|---|---|---|---|
| PH (cm) | 107.33 | 141.14 * | 31.50 | 141.91 * | 32.22 |
| SD (cm) | 0.62 | 0.86 * | 38.71 | 0.94 ** | 51.61 |
| MS | 18.73 | 23.27 | 24.24 | 16.30 | −12.97 |
| Brix (%) | 13.15 | 14.66 | 11.48 | 14.32 | 8.90 |
| LW (cm) | 0.93 | 1.32 * | 41.94 | 1.37 * | 47.31 |
| SSW (kg) | 0.04 | 0.09 * | 125.00 | 0.10 ** | 150.00 |
| MSPH | 283,830 | 352,560 | 24.24 | 247,035 | −12.97 |
| YPH (kg) | 9695.55 | 24,821.25 ** | 156.01 | 24,977.70 ** | 157.62 |
| Name | Type | PH (cm) | SD (cm) | MS | Brix (%) | LW (cm) | SSW (kg) | MSPH (strip/hectare) | YLD (kg/hectare) |
|---|---|---|---|---|---|---|---|---|---|
| YN2017-12-165 | S0 | 142 | 0.90 | 13 | 11.55 | 0.97 | 0.09 | 199,995 | 18,186.00 |
| YN2017-22 | S0 | 98 | 0.59 | 12 | 11.42 | 0.88 | 0.03 | 180,300 | 4847.25 |
| YN2017-41 | S0 | 66 | 0.42 | 11 | 12.65 | 0.67 | 0.01 | 173,220 | 1646.25 |
| VN2 | S0 | 126 | 0.63 | 25 | 16.00 | 1.14 | 0.04 | 373,095 | 14,829.15 |
| YN8 | S0 | 85 | 0.40 | 38 | 13.89 | 1.01 | 0.01 | 573,780 | 6201.00 |
| YN82-1 | S0 | 124 | 0.79 | 13 | 13.36 | 0.90 | 0.06 | 202,575 | 12,463.95 |
| 2018-1 | S1 | 145 | 1.04 | 15 | 13.52 | 1.25 | 0.12 | 226,260 | 28,020.90 |
| 2018-2 | S1 | 153 | 0.95 | 16 | 15.41 | 1.52 | 0.11 | 239,520 | 25,509.15 |
| 2018-3 | S1 | 124 | 0.60 | 39 | 15.04 | 1.19 | 0.03 | 591,915 | 20,933.85 |
| 2020-1 | S2 | 144 | 1.06 | 14 | 14.08 | 1.35 | 0.13 | 204,915 | 26,401.50 |
| 2020-3 | S2 | 141 | 0.92 | 12 | 12.75 | 1.03 | 0.09 | 184,725 | 17,762.70 |
| 2020-4 | S2 | 140 | 0.95 | 18 | 14.44 | 1.25 | 0.10 | 279,165 | 27,625.20 |
| 2020-5 | S2 | 151 | 0.99 | 14 | 14.09 | 1.32 | 0.12 | 219,180 | 25,460.70 |
| 2020-7 | S2 | 147 | 0.97 | 25 | 15.90 | 1.88 | 0.11 | 378,030 | 41,005.95 |
| 2020-9 | S2 | 125 | 0.74 | 14 | 14.69 | 1.42 | 0.05 | 216,150 | 11,610.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Ran, M.; Zhao, L.; Tao, L.; Zan, F.; Yao, L.; Hu, X.; Ren, S.; Zhao, Y.; Xia, H.; et al. The Enhancement of a Saccharum spontaneum Population and a Genetic Impact Analysis of the Agronomic and Yield Traits of Its Progeny. Plants 2025, 14, 1750. https://doi.org/10.3390/plants14121750
Liu J, Ran M, Zhao L, Tao L, Zan F, Yao L, Hu X, Ren S, Zhao Y, Xia H, et al. The Enhancement of a Saccharum spontaneum Population and a Genetic Impact Analysis of the Agronomic and Yield Traits of Its Progeny. Plants. 2025; 14(12):1750. https://doi.org/10.3390/plants14121750
Chicago/Turabian StyleLiu, Jiayong, Maoyong Ran, Liping Zhao, Lianan Tao, Fenggang Zan, Li Yao, Xin Hu, Shenglin Ren, Yong Zhao, Hongming Xia, and et al. 2025. "The Enhancement of a Saccharum spontaneum Population and a Genetic Impact Analysis of the Agronomic and Yield Traits of Its Progeny" Plants 14, no. 12: 1750. https://doi.org/10.3390/plants14121750
APA StyleLiu, J., Ran, M., Zhao, L., Tao, L., Zan, F., Yao, L., Hu, X., Ren, S., Zhao, Y., Xia, H., Zhang, J., Pu, X., Zhang, Z., & Deng, Z. (2025). The Enhancement of a Saccharum spontaneum Population and a Genetic Impact Analysis of the Agronomic and Yield Traits of Its Progeny. Plants, 14(12), 1750. https://doi.org/10.3390/plants14121750





