Vegetation Structure and Environmental Correlates of Climbing Behavior for Desert Shrub Ochradenus baccatus
Abstract
1. Introduction
2. Results
2.1. Associated Floristic Composition
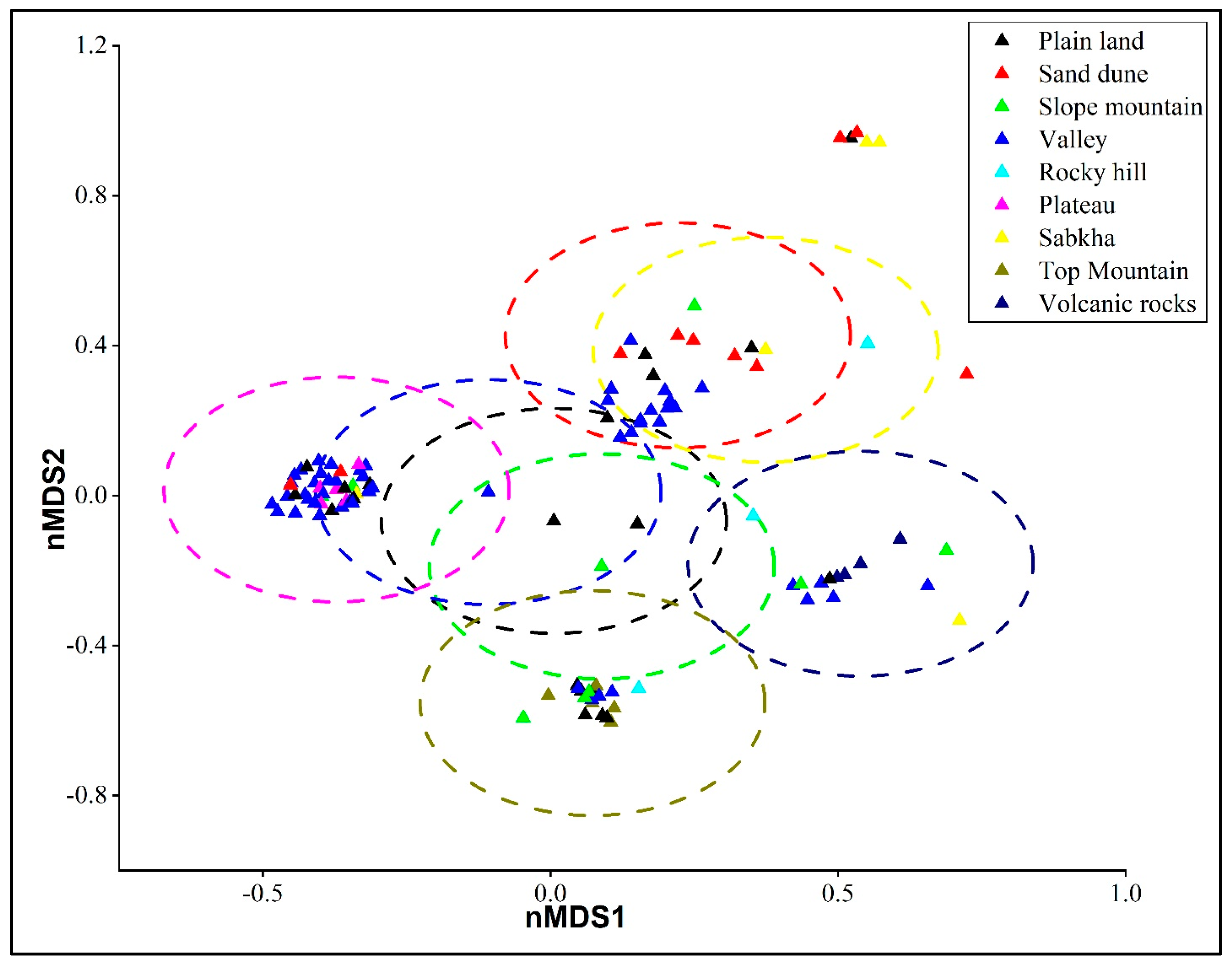
2.2. Structure and Habitat Differentiation
2.3. Influence of Altitude on Diversity Indices by Growth Behavior
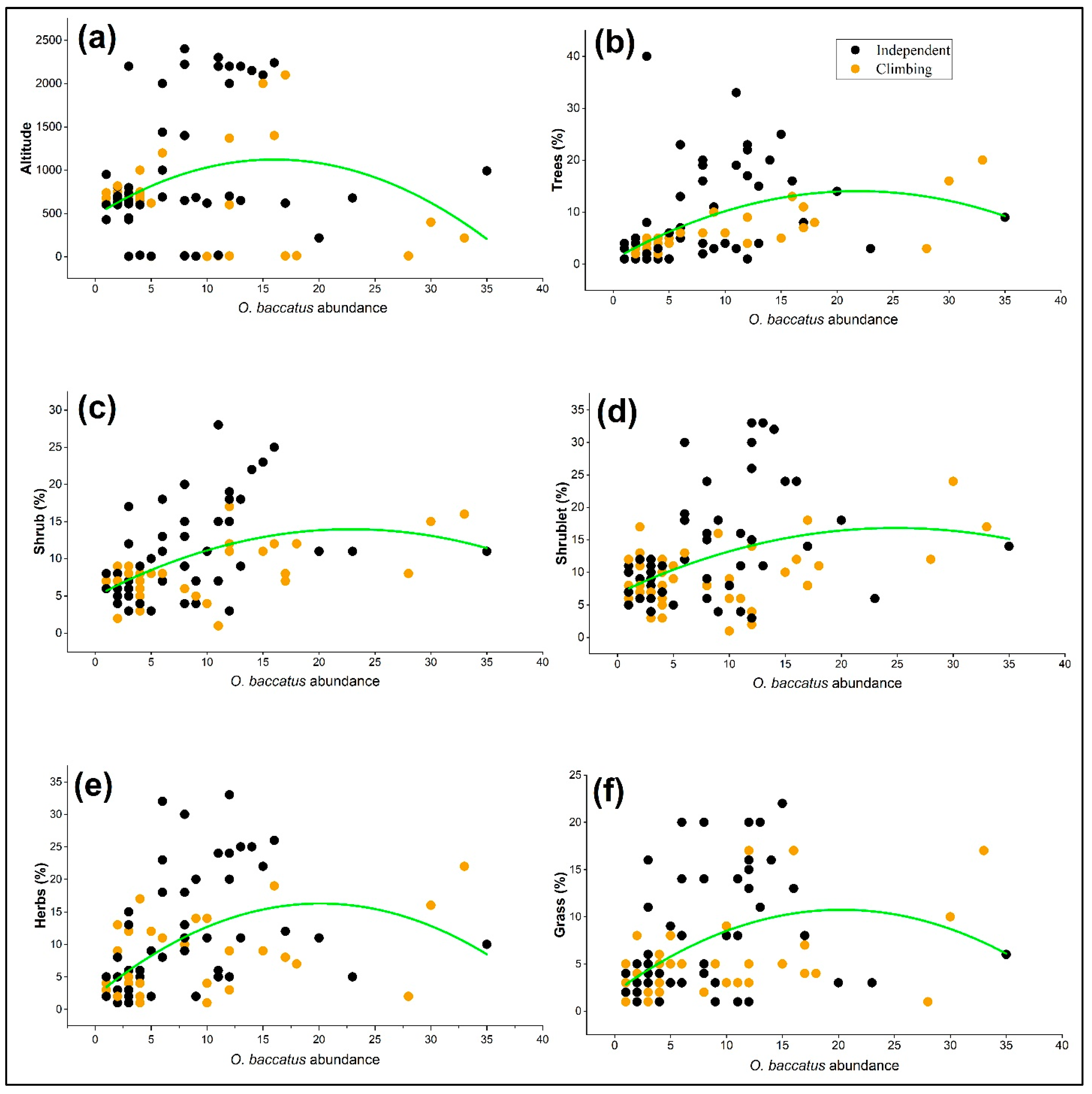
2.4. Association of Growth Behavior with Vegetation Structure and Altitude
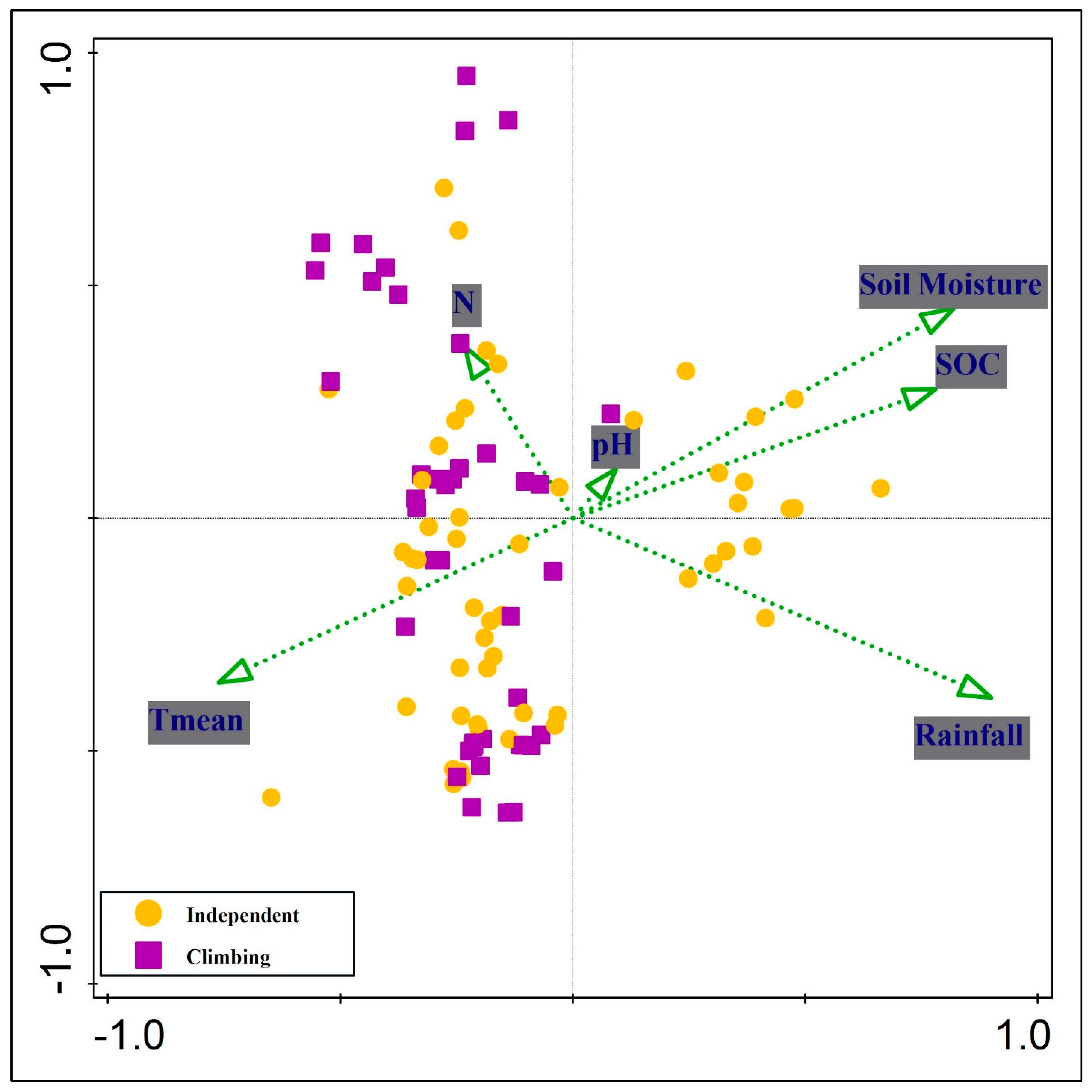
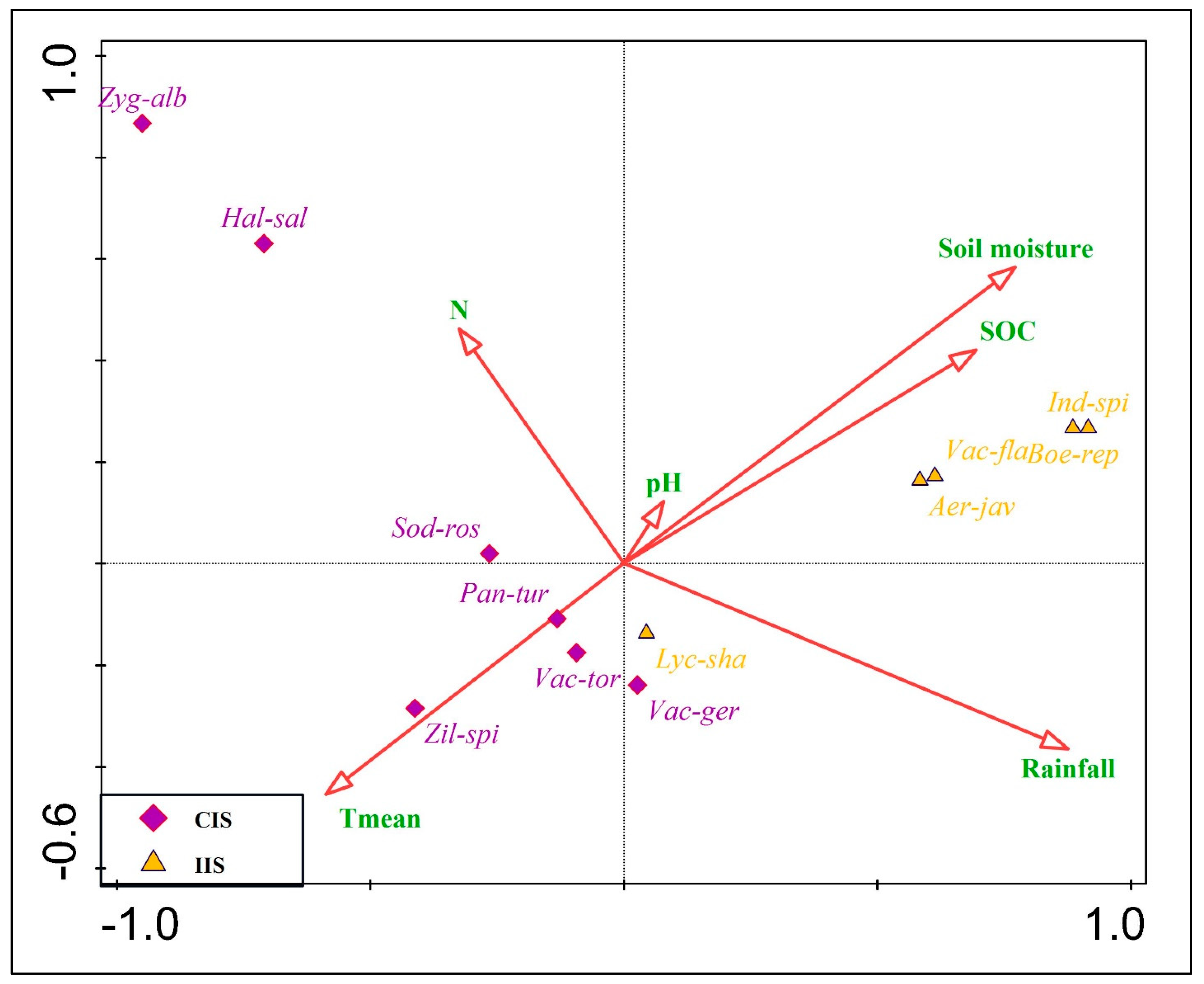
2.5. Environmental Drivers of Growth Behavior
2.6. Indicator Species Associated with Both Behavior
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Field Survey and Vegetation Sampling
4.3. Environmental Data Acquisition
4.4. Data Analaysis
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Tariq, A.; Hughes, A.C.; Hong, D.; Wei, F.; Sun, H.; Sardans, J.; Peñuelas, J.; Perry, G.; Qiao, J.; et al. Challenges and solutions to biodiversity conservation in arid lands. Sci. Total Environ. 2023, 857, 159695. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.J.; Jobson, P.; Köhler, F.; Nano, C.E.; Oliver, P.M. The living heart: Climate gradients predict desert mountain endemism. Ecol. Evol. 2021, 11, 4366–4378. [Google Scholar] [CrossRef] [PubMed]
- Hassanen, S.; Eldin, E.G.; Kamel, W.; Zaghloul, M.S.; Hassan, Y.M. Floral diversity and conservation status of vascular plants in arid mountainous areas. BMC Ecol. Evol. 2025, 25, 3. [Google Scholar] [CrossRef]
- Hanan, N.P.; Milne, E.; Aynekulu, E.; Yu, Q.; Anchang, J. A role for drylands in a carbon neutral world? Front. Environ. Sci. 2021, 9, 786087. [Google Scholar] [CrossRef]
- Sadia, S.; Waheed, M.; Arshad, F.; Al-Andal, A.; Munir, M.; Jabeen, A.; Aslam, S. Adaptive floristic diversity and ecological responses to environmental gradients in the saline soil ecosystem. J. Nat. Conserv. 2025, 84, 126862. [Google Scholar] [CrossRef]
- Arshad, F.; Iqbal, M.; Riaz, A.; Haq, S.M.; Waheed, M.; Qadeer, S.; Bussmann, R.W.; Shoaib, M.; Hashem, A.; Fathi Abd-Allah, E. Road corridors vegetation in the semi-arid region: Functional trait diversity and dynamics. Sci. Rep. 2024, 14, 25212. [Google Scholar] [CrossRef]
- Waheed, M.; Arshad, F. Adaptive convergence and divergence underpin the diversity of Asteraceae in a semi-arid lowland region. Flora 2024, 317, 152554. [Google Scholar] [CrossRef]
- Akram, M.A.; Wang, X.; Shrestha, N.; Zhang, Y.; Sun, Y.; Yao, S.; Li, J.; Hou, Q.; Hu, W.; Ran, J.; et al. Variations and driving factors of leaf functional traits in the dominant desert plant species along an environmental gradient in the drylands of China. Sci. Total Environ. 2023, 897, 165394. [Google Scholar] [CrossRef]
- Jiang, L.; Lv, G.; Gong, Y.; Li, Y.; Wang, H.; Wu, D. Characteristics and driving mechanisms of species beta diversity in desert plant communities. PLoS ONE 2021, 16, e0245249. [Google Scholar] [CrossRef]
- Islam, W.; Zeng, F.; Almoallim, H.S.; Ansari, M.J. Unveiling soil animal community dynamics beneath dominant shrub species in natural desert environment: Implications for ecosystem management and conservation. J. Environ. Manag. 2024, 366, 121697. [Google Scholar] [CrossRef]
- Gao, D.; Wang, S.; Wei, F.; Wu, X.; Zhou, S.; Wang, L.; Li, Z.; Chen, P.; Fu, B. The vulnerability of ecosystem structure in the semi-arid area revealed by the functional trait networks. Ecol. Indic. 2022, 139, 108894. [Google Scholar] [CrossRef]
- Beger, M.; Metaxas, A.; Balbar, A.C.; McGowan, J.A.; Daigle, R.; Kuempel, C.D.; Treml, E.A.; Possingham, H.P. Demystifying ecological connectivity for actionable spatial conservation planning. Trends Ecol. Evol. 2022, 37, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, A.K.; Lovett-Doust, J.; El Adawy, H.A. Size-and season-related sex expression and reproductive performance in gynodioecious Ochradenus baccatus Delile (Resedaceae), at Wadi Degla, Egypt. Flora 2011, 206, 1002–1011. [Google Scholar] [CrossRef]
- Khan, S.; Al-Qurainy, F.; Nadeem, M.; Tarroum, M. Development of genetic markers for Ochradenus arabicus (Resedaceae), an endemic medicinal plant of Saudi Arabia. Genet. Mol. Res. 2012, 11, 1300–1308. [Google Scholar] [CrossRef]
- Bhatt, A.; Pérez-García, F. Seed dormancy of Ochradenus baccatus (Resedaceae), a shrubby species from Arabian desert regions. Rev. Biol. Trop. 2016, 64, 965–974. [Google Scholar] [CrossRef]
- Sallam, H.; Alzain, M.N.; Abuzaid, A.O.; Loutfy, N.; Badry, M.O.; Osman, A.K.; Hammad, S.A. Wild plant diversity and soil characteristics of desert roadside vegetation in the eastern desert. Diversity 2023, 15, 874. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Ragab, E.A.; Khan, R.A. Taily Weed (Ochradenus baccatus Delile) as a Middle Eastern Herb: Chemical Profile, Biological Activities, and Traditional Uses. In Ancient and Traditional Foods, Plants, Herbs and Spices used in the Middle East; CRC Press: Boca Raton, FL, USA, 2023; pp. 293–306. [Google Scholar]
- Al-Mutairi, K.; Al-Shaami, S.A.; Khorshid, Z.; Moawed, M.M. Floristic diversity of Tabuk province, north Saudi Arabia. JAPS J. Anim. Plant Sci. 2016, 26, 1019–1025. [Google Scholar]
- Rowe, N.; Isnard, S.; Speck, T. Diversity of mechanical architectures in climbing plants: An evolutionary perspective. J. Plant Growth Regul. 2004, 23, 108–128. [Google Scholar] [CrossRef]
- Lotan, A.; Izhaki, I. Could abiotic environment shape fleshy fruit traits? A field study of the desert shrub Ochradenus baccatus. J. Arid Environ. 2013, 92, 34–41. [Google Scholar] [CrossRef]
- Munson, S.M.; Muldavin, E.H.; Belnap, J.; Peters, D.P.; Anderson, J.P.; Reiser, M.H.; Gallo, K.; Melgoza-Castillo, A.; Herrick, J.E.; Christiansen, T.A. Regional signatures of plant response to drought and elevated temperature across a desert ecosystem. Ecology 2013, 94, 2030–2041. [Google Scholar] [CrossRef]
- Filazzola, A.; Liczner, A.R.; Westphal, M.; Lortie, C.J. Shrubs indirectly increase desert seedbanks through facilitation of the plant community. PLoS ONE 2019, 14, e0215988. [Google Scholar] [CrossRef] [PubMed]
- Sher, H.; Al-Yemeny, M.N. Ecological investigation of the weed flora in arable and non arable lands of Al-kharj Area, Saudi Arabia. Afr. J. Agric. Res. 2011, 6, 901–906. [Google Scholar]
- Abbas, A.M.; Al-Kahtani, M.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Badry, M.O. Floristic diversity and phytogeography of Jabal Fayfa: A subtropical dry zone, south-west Saudi Arabia. Diversity 2020, 12, 345. [Google Scholar] [CrossRef]
- Loxdale, H.D.; Harvey, J.A. Generalism in nature: A community ecology perspective. Community Ecol. 2023, 24, 113–125. [Google Scholar] [CrossRef]
- Polis, G.A. Desert communities: An overview of patterns and processes. Ecol. Desert Communities 1991, 456, 1–26. [Google Scholar]
- Lichter-Marck, I.H.; Baldwin, B.G. Edaphic specialization onto bare, rocky outcrops as a factor in the evolution of desert angiosperms. Proc. Natl. Acad. Sci. USA 2023, 120, e2214729120. [Google Scholar] [CrossRef]
- Chesson, P.; Gebauer, R.L.; Schwinning, S.; Huntly, N.; Wiegand, K.; Ernest, M.S.; Sher, A.; Novoplansky, A.; Weltzin, J.F. Resource pulses, species interactions, and diversity maintenance in arid and semi-arid environments. Oecologia 2004, 141, 236–253. [Google Scholar] [CrossRef]
- Berdugo, M.; Maestre, F.T.; Kéfi, S.; Gross, N.; Le Bagousse-Pinguet, Y.; Soliveres, S. Aridity preferences alter the relative importance of abiotic and biotic drivers on plant species abundance in global drylands. J. Ecol. 2019, 107, 190–202. [Google Scholar] [CrossRef]
- El-Barougy, R.F.; Bersier, L.F.; Gray, S.M.; El-Keblawy, A.; Galal, T.; Ullah, F.; Elgamal, I.A.; Dakhil, M.A. Shaping beta diversity in arid landscape through native plant species contributions: Synergy of climate, soil, and species traits. Front. Plant Sci. 2025, 16, 1521596. [Google Scholar] [CrossRef]
- Hussein, E.A.; Abd El-Ghani, M.M.; Hamdy, R.S.; Shalabi, L.F. Do anthropogenic activities affect floristic diversity and vegetation structure more than natural soil properties in hyper-arid desert environments? Diversity 2021, 13, 157. [Google Scholar] [CrossRef]
- Al-Bakre, D.A. Diversity of Indicator and Dominant Plant Species along Elevation Gradients in Prince Mohammad Bin Salman Nature Reserve, KSA. Diversity 2023, 15, 1081. [Google Scholar] [CrossRef]
- Alghanem, S.M. Ecological and botanical diversity in Haloxylon persicum community at Al-Qassim Region in Kingdom of Saudi Arabia. Am. J. Environ. Prot 2018, 6, 43–49. [Google Scholar] [CrossRef][Green Version]
- Liu, J.; Hu, D.; Wang, H.; Jiang, L.; Lv, G. Scale effects on the relationship between plant diversity and ecosystem multifunctionality in arid desert areas. Forests 2022, 13, 1505. [Google Scholar] [CrossRef]
- Shu, Y.; Jiang, L.; Liu, F.; Lv, G. Effects of plant diversity and abiotic factors on the multifunctionality of an arid desert ecosystem. PLoS ONE 2022, 17, e0266320. [Google Scholar] [CrossRef]
- Jiang, L.M.; Sattar, K.; Lü, G.H.; Hu, D.; Zhang, J.; Yang, X.D. Different contributions of plant diversity and soil properties to the community stability in the arid desert ecosystem. Front. Plant Sci. 2022, 13, 969852. [Google Scholar] [CrossRef]
- Moeslund, J.E.; Arge, L.; Bøcher, P.K.; Dalgaard, T.; Svenning, J.C. Topography as a driver of local terrestrial vascular plant diversity patterns. Nord. J. Bot. 2013, 31, 129–144. [Google Scholar] [CrossRef]
- Opedal, Ø.H.; Armbruster, W.S.; Graae, B.J. Linking small-scale topography with microclimate, plant species diversity and intra-specific trait variation in an alpine landscape. Plant Ecol. Divers. 2015, 8, 305–315. [Google Scholar] [CrossRef]
- Garbin, M.L.; Carrijo, T.T.; Sansevero, J.B.B.; Sánchez-Tapia, A.; Scarano, F.R. Subordinate, not dominant, woody species promote the diversity of climbing plants. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 257–265. [Google Scholar] [CrossRef]
- Quintana, C.; Balslev, H.; Valencia, R. Species Composition and Distribution of Terrestrial Herbs in a High Montane Forest in Ecuador. Diversity 2024, 16, 262. [Google Scholar] [CrossRef]
- Reding, J.M.; Davies, G.M.; Klips, R.A. Rock climbing disturbance severity and abiotic gradients interact to determine cryptogam diversity and community structure. Appl. Veg. Sci. 2022, 25, e12680. [Google Scholar] [CrossRef]
- McCluney, K.E.; Belnap, J.; Collins, S.L.; González, A.L.; Hagen, E.M.; Nathaniel Holland, J.; Kotler, B.P.; Maestre, F.T.; Smith, S.D.; Wolf, B.O. Shifting species interactions in terrestrial dryland ecosystems under altered water availability and climate change. Biol. Rev. 2012, 87, 563–582. [Google Scholar] [CrossRef] [PubMed]
- Silvertown, J. Plant coexistence and the niche. Trends Ecol. Evol. 2004, 19, 605–611. [Google Scholar] [CrossRef]
- Al-Aklabi, A.; Al-Khulaidi, A.W.; Hussain, A.; Al-Sagheer, N. Main vegetation types and plant species diversity along an altitudinal gradient of Al Baha region, Saudi Arabia. Saudi J. Biol. Sci. 2016, 23, 687–697. [Google Scholar] [CrossRef]
- Almalki, K.A.; Al Mosallam, M.S.; Aldaajani, T.Z.; Al-Namazi, A.A. Landforms characterization of Saudi Arabia: Towards a geomorphological map. Int. J. Appl. Earth Obs. Geoinf. 2022, 112, 102945. [Google Scholar] [CrossRef]
- Dias, A.S.; Oliveira, R.S.; Martins, F.R.; Bongers, F.; Anten, N.P.; Sterck, F.J. Climbing mechanisms as a central trait to understand the ecology of lianas across the tropics. Glob. Ecol. Biogeogr. 2024, 33, e13846. [Google Scholar] [CrossRef]
- Mena-Jiménez, F.; Valencia-Díaz, S.; Corona-López, A.M.; Flores-Palacios, A. A case of accidental epiphytes that supports the notion of the evolution of epiphytes from ancestors living in open environments. Flora 2024, 317, 152553. [Google Scholar] [CrossRef]
- Büchi, L.; Vuilleumier, S. Ecological strategies in stable and disturbed environments depend on species specialisation. Oikos 2016, 125, 1408–1420. [Google Scholar] [CrossRef]
- Kumar, A.; Memo, M.; Mastinu, A. Plant behaviour: An evolutionary response to the environment? Plant Biol. 2020, 22, 961–970. [Google Scholar] [CrossRef]
- Durigon, J.; Durán, S.M.; Gianoli, E. Global distribution of root climbers is positively associated with precipitation and negatively associated with seasonality. J. Trop. Ecol. 2013, 29, 357–360. [Google Scholar] [CrossRef]
- Gallagher, R.V.; Leishman, M.R. A global analysis of trait variation and evolution in climbing plants. J. Biogeogr. 2012, 39, 1757–1771. [Google Scholar] [CrossRef]
- Kumar, J.; Choudhary, A.K.; Gupta, D.S.; Kumar, S. Towards exploitation of adaptive traits for climate-resilient smart pulses. Int. J. Mol. Sci. 2019, 20, 2971. [Google Scholar] [CrossRef] [PubMed]
- Van Horn, D.J.; Van Horn, M.L.; Barrett, J.E.; Gooseff, M.N.; Altrichter, A.E.; Geyer, K.M.; Zeglin, L.H.; Takacs-Vesbach, C.D. Factors controlling soil microbial biomass and bacterial diversity and community composition in a cold desert ecosystem: Role of geographic scale. PLoS ONE 2013, 8, e66103. [Google Scholar] [CrossRef] [PubMed]
- Palpurina, S.; Wagner, V.; von Wehrden, H.; Hájek, M.; Horsák, M.; Brinkert, A.; Hölzel, N.; Wesche, K.; Kamp, J.; Hájková, P.; et al. The relationship between plant species richness and soil pH vanishes with increasing aridity across Eurasian dry grasslands. Glob. Ecol. Biogeogr. 2017, 26, 425–434. [Google Scholar] [CrossRef]
- Li, X.R.; Song, G.; Hui, R.; Wang, Z.R. Precipitation and topsoil attributes determine the species diversity and distribution patterns of crustal communities in desert ecosystems. Plant Soil 2017, 420, 163–175. [Google Scholar] [CrossRef]
- Gianoli, E. The behavioural ecology of climbing plants. AoB Plants 2015, 7, plv013. [Google Scholar] [CrossRef]
- Zahran, M.A.; Younes, H.A.; Al-Tawil, B.A. Ecology of four community types: Red Sea coastal desert, Saudi Arabia. J. Coast. Res. 1985, 279–288. [Google Scholar]
- Zhang, Z.H.; Hu, G.; Zhu, J.D.; Luo, D.H.; Ni, J. Spatial patterns and interspecific associations of dominant tree species in two old-growth karst forests, SW China. Ecol. Res. 2010, 25, 1151–1160. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Z.; Lin, H.; Deng, R.; Liang, Z.; Li, Y.; Liang, S. Trait-based community assembly and functional strategies across three subtropical karst forests, Southwestern China. Front. Plant Sci. 2024, 15, 1451981. [Google Scholar] [CrossRef]
- Salim, E.; Mourey, J.; Crépeau, A.S.; Ravanel, L. Climbing the Alps in a warming world: Perspective of climate change impacts on high mountain areas influences alpinists’ behavioural adaptations. J. Outdoor Recreat. Tour. 2023, 44, 100662. [Google Scholar] [CrossRef]
- El-Sheikh, M.A.; Thomas, J.; Arif, I.A.; El-Sheikh, H.M. Ecology of inland sand dunes “nafuds” as a hyper-arid habitat, Saudi Arabia: Floristic and plant associations diversity. Saudi J. Biol. Sci. 2021, 28, 1503–1513. [Google Scholar] [CrossRef]
- Youssef, S.; Miara, M.D.; Boivin, S.; Sallio, R.; Nespoulous, J.; Boukcim, H.; Almalki, S.D.; Rees, S.K.; Lee, B.P.H.; Mohamed, A.H. Recovery of Perennial Plant Communities in Disturbed Hyper-Arid Environments (Sharaan Nature Reserve, Saudi Arabia). Land 2024, 13, 2033. [Google Scholar] [CrossRef]
- Fynn, R.W.S.; Morris, C.D.; Kirkman, K.P. Plant strategies and trait trade-offs influence trends in competitive ability along gradients of soil fertility and disturbance. J. Ecol. 2005, 93, 384–394. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, C.; Li, F.; Shen, P.; Liu, L.; Hu, Y. Distribution Patterns of Urban Spontaneous Vegetation Diversity and Their Response to Habitat Heterogeneity: A Case Study of Five Cities in Heilongjiang Province, China. Plants 2024, 13, 2982. [Google Scholar] [CrossRef]
- Mallick, J.; Al-Wadi, H.; Rahman, A.; Ahmed, M. Landscape dynamic characteristics using satellite data for a mountainous watershed of Abha, Kingdom of Saudi Arabia. Environ. Earth Sci. 2014, 72, 4973–4984. [Google Scholar] [CrossRef]
- Al-Dosary, N.M.N. Evaluation of soil characteristics for agricultural machinery management and cropping requirements in AL Aflaj Oasis, Saudi Arabia. Sustainability 2022, 14, 7991. [Google Scholar] [CrossRef]
- Alghamdi, A.S.; Alzhrani, A.I.; Alanazi, H.H. Local climate zones and thermal characteristics in Riyadh City, Saudi Arabia. Remote Sens. 2021, 13, 4526. [Google Scholar] [CrossRef]
- Alharthi, S.T.; El-Shiekh, M.A.; Alfarhan, A.A. Alien plant invasions of the natural habitat in the Western Region of Saudi Arabia: Floristic Diversity and Vegetation Structure. Diversity 2023, 15, 309. [Google Scholar] [CrossRef]
- Assaeed, A.M.; Al-Rowaily, S.L.; El-Bana, M.I.; Abood, A.A.; Dar, B.A.; Hegazy, A.K. Impact of off-road vehicles on soil and vegetation in a desert rangeland in Saudi Arabia. Saudi J. Biol. Sci. 2018, 26, 1187. [Google Scholar] [CrossRef]
- Waheed, M.; Arshad, F.; Majeed, M.; Fatima, S.; Mukhtar, N.; Aziz, R.; Mangrio, W.M.; Almohamad, H.; Al Dughairi, A.A.; Al-Mutiry, M.; et al. Community structure and distribution pattern of woody vegetation in response to soil properties in semi-arid lowland District Kasur Punjab, Pakistan. Land 2022, 11, 2145. [Google Scholar] [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M.A.S.S. The vegan package. Community Ecol. Package 2007, 10, 719. [Google Scholar]
- De Caceres, M.; Jansen, F.; De Caceres, M.M. Package ‘indicspecies’. Indicators 2016, 8. [Google Scholar]
- Hammer, Ø.; Harper, D.A. Past: Paleontological statistics software package for educaton and data anlysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]
- ter Braak, C.J.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Palo Alto, CA, USA, 2012.
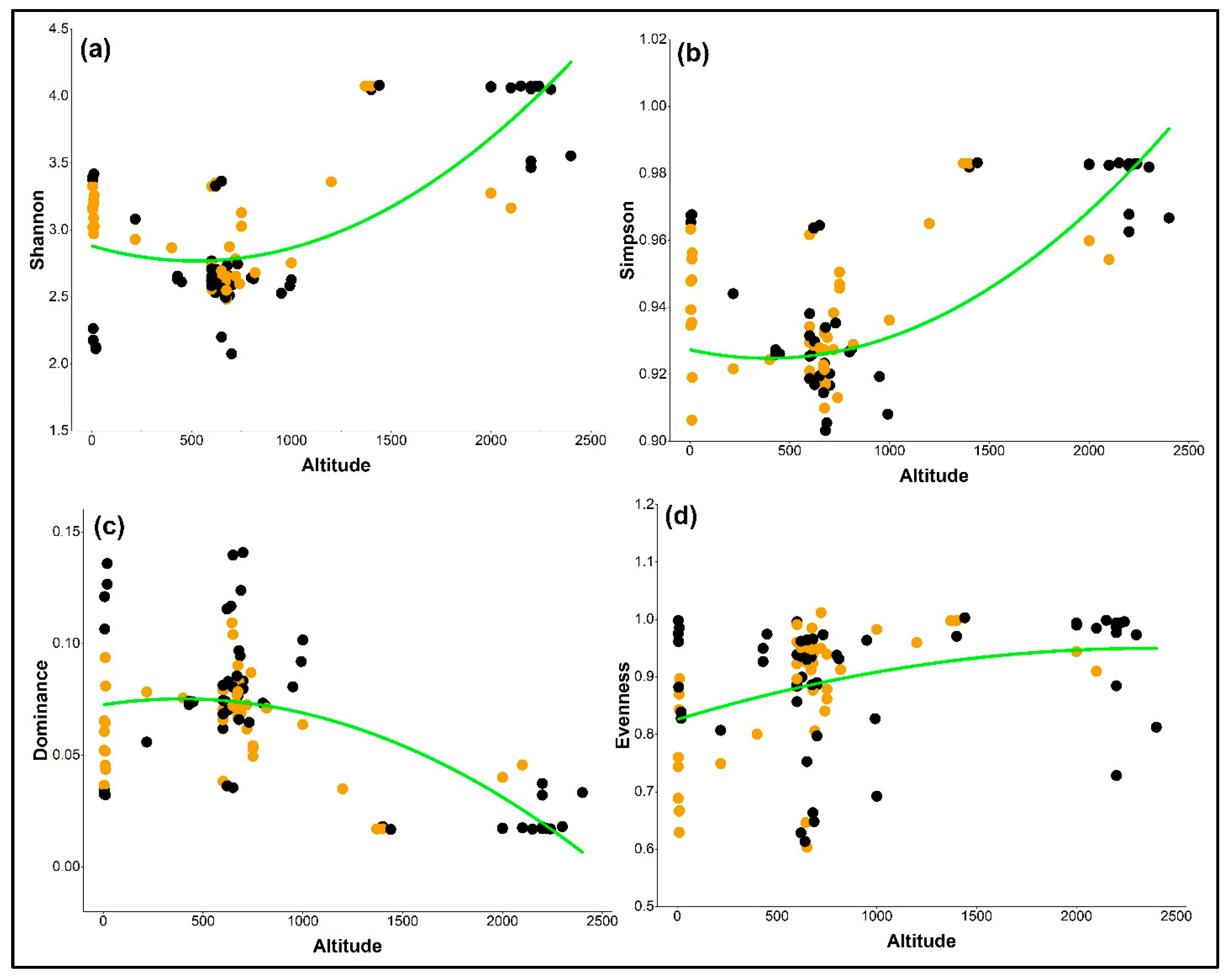


| Diversity Index | Linear Coefficient | SE (±) | R2 | p-Value |
|---|---|---|---|---|
| Shannon | −0.000436 | 0.00019 | 0.376 | <0.001 |
| Simpson | −0.000013 | 1.30 × 10−5 | 0.399 | <0.0001 |
| Dominance | −0.000028 | 1.30 × 10−5 | 0.418 | <0.0001 |
| Evenness | 0.000031 | 1.20 × 10−5 | 0.193 | <0.001 |
| Variable | Linear Coefficient | SE (±) | R2 | p-Value |
|---|---|---|---|---|
| Altitude | 81.16364 | 24.78134 | 0.09694 | <0.001 |
| Trees (%) | 1.20723 | 0.25785 | 0.24774 | <0.0001 |
| Shrub (%) | 0.7897 | 0.1755 | 0.24784 | <0.0001 |
| Shrublet (%) | 0.81054 | 0.24799 | 0.16692 | <0.001 |
| Herbs (%) | 1.41565 | 0.2623 | 0.27492 | <0.0001 |
| Grass (%) | 0.86468 | 0.19302 | 0.20916 | <0.0001 |
| Statistic | Axis 1 | Axis 2 | Axis 3 | Axis 4 |
|---|---|---|---|---|
| Eigenvalues | 0.6448 | 0.4849 | 0.2527 | 0.0588 |
| Explained variation (cumulative) | 14.08 | 24.68 | 30.2 | 31.48 |
| Pseudo-canonical correlation | 0.8918 | 0.8336 | 0.6011 | 0.524 |
| Explained fitted variation (cumulative) | 44.18 | 77.4 | 94.72 | 98.75 |
| Variable | Simple Effect (%) | Simple Pseudo-F | Simple p-Value | Conditional Effect (%) | Conditional Pseudo-F | Conditional p-Value |
|---|---|---|---|---|---|---|
| Rainfall | 13 | 15.1 | 0.002 | 13 | 15.1 | 0.002 |
| Soil Moisture | 11.8 | 13.5 | 0.002 | 10 | 13 | 0.002 |
| Temperature mean | 11 | 12.4 | 0.002 | 5.4 | 7.5 | 0.002 |
| SOC | 9.6 | 10.8 | 0.002 | 1.1 | 1.6 | 0.15 |
| N | 3.6 | 3.7 | 0.012 | 2.1 | 3 | 0.048 |
| pH | 0.4 | 0.4 | 0.74 | 0.2 | 0.3 | 0.716 |
| Species | Climbing | Independent | ||
|---|---|---|---|---|
| p-Value | IndVal% | p-Value | IndVal% | |
| Aerva javanica (Burm.f.) Juss. ex Schult. | 0.2755 | 0.8121 | 0.0281 | 2.196 |
| Boerhavia repens L. | 0.3373 | 0.6526 | 0.0649 | 2.053 |
| Haloxylon salicornicum (Moq.) Bunge ex Boiss. | 0.0278 | 3.35 | 0.082 | 1.589 |
| Indigofera spinosa Forssk. | 0.4318 | 0.4785 | 0.0363 | 2.183 |
| Lycium shawii Roem. & Schult. | 0.0424 | 2.813 | 0.0124 | 2.824 |
| Panicum turgidum Forssk. | 0.0368 | 3.016 | 0.0513 | 2.074 |
| Soda rosmarinus (Bunge ex Boiss.) Akhani | 1 | 0 | 0.576 | 0.4911 |
| Vachellia flava (Forssk.) Kyal. & Boatwr. | 0.1912 | 0.9571 | 0.0222 | 2.244 |
| Vachellia gerrardi (Benth.) P.J.H.Hurter | 0.0408 | 2.726 | 0.0422 | 2.162 |
| Vachellia tortilis (Forssk.) Galasso & Banfi | 0.0219 | 3.393 | 0.0626 | 2.053 |
| Zilla spinosa (L.) Prantl | 0.0464 | 2.581 | 0.4153 | 0.9072 |
| Zygophyllum album L.f. | 0.0148 | 3.582 | 0.9304 | 0.04775 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Bakre, D.A. Vegetation Structure and Environmental Correlates of Climbing Behavior for Desert Shrub Ochradenus baccatus. Plants 2025, 14, 1696. https://doi.org/10.3390/plants14111696
Al-Bakre DA. Vegetation Structure and Environmental Correlates of Climbing Behavior for Desert Shrub Ochradenus baccatus. Plants. 2025; 14(11):1696. https://doi.org/10.3390/plants14111696
Chicago/Turabian StyleAl-Bakre, Dhafer A. 2025. "Vegetation Structure and Environmental Correlates of Climbing Behavior for Desert Shrub Ochradenus baccatus" Plants 14, no. 11: 1696. https://doi.org/10.3390/plants14111696
APA StyleAl-Bakre, D. A. (2025). Vegetation Structure and Environmental Correlates of Climbing Behavior for Desert Shrub Ochradenus baccatus. Plants, 14(11), 1696. https://doi.org/10.3390/plants14111696






