Overexpression of NTPCS1 Enhances Zn Tolerance in Tobacco
Abstract
1. Introduction
2. Results
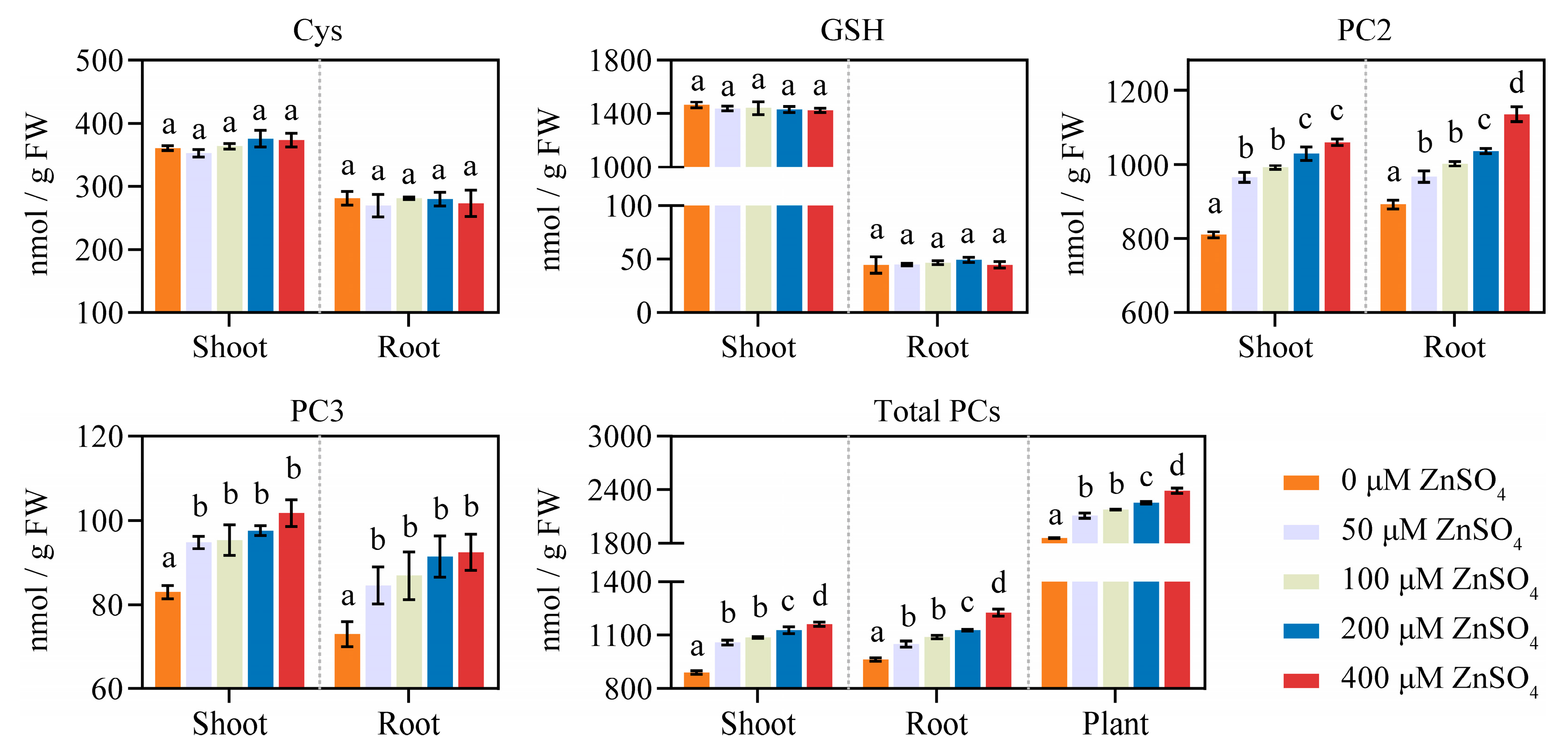
2.1. Zn Induces an Increase in NtPCS1 Expression and PCs Synthesis
2.2. Overexpression of NtPCS1 Enhances Zn Tolerance in Tobacco
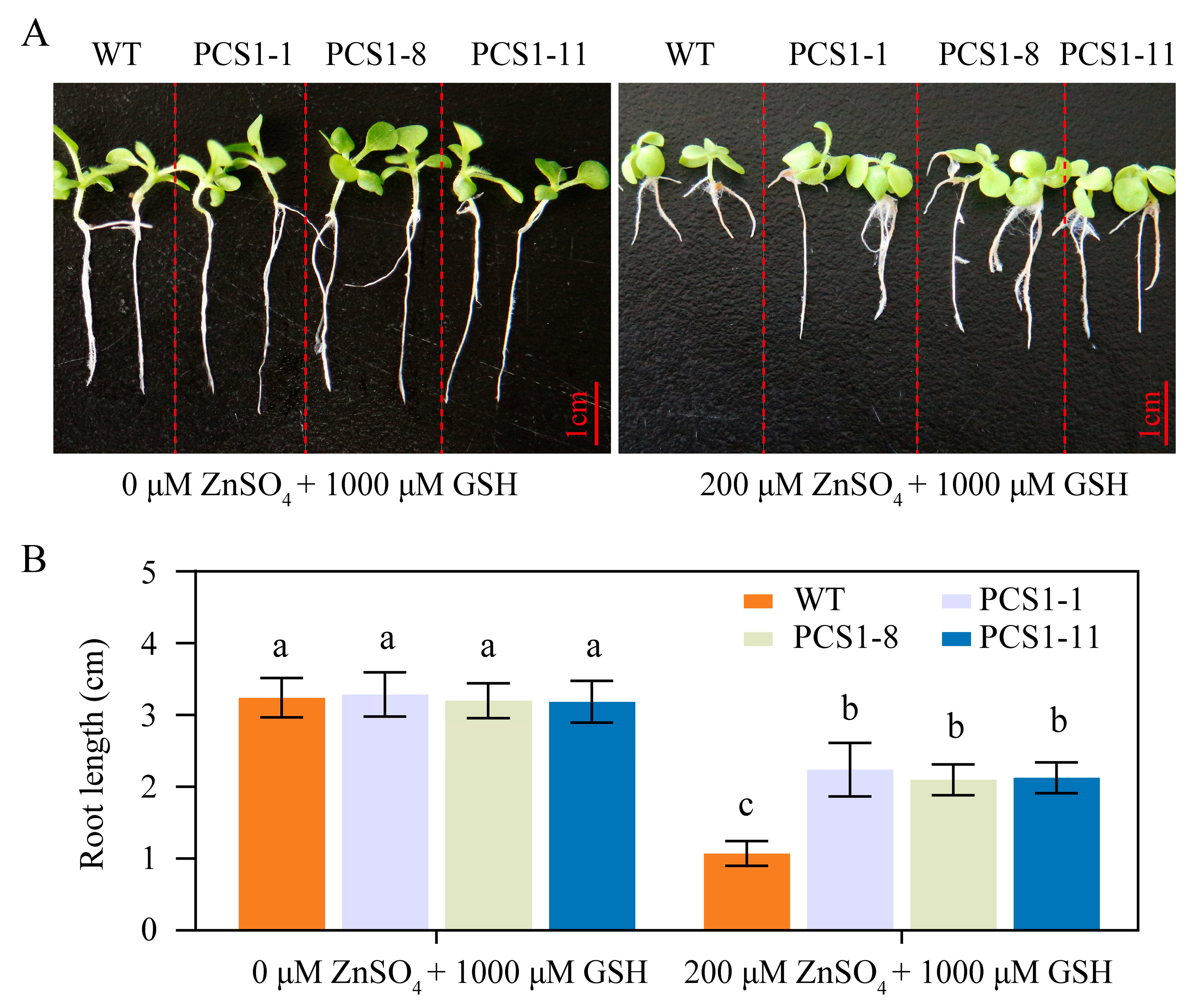
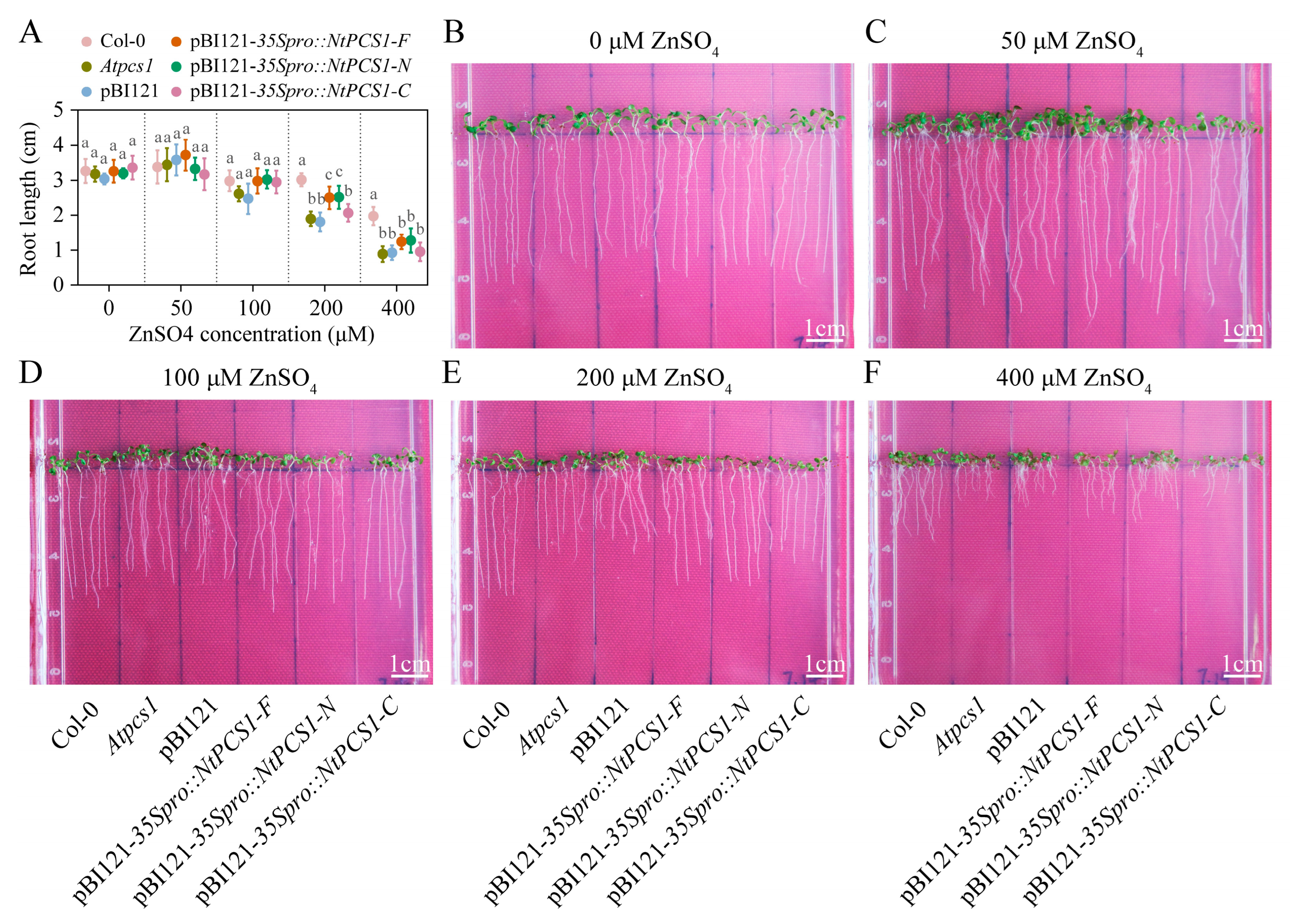
2.3. The N-Terminal Domain of the Tobacco NtPCS1 Protein Is Crucial for Zn Tolerance
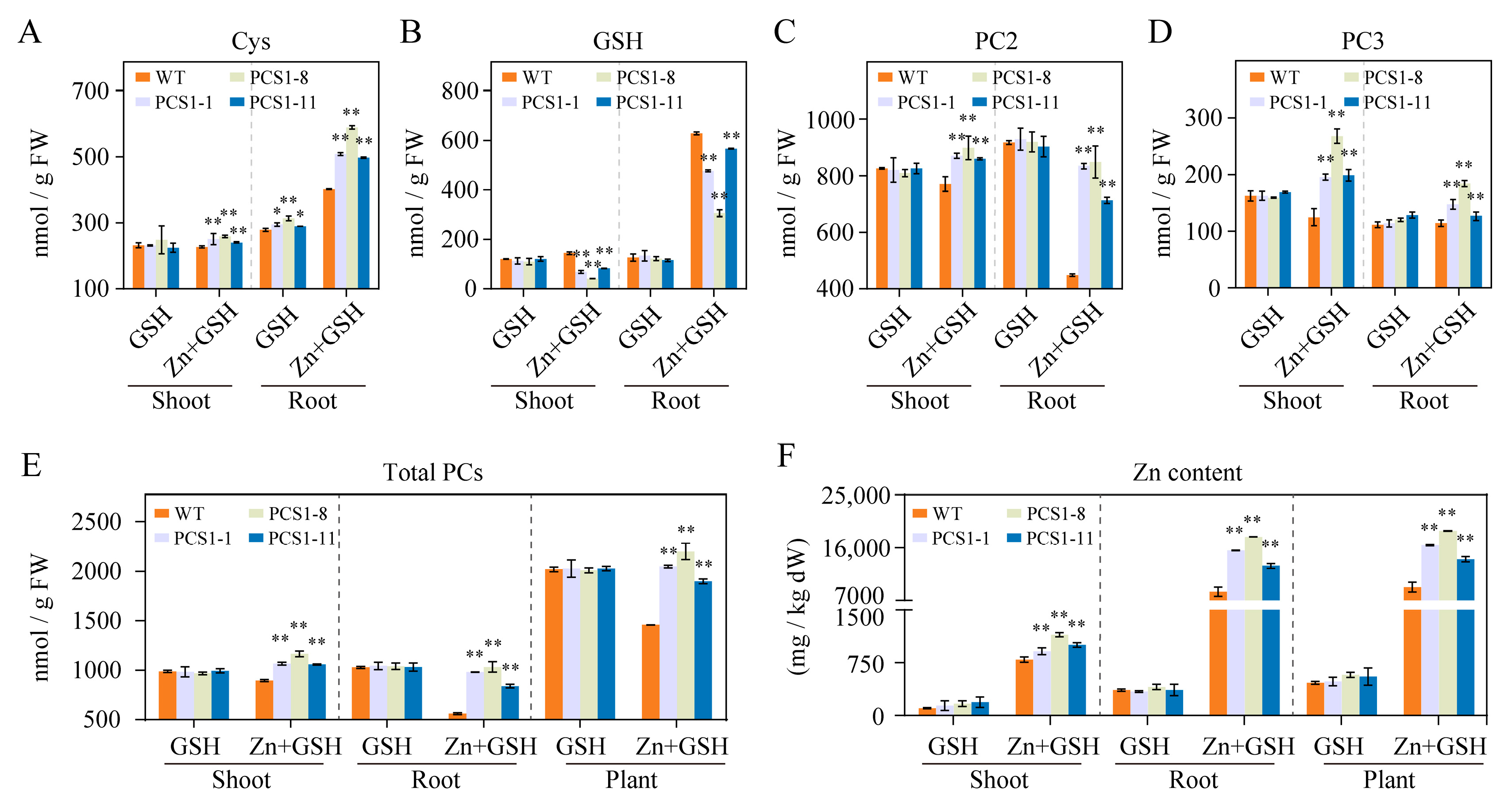
2.4. Overexpression of NtPCS1 Results in Increased PC and Zn Content in Tobacco
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Plant Expression Constructs and Transgenic Plants
4.3. Tobacco Seedling Experiments
- (i)
- To analyze the effect of Zn treatment on the expression of NtPCS1 and PC content in tobacco, the WT tobacco seeds were germinated on a foam sheet. After 28 days, seedlings were transferred to a hydroponic system: roots were submerged in 1/2 Hoagland solution with renewal every 2–3 days. Following a 7-day acclimatization period, plants were transferred to renewed Hoagland solution containing ZnSO4 at final concentrations of 0, 50, 100, 200, or 400 µM. After 48 h, 100 mg samples of shoots and roots were collected for qRT-PCR to detect the transcript level of NtPCS1. Additionally, 1 g samples of shoots and roots were used for the detection of PCs content by high-performance liquid chromatography (HPLC).
- (ii)
- To investigate the effect of Zn treatment on the activity of the NtPCS1 promoter in tobacco, homozygous transgenic tobacco seeds, which were transformed with the pBI121-NtPCS1pro::GUS construct, were germinated on 1/2 MS medium supplemented with either 0 or 200 µM ZnSO4. After 10, 20, and 30 days of cultivation, 10 seedlings were harvested for GUS staining, and 1 g of seedlings was collected for enzymatic activity analysis.
- (iii)
- In the root length experiment, seeds from both WT and homozygous PCS1 lines were germinated on 1/2 MS medium. The medium was supplemented with 0 or 200 μM ZnSO4, either with or without the addition of 1000 μM GSH. The seedlings were grown in a vertical orientation. After 21 days, the root lengths of the 24 tobacco seedlings were measured.
- (iv)
- Seeds from both WT and homozygous PCS1 lines were germinated on a foam sheet. After 28 days, the seedlings were transferred to 1/2 Hoagland solution with renewal every 2–3 days. Following a 7-day acclimatization period, the tobacco seedlings were moved to a fresh solution containing either 0 or 200 µM ZnSO4, with or without the presence of 1000 µM GSH. After 14 days, 1 g and 5 g samples of both shoots and roots were collected for HPLC and absorption spectroscopy analysis (AAS) analyses, respectively, to quantify PCs and Zn contents.
4.4. Arabidopsis Atpcs1 Mutant Complementation
4.5. RNA Extract and qRT-PCR
4.6. GUS Staining
4.7. GUS Enzymatic Activity Analysis
4.8. PCs Content Analysis
4.9. Zn Content Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaur, H.; Garg, N. Zinc toxicity in plants: A review. Planta 2021, 253, 129. [Google Scholar] [CrossRef] [PubMed]
- Angulo-Bejarano, P.I.; Puente-Rivera, J.; Cruz-Ortega, R. Metal and Metalloid Toxicity in Plants: An Overview on Molecular Aspects. Plants 2021, 10, 635. [Google Scholar] [CrossRef] [PubMed]
- Seregin, I.V.; Kozhevnikova, A.D. Phytochelatins: Sulfur-Containing Metal(loid)-Chelating Ligands in Plants. Int. J. Mol. Sci. 2023, 24, 2430. [Google Scholar] [CrossRef]
- Rea, P.A. Phytochelatin synthase: Of a protease a peptide polymerase made. Physiol. Plant. 2012, 145, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Tsuji, N.; Miyamoto, K. Biosynthetic regulation of phytochelatins, heavy metal-binding peptides. J. Biosci. Bioeng. 2005, 100, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Shine, A.M.; Shakya, V.P.; Idnurm, A. Phytochelatin synthase is required for tolerating metal toxicity in a basidiomycete yeast and is a conserved factor involved in metal homeostasis in fungi. Fungal Biol. Biotechnol. 2015, 2, 3. [Google Scholar] [CrossRef]
- Olsson, S.; Penacho, V.; Puente-Sánchez, F.; Díaz, S.; Gonzalez-Pastor, J.E.; Aguilera, A. Horizontal Gene Transfer of Phytochelatin Synthases from Bacteria to Extremophilic Green Algae. Microb. Ecol. 2017, 73, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Shri, M.; Dave, R.; Diwedi, S.; Shukla, D.; Kesari, R.; Tripathi, R.D.; Trivedi, P.K.; Chakrabarty, D. Heterologous expression of Ceratophyllum demersum phytochelatin synthase, CdPCS1, in rice leads to lower arsenic accumulation in grain. Sci. Rep. 2014, 4, srep05784. [Google Scholar] [CrossRef]
- Kühnlenz, T.; Schmidt, H.; Uraguchi, S.; Clemens, S. Arabidopsis thaliana phytochelatin synthase 2 is constitutively active in vivo and can rescue the growth defect of the PCS1-deficient cad1-3 mutant on Cd-contaminated soil. J. Exp. Bot. 2014, 65, 4241–4253. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Kim, S.H.; Lee, H.S.; Marinoia, E.; Song, W.Y. Characterization of Brassica rapa metallothionein and phytochelatin synthase genes potentially involved in heavy metal detoxification. PLoS ONE 2021, 16, e0252899. [Google Scholar] [CrossRef]
- Park, H.C.; Hwang, J.E.; Jiang, Y.; Kim, Y.J.; Kim, S.H.; Nguyen, X.C.; Kim, C.Y.; Chung, W.S. Functional characterisation of two phytochelatin synthases in rice (Oryza sativa cv. Milyang 117) that respond to cadmium stress. Plant Biol. 2019, 21, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Z.; Zhu, C. Heteroexpression of the wheat phytochelatin synthase gene (TaPCS1) in rice enhances cadmium sensitivity. Acta Biochim. Biophys. Sin. 2012, 44, 886–893. [Google Scholar] [CrossRef]
- Kühnlenz, T.; Westphal, L.; Schmidt, H.; Scheel, D.; Clemens, S. Expression of Caenorhabditis elegans PCS in the AtPCS1-deficient Arabidopsis thaliana cad1-3 mutant separates the metal tolerance and non-host resistance functions of phytochelatin synthases. Plant Cell Environ. 2015, 38, 2239–2247. [Google Scholar] [CrossRef]
- Rigouin, C.; Nylin, E.; Cogswell, A.A.; Schaumlöffel, D.; Dobritzsch, D.; Williams, D.L. Towards an understanding of the function of the phytochelatin synthase of Schistosoma mansoni. PLoS Negl. Trop. Dis. 2013, 7, e2037. [Google Scholar] [CrossRef] [PubMed]
- Rigouin, C.; Vermeire, J.J.; Nylin, E.; Williams, D.L. Characterization of the phytochelatin synthase from the human parasitic nematode Ancylostoma ceylanicum. Mol. Biochem. Parasitol. 2013, 191, 1–6. [Google Scholar] [CrossRef]
- Lee, S.; Korban, S.S. Transcriptional regulation of Arabidopsis thaliana phytochelatin synthase (AtPCS1) by cadmium during early stages of plant development. Planta 2002, 215, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Heiss, S. Phytochelatin synthase (PCS) protein is induced in Brassica juncea leaves after prolonged Cd exposure. J. Exp. Bot. 2003, 54, 1833–1839. [Google Scholar] [CrossRef]
- Zha, Y.Q.; Zhang, K.K.; Pan, F.; Liu, X.; Han, S.M.; Guan, P. Cloning of PCS gene (TpPCS1) from Tagetes patula L. and expression analysis under cadmium stress. Plant Biol. 2021, 23, 508–516. [Google Scholar] [CrossRef]
- Azizi, N.; Shahpiri, A. Functional characterization of Helianthus annuus phytochelatin synthase (HaPCS): Gene expression and protein profiles of HaPCS responding to arsenic and evaluation of arsenic accumulation in engineered bacteria expressing HaPCS. Environ. Exp. Bot. 2021, 187, 104470. [Google Scholar] [CrossRef]
- Das, N.; Bhattacharya, S.; Bhattacharyya, S.; Maiti, M.K. Identification of alternatively spliced transcripts of rice phytochelatin synthase 2 gene OsPCS2 involved in mitigation of cadmium and arsenic stresses. Plant Mol. Biol. 2017, 94, 167–183. [Google Scholar] [CrossRef]
- Yamazaki, S.; Ueda, Y.; Mukai, A.; Ochiai, K.; Matoh, T. Rice phytochelatin synthases OsPCS1 and OsPCS2 make different contributions to cadmium and arsenic tolerance. Plant Direct 2018, 2, e00034. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.A.; Gupta, M. Exposure of Brassica juncea (L) to arsenic species in hydroponic medium: Comparative analysis in accumulation and biochemical and transcriptional alterations. Environ. Sci. Pollut. Res. 2013, 20, 8141–8150. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, C.; Tan, Z.; Liu, J.; Zhuang, L.; Yang, Z.; Huang, B. Functional Identification and Characterization of Genes Cloned from Halophyte Seashore Paspalum Conferring Salinity and Cadmium Tolerance. Front. Plant Sci. 2016, 7, 102. [Google Scholar] [CrossRef]
- Yousefi, Z.; Kolahi, M.; Majd, A.; Jonoubi, P. Effect of cadmium on morphometric traits, antioxidant enzyme activity and phytochelatin synthase gene expression (SoPCS) of Saccharum officinarum var. cp48-103 in vitro. Ecotoxicol. Environ. Saf. 2018, 157, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Kısa, D. Responses of phytochelatin and proline-related genes expression associated with heavy metal stress in Solanum lycopersicum. Acta Bot. Croat. 2019, 78, 9–16. [Google Scholar] [CrossRef]
- Fan, W.; Guo, Q.; Liu, C.; Liu, X.; Zhang, M.; Long, D.; Xiang, Z.; Zhao, A. Two mulberry phytochelatin synthase genes confer zinc/cadmium tolerance and accumulation in transgenic Arabidopsis and tobacco. Gene 2018, 645, 95–104. [Google Scholar] [CrossRef]
- Talebi, M.; Tabatabaei, B.E.S.; Akbarzadeh, H. Hyperaccumulation of Cu, Zn, Ni, and Cd in Azolla species inducing expression of methallothionein and phytochelatin synthase genes. Chemosphere 2019, 230, 488–497. [Google Scholar] [CrossRef]
- Cao, Z.Z.; Qin, M.L.; Lin, X.Y.; Zhu, Z.W.; Chen, M.X. Sulfur supply reduces cadmium uptake and translocation in rice grains (Oryza sativa L.) by enhancing iron plaque formation, cadmium chelation and vacuolar sequestration. Environ. Pollut. 2018, 238, 76–84. [Google Scholar] [CrossRef]
- Sauge-Merle, S.; Cuiné, S.P.; Carrier, P.; Lecomte-Pradines, C.; Luu, D.-T.; Peltier, G. Enhanced Toxic Metal Accumulation in Engineered Bacterial Cells Expressing Arabidopsis thaliana Phytochelatin Synthase. Appl. Environ. Microbiol. 2003, 69, 490–494. [Google Scholar] [CrossRef]
- Vatamaniuk, O.K.; Mari, S.; Lu, Y.P.; Rea, P.A. AtPCS1, a phytochelatin synthase from Arabidopsis: Isolation and in vitro reconstitution. Proc. Natl. Acad. Sci. USA 1999, 96, 7110–7115. [Google Scholar] [CrossRef]
- Zhu, S.; Shi, W.; Jie, Y. Overexpression of BnPCS1, a Novel Phytochelatin Synthase Gene from Ramie (Boehmeria nivea), Enhanced Cd Tolerance, Accumulation, and Translocation in Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 639189. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wang, X.; Wang, R.; Wang, J.; Le, S.; Zhao, Y. Overexpression of Three Duplicated BnPCS Genes Enhanced Cd Accumulation and Translocation in Arabidopsis thaliana Mutant cad1-3. Bull. Environ. Contam. Toxicol. 2018, 102, 146–152. [Google Scholar] [CrossRef]
- Gasic, K.; Korban, S.S. Transgenic Indian mustard (Brassica juncea) plants expressing an Arabidopsis phytochelatin synthase (AtPCS1) exhibit enhanced As and Cd tolerance. Plant Mol. Biol. 2007, 64, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; Bernal, P.; Almela, C.; Vélez, D.; García-Agustín, P.; Serrano, R.; Navarro-Aviñó, J. An engineered plant that accumulates higher levels of heavy metals than Thlaspi caerulescens, with yields of 100 times more biomass in mine soils. Chemosphere 2006, 64, 478–485. [Google Scholar] [CrossRef]
- Lee, B.D.; Hwang, S. Tobacco phytochelatin synthase (NtPCS1) plays important roles in cadmium and arsenic tolerance and in early plant development in tobacco. Plant Biotechnol. Rep. 2015, 9, 107–114. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, J.; Chen, M.; Liu, J.; Tang, Y. Characterization of a Nicotiana tabacum phytochelatin synthase 1 and its response to cadmium stress. Front. Plant Sci. 2024, 15, 1418762. [Google Scholar] [CrossRef] [PubMed]
- Wojas, S.; Clemens, S.; Hennig, J.; Sklodowska, A.; Kopera, E.; Schat, H.; Bal, W.; Antosiewicz, D.M. Overexpression of phytochelatin synthase in tobacco: Distinctive effects of AtPCS1 and CePCS genes on plant response to cadmium. J. Exp. Bot. 2008, 59, 2205–2219. [Google Scholar] [CrossRef]
- Pomponi, M.; Censi, V.; Di Girolamo, V.; De Paolis, A.; di Toppi, L.S.; Aromolo, R.; Costantino, P.; Cardarelli, M. Overexpression of Arabidopsis phytochelatin synthase in tobacco plants enhances Cd2+ tolerance and accumulation but not translocation to the shoot. Planta 2006, 223, 180–190. [Google Scholar] [CrossRef]
- Cobbett, C.S. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000, 123, 825–832. [Google Scholar] [CrossRef]
- Kühnlenz, T.; Hofmann, C.; Uraguchi, S.; Schmidt, H.; Schempp, S.; Weber, M.; Lahner, B.; Salt, D.E.; Clemens, S. Phytochelatin Synthesis Promotes Leaf Zn Accumulation of Arabidopsis thaliana Plants Grown in Soil with Adequate Zn Supply and is Essential for Survival on Zn-Contaminated Soil. Plant Cell Physiol. 2016, 57, 2342–2352. [Google Scholar] [CrossRef]
- Romanyuk, N.D.; Rigden, D.J.; Vatamaniuk, O.K.; Lang, A.; Cahoon, R.E.; Jez, J.M.; Rea, P.A. Mutagenic Definition of a Papain-Like Catalytic Triad, Sufficiency of the N-Terminal Domain for Single-Site Core Catalytic Enzyme Acylation, and C-Terminal Domain for Augmentative Metal Activation of a Eukaryotic Phytochelatin Synthase. Plant Physiol. 2006, 141, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Ruotolo, R.; Peracchi, A.; Bolchi, A.; Infusini, G.; Amoresano, A.; Ottonello, S. Domain organization of phytochelatin synthase: Functional properties of truncated enzyme species identified by limited proteolysis. J. Biol. Chem. 2004, 279, 14686–14693. [Google Scholar] [CrossRef] [PubMed]
- Uraguchi, S.; Sone, Y.; Ohta, Y.; Ohkama-Ohtsu, N.; Hofmann, C.; Hess, N.; Nakamura, R.; Takanezawa, Y.; Clemens, S.; Kiyono, M. Identification of C-terminal Regions in Arabidopsis thaliana Phytochelatin Synthase 1 Specifically Involved in Activation by Arsenite. Plant Cell Physiol. 2018, 59, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Barbaro, E.; Bellini, E.; Saba, A.; Sanità di Toppi, L.; Varotto, C.; Cuypers, A. Ancestral function of the phytochelatin synthase C-terminal domain in inhibition of heavy metal-mediated enzyme overactivation. J. Exp. Bot. 2020, 71, 6655–6669. [Google Scholar] [CrossRef]
- Sneller, F.E.; Heerwaarden, L.M.; Koevoets, P.L.; Vooijs, R.; Schat, H.; Verkleij, J.A. Derivatization of phytochelatins from Silene vulgaris, induced upon exposure to arsenate and cadmium: Comparison of derivatization with Ellman’s reagent and monobromobimane. J. Agric. Food Chem. 2000, 48, 4014–4019. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Zhang, J. Overexpression of NTPCS1 Enhances Zn Tolerance in Tobacco. Plants 2025, 14, 1688. https://doi.org/10.3390/plants14111688
Wu C, Zhang J. Overexpression of NTPCS1 Enhances Zn Tolerance in Tobacco. Plants. 2025; 14(11):1688. https://doi.org/10.3390/plants14111688
Chicago/Turabian StyleWu, Chanjuan, and Jie Zhang. 2025. "Overexpression of NTPCS1 Enhances Zn Tolerance in Tobacco" Plants 14, no. 11: 1688. https://doi.org/10.3390/plants14111688
APA StyleWu, C., & Zhang, J. (2025). Overexpression of NTPCS1 Enhances Zn Tolerance in Tobacco. Plants, 14(11), 1688. https://doi.org/10.3390/plants14111688





