Biogeographical and Ecological Patterns of the Bryophytic Flora Inhabiting the Small Islands Surrounding the Italian Peninsula, Sicily and Sardinia
Abstract
1. Introduction
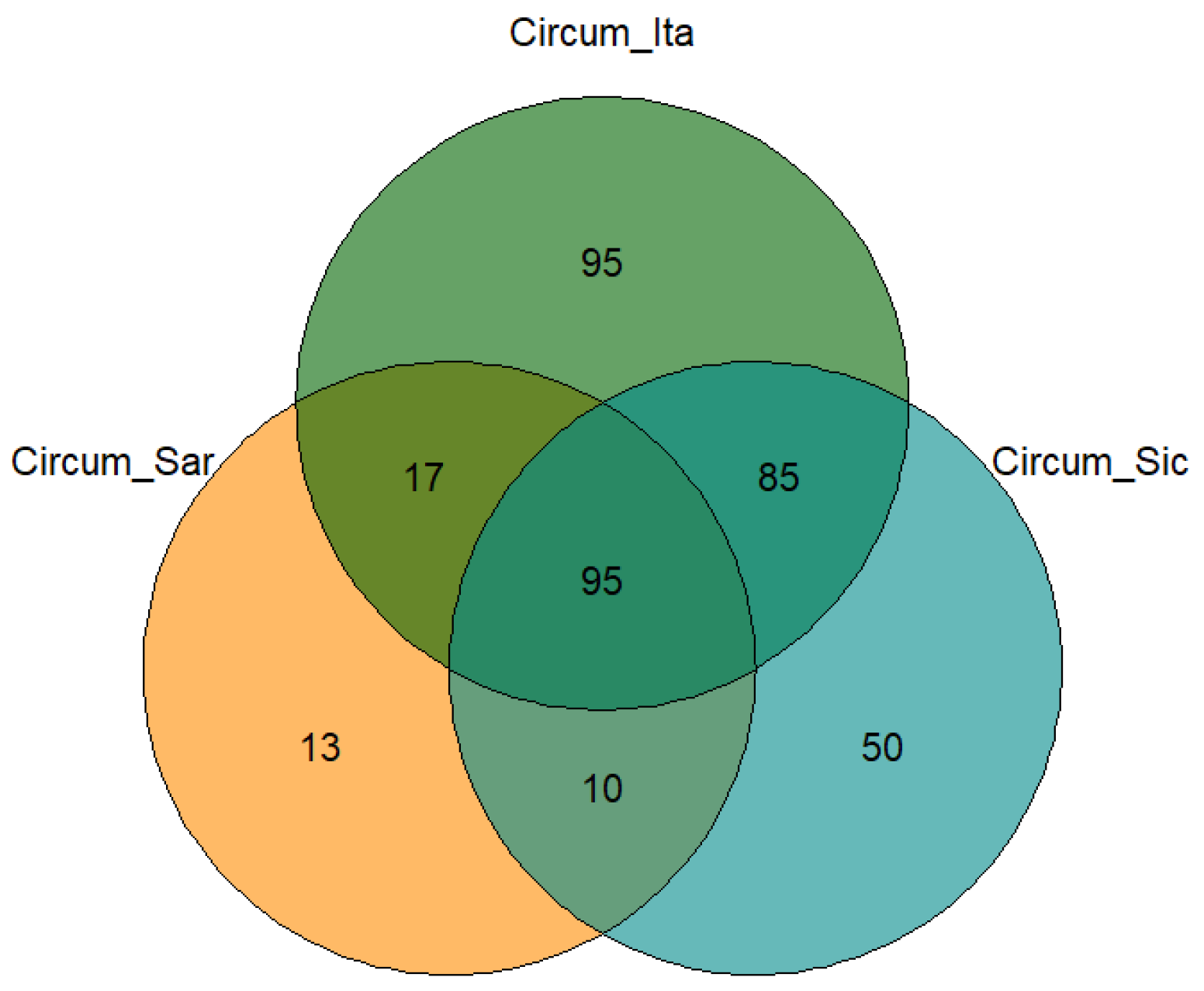
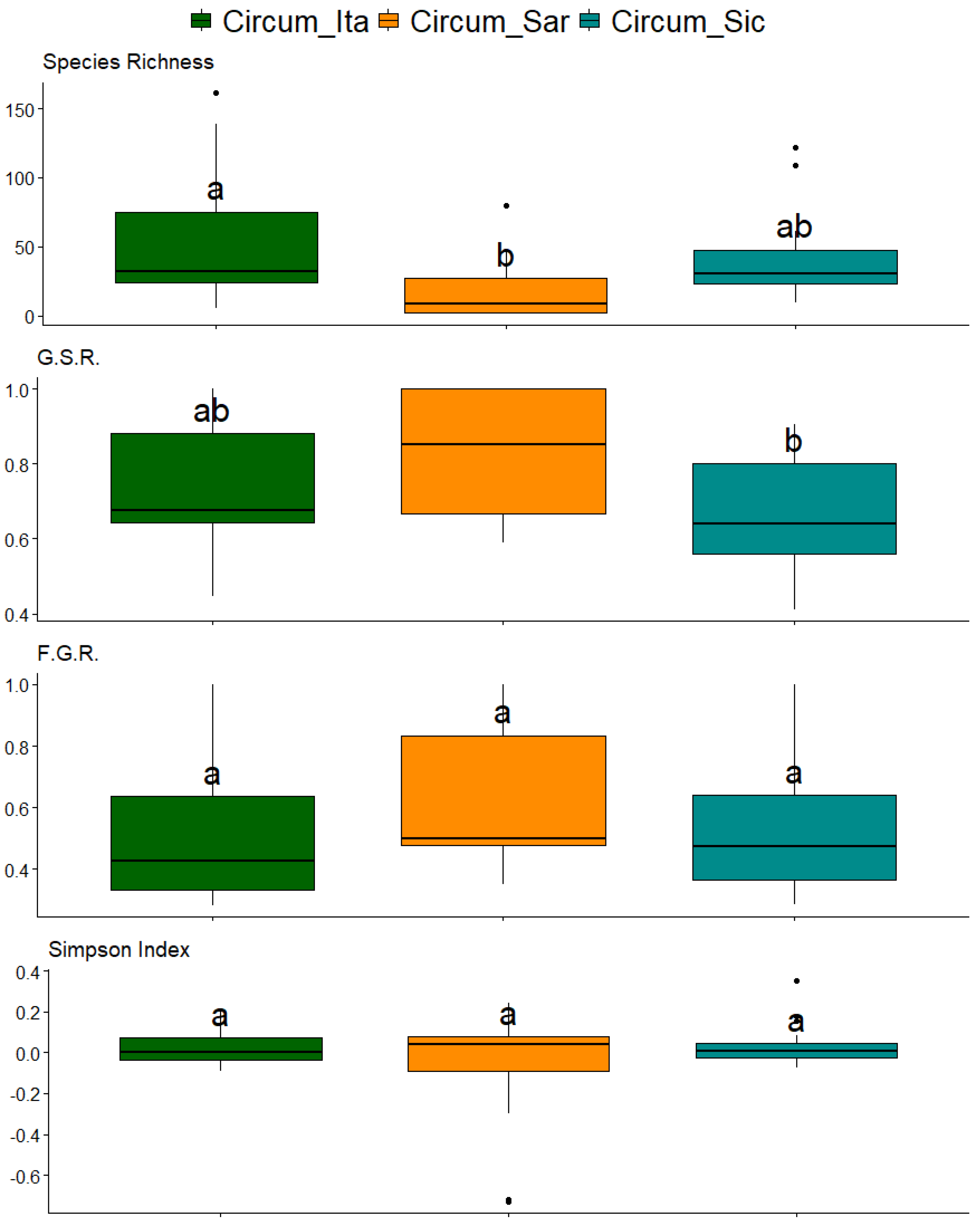
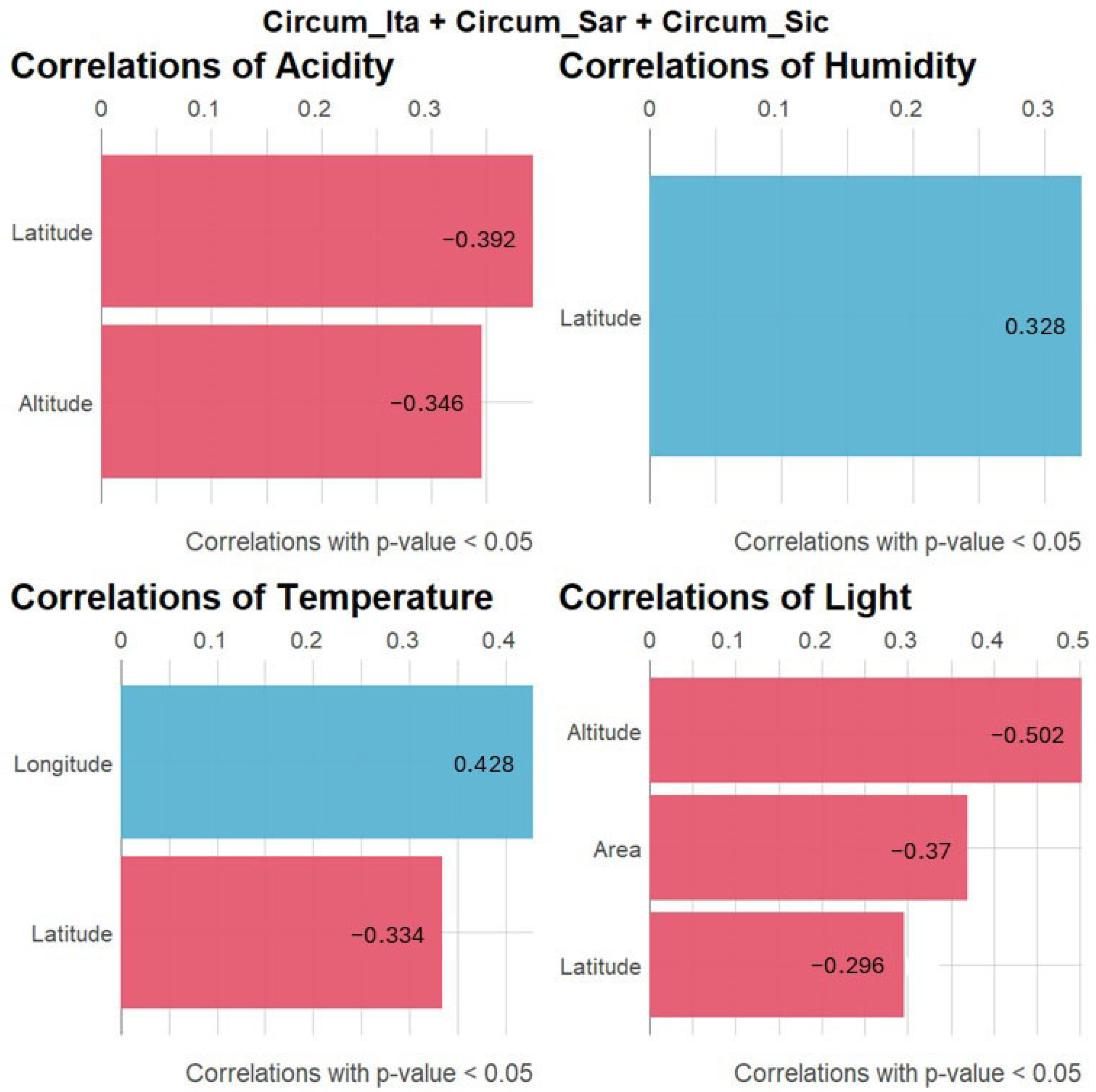
2. Results
3. Materials and Methods
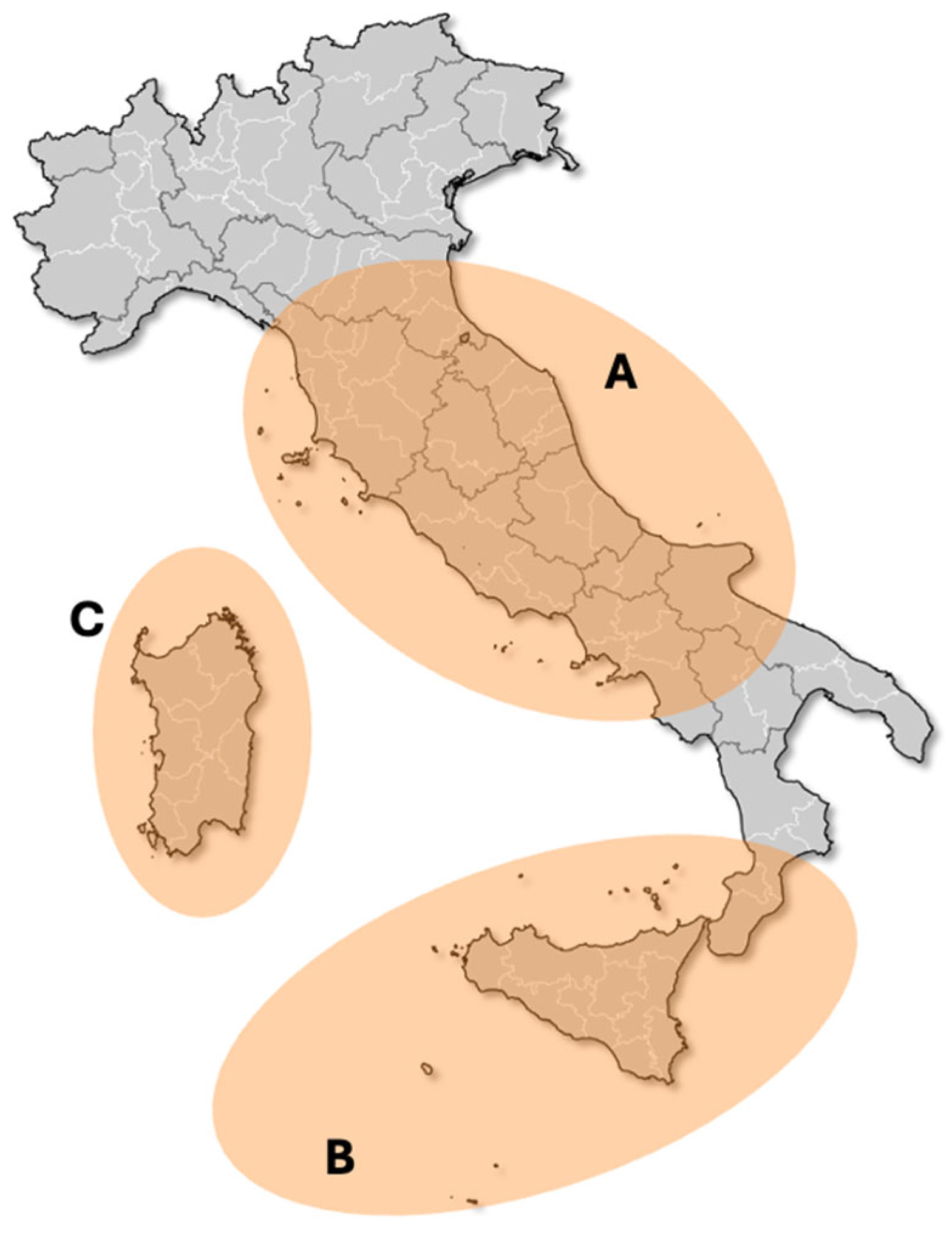
3.1. Geology of Study Sites
3.2. Data and Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Whittaker, R.J.; Fernández-Palacios, J.M. Island Biogeography: Ecology, Evolution, and Conservation; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Manes, S.; Costello, M.J.; Beckett, H.; Debnath, A.; Devenish-Nelson, E.; Grey, K.A.; Jenkins, R.; Khan, T.M.; Kiessling, W.; Krause, C.; et al. Endemism increases species’ climate change risk in areas of global biodiversity importance. Biol. Conserv. 2021, 257, 109070. [Google Scholar] [CrossRef]
- Barrett, S.C.H. The reproductive biology and genetics of island plants. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1996, 351, 725–733. [Google Scholar]
- Glime, J.M. Volume 1, Chapter 4–7: Adaptive Strategies: Vegetative vs Sexual Diaspores; Michigan Technological University: Houghton, MI, USA, 2013; on-line. [Google Scholar]
- Cuena-Lombraña, A.; Fois, M.; Cogoni, A.; Bacchetta, G. Where we come from and where to go: Six decades of botanical studies in the Mediterranean wetlands, with Sardinia (Italy) as a case study. Wetlands 2021, 41, 69. [Google Scholar] [CrossRef]
- De Notaris, G. Cronaca della briologia italiana. Comment. Soc. Crittogamologica Ital. 1865–1867, 2, 89–113+269–312. [Google Scholar]
- Giacomini, V. Contributo alla conoscenza della flora briologica della Sardegna. Nuovo G. Bot. Ital. 1938, 45, 567–571. [Google Scholar] [CrossRef]
- Giacomini, V. Eine heteroropische, postglaziale Bryophyten-kolonie aus der Adamellogruppe (Italienische Zentralalpen). Ann. Bryol. 1938, 11, 68–75. [Google Scholar]
- Cogoni, A.; Scrugli, A. Acaulon fontiquerianum Casas et Sérgio (Musci, Pottiaceae) new to Sardinia (Italy). Cryptogam. Bryol. 2000, 21, 285–288. [Google Scholar]
- Cogoni, A.; Flore, F.; Scrugli, A. The bryological flora of Isola dei Cavoli SE-Sardinia, Italy. Flora Mediterr. 2004, 14, 115–127. [Google Scholar]
- Cogoni, A.; Flore, F.; Adamo, C.; Scrugli, A. The bryophytic flora of the Molara Island (Northeastern Sardinia). Flora Mediterr. 2007, 17, 185–204. [Google Scholar]
- Cogoni, A.; Scrugli, A. Aggiornamento della brioflora di alcune isole minori della Sardegna. In Proceedings of the Abstract 103 Congresso Nazionale della Società Botanica Italiana, Reggio Calabria, Italy, 17–19 September 2008; p. 35. [Google Scholar]
- Cogoni, A.; Scrugli, A.; Flore, F.; Cortis, P.; Aleffi, M. The bryophyte flora of the Asinara Island (northwest Sardinia, Italy). Cryptogam. Bryol. 2009, 30, 79–89. [Google Scholar]
- Privitera, M.; Puglisi, M. On the occurrence of some interesting mosses on Linosa (Pelagian arcipelago, Sicily). Flora Mediterr. 2006, 16, 5–9. [Google Scholar]
- Privitera, M.; Campisi, P.; Carratello, A.; Cogoni, A.; Flore, F.; Gueli, L.; Lo Giudice, R.; Provenzano, F.; Petraglia, A.; Sguazzin, F.; et al. La Flora briofitica dell’Isola di Lipari (Arcipelago delle Eolie, Sicilia). Inf. Bot. Ital. 2008, 40, 3–13. [Google Scholar]
- Privitera, M.; Puglisi, M. The circum-Sicilian islands as important refuge areas for some remarkable bryophytes. Plant Biosyst. 2009, 143, S126–S135. [Google Scholar] [CrossRef]
- Puglisi, M.; Privitera, M. New moss records for the Mediterranean islands. Cryptogam. Bryol. 2018, 39, 177–183. [Google Scholar] [CrossRef]
- Aleffi, M.; Cogoni, A.; Poponessi, S. An updated checklist of the bryophytes of Italy, including the Republic of San Marino and Vatican City State. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2023, 157, 1259–1307. [Google Scholar] [CrossRef]
- Dierßen, K. Distribution ecological amplitude and phytosociological characterization of European bryophytes. Bryophyt. Biblioth. 2001, 56, 1–289. [Google Scholar]
- Chiarabba, C.; De Gori, P.; Speranza, F. The southern Tyrrhenian subduction zone: Deep geometry, magmatism and Plio-Pleistocene evolution. Earth Planet. Sci. Lett. 2008, 268, 408–423. [Google Scholar] [CrossRef]
- Franceschelli, M.; Puxeddu, M.; Cruciani, G. Variscan metamorphism in Sardinia, Italy: Review and discussion. J. Virtual Explor. 2005, 19, 2–36. [Google Scholar] [CrossRef]
- Martín-Queller, E.; Albert, C.H.; Dumas, P.J.; Saatkamp, A. Islands, mainland, and terrestrial fragments: How isolation shapes plant diversity. Ecol. Evol. 2017, 7, 6904–6917. [Google Scholar] [CrossRef]
- Puglisi, M.; Kürschner, H.; Privitera, M. Syntaxonomy, life forms and life strategies of the bryophyte vegetation of the Carnic Alps (NE Italy). Nova Hedwig. 2013, 96, 325–349. [Google Scholar] [CrossRef]
- Bottini, A. Sulla briologia delle isole italiane. Webbia 1907, 2, 345–402. [Google Scholar] [CrossRef]
- Aleffi, M.; Cortini Pedrotti, C.; Gafta, D. Bryogeographic patterns in the small islands surrounding the ltalian peninsula, Sicily and Sardinia. Bocconea 2003, 16, 93–103. [Google Scholar]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 1967. [Google Scholar]
- Buck, W.R.; Privitera, M. Taxonomic remarks on Rhynchostegium strongylense (Bott.) comb. nov., rare endemic from the Mediterranean area. Cryptogam. Bryol. 1999, 20, 11–15. [Google Scholar] [CrossRef]
- Privitera, M.; Puglisi, M. Osservazioni sulla flora e vegetazione briofitica dell’isola di Pantelleria. Boll. Accad. Gioenia Sci. Nat. Catania 1989, 22, 67–104. [Google Scholar]
- Puglisi, M.; Campisi, P.; Aleffi, M.; Bacilliere, G.; Bonini, I.; Cogoni, A.; Dia, M.G.; Miserere, L.; Privitera, M.; Tiburtini, M.; et al. Red-list of Italian bryophytes. 2. Mosses. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2024, 158, 1031–1056. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poponessi, S.; Aleffi, M.; Cogoni, A.; De Agostini, A. Biogeographical and Ecological Patterns of the Bryophytic Flora Inhabiting the Small Islands Surrounding the Italian Peninsula, Sicily and Sardinia. Plants 2025, 14, 1618. https://doi.org/10.3390/plants14111618
Poponessi S, Aleffi M, Cogoni A, De Agostini A. Biogeographical and Ecological Patterns of the Bryophytic Flora Inhabiting the Small Islands Surrounding the Italian Peninsula, Sicily and Sardinia. Plants. 2025; 14(11):1618. https://doi.org/10.3390/plants14111618
Chicago/Turabian StylePoponessi, Silvia, Michele Aleffi, Annalena Cogoni, and Antonio De Agostini. 2025. "Biogeographical and Ecological Patterns of the Bryophytic Flora Inhabiting the Small Islands Surrounding the Italian Peninsula, Sicily and Sardinia" Plants 14, no. 11: 1618. https://doi.org/10.3390/plants14111618
APA StylePoponessi, S., Aleffi, M., Cogoni, A., & De Agostini, A. (2025). Biogeographical and Ecological Patterns of the Bryophytic Flora Inhabiting the Small Islands Surrounding the Italian Peninsula, Sicily and Sardinia. Plants, 14(11), 1618. https://doi.org/10.3390/plants14111618






