Effect of Nitrogen and Phosphorus Fertilizers on Dry Matter Accumulation and Translocation of Two Amylose Content Indica Rice on Yield
Abstract
1. Introduction
2. Results
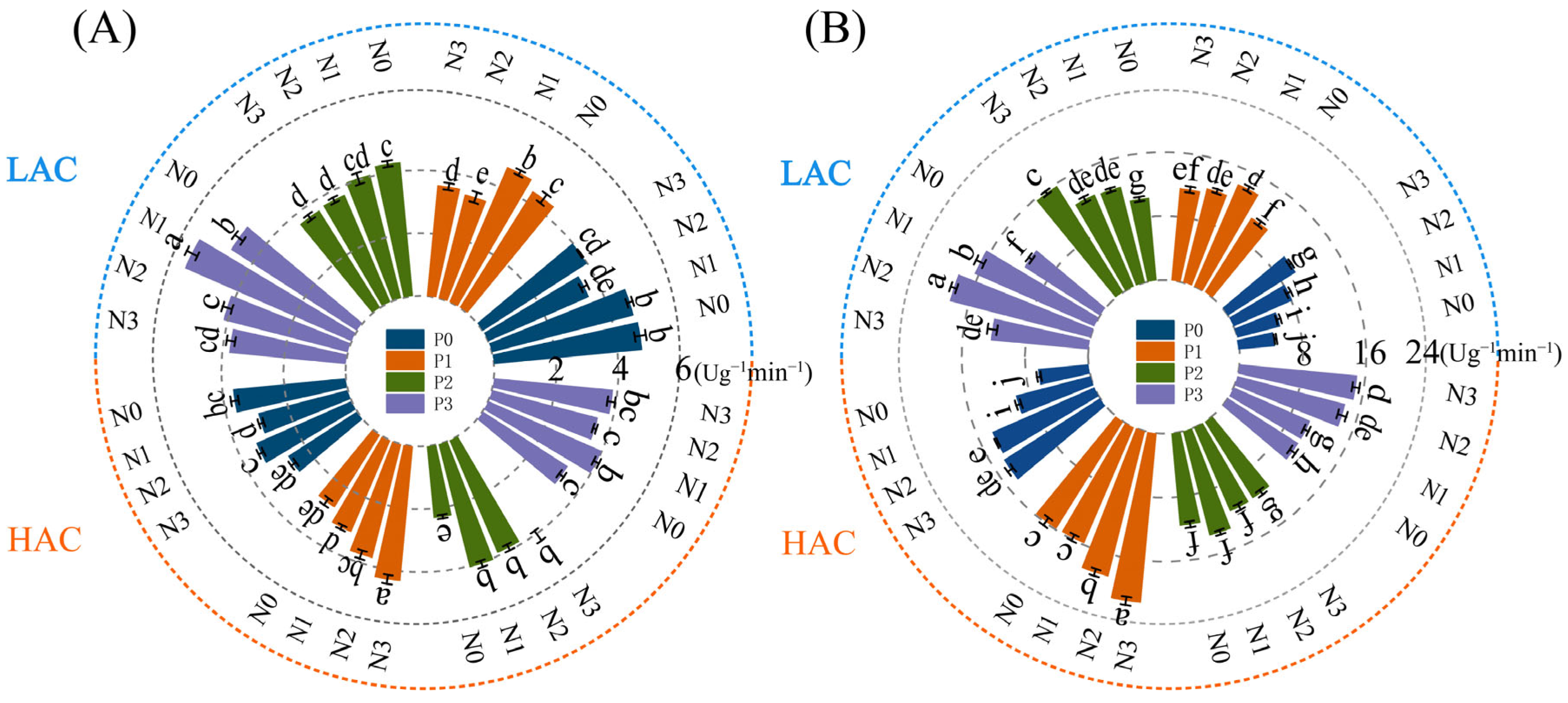
2.1. Effect of Nitrogen and Phosphorus Fertilizers on Dry Matter Accumulation in Rice
2.2. Effects of Nitrogen and Phosphorus Fertilizers on Dry Matter Transport in Various Organs of Rice at the Tassel Stage–Maturity Stage
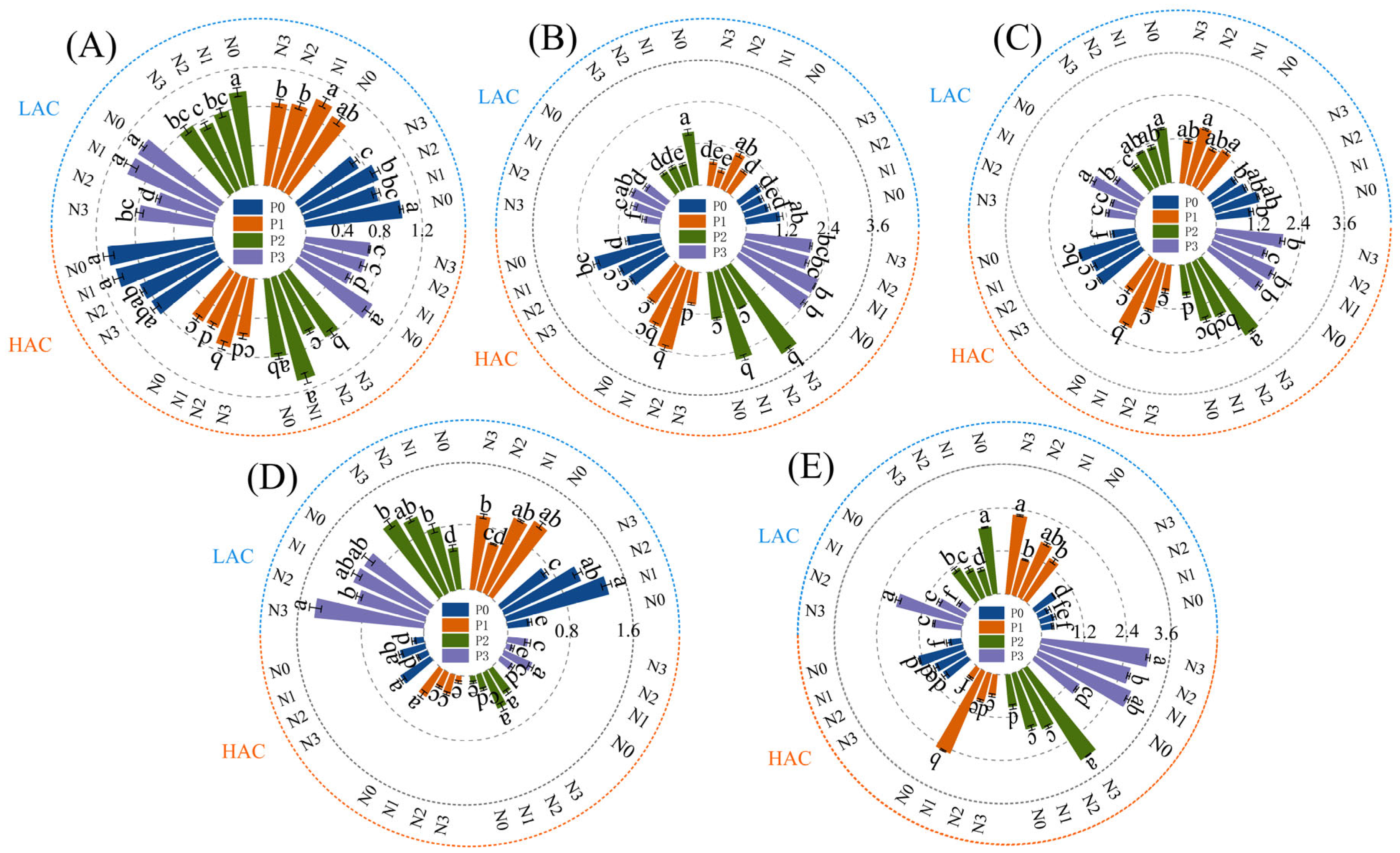
2.3. Effect of Nitrogen and Phosphorus Fertilizers on Fertilizer Utilization in Rice
2.4. Effect of Nitrogen and Phosphorus Fertilizers on Rice Yield and Yield Components
2.5. Effects of Nitrogen and Phosphorus Fertilizers on Enzyme Activities Related to Nitrogen Metabolism in Rice
2.6. Effect of Nitrogen and Phosphorus Fertilizers on the Relative Expression of Nitrogen Metabolism Genes in Rice
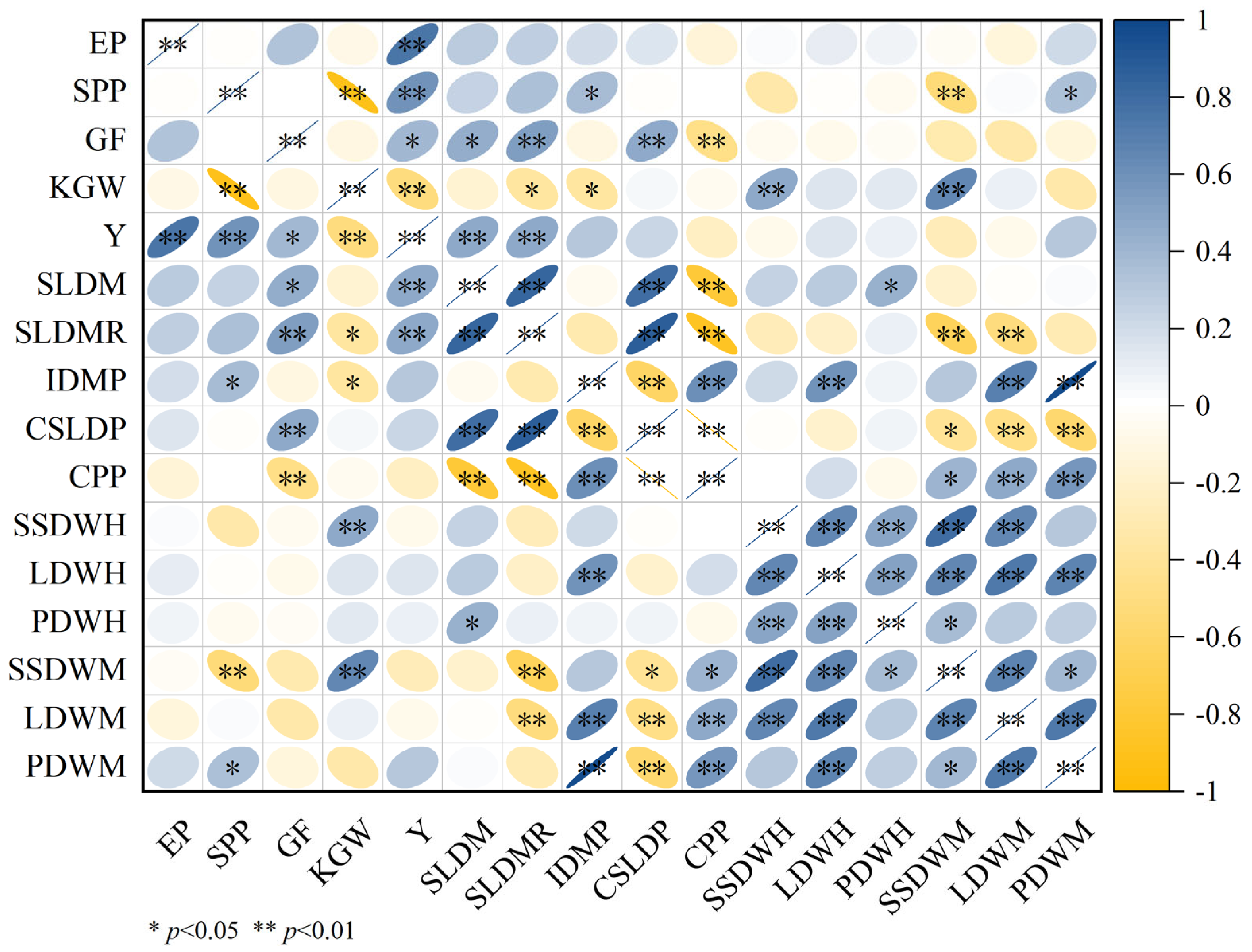
2.7. Correlation Analysis Between Rice Yield Components and Dry Matter Accumulation Transit
3. Discussion
3.1. Effect of Nitrogen and Phosphorus Fertilizers on Dry Matter Accumulation and Nitrogen and Phosphorus Utilization of Indica Rice with Different Straight Chain Starch Contents
3.2. Effect of Nitrogen and Phosphorus Fertilizers on Yield of Indica Rice with Different Linear Amylose Contents
4. Materials and Methods
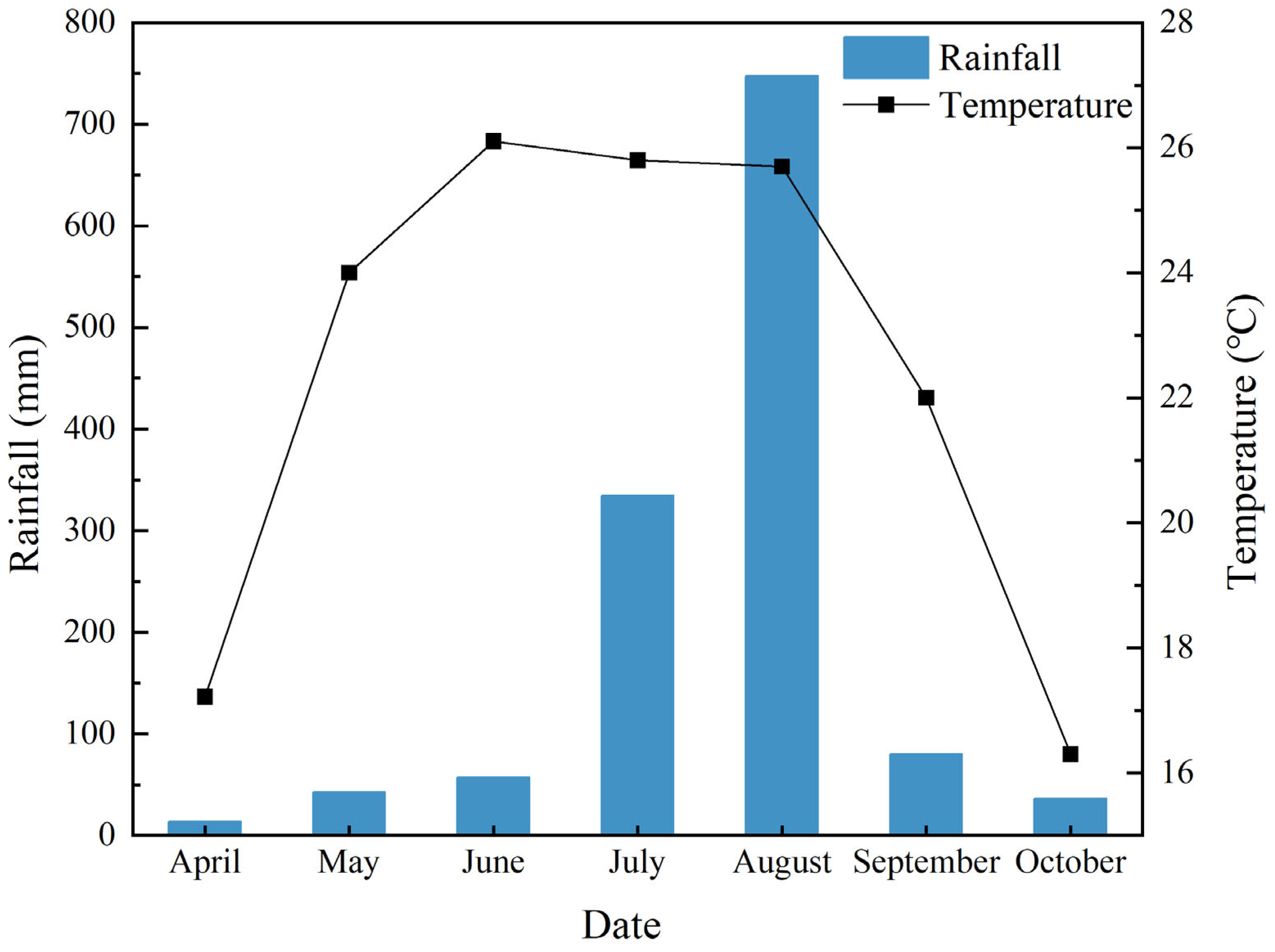
4.1. Test Materials and Sites
4.2. Experimental Design
4.3. Determination of Content and Methods
4.3.1. Rice Yield and Yield Components
4.3.2. Dry Matter Accumulation and Translocation
4.3.3. Nitrogen and Phosphorus Utilization Rate
4.3.4. Measurement of Enzyme Activity
4.3.5. Relative Expression of Rice Nitrogen Metabolism Genes
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, Y.Y.; Gu, Q.Q.; Dong, Q.; Zhang, Z.H.; Lin, C.; Hu, W.M.; Pan, R.H.; Guan, Y.J.; Hu, J. Spermidine enhances heat tolerance of rice seeds by modulating endogenous starch and polyamine metabolism. Molecules 2019, 24, 1395. [Google Scholar] [CrossRef]
- Kakar, K.; Xuan, T.D.; Noori, Z.; Aryan, S.; Gulab, G. Effects of organic and inorganic fertilizer application on growth, yield, and grain quality of rice. Agriculture 2020, 10, 544. [Google Scholar] [CrossRef]
- Wu, H.; Ge, Y. Excessive application of fertilizer, agricultural non-point source pollution, and farmers’ policy choice. Sustainability 2019, 11, 1165. [Google Scholar] [CrossRef]
- Liu, L.Y.; Zheng, X.Q.; Wei, X.C.; Kai, Z.; Xu, Y. Excessive application of chemical fertilizer and organophosphorus pesticides induced total phosphorus loss from planting causing surface water eutrophication. Sci. Rep. 2021, 11, 23015. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.K.; Liu, Z.G.; Zhang, M.; Shi, Y.F.; Zhou, Q.; Sun, Y.B.; Zhou, H.Y.; Li, C.L.; Yang, Y.C.; Geng, J.B. Improving crop yields, nitrogen use efficiencies, and profits by using mixtures of coated controlled-released and uncoated urea in a wheat-maize system. Field Crops Res. 2017, 205, 106–115. [Google Scholar] [CrossRef]
- Ma, Q.; Tao, R.; Jia, W.; Zhu, M.; Ding, J.F.; Li, C.Y.; Guo, W.S.; Zhou, G.S.; Zhu, X.K. Split application of polymer-coated urea combined with common urea improved nitrogen efficiency without sacrificing wheat yield and benefits while saving 20% nitrogen input. Front. Plant Sci. 2024, 15, 1321900. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Q.; Liu, K.; Zhuo, X.X.; Wang, W.L.; Zhang, W.Y.; Zhang, H.; Gu, J.F.; Yang, J.C.; Liu, L.J. Optimizing nitrogen regime improves dry matter and nitrogen accumulation during grain filling to increase rice yield. Agronomy 2023, 13, 1983. [Google Scholar] [CrossRef]
- Farooq, M.S.; Majeed, A.; Ghazy, A.H.; Fatima, H.; Uzair, M.; Ahmed, S.; Murtaza, M.; Fiaz, S.; Khan, M.R.; Al-Doss, A.; et al. Partial replacement of inorganic fertilizer with organic inputs for enhanced nitrogen use efficiency, grain yield, and decreased nitrogen losses under rice-based systems of mid-latitudes. BMC Plant Biol. 2024, 24, 919. [Google Scholar] [CrossRef]
- Djaman, K.; Bado, B.V.; Mel, V.C. Effect of nitrogen fertilizer on yield and nitrogen use efficiency of four aromatic rice varieties. Emir. J. Food Agric. 2016, 28, 126–135. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; Liu, G.; Li, J.; Siddique, K.H.M.; Zhao, D.Y. Agronomic Traits, Nutrient Accumulation, and Their Correlations in Wheat, as Affected by Nitrogen Supply in Rainfed Coastal Saline Soils. Plants 2025, 14, 1022. [Google Scholar] [CrossRef]
- Fan, X.T.; Lu, C.S.; Khan, Z.; Li, Z.H.; Duan, S.P.; Shen, H.; Fu, Y.Q. Mixed ammonium-nitrate nutrition regulates enzymes, gene expression, and metabolic pathways to improve nitrogen uptake, partitioning, and utilization efficiency in rice. Plants 2025, 14, 611. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.D.; Zhao, Q.Y.; Zhao, C.F.; Zhang, Z.; Chen, T.; Lu, K.; He, L.; Zhou, L.H.; Huang, S.H.; Li, Y.S.; et al. Moderate Nitrogen Application Synergistically Improved Yield and Quality of Nanjing Series japonica Rice Varieties with Good Taste. Plants 2025, 14, 940. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.Y.; Yang, D.M.; Deng, L.C.; Zhang, Y.X.; Zhou, J.J.; Chen, Z.C.; Ma, X.Q.; Guo, M.; Lu, Z.H.; Ma, L.Y. Phosphorus uptake, transport, and signaling in woody and model plants. For. Res. 2024, 4, e017. [Google Scholar] [CrossRef]
- Sun, Z.W.; Qiao, S.F.; Xu, Y.; Ji, D.L.; Zhang, W.Y.; Gu, J.F.; Zhu, K.Y.; Wang, Z.Q.; Zhang, J.H.; Yang, J.C. Agronomic and physiological performance of the Indica rice varieties differing in tolerance to low phosphorus. Agronomy 2023, 14, 41. [Google Scholar] [CrossRef]
- Tian, G.; Gao, L.; Kong, Y.; Hu, X.; Xie, K.; Zhang, R.; Ling, N.; Shen, Q.; Guo, S. Improving rice population productivity by reducing nitrogen rate and increasing plant density. PLoS ONE 2017, 12, e0182310. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Zhang, W.; Liang, X.Y.; Liu, Y.M.; Xu, S.J.; Zhao, Q.Y.; Du, Y.F.; Zhang, L.; Chen, X.P.; Zou, C.Q. Physiological and developmental traits associated with the grain yield of winter wheat as affected by phosphorus fertilizer management. Sci. Rep. 2019, 9, 16580. [Google Scholar] [CrossRef]
- Madan, B.; Raghuram, N. Phenotypic, Physiological, and Gene Expression Analysis for Nitrogen and Phosphorus Use Efficienies in Three Popular Genotypes of Rice (Oryza sativa Indica). Plants 2024, 13, 2567. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, J.; Zhong, M.; Zhang, W.Z. Effects of Phosphorus and Potassium Supply on Photosynthetic Nitrogen Metabolism, Nitrogen Absorption, and Nitrogen Utilization of Hydroponic Rice. Agronomy 2024, 14, 1726. [Google Scholar] [CrossRef]
- Chen, G.Y.; Duan, Q.; Wu, C.Y.; He, X.M.; Hu, M.M.; Li, C.M.; Ouyang, Y.Y.; Peng, L.G.; Yang, H.; Zhang, Q.Q.; et al. Optimizing rice yield, quality and nutrient use efficiency through combined application of nitrogen and potassium. Front. Plant Sci. 2024, 15, 1335744. [Google Scholar] [CrossRef]
- Zhu, H.J.; He, X.; Wang, X.H.; Long, P. Increasing hybrid rice yield, water productivity, and nitrogen use efficiency: Optimization strategies for irrigation and fertilizer management. Plants 2024, 13, 1717. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhang, X.Q.; Xiao, Y.; Chen, H.; Wang, X.C.; Hu, Y.G. Effect of nitrogen fertilizer on the quality traits of Indica rice with different amylose contents. J. Sci. Food Agric. 2024, 104, 8492–8499. [Google Scholar] [CrossRef]
- Li, Y.M.; Liang, C.; Liu, J.F.; Zhou, C.C.; Wu, Z.Z.; Guo, S.M.; Liu, J.X.; A, N.; Wang, S.; Xin, G.; et al. Moderate reduction in nitrogen fertilizer results in improved rice quality by affecting starch properties without causing yield loss. Foods 2023, 1, 2601. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.P.; Li, X.K. Effects of optimized nitrogen fertilizer management on the yield, nitrogen uptake, and ammonia volatilization of direct-seeded rice. J. Sci. Food Agric. 2023, 103, 4553–4561. [Google Scholar] [CrossRef]
- Islam, S.M.M.; Gaihre, Y.K.; Biswas, J.C.; Biswas, J.C.; Jahan, M.J.; Sinjoy, U.S.; Adhikary, S.K.; Satter, M.A.; Saleque, M.A. Different nitrogen rates and methods of application for dry season rice cultivation with alternate wetting and drying irrigation: Fate of nitrogen and grain yield. Agric. Water Manag. 2018, 196, 144–153. [Google Scholar] [CrossRef]
- Yan, J.Y.; Ren, T.; Wang, K.K.; Li, X.K.; Cong, R.K. Improved crop yield and phosphorus uptake through the optimization of phosphorus fertilizer rates in an oilseed rape-rice cropping system. Field Crops Res. 2022, 286, 108614. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhang, W.; Wu, M.; Liu, G.S.; Zhang, Z.J.; Yang, J.C. Effects of irrigation schedules and phosphorus fertilizer rates on grain yield and quality of upland rice and paddy rice. Environ. Exp. Bot. 2021, 186, 104465. [Google Scholar] [CrossRef]
- Zheng, Y.T.; Chen, H.; Yang, G.T.; Wang, R.D.; Farhan, N.; Li, C.; Liang, C.; Wang, X.C.; Hu, Y.G. Combined effect of nitrogen and phosphorous fertiliser on nitrogen absorption and utilisation in rice. Plant Soil Environ. 2023, 69, 25–37. [Google Scholar] [CrossRef]
- Padhan, B.K.; Sathee, L.; Kumar, S.; Chinnusamy, V.; Kumar, A. Variation in nitrogen partitioning and reproductive stage nitrogen remobilization determines nitrogen grain production efficiency (NUEg) in diverse rice genotypes under varying nitrogen supply. Front. Plant Sci. 2023, 14, 1093581. [Google Scholar] [CrossRef]
- Ma, P.; Liao, X.H.; Zhang, K.Y.; Aer, L.; Deng, J.; Yang, E.L.; Zhang, R.P. Effects of Compound Fertilizer and Branch Fertilizer on Population Construction and Yield of Machine-Transplanted Rice. Plants 2024, 13, 2436. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Yuan, X.j.; Chen, K.; Wang, H.Y.; Luo, Y.H.; Guo, C.C.; Wang, Z.L.; Shu, C.H.; Yang, Y.G.; Weng, Z.L.; et al. Improving the yield and nitrogen use efficiency of hybrid rice through rational use of controlled-release nitrogen fertilizer and urea topdressing. Front. Plant Sci. 2023, 14, 1240238. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, G.M.; Chen, Y.; Jang, Y.; Shi, Y.; Zhao, L.T.; Liao, P.Q.; Wang, W.L.; Xu, K.; Dai, Q.G.; et al. Excessive nitrogen application leads to lower rice yield and grain quality by inhibiting the grain filling of inferior grains. Agriculture 2022, 12, 962. [Google Scholar] [CrossRef]
- Yang, Q.R.; Zhang, H.Y.; Zhang, X.; Geng, S.N.; Zhang, Y.J.; Miao, Y.H.; Li, L.T.; Wang, Y.L. Optimized Phosphorus Application Enhances Canopy Photothermal Responses, Phosphorus Accumulation, and Yield in Summer Maize. Agronomy 2025, 15, 514. [Google Scholar] [CrossRef]
- Ye, C.; Ma, H.Y.; Huang, X.; Xu, C.M.; Chen, S.; Chu, G.; Zhang, X.F.; Wang, D. Effects of increasing panicle-stage N on yield and N use efficiency of indica rice and its relationship with soil fertility. Crop J. 2022, 10, 1784–1797. [Google Scholar] [CrossRef]
- Zhu, H.J.; Nie, L.L.; He, X.E.; Wang, X.H.; Long, P.; Chen, H.Y. Water and fertilizer management is an important way to synergistically enhance the yield, rice quality and lodging resistance of hybrid rice. Plants 2024, 13, 2518. [Google Scholar] [CrossRef] [PubMed]
- He, X.E.; Zhu, H.J.; Shi, A.L.; Wang, X.H. Optimizing nitrogen fertilizer management enhances rice yield, dry matter, and nitrogen use efficiency. Agronomy 2024, 14, 919. [Google Scholar] [CrossRef]
- Yang, H.S.; Feng, J.X.; Weih, M.; Meng, Y.; Li, Y.F.; Zhai, S.; Zhang, W.Y. Yield reduction of direct-seeded rice under returned straw can be mitigated by appropriate water management improving soil phosphorus availability. Crop Pasture Sci. 2020, 71, 134–146. [Google Scholar] [CrossRef]
- Zhao, L.M.; Zhou, H.; Tang, L.; Na, Y.Y.; Duan, S.B.; Zheng, D.F.; Feng, N.J.; Shen, X.F. Optimizing Nitrogen Dosage and Planting Density to Improve Japonica Rice Yield. Agronomy 2024, 14, 1738. [Google Scholar] [CrossRef]
- Deng, S.Y.; Ashraf, U.; Nawaz, M.; Abbas, G.; Tang, X.R.; Mo, Z.W. Water and nitrogen management at the booting stage affects yield, grain quality, nutrient uptake, and use efficiency of fragrant rice under the agroclimatic conditions of South China. Front. Plant Sci. 2022, 13, 907231. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ail, M.; Youssef, M.; Roumia, A.; Seymour, D.; Yuan, Z.-C. Nitrogen journey in plants: From uptake to metabolism, stress response, and microbe interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef]
- Tantray, A.Y.; Hazzazi, Y.; Ahmad, A. Physiological, agronomical, and proteomic studies reveal crucial players in rice nitrogen use efficiency under low nitrogen supply. Int. J. Mol. Sci. 2022, 23, 6410. [Google Scholar] [CrossRef]
- Sharma, N.; Kumari, S.; Jaiswal, D.K.; Raghuram, N. Comparative transcriptomic analyses of nitrate-response in rice genotypes with contrasting nitrogen use efficiency reveals common and genotype-specific processes, molecular targets and nitrogen use efficiency-candidates. Front. Plant Sci. 2022, 13, 881204. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.F.; Chen, Y.Y.; Ashraf, U.; Zhang, M.H.; Mo, Z.W.; Duan, M.Y.; Wang, Z.M.; Tang, X.G.; Pan, S.G. Effects of different fertilization methods on grain yield, photosynthetic characteristics and nitrogen synthetase enzymatic activities of direct-seeded rice in South China. J. Plant Growth Regul. 2022, 41, 1642–1653. [Google Scholar] [CrossRef]
- The, S.V.; Snyder, R.; Tegeder, M. Targeting nitrogen metabolism and transport processes to improve plant nitrogen use efficiency. Front. Plant Sci. 2021, 11, 628366. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Ma, Y.D.; Zhao, L.; Yin, Y.G.; Wang, G.D.; Li, Y.X. Effects of water and nitrogen management on root morphology, nitrogen metabolism enzymes, and yield of rice under drip irrigation. Agronomy 2023, 13, 1118. [Google Scholar] [CrossRef]
- Moll, R.H.; Kamprath, E.J.; Jackson, W.A. Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization. Agron. J. 1982, 74, 562–564. [Google Scholar] [CrossRef]
- Wolf, B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Chai, X.Q.; Yin, L.P.; Liu, X.L.; Yu, B.X.; Han, Q.E.; Hong, J.M.; Qiu, Z.S. Influence of Different Concentrations of NO-3 and NH+4 on the Activity of Glutamine Synthetase and Other Relevant Enzymes of Nitrogen Metabolism in Wheat Roots. J. Integr. Plant Biol. 1996, 38, 803–808. [Google Scholar]
- Haghighat, N. Estrogen (17β-Estradiol) enhances glutamine synthetase activity in C6-glioma cells. Neurochem. Res. 2005, 30, 661–667. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, X.J.; Sun, F.; Hu, J.H.; Su, W.; Yang, J.S. Overexpressing lncRNA LAIR increases grain yield and regulates neighbouring gene cluster expression in rice. Nat. Commun. 2018, 9, 3516. [Google Scholar] [CrossRef]

| Treatment | LAC | HAC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SLDM/(t/hm2) | SLDMR/% | IDMP/ (t/hm2) | CSLDP/% | CPP/% | SLDM/(t/hm2) | SLDMR/% | IDMP/(t/hm2) | CSLDP/% | CPP/% | |
| N0P0 | 1.67 ± 0.44 f | 15.22 ± 3.63 f | 7.81 ± 0.86 b | 15.02 ± 3.81 e | 84.98 ± 3.81 a | 2.05 ± 0.72 e | 22.49 ± 6.62 ef | 6.34 ± 0.38 d | 19.21 ± 5.40 e | 80.79 ± 5.40 a |
| N1P0 | 2.66 ± 0.68 c | 23.26 ± 5.95 cd | 7.10 ± 0.37 bc | 23.9 ± 6.54 cde | 76.10 ± 6.57 abc | 2.49 ± 0.32 de | 26.54 ± 2.45 d | 7.41 ± 0.73 cd | 23.53 ± 4.85 d | 76.47 ± 4.85 b |
| N2P0 | 3.14 ± 0.48 ab | 25.09 ± 4.96 bc | 7.17 ± 0.64 bc | 26.57 ± 5.02 bcd | 73.43 ± 5.02 bcd | 2.60 ± 0.42 de | 25.26 ± 2.36 d | 8.74 ± 0.73 ab | 20.19 ± 3.15 de | 79.81 ± 3.15 ab |
| N3P0 | 2.73 ± 0.64 bc | 21.18 ± 4.56 de | 8.72 ± 0.41 ab | 20.70 ± 3.92 cde | 79.30 ± 3.92 abc | 3.58 ± 0.36 ab | 32.27 ± 2.47 b | 8.39 ± 0.22 abc | 29.66 ± 4.61 bc | 70.34 ± 4.61 cd |
| N0P1 | 2.30 ± 0.58 de | 18.58 ± 3.17 ef | 7.35 ± 0.43 bc | 19.47 ± 4.02 cd | 80.53 ± 4.40 ab | 2.34 ± 0.08 e | 19.90 ± 2.09 f | 9.96 ± 0.17 a | 16.68 ± 2.66 f | 83.32 ± 2.66 a |
| N1P1 | 2.79 ± 0.19 bc | 25.64 ± 2.78 bc | 6.32 ± 0.47 cd | 26.32 ± 5.33 bcd | 73.68 ± 5.33 bcd | 3.09 ± 0.49 bc | 34.65 ± 0.98 ab | 6.46 ± 0.41 cd | 31.69 ± 8.05 ab | 68.31 ± 8.05 de |
| N2P1 | 3.33 ± 0.76 a | 28.08 ± 6.25 ab | 5.94 ± 0.48 de | 30.98 ± 7.28 abc | 69.02 ± 7.28 cde | 4.14 ± 0.20 a | 35.27 ± 1.39 a | 7.04 ± 0.16 bcd | 34.84 ± 3.18 a | 65.16 ± 3.18 de |
| N3P1 | 2.33 ± 0.42 cd | 27.48 ± 4.88 b | 4.81 ± 0.71 de | 30.10 ± 9.16 abc | 69.90 ± 9.16 cde | 3.33 ± 0.36 bc | 29.11 ± 1.12 bc | 7.46 ± 0.48 bcd | 27.50 ± 1.93 cd | 72.50 ± 1.93 bc |
| N0P2 | 2.14 ± 0.21 e | 17.02 ± 1.24 ef | 9.28 ± 0.58 a | 16.36 ± 1.00 e | 83.64 ± 1.00 a | 2.64 ± 0.34 de | 23.86 ± 4.03 e | 7.40 ± 0.85 bcd | 21.58 ± 3.50 de | 78.42 ± 3.50 ab |
| N1P2 | 2.37 ± 0.29 cd | 20.59 ± 3.26 de | 6.45 ± 0.14 cd | 21.81 ± 4.05 cde | 78.19 ± 4.05 abc | 3.07 ± 0.29 cd | 31.36 ± 3.89 bc | 5.96 ± 0.87 d | 30.44 ± 4.22 b | 69.56 ± 4.22 d |
| N2P2 | 3.05 ± 0.31 b | 23.69 ± 3.78 cd | 7.48 ± 0.79 bc | 27.15 ± 4.75 bcd | 72.80 ± 4.76 bcd | 3.87 ± 0.26 ab | 30.32 ± 3.05 bc | 8.75 ± 0.18 ab | 30.21 ± 4.70 b | 69.79 ± 4.70 d |
| N3P2 | 3.01 ± 0.28 b | 32.28 ± 2.72 a | 5.16 ± 0.46 cde | 36.26 ± 3.56 a | 63.74 ± 3.56 de | 3.13 ± 0.22 cd | 28.32 ± 2.43 cd | 8.74 ± 0.60 ab | 23.94 ± 3.47 d | 76.06 ± 3.47 b |
| N0P3 | 2.70 ± 0.16 bc | 23.38 ± 1.69 cd | 5.20 ± 0.33 cde | 29.97 ± 0.60 abc | 70.03 ± 0.60 cde | 2.10 ± 0.15 e | 23.94 ± 1.08 e | 7.21 ± 0.14 bcd | 19.93 ± 1.63 e | 80.07 ± 1.63 a |
| N1P3 | 3.06 ± 0.23 b | 28.68 ± 1.99 ab | 4.48 ± 0.28 e | 34.28 ± 2.65 ab | 65.72 ± 2.65 de | 2.53 ± 0.41 de | 23.63 ± 2.84 e | 8.56 ± 0.07 ab | 20.68 ± 3.22 de | 79.32 ± 3.22 ab |
| N2P3 | 3.38 ± 0.40 a | 28.60 ± 3.20 ab | 6.86 ± 0.31 cd | 29.63 ± 4.07 abc | 70.37 ± 4.07 cde | 3.68 ± 0.15 ab | 32.19 ± 2.50 b | 7.40 ± 0.34 bcd | 31.51 ± 1.52 ab | 68.49 ± 1.52 de |
| N3P3 | 3.19 ± 0.26 ab | 28.62 ± 1.15 ab | 6.88 ± 0.72 cd | 26.25 ± 1.49 bcd | 73.75 ± 1.49 bcd | 3.31 ± 0.44 cd | 29.91 ± 2.49 bc | 7.66 ± 0.56 bcd | 28.71 ± 4.67 c | 71.29 ± 4.67 bc |
| N | 11.382 ** | 12.74 ** | 9.53 ** | 6.50 ** | 6.50 ** | 31.15 ** | 18.42 ** | 2.08 | 13.31 ** | 13.31 ** |
| P | 3.51 * | 5.17 ** | 4.28 ** | 8.09 ** | 8.09 ** | 6.04 ** | 2.36 | 0.01 | 2.68 | 2.69 |
| N × P | 1.11 | 1.82 * | 5.17 ** | 3.23 ** | 3.23 ** | 3.19 ** | 4.49 ** | 4.48 ** | 3.75 ** | 3.75 ** |
| Treatment | LAC | HAC | ||||||
|---|---|---|---|---|---|---|---|---|
| NHI/% | NAE/(kg/kg) | NCR/% | NUE/(kg/kg) | NHI/% | NAE/(kg/kg) | NCR/% | NUE/(kg/kg) | |
| N0P0 | 64.57 ± 1.21 bcde | -- | -- | -- | 72.86 ± 4.74 bc | -- | -- | -- |
| N1P0 | 68.77 ± 1.62 ab | 9.05 ± 0.02 bc | 10.81 ± 0.27 d | 11.51 ± 0.19 cd | 70.91 ± 2.90 bcd | 6.96 ± 0.44 d | 6.74 ± 0.18 d | 7.32 ± 0.23 d |
| N2P0 | 67.02 ± 1.33 bcd | 10.83 ± 0.29 b | 9.65 ± 0.32 e | 13.10 ± 0.03 bc | 71.13 ± 3.16 bcd | 9.97 ± 0.01 bc | 15.1 ± 0.10 bcd | 10.8 ± 0.36 bc |
| N3P0 | 65.88 ± 2.26 bcde | 7.07 ± 0.07 cd | 3.25 ± 0.08 f | 14.82 ± 0.59 ab | 70.47 ± 1.74 bcd | 5.57 ± 0.20 d | 14.79 ± 0.12 bcd | 11.60 ± 0.34 bc |
| N0P1 | 52.03 ± 3.67 f | -- | -- | -- | 56.58 ± 1.75 f | -- | -- | -- |
| N1P1 | 63.12 ± 2.30 cde | 2.91 b ± 0.09 f | 3.37 ± 0.06 f | 7.47 ± 0.22 e | 81.15 ± 1.70 a | 7.25 ± 0.07 d | 11.01 ± 0.01 cd | 10.23 ± 0.22 c |
| N2P1 | 65.36 ± 2.80 bcde | 5.88 ± 0.07 de | 10.53 ± 0.16 d | 10.28 ± 0.09 d | 66.07 ± 2.82 de | 13.17 ± 0.16 bc | 18.41 ± 0.57 ab | 10.04 ± 0.40 c |
| N3P1 | 70.94 ± 3.76 a | 6.77 ± 0.19 d | 21.42 ± 0.16 ab | 15.71 ± 0.15 a | 74.83 ± 6.82 b | 6.08 ± 0.07 d | 15.77 ± 0.10 bcd | 9.17 ± 0.34 c |
| N0P2 | 67.27 ± 1.44 abc | -- | -- | -- | 71.73 ± 2.24 bcd | -- | -- | -- |
| N1P2 | 64.16 ± 2.81 cde | 5.93 ± 0.07 de | 6.19 ± 0.06 ab | 9.22 ± 0.18 de | 69.17 ± 2.16 bcd | 9.63 ± 0.15 bc | 9.29 ± 0.28 cd | 9.67 ± 0.1 c |
| N2P2 | 61.69 ± 2.29 e | 10.75 ± 0.21 b | 16.65 ± 0.21 c | 14.69 ± 0.23 ab | 60.90 ± 2.16 e | 12.64 ± 0.22 bc | 18.31 ± 0.5 ab | 12.17 ± 0.46 b |
| N3P2 | 65.92 ± 2.24 bcde | 3.05 ± 0.04 ef | 9.27 ± 0.07 e | 13.81 ± 0.14 bc | 71.18 ± 0.99 bcd | 7.56 ± 0.11 d | 19.45 ± 0.25 a | 16.94 ± 0.04 a |
| N0P3 | 62.70 ± 1.74 de | -- | -- | -- | 70.58 ± 4.51 bcd | -- | -- | -- |
| N1P3 | 64.67 ± 2.10 cde | 28.72 ± 0.47 a | 28.13 ± 0.16 a | 9.25 ± 0.04 de | 67.44 ± 2.19 bcd | 23.52 ± 0.19 a | 20.99 ± 0.18 a | 9.45 ± 0.23 c |
| N2P3 | 66.48 ± 1.62 bcd | 11.00 ± 0.20 b | 20.00 ± 0.39 ab | 13.24 ± 0.08 bc | 66.92 ± 5.28 cd | 13.02 ± 0.20 bc | 19.62 ± 0.11 a | 12.41 ± 0.09 b |
| N3P3 | 61.76 ± 1.01 e | 5.86 ± 0.12 de | 19.3 ± 0.29 bc | 14.12 ± 0.14 ab | 69.19 ± 2.74 bcd | 7.20 ± 0.12 bc | 19.53 ± 0.57 a | 12.69 ± 0.26 b |
| N | 12.55 ** | 3.38 * | 5.37 ** | 7.56 ** | 9.07 ** | 159.17 ** | 6.32 ** | 2.29 |
| P | 8.710 ** | 175.04 ** | 12.17 ** | 2.61 * | 2.11 | 1711.46 ** | 5.80 ** | 10.08 ** |
| N × P | 16.36 ** | 7.65 ** | 5.11 ** | 5.59 ** | 11.29 ** | 194.75 ** | 0.63 | 2.13 |
| Treatment | LAC | HAC | ||||||
|---|---|---|---|---|---|---|---|---|
| PHI/% | PAE/(kg/kg) | PCR/% | PUE/(kg/kg) | PHI/% | PAE/(kg/kg) | PCR/% | PUE/(kg/kg) | |
| N0P0 | 62.28 ± 2.32 ab | -- | -- | -- | 62.04 ± 2.55 bc | -- | -- | -- |
| N1P0 | 57.77 ± 3.19 abcd | -- | -- | -- | 72.02 ± 3.99 a | -- | -- | -- |
| N2P0 | 55.13 ± 2.01 abcd | -- | -- | -- | 62.20 ± 1.53 bc | -- | -- | -- |
| N3P0 | 60.43 ± 6.72 abc | -- | -- | -- | 70.68 ± 4.54 ab | -- | -- | -- |
| N0P1 | 49.47 ± 5.37 cd | 11.57 ± 1.1 ab | 3.18 ± 0.10 g | 3.36 ± 0.08 cd | 60.37 ± 0.80 c | 7.66 ± 0.32 ab | 4.33 ± 0.12 e | 2.80 ± 0.02 c |
| N1P1 | 61.98 ± 2.81 ab | 13.73 ± 0.71 a | 4.55 ± 0.02 g | 3.97 ± 0.02 bc | 61.60 ± 1.23 bc | 8.73 ± 0.55 ab | 8.22 ± 0.23 c | 4.46 ± 0.06 b |
| N2P1 | 65.89 ± 0.29 a | 14.13 ± 0.40 a | 3.22 ± 0.12 g | 4.55 ± 0.06 a | 62.56 ± 2.25 bc | 11.88 ± 0.4 ab | 11.74 ± 0.48 b | 4.14 ± 0.10 b |
| N3P1 | 55.48 ± 5.30 abcd | 10.91 ± 0.39 ab | 9.93 ± 0.07 b | 5.45 ± 0.09 a | 60.19 ± 1.43 c | 10.04 ± 0.09 ab | 2.89 ± 0.04 f | 5.51 ± 0.06 a |
| N0P2 | 60.81 ± 4.44 abc | 13.14 ± 0.42 ab | 7.75 ± 0.2 cd | 1.63 ± 0.04 gh | 61.10 ± 2.03 c | 9.42 ± 0.20 ab | 6.58 ± 0.10 d | 1.87 ± 0.08 de |
| N1P2 | 48.76 ± 2.43 d | 14.58 ± 0.26 a | 12.2 ± 0.22 a | 2.42 ± 0.08 efg | 60.57 ± 2.31 c | 11.59 ± 0.10 ab | 3.13 ± 0.15 f | 2.44 ± 0.06 cd |
| N2P2 | 52.34 ± 2.07 bcd | 15.42 ± 0.71 a | 13.69 ± 0.25 a | 2.79 ± 0.09 de | 62.20 ± 4.43 bc | 15.45 ± 0.42 a | 8.58 ± 0.22 c | 2.67 ± 0.05 cd |
| N3P2 | 60.60 ± 3.34 abc | 11.09 ± 0.43 ab | 8.51 ± 0.21 bc | 2.64 ± 0.06 def | 68.48 ± 2.62 abc | 13.12 ± 0.38 ab | 4.52 ± 0.08 e | 2.7 ± 0.06 cd |
| N0P3 | 52.25 ± 6.50 bcd | 6.44 ± 0.06 b | 5.70 ± 0.15 ef | 1.25 ± 0.04 h | 67.64 ± 2.33 abc | 6.07 ± 0.40 b | 11.36 ± 0.36 b | 1.17 ± 0.02 e |
| N1P3 | 61.09 ± 1.53 ab | 9.66 ± 0.54 ab | 8.53 ± 0.22 bc | 1.50 ± 0.06 h | 64.58 ± 4.41 abc | 8.44 ± 0.35 ab | 9.41 ± 0.07 c | 1.52 ± 0.04 e |
| N2P3 | 53.65 ± 3.81 bcd | 10.08 ± 0.08 ab | 6.42 ± 0.15 de | 1.96 ± 0.05 fgh | 65.00 ± 4.50 abc | 8.13 ± 0.49 ab | 12.51 ± 0.06 a | 1.54 ± 0.03 e |
| N3P3 | 54.91 ± 2.44 abcd | 8.99 ± 0.02 ab | 3.56 ± 0.10 f | 1.86 ± 0.01 fgh | 59.13 ± 7.11 c | 7.38 ± 0.44 b | 11.15 ± 0.50 b | 1.50 ± 0.02 e |
| N | 0.19 | 1.57 | 17.22 ** | 14.17 ** | 0.53 | 1.54 | 108.33 ** | 3.66 ** |
| P | 1.11 | 5.83 | 120.66 ** | 121.38 ** | 2.71 | 3.51 * | 279.81 ** | 11.49 ** |
| N × P | 3.31 ** | 0.23 ** | 30.47 ** | 2.06 | 2.19 * | 0.26 * | 724.44 ** | 105.62 ** |
| Treatment | LAC | HAC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Effective Panicles /(×104/hm2) | Spikelets Per Panicle | Grain Filling /% | 1000-Grain Weight /g | Yield /(t/hm2) | Effective Panicles /(×104/hm2) | Spikelets Per Panicle | Grain Filling /% | 1000-Grain Weight/g | Yield /(t/hm2) | |
| N0P0 | 143.96 ±0.01 e | 148.17 ±3.67 bc | 88.02 ±3.28 bcdef | 36.54 ±0.43 bcd | 6.86 ±0.32 fg | 161.88 ±0.05 c | 195.76 ±2.59 de | 90.66 ±0.23 abc | 30.30 ±0.63 cdef | 8.71 ±0.35 ef |
| N1P0 | 165.25 ±3.51 d | 136.8 ±0.92 f | 88.81 ±3.89 bcdef | 36.98 ±0.27 bcd | 7.42 ±0.20 ef | 177.91 ±3.26 b | 199.00 ±5.37 cde | 93.30 ±1.36 ab | 31.14 ±0.91 bc | 10.29 ±0.22 bc |
| N2P0 | 177.89 ±3.45 c | 146.11 ±3.20 bc | 92.00 ±0.72 abc | 37.25 ±0.70 bcd | 8.91 ±0.30 bc | 166.25 ±0.58 c | 195.78 ±2.31 de | 90.76 ±0.54 abc | 30.89 ±0.68 bcd | 9.13 ±0.03 de |
| N3P0 | 197.23 ±1.15 b | 152.05 ±5.41 b | 90.48 ±2.85 abcd | 36.97 ±0.70 bcd | 10.03 ±0.19 a | 179.90 ±0.02 b | 196.83 ±1.07 de | 92.28 ±2.98 ab | 29.89 ±0.69 ef | 9.77 ±0.05 cd |
| N0P1 | 161.92 ±0.02 d | 135.41 ±1.80 f | 88.55 ±1.64 bcdef | 36.62 ±0.75 bcd | 7.11 ±0.34 f | 159.92 ±3.58 c | 193.82 ±0.54 e | 89.93 ±0.88 bc | 30.83 ±0.03 bcde | 8.59 ±0.07 f |
| N1P1 | 161.92 ±0.02 d | 135.56 ±3.97 f | 93.25 ±0.90 a | 37.03 ±0.42 bcd | 7.58 ±0.19 ef | 161.90 ±0.03 c | 182.74 ±1.29 f | 93.70 ±2.14 a | 31.26 ±0.33 bc | 8.67 ±0.04 ef |
| N2P1 | 163.72 ±3.65 d | 141.59 ±4.50 cde | 89.22 ±1.05 abcde | 37.30 ±0.45 bcd | 7.71 ±0.26 ef | 160.92 ±1.73 c | 219.11 ±0.19 a | 93.18 ±1.22 ab | 31.34 ±0.45 b | 10.30 ±0.27 bc |
| N3P1 | 203.91 ±0.01 a | 134.99 ±2.99 ef | 92.22 ±0.57 abc | 36.71 ±0.22 bcd | 9.32 ±0.18 b | 213.89 ±3.46 a | 184.73 ±2.94 f | 90.75 ±2.24 abc | 31.36 ±0.54 b | 11.24 ±0.12 a |
| N0P2 | 161.91 ±0.01 d | 159.47 ±1.82 def | 85.63 ±0.47 ef | 36.61 ±0.61 bcd | 8.09 ±0.26 cd | 161.92 ±0.02 c | 193.43 ±3.08 e | 87.66 ±2.36 bc | 30.95 ±0.53 bcd | 8.50 ±0.21 fg |
| N1P2 | 163.25 ±2.31 d | 143.06 ±4.18 cd | 88.29 ±3.22 cdef | 38.79 ±0.96 a | 8.00 ±0.38 de | 159.82 ±3.77 c | 206.79 ±1.05 b | 91.57 ±0.41 ab | 30.92 ±0.65 ab | 9.36 ±0.02 d |
| N2P2 | 181.91 ±1.73 c | 146.35 ±6.47 bc | 92.56 ±2.12 ab | 37.71 ±0.15 b | 9.29 ±0.09 b | 177.82 ±3.38 b | 204.70 ±1.51 bc | 92.23 ±2.96 ab | 33.02 ±0.79 ba | 11.09 ±0.08 ab |
| N3P2 | 177.70 ±3.36 c | 136.56 ±5.17 bc | 91.83 ±0.9 abc | 36.38 ±0.29 d | 8.11 ±0.36 cd | 213.92 ±3.40 a | 200.68 ±2.58 cd | 87.76 ±1.94 cd | 29.61 ±0.16 f | 11.16 ±0.35 a |
| N0P3 | 125.94 ±0.01 f | 170.60 ±2.95 a | 84.83 ±2.67 f | 36.14 ±0.78 cd | 6.59 ±0.25 g | 143.92 ±0.02 d | 204.057 ±1.13 bc | 84.65 ±1.08 d | 30.06 ±0.28 def | 7.47 ±0.09 g |
| N1P3 | 164.58 ±4.61 d | 172.39 ±5.55 a | 89.23 ±1.11 abcde | 36.41 ±0.92 cd | 9.22 ±0.25 b | 184.24 ±5.85 b | 220.68 ±2.07 a | 86.01 ±1.02 d | 31.05 ±0.23 bcd | 10.86 ±0.20 abc |
| N2P3 | 177.58 ±3.58 c | 143.95 ±3.76 b | 89.54 ±1.65 abcde | 37.45 ±0.54 bc | 8.57 ±0.32 cd | 177.87 ±3.54 b | 185.36 ±0.31 f | 89.73 ±3.33 bc | 31.30 ±0.66 bc | 9.26 ±0.07 d |
| N3P3 | 163.59 ±3.60 d | 155.21 ±5.50 ef | 87.24 ±2.78 def | 36.71 ±0.98 bcd | 8.13 ±0.20 cd | 161.92 ±0.01 c | 209.15 ±1.22 b | 91.53 ±0.97 ab | 29.45 ±0.25 f | 9.13 ±0.19 de |
| N | 477.374 ** | 6.477 ** | 4.772 * | 8.408 ** | 66.717 ** | 131.160 ** | 0.181 | 7.471 ** | 20.944 ** | 10.207 ** |
| P | 87.995 ** | 2.501 | 51.708 ** | 4.421 ** | 4.478* | 15.270 ** | 0.701 | 11.831 ** | 4.857 ** | 1.737 |
| N × P | 78.215 ** | 2.225 * | 12.152 ** | 2.157 * | 21.490 ** | 42.651 ** | 1.32 | 3.610 ** | 4.153 ** | 2.322 * |
| Gene Name | Primer |
|---|---|
| OsGS1;1 | F: CAAGTCTTTTGGGCGTGATATTGTTGAC |
| R: CACCTGATCACCGGCAGAAATGCCGACA | |
| OsGS1;2 | F: AAAGGCGTTCGGCCGCGACATCGTGGAC |
| R: CACTTGGTCAGCAGCGGCGATGCCAACT | |
| OsGS2 | F: AGAACTTGGACGATGAATCGGGGC |
| R: GAGGGAAGGACGCAGGACTGAAGA | |
| OsAMT2;1 | F: GATGAATCACGCCGAAACAC |
| R: GCACGGACGAATCGCTACTT | |
| OsNPF2;2 | F: GTCGCAGGAGCAAACTAAGCTG |
| R: TTTCGCATGTCTCGTTCCCTATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.; Rao, X.; Liu, H.; Hong, J.; Tang, W.; Yan, S.; Yang, G.; Chen, H.; Hu, Y. Effect of Nitrogen and Phosphorus Fertilizers on Dry Matter Accumulation and Translocation of Two Amylose Content Indica Rice on Yield. Plants 2025, 14, 1536. https://doi.org/10.3390/plants14101536
Qin X, Rao X, Liu H, Hong J, Tang W, Yan S, Yang G, Chen H, Hu Y. Effect of Nitrogen and Phosphorus Fertilizers on Dry Matter Accumulation and Translocation of Two Amylose Content Indica Rice on Yield. Plants. 2025; 14(10):1536. https://doi.org/10.3390/plants14101536
Chicago/Turabian StyleQin, Xiaohong, Xinyue Rao, Hongjing Liu, Jiale Hong, Wanlin Tang, Shengmin Yan, Guotao Yang, Hong Chen, and Yungao Hu. 2025. "Effect of Nitrogen and Phosphorus Fertilizers on Dry Matter Accumulation and Translocation of Two Amylose Content Indica Rice on Yield" Plants 14, no. 10: 1536. https://doi.org/10.3390/plants14101536
APA StyleQin, X., Rao, X., Liu, H., Hong, J., Tang, W., Yan, S., Yang, G., Chen, H., & Hu, Y. (2025). Effect of Nitrogen and Phosphorus Fertilizers on Dry Matter Accumulation and Translocation of Two Amylose Content Indica Rice on Yield. Plants, 14(10), 1536. https://doi.org/10.3390/plants14101536






