Screening and Identification of Drought-Tolerant Genes in Tomato (Solanum lycopersicum L.) Based on RNA-Seq Analysis
Abstract
1. Introduction
2. Results
2.1. Growth Indicator Determination of “FQ118” and “FQ119” Under Drought Stress
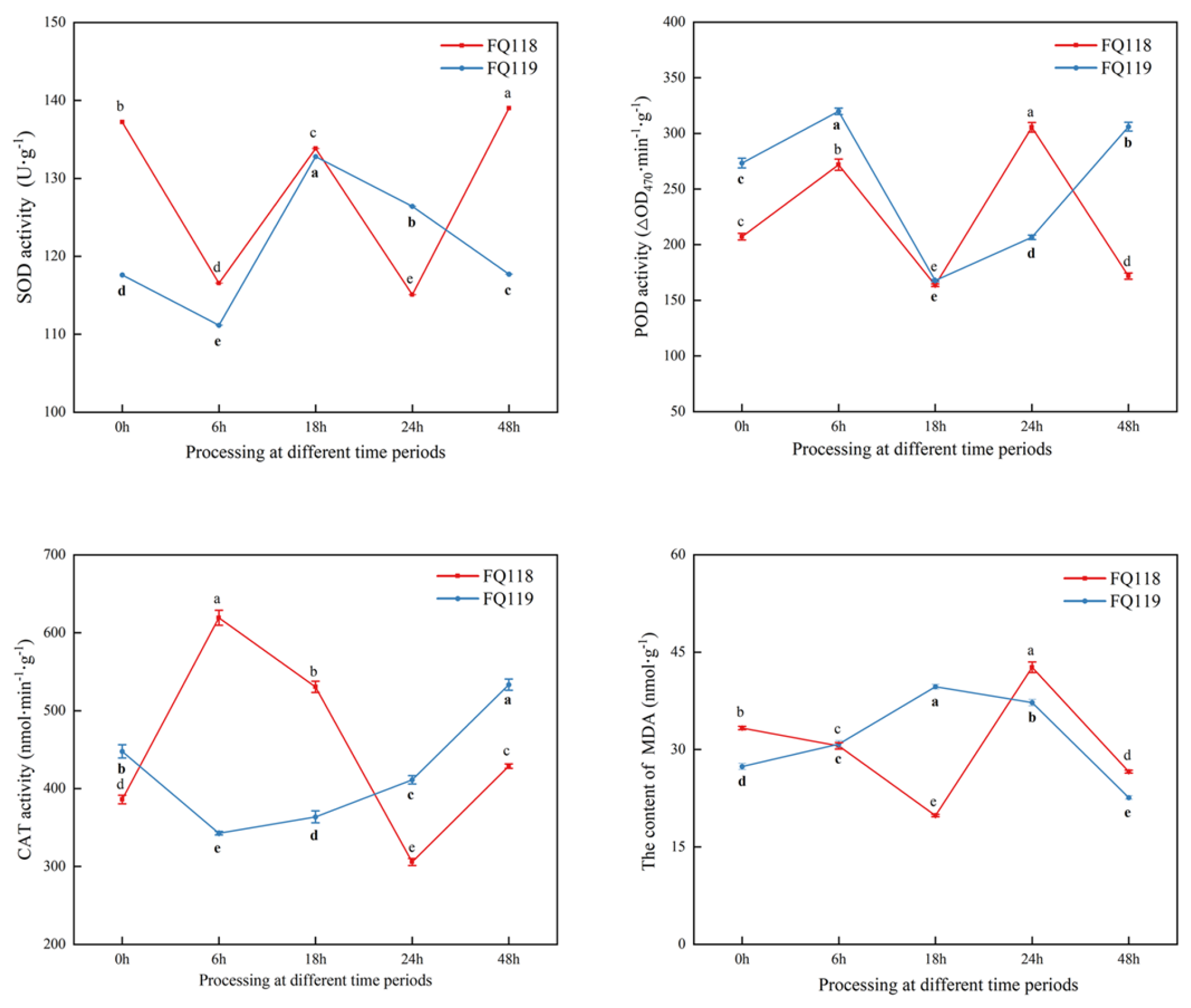
2.2. ROS Indicators Determination of “FQ118” and “FQ119” Under Drought Stress
2.3. Transcriptome Analysis of “FQ118” and “FQ119” Under Drought Stress
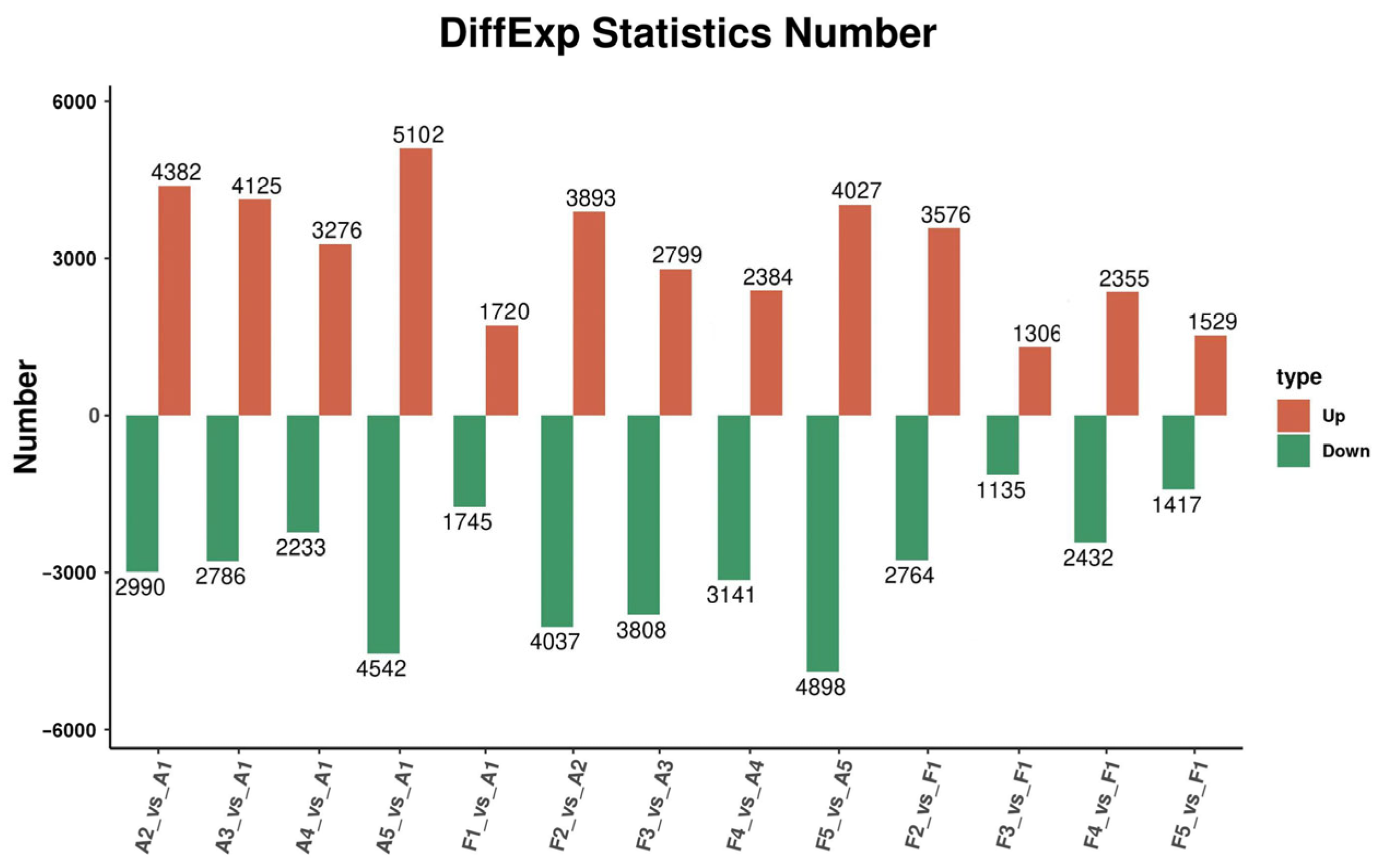
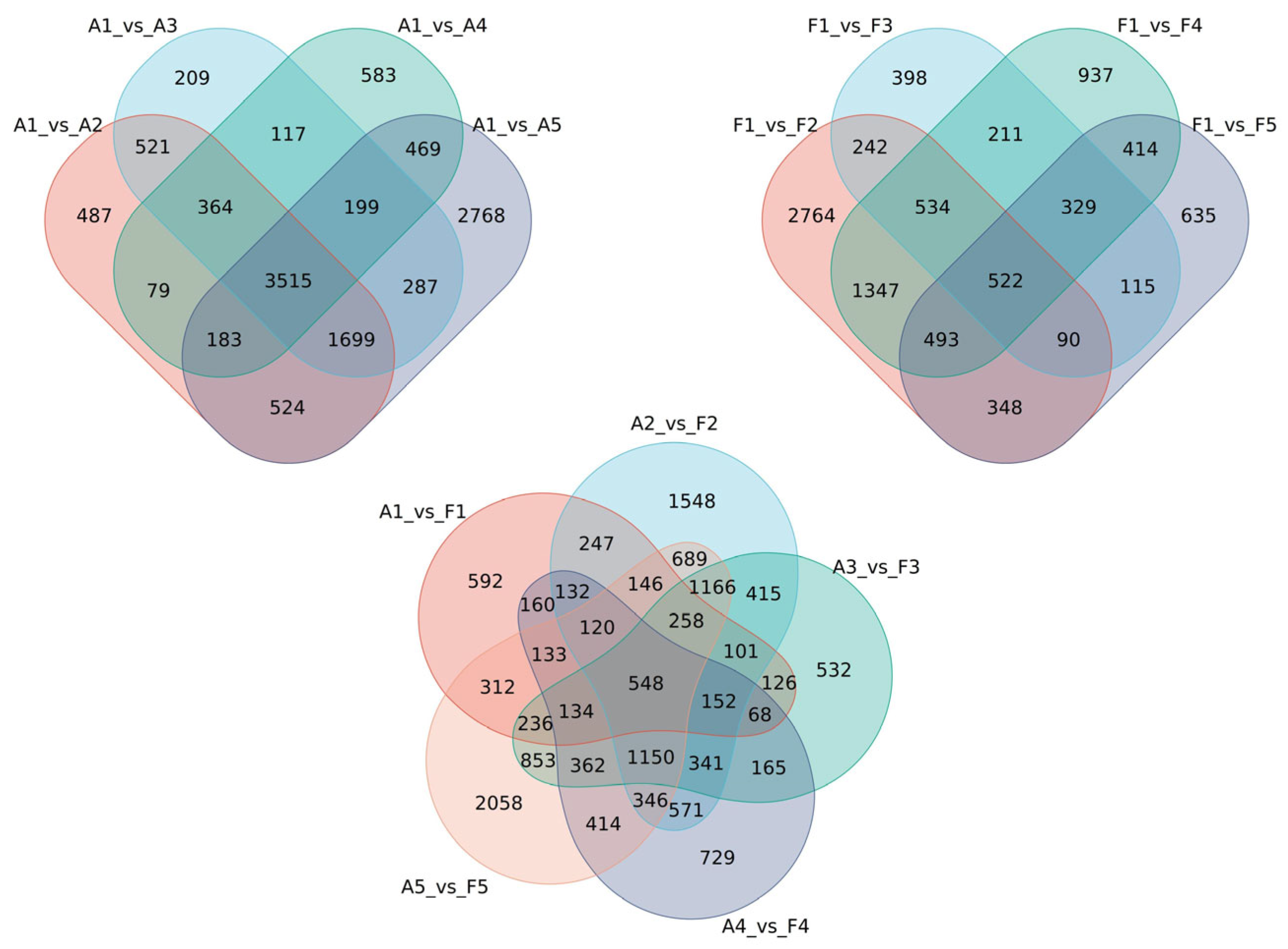
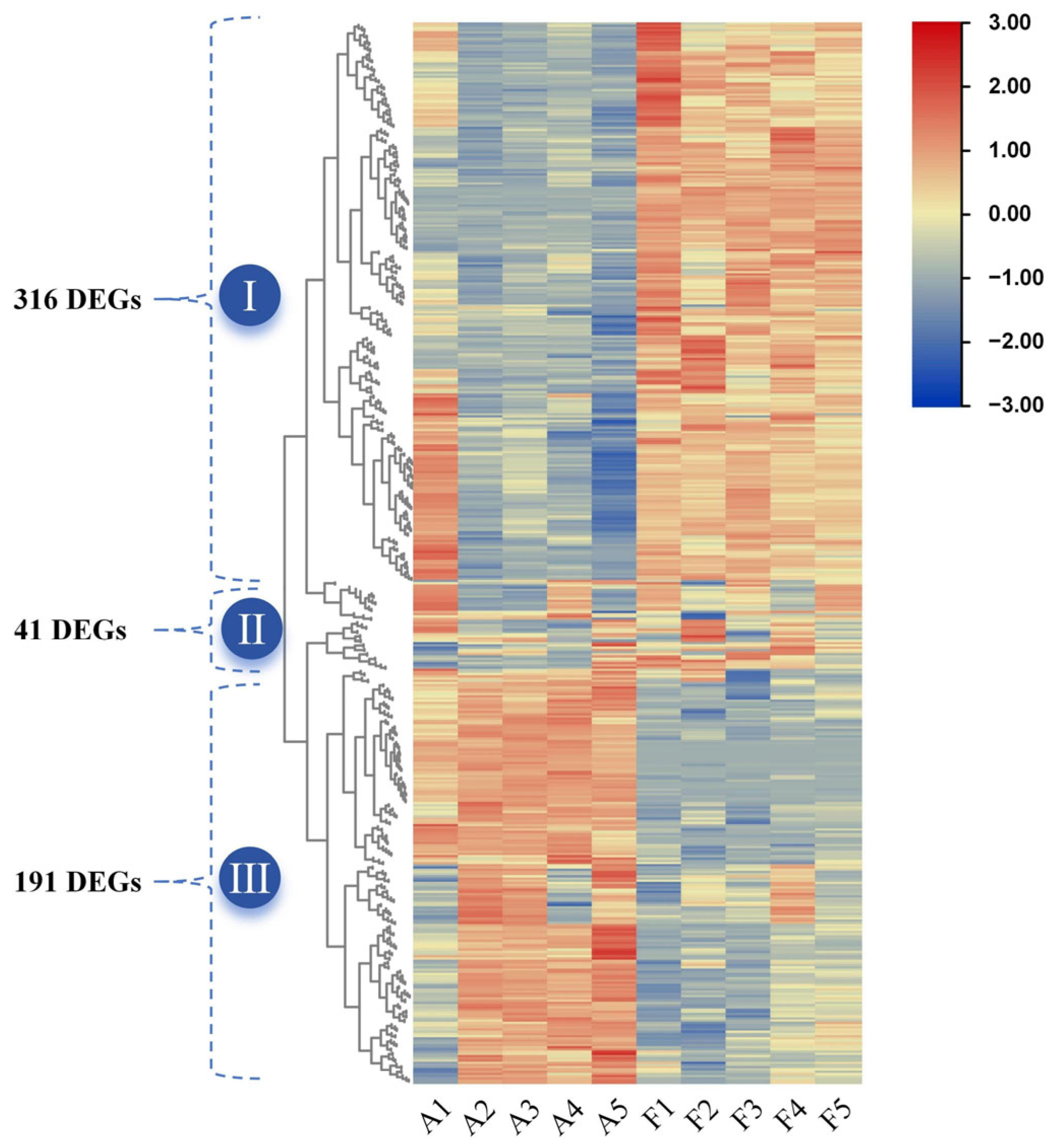
2.4. Analysis of DEG Response to Soil Drought Stress
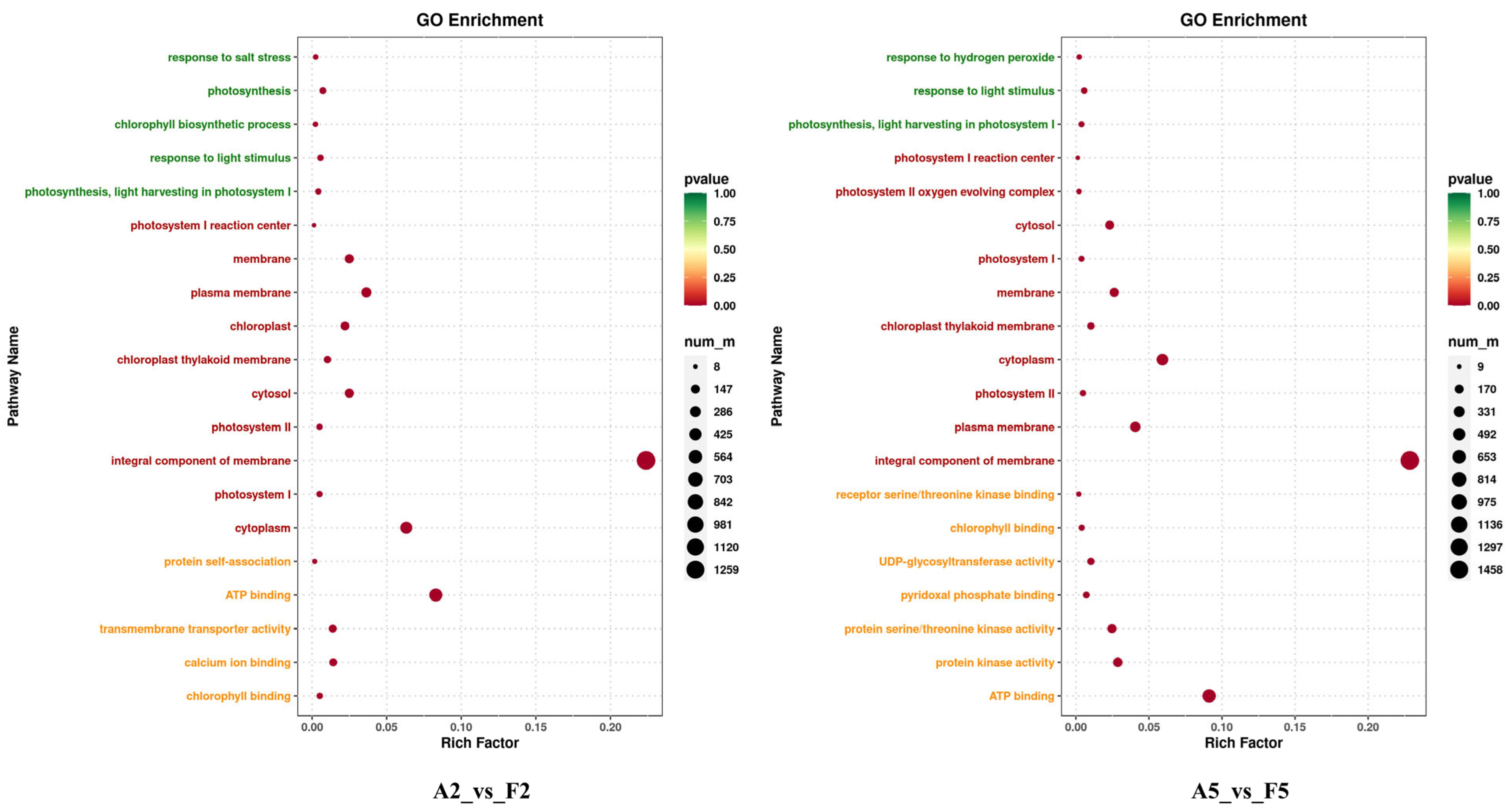
2.5. GO and KEGG Pathway Analysis of DEGs
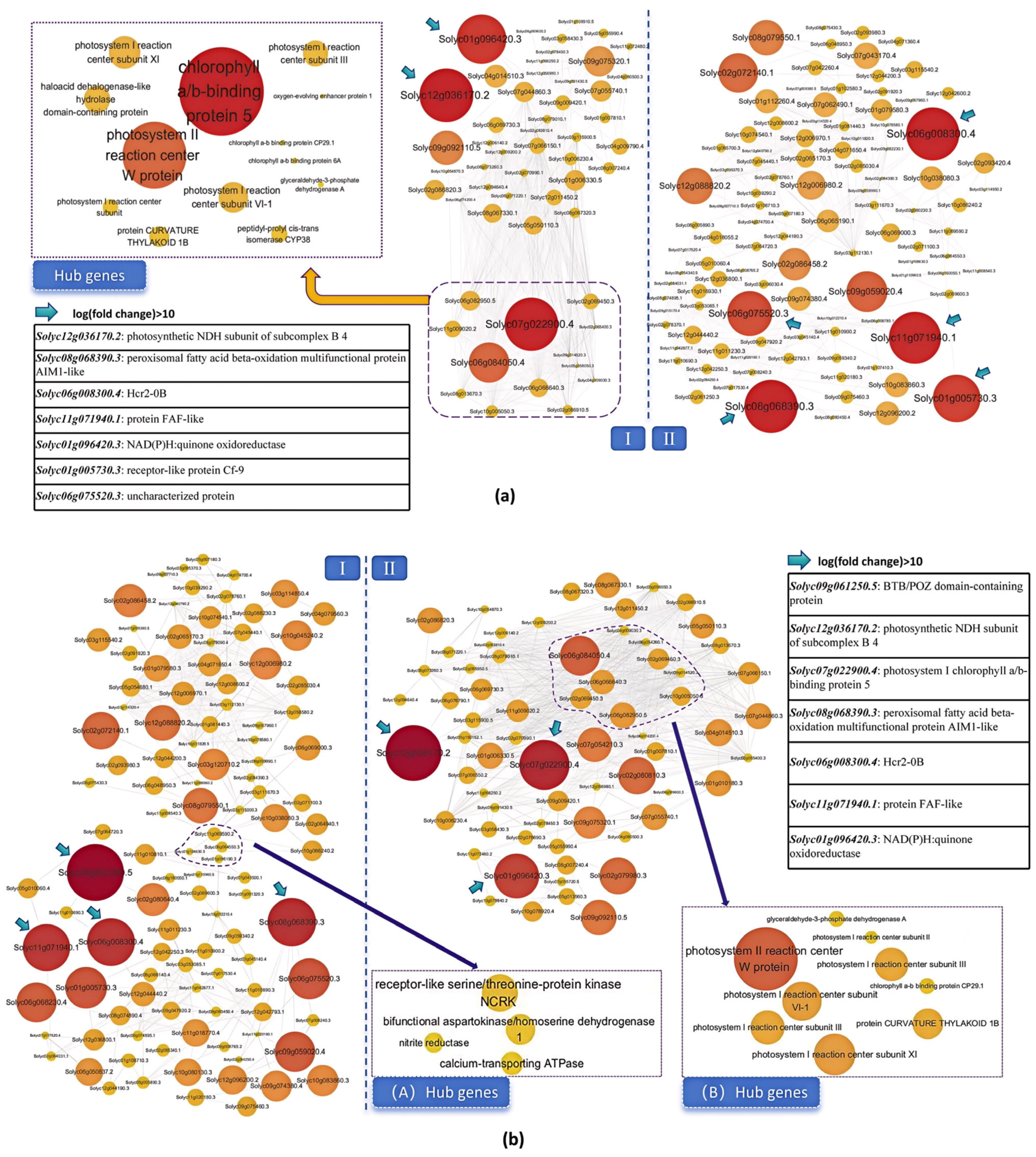
2.6. Analysis of Drought Resistance-Related Gene Expression Patterns and Establishment of Co-Expression Network
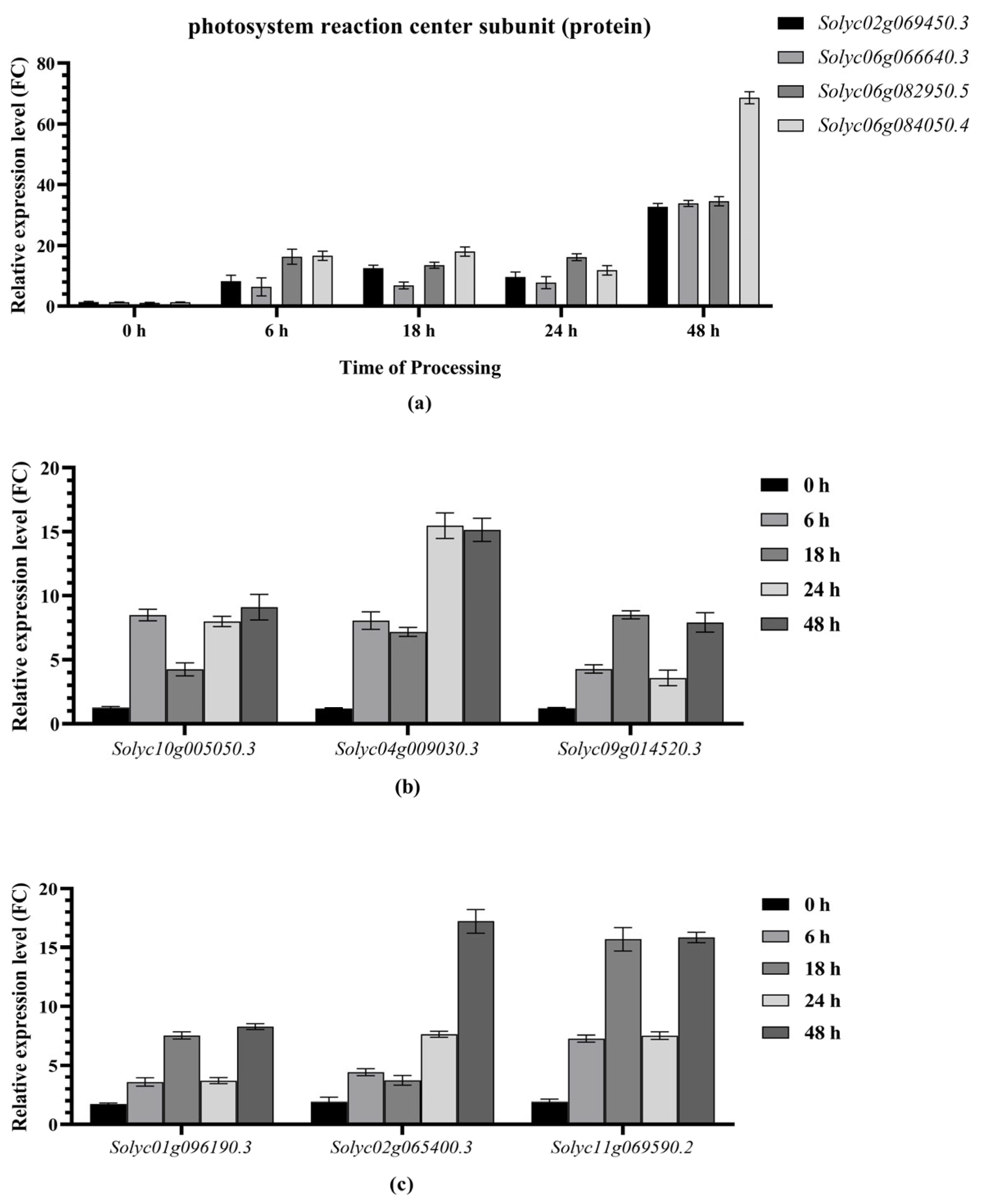
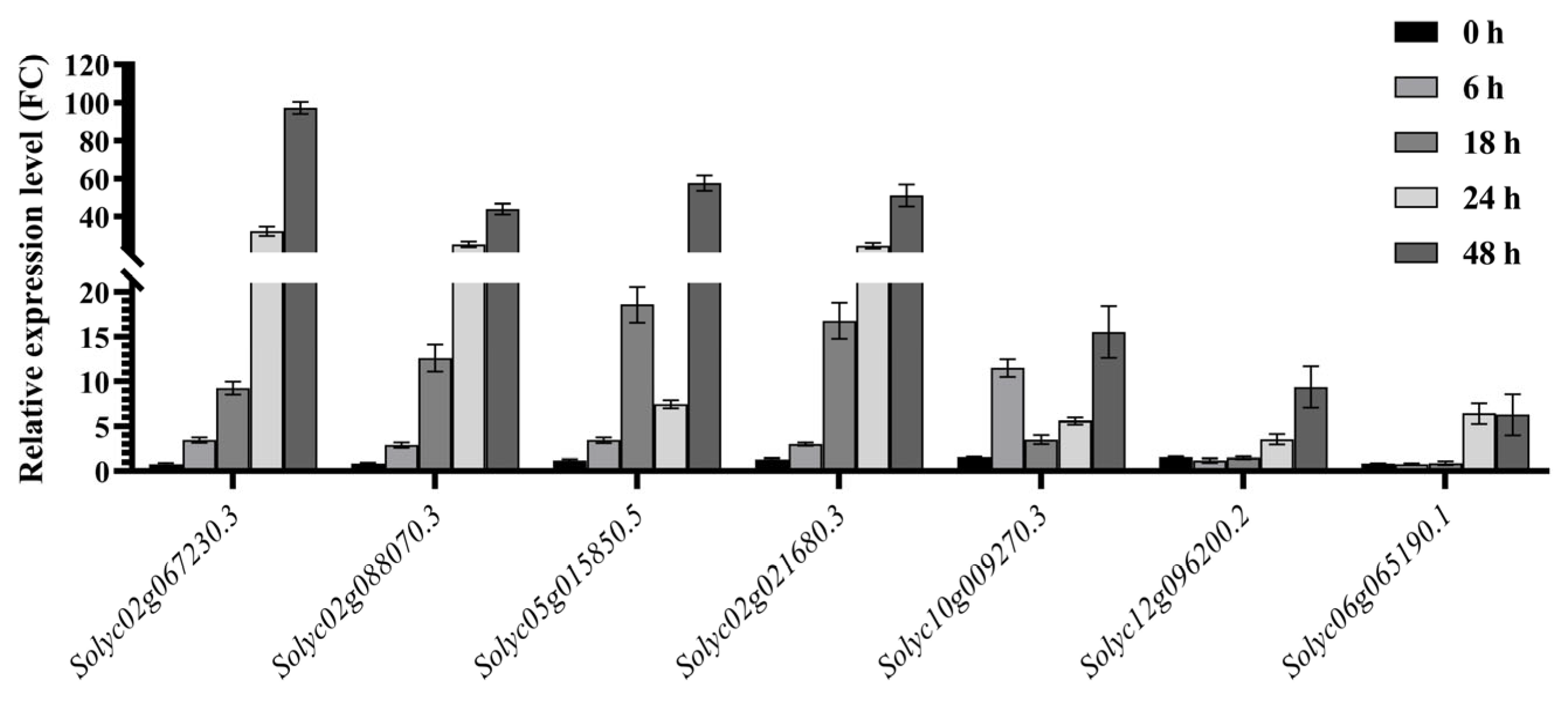
2.7. Expression Trends of Drought Resistance-Related Genes Identified via RT‒qPCR
2.8. Function Validation of Genes Related to Drought Resistance by VIGS
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth
4.2. Drought Treatments and RNA-Seq Assay
4.3. Construction of RNAi Vectors
4.4. RNAi Line Construction
4.5. RT‒qPCR
4.6. Growth Indicator Determination
4.7. Physiological Indicator Determination
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, N.; Shen, G.; Zhang, H. Genetic manipulation for abiotic stress resistance traits in crops. Front. Plant Sci. 2022, 13, 1011985. [Google Scholar] [CrossRef] [PubMed]
- Trono, D.; Pecchioni, N. Candidate Genes Associated with Abiotic Stress Response in Plants as Tools to Engineer Tolerance to Drought, Salinity and Extreme Temperatures in Wheat: An Overview. Plants 2022, 11, 3358. [Google Scholar] [CrossRef]
- Supriya, L.; Durgeshwar, P.; Muthamilarasan, M.; Padmaja, G. Melatonin Mediated Differential Regulation of Drought Tolerance in Sensitive and Tolerant Varieties of Upland Cotton (Gossypium hirsutum L.). Front. Plant Sci. 2022, 13, 821353. [Google Scholar] [CrossRef]
- Li, Z.; Lian, Y.; Gong, P.; Song, L.; Hu, J.; Pang, H.; Ren, Y.; Xin, Z.; Wang, Z.; Lin, T. Network of the transcriptome and metabolomics reveals a novel regulation of drought resistance during germination in wheat. Ann. Bot. 2022, 130, 717–735. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Jing, D.W.; Xing, S.J.; Du, Z.Y.; Liu, F.C. Effects of drought stress on the growth, photosynthetic characteristics, and active oxygen metabolism of poplar seedlings. Ying Yong Sheng Tai Xue Bao 2013, 24, 1809–1816. [Google Scholar]
- Kang, H.K.; Nam, K.H. Reverse function of ROS-induced CBL10 during salt and drought stress responses. Plant Sci. 2016, 243, 49–55. [Google Scholar] [CrossRef]
- Li, S. Novel insight into functions of ascorbate peroxidase in higher plants: More than a simple antioxidant enzyme. Redox Biol. 2023, 64, 102789. [Google Scholar] [CrossRef]
- Chen, J.; Li, N.; Wang, X.; Meng, X.; Cui, X.; Chen, Z.; Ren, H.; Ma, J.; Liu, H. Late embryogenesis abundant (LEA) gene family in Salvia miltiorrhiza: Identification, expression analysis, and response to drought stress. Plant Signal Behav. 2021, 16, 1891769. [Google Scholar] [CrossRef]
- Gu, X.; Gao, Z.; Yan, Y.; Wang, X.; Qiao, Y.; Chen, Y. RdreB1BI enhances drought tolerance by activating AQP-related genes in transgenic strawberry. Plant Physiol Biochem. 2017, 119, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Aneja, B.; Yadav, N.R.; Kumar, N.; Yadav, R.C. Hsp transcript induction is correlated with physiological changes under drought stress in Indian mustard. Physiol. Mol. Biol. Plants 2015, 21, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.J.; Li, J.M.; Zong, J.W.; Yao, Y.Z.; Chen, K.L. Effects of sub-low temperature and drought stress on root morphology and leaf structure of tomato seedlings. Ying Yong Sheng Tai Xue Bao 2012, 23, 3027–3032. [Google Scholar] [PubMed]
- Li, F.; Chen, X.; Zhou, S.; Xie, Q.; Wang, Y.; Xiang, X.; Hu, Z.; Chen, G. Overexpression of SlMBP22 in Tomato Affects Plant Growth and Enhances Tolerance to Drought Stress. Plant Sci. 2020, 301, 110672. [Google Scholar] [CrossRef]
- Takahashi, F.; Kuromori, T.; Urano, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Drought Stress Responses and Resistance Plants: From Cellular Responses to Long-Distance Intercellular Communication. Front. Plant Sci. 2020, 11, 556972. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Ottosen, C.O.; Rosenqvist, E.; Zhao, L.; Wang, Y.; Yu, W.; Zhao, T.; Wu, Z. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017, 17, 24. [Google Scholar] [CrossRef]
- Suzuki, N.; Miller, G.; Morales, J.; Shulaev, V.; Torres, M.A.; Mittler, R. Respiratory burst oxidases: The engines of ROS signaling. Curr Opin Plant Biol. 2011, 14, 691–699. [Google Scholar] [CrossRef]
- Ma, X.; Xia, H.; Liu, Y.; Wei, H.; Zheng, X.; Song, C.; Chen, L.; Liu, H.; Luo, L. Transcriptomic and Metabolomic Studies Disclose Key Metabolism Pathways Contributing to Well-maintained Photosynthesis under the Drought and the Consequent Drought-Tolerance in Rice. Front. Plant Sci. 2016, 7, 1886. [Google Scholar] [CrossRef]
- Geng, S.; Gao, W.; Li, S.; Chen, Q.; Jiao, Y.; Zhao, J.; Wang, Y.; Wang, T.; Qu, Y.; Chen, Q. Rapidly mining candidate cotton drought resistance genes based on key indicators of drought resistance. BMC Plant Biol. 2024, 24, 129. [Google Scholar] [CrossRef]
- Armbruster, U.; Labs, M.; Pribil, M.; Viola, S.; Xu, W.; Scharfenberg, M.; Hertle, A.P.; Rojahn, U.; Jensen, P.E.; Rappaport, F.; et al. Arabidopsis CURVATURE THYLAKOID1 proteins modify thylakoid architecture by inducing membrane curvature. Plant Cell 2013, 25, 2661–2678. [Google Scholar] [CrossRef]
- Yu, A.; Xie, Y.; Pan, X.; Zhang, H.; Cao, P.; Su, X.; Chang, W.; Li, M. Photosynthetic Phosphoribulokinase Structures: Enzymatic Mechanisms and the Redox Regulation of the Calvin-Benson-Bassham Cycle. Plant Cell 2020, 32, 1556–1573. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.; Takeda, S.; Kobayashi, M.J.; Kimura, S.; Iizuka, A.; Imai, A.; Hishinuma, H.; Kawarazaki, T.; Mori, K.; Yamamoto, Y.; et al. Comparative analysis of the reactive oxygen species-producing enzymatic activity of Arabidopsis NADPH oxidases. Plant J. 2019, 98, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Irshad, Z.; Dimitri, F.; Christian, M.; Zammit, V.A. Diacylglycerol acyltransferase 2 links glucose utilization to fatty acid oxidation in the brown adipocytes. J. Lipid Res. 2017, 58, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Guo, X.; Hu, L.; Li, S.; Chen, Y.; Wang, J.; Wang, R.R.; Fan, C.; Hu, Z. Genome-Wide Characterization of DGATs and Their Expression Diversity Analysis in Response to Abiotic Stresses in Brassica napus. Plants 2022, 11, 1156. [Google Scholar] [CrossRef] [PubMed]
- Sree, B.K.; Rajendrakumar, C.S.V.; Reddy, A.R. Aldose reductase in rice (Oryza sativa L.): Stress response and developmental specificity. Plant Sci. 2001, 160, 149–157. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, K.; Guo, X.; Pei, Y.; Zhao, M.; Song, X.; Li, Y.; Liu, S.; Li, J. Constitutive expression of aldose reductase 1 from Zea mays exacerbates salt and drought sensitivity of transgenic Escherichia coli and Arabidopsis. Plant Physiol. Biochem. 2020, 156, 436–444. [Google Scholar] [CrossRef]
- Tan, Y.; Han, M.; Zhang, C.; Chang, P.; Xu, W. Effects of overexpression of apple glycerin diester kinase gene MpDGK7 on drought resistance of Arabidopsis plants. J. Nucl. Agron. 2023, 37, 1729–1734. (In Chinese) [Google Scholar]
- Zhang, Y.; Xiao, Y.; Zhang, Y.; Dong, Y.; Liu, Y.; Liu, L.; Wan, S.; He, J.; Yu, Y. Accumulation of Galactinol and ABA Is Involved in Exogenous EBR-Induced Drought Tolerance in Tea Plants. J. Agric. Food Chem. 2022, 70, 13391–13403. [Google Scholar] [CrossRef]
- Taji, T.; Ohsumi, C.; Iuchi, S.; Seki, M.; Shinozaki, K. Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J. 2002, 29, 417–426. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Feng, S.; Zhang, J.; Zhang, F.; Wang, W.; Hu, H.; Zhang, W.; Bao, M. The C2H2-type zinc finger protein PhZFP1 regulates cold stress tolerance by modulating galactinol synthesis in Petunia hybrida. J. Exp. Bot. 2022, 73, 6434–6448. [Google Scholar] [CrossRef]
- Gu, L.; Jiang, T.; Zhang, C.; Li, X.; Zhao, T. Maize HSFA2 and HSBP2 antagonistically modulate raffinose biosynthesis and heat tolerance in Arabidopsis. Plant J. 2019, 100, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, Y.; Liu, Y.; Li, X.; Hao, G.; Han, Q.; Dirk, L.M.A.; Downie, A.B.; Ruan, Y.L.; Wang, J. Raffinose synthase enhances drought tolerance through raffinose synthesis or galactinol hydrolysis in maize and Arabidopsis plants. J. Biol. Chem. 2020, 295, 8064–8077. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Gao, Y.; Zhang, Y.; Li, D.; Dirk, L.M.; Downie, A.B.; Zhao, T. Raffinose catabolism enhances maize waterlogging tolerance by stimulating adventitious root growth and development. J. Exp. Bot. 2024, 18, 5955–5970. [Google Scholar] [CrossRef] [PubMed]
- Yokono, M.; Takabayashi, A.; Akimoto, S.; Tanaka, A. A megacomplex composed of both photosystem reaction centres in higher plants. Nat. Commun. 2015, 6, 6675. [Google Scholar] [CrossRef]
- Pu, X.; Fu, Y.; Xu, C.; Li, X.; Wang, W.; De, K.; Wei, X.; Yao, X. Transcriptomic analyses provide molecular insight into the cold stress response of cold-tolerant alfalfa. BMC Plant Biol. 2024, 24, 741. [Google Scholar] [CrossRef]
- Wang, L.; Wei, J.; Shi, X.; Qian, W.; Mehmood, J.; Yin, Y.; Jia, H. Identification of the Light-Harvesting Chlorophyll a/b Binding Protein Gene Family in Peach (Prunus persica L.) and Their Expression under Drought Stress. Genes 2023, 14, 1475. [Google Scholar] [CrossRef]
- Trotta, A.; Bajwa, A.A.; Mancini, I.; Paakkarinen, V.; Pribil, M.; Aro, E.M. The Role of Phosphorylation Dynamics of CURVATURE THYLAKOID 1B in Plant Thylakoid Membranes. Plant Physiol. 2019, 181, 1615–1631. [Google Scholar] [CrossRef]
- Höhner, R.; Pribil, M.; Herbstová, M.; Lopez, L.S.; Kunz, H.H.; Li, M.; Wood, M.; Svoboda, V.; Puthiyaveetil, S.; Leister, D.; et al. Plastocyanin is the long-range electron carrier between photosystem II and photosystem I in plants. Proc. Natl. Acad. Sci. USA 2020, 117, 15354–15362. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dwivedi, S.; Bhagavatula, L.; Datta, S. Integration of light and ABA signaling pathways to combat drought stress in plants. Plant Cell Rep. 2023, 42, 829–841. [Google Scholar] [CrossRef]
- Nicholls, C.; Pinto, A.R.; Li, H.; Li, L.; Wang, L.; Simpson, R.; Liu, J.P. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) induces cancer cell senescence by interacting with telomerase RNA component. Proc. Natl. Acad. Sci. USA 2012, 109, 13308–13313. [Google Scholar] [CrossRef]
- Niu, E.; Ye, C.; Zhao, W.; Kondo, H.; Wu, Y.; Chen, J.; Andika, I.B.; Sun, L. Coat protein of Chinese wheat mosaic virus upregulates and interacts with cytosolic glyceraldehyde-3-phosphate dehydrogenase, a negative regulator of plant autophagy, to promote virus infection. J. Integr. Plant Biol. 2022, 64, 1631–1645. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Guo, L.; Wang, X. Nuclear moonlighting of cytosolic glyceraldehyde-3-phosphate dehydrogenase regulates Arabidopsis response to heat stress. Nat. Commun. 2020, 11, 3439. [Google Scholar] [CrossRef] [PubMed]
- Oh, C. Genome-Wide Characterization of Glyceraldehyde-3-Phosphate Dehydrogenase Genes and Their Expression Profile under Drought Stress in Quercus rubra. Plants 2024, 13, 2312. [Google Scholar] [CrossRef]
- Geng, S.; Li, S.; Zhao, J.; Gao, W.; Chen, Q.; Zheng, K.; Wang, Y.; Jiao, Y.; Long, Y.; Liu, P.; et al. Glyceraldehyde-3-phosphate dehydrogenase Gh_GAPDH9 is associated with drought resistance in Gossypium hirsutum. PeerJ 2023, 11, e16445. [Google Scholar] [CrossRef]
- Kim, E.Y.; Choi, Y.H.; Lee, J.I.; Kim, I.H.; Nam, T.J. Antioxidant Activity of Oxygen Evolving Enhancer Protein 1 Purified from Capsosiphon fulvescens. J. Food Sci. 2015, 80, H1412–H1417. [Google Scholar] [CrossRef]
- Mayfield, S.P. Over-expression of the oxygen-evolving enhancer 1 protein and its consequences on photosystem II accumulation. Planta 1991, 185, 105–110. [Google Scholar] [CrossRef]
- Liu, R.; Chen, T.; Yin, X.; Xiang, G.; Peng, J.; Fu, Q.; Li, M.; Shang, B.; Ma, H.; Liu, G.; et al. A Plasmopara viticola RXLR effector targets a chloroplast protein PsbP to inhibit ROS production in grapevine. Plant J. 2021, 106, 1557–1570. [Google Scholar] [CrossRef]
- Xu, M.; Li, Y.; Zheng, Z.; Dai, Z.; Tao, Y.; Deng, X. Transcriptional Analyses of Mandarins Seriously Infected by ‘Candidatus Liberibacter asiaticus’. PLoS ONE 2015, 10, e0133652. [Google Scholar] [CrossRef]
- Saleethong, P.; Roytrakul, S.; Kong-Ngern, K.; Theerakulpisut, P. Differential Proteins Expressed in Rice Leaves and Grains in Response to Salinity and Exogenous Spermidine Treatments. Rice Sci. 2016, 23, 9–21. [Google Scholar] [CrossRef]
- Ghatak, A.; Chaturvedi, P.; Weckwerth, W. Cereal Crop Proteomics: Systemic Analysis of Crop Drought Stress Responses Towards Marker-Assisted Selection Breeding. Front. Plant Sci. 2017, 8, 757. [Google Scholar] [CrossRef]
- Rahimi, Y.; Ingvarsson, P.K.; Bihamta, M.R.; Alipour, H.; Taleei, A.; Khoshnoodi Jabar Abadi, S. Characterization of Dynamic Regulatory Gene and Protein Networks in Wheat Roots Upon Perceiving Water Deficit Through Comparative Transcriptomics Survey. Front. Plant Sci. 2021, 12, 710867. [Google Scholar] [CrossRef] [PubMed]
- Falhof, J.; Pedersen, J.T.; Fuglsang, A.T.; Palmgren, M. Plasma Membrane H(+)-ATPase Regulation in the Center of Plant Physiology. Mol. Plant 2016, 9, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.J.; Wood, A.J.; Lightfoot, D.A. Plant receptor-like serine threonine kinases: Roles in signaling and plant defense. Mol. Plant-Microbe Interact. 2008, 21, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Ng, D.W.; Abeysinghe, J.K.; Kamali, M. Regulating the Regulators: The Control of Transcription Factors in Plant Defense Signaling. Int. J. Mol. Sci. 2018, 19, 3737. [Google Scholar] [CrossRef]
- Yu, Q.; Li, C.; Zhang, J.; Tian, Y.; Zhang, L. Genome-wide identification and expression analysis of the Dof gene family under drought stress in tea (Camellia sinensis). PeerJ 2020, 8, e9269. [Google Scholar] [CrossRef]
- Shim, Y.; Kim, B.; Choi, Y.; Cho, S.-H.; Kim, Y.; Kim, S.-H.; Yim, Y.; Kang, K.; Paek, N.-C. Rice OsDof12 enhances tolerance to drought stress by activating the phenylpropanoid pathway. Plant J. 2025, 121, e17175. [Google Scholar] [CrossRef]
- Wang, C.T.; Ru, J.N.; Liu, Y.W.; Yang, J.F.; Li, M.; Xu, Z.S.; Fu, J.D. The Maize WRKY Transcription Factor ZmWRKY40 Confers Drought Resistance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2018, 19, 2580. [Google Scholar] [CrossRef]
- Chen, T.; Li, W.; Hu, X.; Guo, J.; Liu, A.; Zhang, B. A Cotton MYB Transcription Factor, GbMYB5, is Positively Involved in Plant Adaptive Response to Drought Stress. Plant Cell Physiol. 2015, 56, 917–929. [Google Scholar] [CrossRef]
- Feng, H.L.; Ma, N.N.; Meng, X.; Zhang, S.; Wang, J.R.; Chai, S.; Meng, Q.W. A novel tomato MYC-type ICE1-like transcription factor, SlICE1a, confers cold, osmotic and salt tolerance in transgenic tobacco. Plant Physiol. Biochem. 2013, 73, 309–320. [Google Scholar] [CrossRef]
- Edris, S.; Abulfaraj, A.A.; Makki, R.M.; Abo-Aba, S.; Algandaby, M.M.; Sabir, J.; Jansen, R.K.; El Domyati, F.M.; Bahieldin, A. Early Fruit Development Regulation-Related Genes Concordantly Expressed with TCP Transcription Factors in Tomato (Solanum lycopersicum). Curr. Issues Mol. Biol. 2023, 45, 2372–2380. [Google Scholar] [CrossRef]
- Ding, S.; Cai, Z.; Du, H.; Wang, H. Genome-Wide Analysis of TCP Family Genes in Zea mays L. Identified a Role for ZmTCP42 in Drought Tolerance. Int. J. Mol. Sci. 2019, 20, 2762. [Google Scholar] [CrossRef] [PubMed]
- Köppel, R.; Bucher, T.B. Duplex real-time PCR for the determination of wasabi (Eutrema wasabi) contents in horseradish (Armoracia rusticana) products applying the ΔΔct-method. Eur. Food Res. Technol. 2016, 242, 1111–1115. [Google Scholar] [CrossRef]
- Ma, Q.J.; Sun, M.H.; Lu, J.; Liu, Y.J.; Hu, D.G.; Hao, Y.J. Transcription Factor AREB2 Is Involved in Soluble Sugar Accumulation by Activating Sugar Transporter and Amylase Genes. Plant Physiol. 2017, 174, 2348–2362. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Li, Y.; Wang, F.; Qing, Q.; Deng, C.; Wang, H.; Song, Y. Screening and Identification of Drought-Tolerant Genes in Tomato (Solanum lycopersicum L.) Based on RNA-Seq Analysis. Plants 2025, 14, 1471. https://doi.org/10.3390/plants14101471
Ma Y, Li Y, Wang F, Qing Q, Deng C, Wang H, Song Y. Screening and Identification of Drought-Tolerant Genes in Tomato (Solanum lycopersicum L.) Based on RNA-Seq Analysis. Plants. 2025; 14(10):1471. https://doi.org/10.3390/plants14101471
Chicago/Turabian StyleMa, Yue, Yushan Li, Fan Wang, Quan Qing, Chengzhu Deng, Hao Wang, and Yu Song. 2025. "Screening and Identification of Drought-Tolerant Genes in Tomato (Solanum lycopersicum L.) Based on RNA-Seq Analysis" Plants 14, no. 10: 1471. https://doi.org/10.3390/plants14101471
APA StyleMa, Y., Li, Y., Wang, F., Qing, Q., Deng, C., Wang, H., & Song, Y. (2025). Screening and Identification of Drought-Tolerant Genes in Tomato (Solanum lycopersicum L.) Based on RNA-Seq Analysis. Plants, 14(10), 1471. https://doi.org/10.3390/plants14101471






