Regulatory Effects of Source–Sink Manipulations on Photosynthesis in Wheat with Different Source–Sink Relationships
Abstract
1. Introduction
2. Results
2.1. Yield, Its Components, and Sink–Source Ratio
2.2. Chlorophyll SPAD
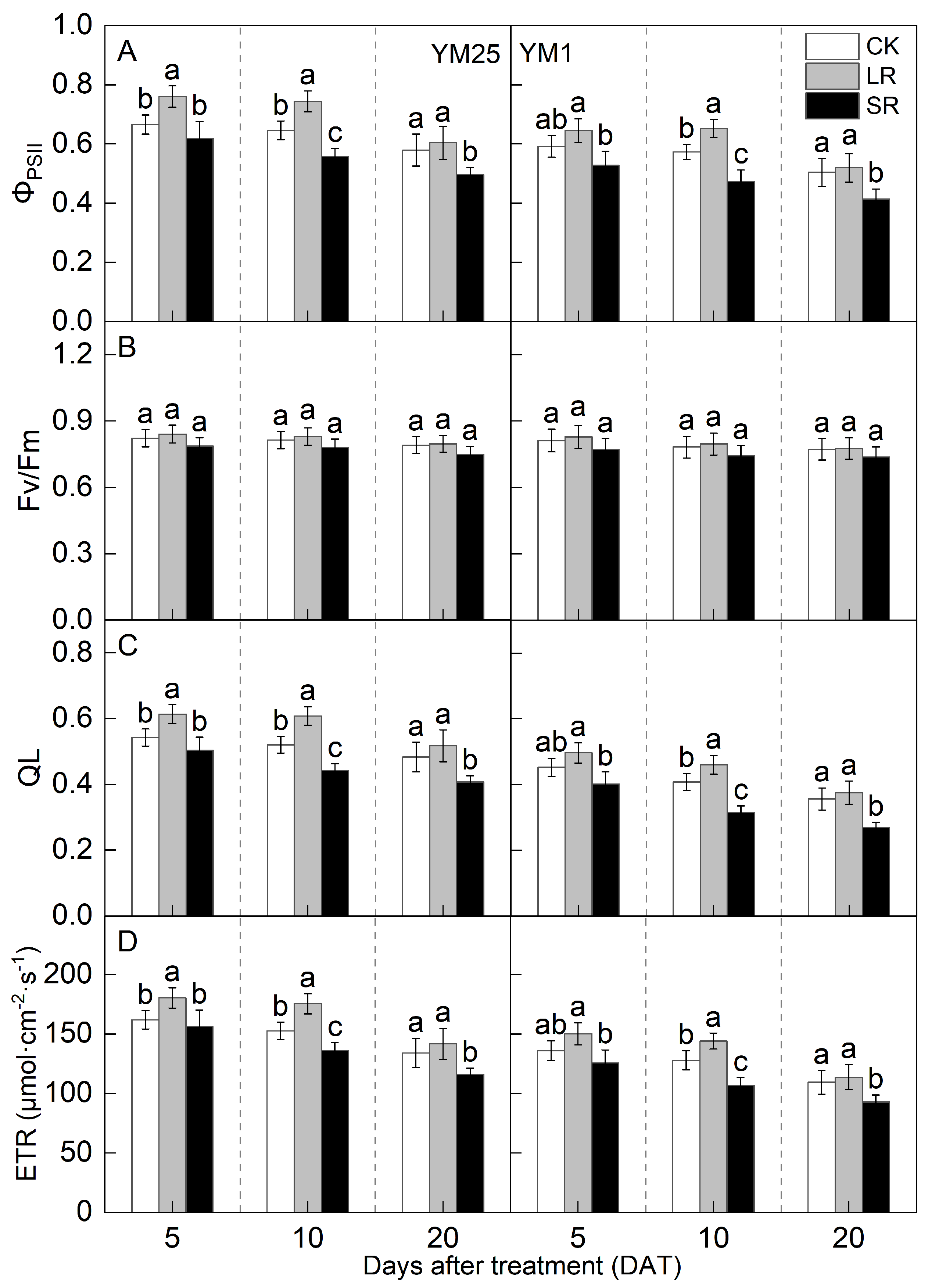
2.3. Pn and Photosynthetic Fluorescence Parameters
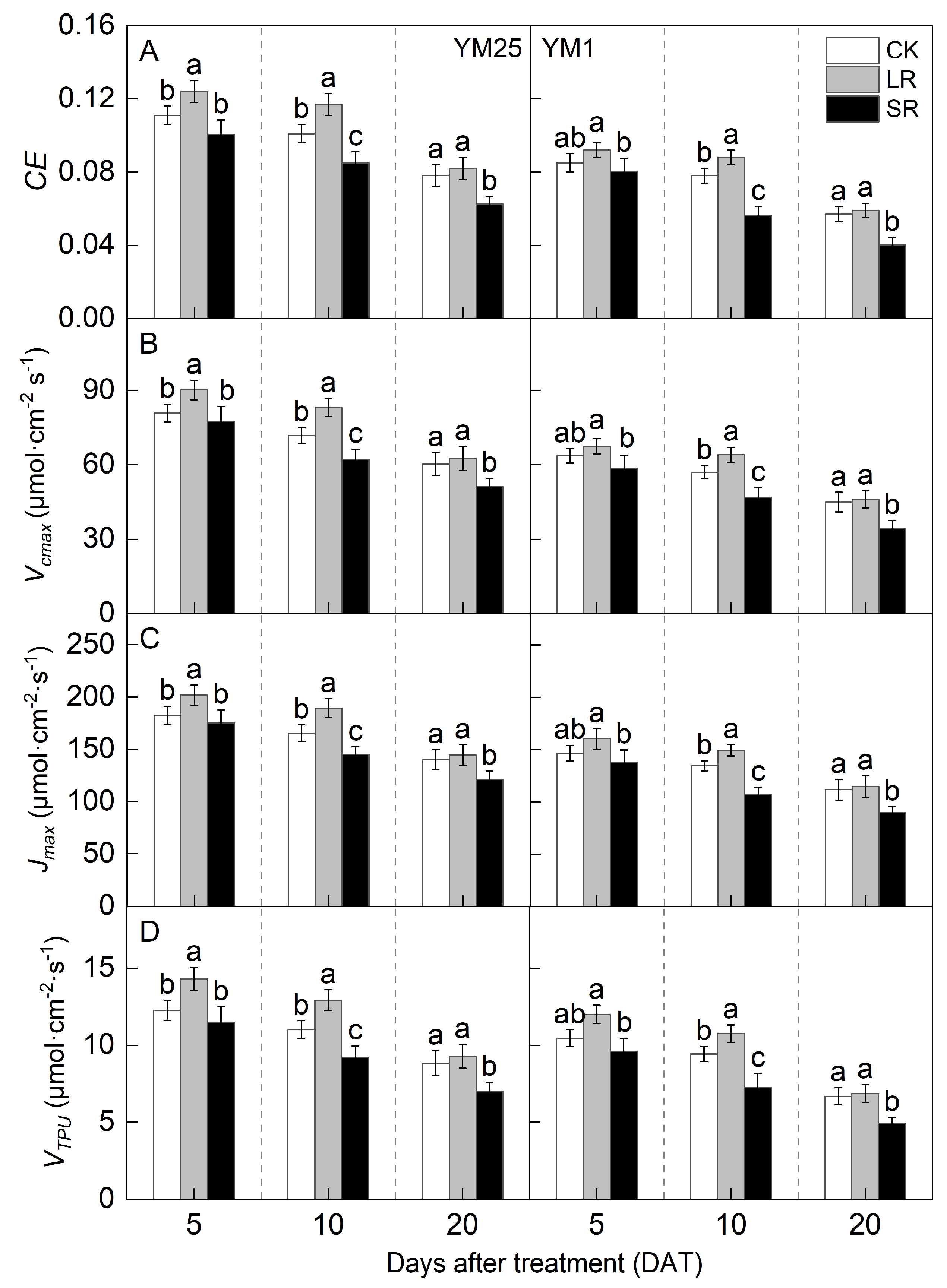
2.4. Carboxylation Efficiency (CE), Maximum Carboxylation Rate (Vcmax), Maximum Electron Transport Rate (Jmax) and Triose Phosphate Utilization Rate (VTPU)
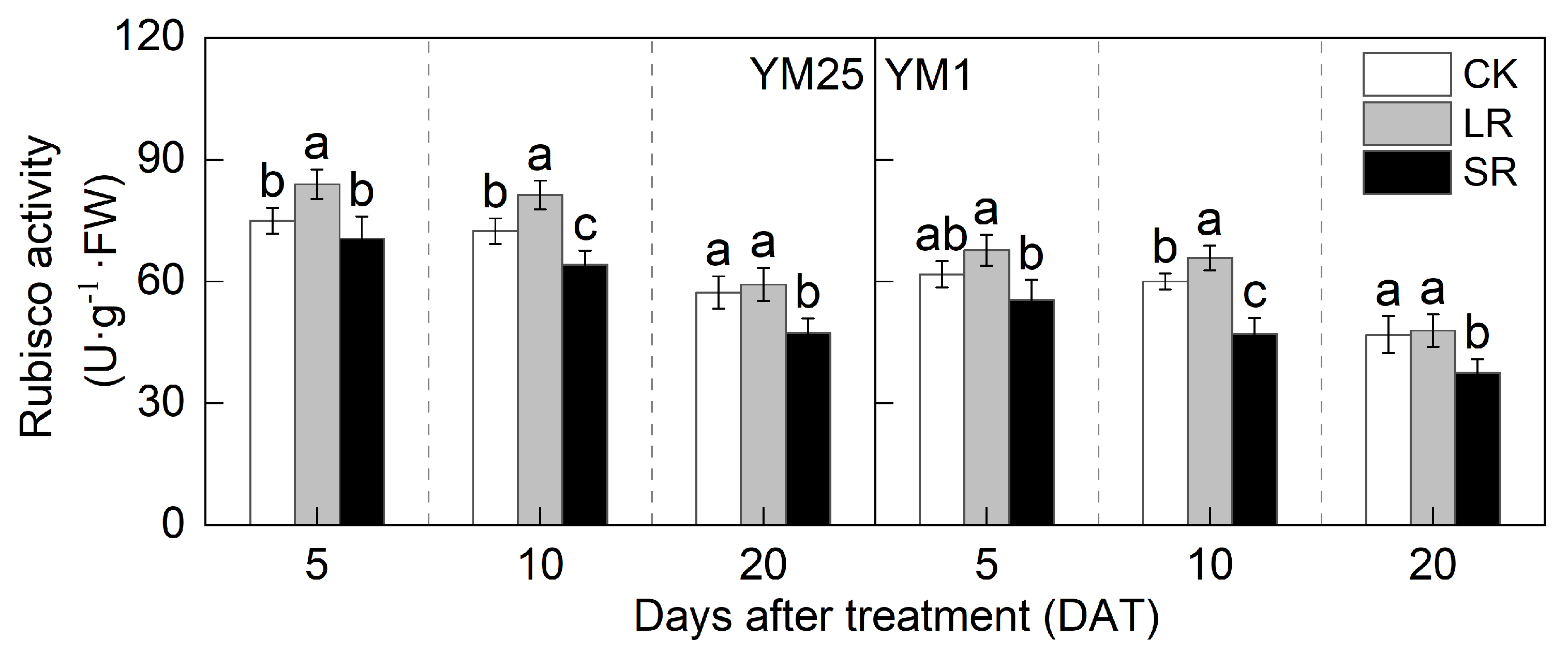
2.5. Rubisco Activity
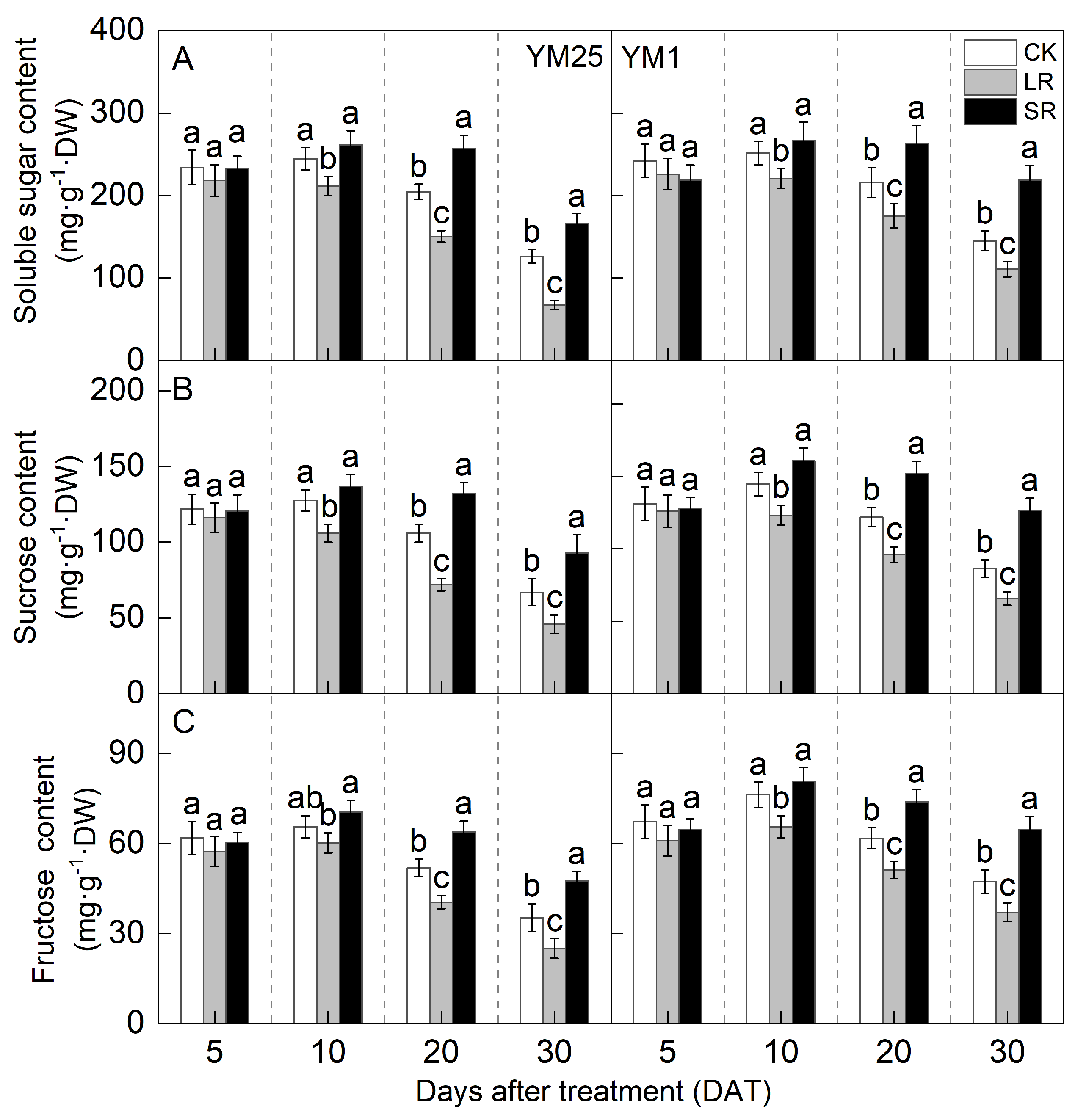
2.6. Soluble Sugar, Sucrose, and Fructose Contents
2.7. Enzyme Activity of Carbohydrate Metabolism
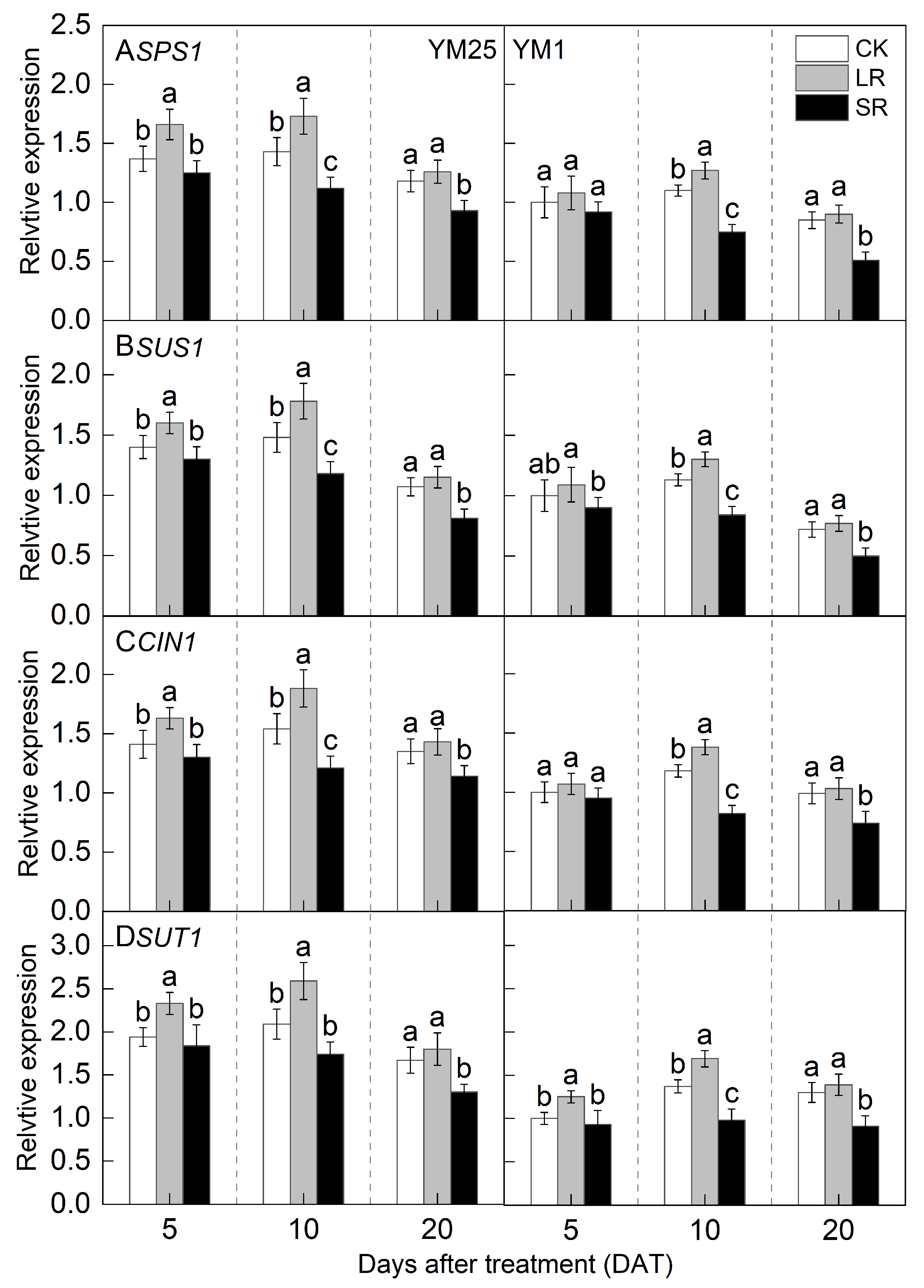
2.8. Relative Expression of SPS1, SUS1, CIN1, and SUT1
3. Discussion
4. Materials and Methods
4.1. Experiment Design
4.2. Yield and Its Components and Sink–Source Ratio
4.3. SPAD, Pn, and Chlorophyll Fluorescence Measurement
4.4. CE, Vcmax, Jmax, and VTPU
4.5. Rubisco Activity
4.6. Soluble Sugar, Sucrose, and Fructose Contents
4.7. Enzyme Activity of Carbohydrate Metabolism
4.8. qRT-PCR Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rempelos, L.; Almuayrifi, M.S.B.; Baranski, M.; Tetard-Jones, C.; Barkla, B.; Cakmak, I.; Ozturk, L.; Cooper, J.; Volakakis, N.; Hall, G.; et al. The effect of agronomic factors on crop health and performance of winter wheat varieties bred for the conventional and the low input farming sector. Field Crops Res. 2020, 254, 107822. [Google Scholar] [CrossRef]
- Alonso, M.P.; Abbate, P.E.; Mirabella, N.E.; Merlos, F.A.; Panelo, J.S.; Pontaroli, A.C. Analysis of sink/source relations in bread wheat recombinant inbred lines and commercial cultivars under a high yield potential environment. Eur. J. Agron. 2018, 93, 82–87. [Google Scholar] [CrossRef]
- Mason, T.G.; Maskell, E.J. Studies on the transport of carbohydrates in the cotton plant: II. The factors determining the rate and the direction of movement of sugars. Ann. Bot. 1928, 42, 571–636. [Google Scholar] [CrossRef]
- Xiao, Y.; Chang, T.; Song, Q.; Wang, S.; Tholen, D.; Wang, Y.; Xin, C.; Zheng, G.; Zhao, H.; Zhu, X.G. ePlant for quantitative and predictive plant science research in the big data era—Lay the foundation for the future model guided crop breeding, engineering and agronomy. Quant. Biol. 2017, 5, 260–271. [Google Scholar] [CrossRef]
- Krahmer, J.; Ganpudi, A.; Abbas, A.; Romanowski, A.; Halliday, K.J. Phytochrome, Carbon Sensing, Metabolism, and Plant Growth Plasticity. Plant Physiol. 2018, 176, 1039–1048. [Google Scholar] [CrossRef]
- Koper, K.; Hwang, S.K.; Singh, S.; Okita, T.W. Source-sink relationships and its effect on plant productivity: Manipulation of primary carbon and starch metabolism. In Genome Engineering for Crop Improvement; Springer: Cham, Switzerland, 2021; pp. 1–31. [Google Scholar] [CrossRef]
- Zhang, H.; Richards, R.; Riffkin, P.; Berger, J.; Christy, B.; O’Leary, G.; Acuna, T.B.; Merry, A. Wheat grain number and yield: The relative importance of physiological traits and source-sink balance in southern Australia. Eur. J. Agron. 2019, 110, 125935. [Google Scholar] [CrossRef]
- Chang, T.-G.; Zhu, X.-G. Source-sink interaction: A century old concept under the light of modern molecular systems biology. J. Exp. Bot. 2017, 68, 4417–4431. [Google Scholar] [CrossRef]
- Burnett, A.C.; Rogers, A.; Rees, M.; Osborne, C.P. Carbon source-sink limitations differ between two species with contrasting growth strategies. Plant Cell Environ. 2016, 39, 2460–2472. [Google Scholar] [CrossRef]
- Fernie, A.R.; Bachem, C.W.B.; Helariutta, Y.; Neuhaus, H.E.; Prat, S.; Ruan, Y.L.; Stitt, M.; Sweetlove, L.J.; Tegeder, M.; Wahl, V.; et al. Synchronization of developmental, molecular and metabolic aspects of source-sink interactions. Nat. Plants 2020, 6, 55–66. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, N.; Hong, J.; Zhang, Q.; Wang, C.; Xue, Q.; Zhou, S.; Huang, Q.; Wang, Z. Effect of source-sink manipulation on photosynthetic characteristics of flag leaf and the remobilization of dry mass and nitrogen in vegetative organs of wheat. J. Integr. Agric. 2014, 13, 1680–1690. [Google Scholar] [CrossRef]
- Asseng, S.; Kassie, B.T.; Labra, M.H.; Amador, C.; Calderini, D.F. Simulating the impact of source-sink manipulations in wheat. Field Crops Res. 2017, 202, 47–56. [Google Scholar] [CrossRef]
- Li, C.; Kraeutler, B. Facile retro-Dieckmann cleavage of a pink phyllobilin: New type of potential downstream steps of natural chlorophyll breakdown. Monatsh. Chem. 2022, 154, 1359–1368. [Google Scholar] [CrossRef]
- Xiao, L.W.; Miao, L.; Chao, S.L.; McHugh, A.D.; Ming, L.; Tao, X.; Yu, B.L.; Yong, L.T. Source-sink relations and responses to sink-source manipulations during grain filling in wheat. J. Integr. Agric. 2022, 21, 1593–1605. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Pellegrineschi, A.; Skovmand, B. Sink-limitation to yield and biomass: A summary of some investigations in spring wheat. Ann. Appl. Biol. 2005, 146, 39–49. [Google Scholar] [CrossRef]
- Alvaro, F.; Royo, C.; del Moral, L.F.G.; Villegas, D. Grain filling and dry matter translocation responses to source-sink modifications in a historical series of durum wheat. Crop Sci. 2008, 48, 1523–1531. [Google Scholar] [CrossRef]
- Rivera-Amado, C.; Trujillo-Negrellos, E.; Molero, G.; Reynolds, M.P.; Sylvester-Bradley, R.; Foulkes, M.J. Optimizing dry-matter partitioning for increased spike growth, grain number and harvest index in spring wheat. Field Crops Res. 2019, 240, 154–167. [Google Scholar] [CrossRef]
- Maydup, M.L.; Antonietta, M.; Guiamet, J.J.; Tambussi, E.A. The contribution of green parts of the ear to grain filling in old and modern cultivars of bread wheat (Triticum aestivum L.): Evidence for genetic gains over the past century. Field Crops Res. 2012, 134, 208–215. [Google Scholar] [CrossRef]
- Ali, M.A.; Hussain, M.; Khan, M.I.; Ali, Z.; Zulkiffal, M.; Anwar, J.; Sabir, W.; Zeeshan, M. Source-sink relationship between photosynthetic organs and grain yield attributes during grain filling stage in spring wheat (Triticum aestivum). Int. J. Agric. Biol. 2010, 12, 509–515. [Google Scholar] [CrossRef]
- Acreche, M.M.; Slafer, G.A. Grain weight, radiation interception and use efficiency as affected by sink-strength in Mediterranean wheats released from 1940 to 2005. Field Crops Res. 2009, 110, 98–105. [Google Scholar] [CrossRef]
- Serrago, R.A.; Alzueta, I.; Savin, R.; Slafer, G.A. Understanding grain yield responses to source-sink ratios during grain filling in wheat and barley under contrasting environments. Field Crops Res. 2013, 150, 42–51. [Google Scholar] [CrossRef]
- Nebauer, S.G.; Renau-Morata, B.; Guardiola, J.L.; Molina, R.V. Photosynthesis down-regulation precedes carbohydrate accumulation under sink limitation in Citrus. Tree Physiol. 2011, 31, 169–177. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Liu, N.; Su, D.; Xue, Q.; Stewart, B.A.; Wang, Z. Effect of source-sink manipulation on accumulation of micronutrients and protein in wheat grains. J. Plant Nutr. Soil Sci. 2012, 175, 622–629. [Google Scholar] [CrossRef]
- Wang, L.; Xia, H.; Li, X.; Qiao, Y.; Xue, Y.; Jiang, X.; Yan, W.; Liu, Y.; Xue, Y.; Kong, L. Source–sink manipulation affects accumulation of zinc and other nutrient elements in wheat grains. Plants 2021, 10, 1032. [Google Scholar] [CrossRef]
- Liang, X.G.; Chen, H.M.; Pan, Y.Q.; Wang, Z.W.; Huang, C.; Chen, Z.Y.; Tang, W.; Chen, X.M.; Shen, S.; Zhou, S.L. Yield more in the shadow: Mitigating shading-induced yield penalty of maize via optimizing source-sink carbon partitioning. Eur. J. Agron. 2025, 162, 127421. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, X.; Li, C.; Yang, W.; Huang, M.; Ma, X.; Li, S. Yield, growth, canopy traits and photosynthesis in high-yielding, synthetic hexaploid-derived wheats cultivars compared with non-synthetic wheats. Crop Pasture Sci. 2017, 68, 115–125. [Google Scholar] [CrossRef]
- Balkan, A.; Gentan, T.; Bilgin, O.J. Effect of removal of some photosynthetic organs on yield components in durum wheat (Triticum aestivum L.). Bangladesh J. Agric. Res. 2011, 36, 1–12. [Google Scholar] [CrossRef]
- Felekari, H.; Ghobadi, M.E.; Ghobadi, M.; Honarmand, S.J.; Saeidi, M.J. The effect of post anthesis source and sink limitation in wheat cultivars under moderate condition. Int. J. Biosci. 2014, 5, 52–59. [Google Scholar] [CrossRef]
- Chanishvili, S.S.; Badridze, G.S.; Barblishvili, T.F.; Dolidze, M.D. Defoliation, photosynthetic rates, and assimilate transport in grapevine plants. Russ. J. Plant Physiol. 2005, 52, 448–453. [Google Scholar] [CrossRef]
- Liu, J.; Ma, Y.; Lv, F.; Chen, J.; Zhou, Z.; Wang, Y.; Abudurezike, A.; Oosterhuis, D.M. Changes of sucrose metabolism in leaf subtending to cotton boll under cool temperature due to late planting. Field Crops Res. 2013, 144, 200–211. [Google Scholar] [CrossRef]
- Mahfoozi, S.; Jasemi, S.J. Study of the possibility of increasing grain yield by increasing grain weight in winter and facultative wheat genotypes with manipulating sink capacity. Iran. J. Crop Sci. 2010, 12, 76–84. [Google Scholar]
- Fabre, D.; Yin, X.; Dingkuhn, M.; Clement-Vidal, A.; Roques, S.; Rouan, L.; Soutiras, A.; Luquet, D. Is triose phosphate utilization involved in the feedback inhibition of photosynthesis in rice under conditions of sink limitation? J. Exp. Bot. 2019, 70, 5773–5785. [Google Scholar] [CrossRef]
- IIqbal, N.; Masood, A.; Khan, N.A. Analyzing the significance of defoliation in growth, photosynthetic compensation and source-sink relations. Photosynthetica 2012, 50, 161–170. [Google Scholar] [CrossRef]
- Xu, B.; You, C.; Ding, Y.; Wang, S. Effect of source-sink manipulation on translocation of carbohydrate and nitrogen, phosphors, potassium in vegetative organs of conventional Japonica rice after anthesis. Sci. Agric. Sin. 2016, 49, 643–656. [Google Scholar] [CrossRef]
- Luo, K.; Zeng, Y.; Hu, Q.; Chen, L.; Li, X. Effects of weak light stress at different stages on sink-source characteristics and protective enzyme activities in leaf of late rice varieties with different tolerance. Chin. J. Rice Sci. 2018, 32, 581. [Google Scholar] [CrossRef]
- Dong, S.; Beckles, D.M. Dynamic changes in the starch-sugar interconversion within plant source and sink tissues promote a better abiotic stress response. J. Plant Physiol. 2019, 234, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Körner, C.; Basel, M.L. Growth Controls Photosynthesis–Mostly. Nova Acta Leopold. 2013, 283, 273–283. [Google Scholar]
- Zhang, S.; Sun, J.; Lu, Y.; Yang, S.; Zhang, Y.; Chai, H.; Jiang, D.; Dai, T.; Tian, Z. Rubisco and sucrose synthesis and translocation are involved in the regulation of photosynthesis in wheat with different source-sink relationships. Physiol. Plant. 2024, 176, e14196. [Google Scholar] [CrossRef]
- Mu, H.; Jiang, D.; Wollenweber, B.; Dai, T.; Jing, Q.; Cao, W. Long-term low radiation decreases leaf photosynthesis, photochemical efficiency and grain yield in winter wheat. J. Agron. Crop Sci. 2010, 196, 38–47. [Google Scholar] [CrossRef]
- Shao, Y.; Li, S.; Gao, L.; Sun, C.; Hu, J.; Ullah, A.; Gao, J.; Li, X.; Liu, S.; Jiang, D.; et al. Magnesium application promotes rubisco activation and contributes to high-temperature stress alleviation in wheat during the grain filling. Front. Plant Sci. 2021, 12, 675582. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The Relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, Y.; Xu, X.; Shen, Q.; Guo, S. Light-saturated photosynthetic rate in high-nitrogen rice (Oryza sativa L.) leaves is related to chloroplastic CO2 concentration. J. Exp. Bot. 2009, 60, 2351–2360. [Google Scholar] [CrossRef]
- Gao, J.; Wang, F.; Sun, J.; Tian, Z.; Hu, H.; Jiang, S.; Luo, Q.; Xu, Y.; Jiang, D.; Cao, W.; et al. Enhanced Rubisco activation associated with maintenance of electron transport alleviates inhibition of photosynthesis under low nitrogen conditions in winter wheat seedlings. J. Exp. Bot. 2018, 69, 5477–5488. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Hu, J.; Tan, Q.; Hu, H.; Sun, C.; Lei, K.; Tian, Z.; Dai, T. Improved chloroplast Pi allocation helps sustain electron transfer to enhance photosynthetic low-phosphorus tolerance of wheat. Plant Physiol. Biochem. 2023, 201, 107880. [Google Scholar] [CrossRef]
- Fales, F.W. The assimilation and degradation of carbohydrates by yeast cells. J. Biol. Chem. 1951, 193, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Borsani, J.; Budde, C.O.; Porrini, L.; Lauxmann, M.A.; Lombardo, V.A.; Murray, R.; Andreo, C.S.; Drincovich, M.F.; Lara, M.V. Carbon metabolism of peach fruit after harvest: Changes in enzymes involved in organic acid and sugar level modifications. J. Exp. Bot. 2009, 60, 1823–1837. [Google Scholar] [CrossRef] [PubMed]
- Miron, D.; Schaffer, A.A. Sucrose phosphate synthase, sucrose synthase, and invertase activities in developing fruit of Lycopersicon esculentum Mill. and the sucrose accumulating Lycopersicon hirsutum Humb. and Bonpl. Plant Physiol. 1991, 95, 623–627. [Google Scholar] [CrossRef]
- Ren, L.P.; Sun, J.; Chen, S.M.; Gao, J.J.; Dong, B.; Liu, Y.N.; Xia, X.L.; Wang, Y.J.; Liao, Y.; Teng, N.J.; et al. A transcriptomic analysis of Chrysanthemum nankingense provides insights into the basis of low temperature tolerance. BMC Genom. 2014, 15, 844. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]




| Cultivars | Treatment | Grain Yield (kg·ha−1) | Kernels Per Spike | 1000-Kernel Weight (g) | Leaf Area (cm2) | Sink–Source Ratio (mg·cm−2) |
|---|---|---|---|---|---|---|
| 2021–2022 | ||||||
| YM25 | CK | 8581 a | 44.55 a | 44.52 b | 88.68 a | 22.36 b |
| LR | 7317 b | 42.56 b | 38.92 d | 58.44 c | 28.35 a | |
| SR | 5125 c | 23.87 e | 49.75 a | 90.54 a | 13.12 d | |
| YM1 | CK | 6967 b | 36.01 c | 38.41 d | 68.96 b | 20.06 c |
| LR | 5522 c | 33.05 d | 32.48 e | 45.71 d | 23.48 b | |
| SR | 3646 d | 17.54 f | 41.48 c | 70.28 b | 10.35 e | |
| F-value | FC | 224.28 ** | 331.25 ** | 181.25 ** | 76.73 ** | 40.49 ** |
| FT | 327.77 ** | 759.29 ** | 124.37 ** | 83.98 ** | 257.19 ** | |
| FC×T | 0.71 | 4.45 * | 1.70 | 1.46 | 2.28 | |
| 2022–2023 | ||||||
| YM25 | CK | 9292 a | 46.90 a | 42.76 b | 90.03 a | 22.28 b |
| LR | 8072 b | 43.40 b | 39.66 c | 58.54 c | 29.40 a | |
| SR | 5460 d | 23.53 e | 48.97 a | 87.01 a | 13.24 d | |
| YM1 | CK | 7792 b | 40.12 c | 37.58 d | 76.73 b | 19.65 c |
| LR | 6308 c | 36.44 d | 33.2 e | 51.54 d | 23.48 b | |
| SR | 4165 e | 20.18 f | 40.03 c | 76.40 b | 10.57 e | |
| F-value | FC | 160.28 ** | 84.48 ** | 182.53 ** | 24.64 ** | 51.12 ** |
| FT | 329.89 ** | 467.21 ** | 84.33 ** | 78.26 ** | 262.43 ** | |
| FC×T | 1.28 | 3.57 | 4.75 * | 0.77 | 4.34 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Chai, H.; Sun, J.; Zhang, Y.; Lu, Y.; Jiang, D.; Dai, T.; Tian, Z. Regulatory Effects of Source–Sink Manipulations on Photosynthesis in Wheat with Different Source–Sink Relationships. Plants 2025, 14, 1456. https://doi.org/10.3390/plants14101456
Zhang S, Chai H, Sun J, Zhang Y, Lu Y, Jiang D, Dai T, Tian Z. Regulatory Effects of Source–Sink Manipulations on Photosynthesis in Wheat with Different Source–Sink Relationships. Plants. 2025; 14(10):1456. https://doi.org/10.3390/plants14101456
Chicago/Turabian StyleZhang, Siqi, Huimin Chai, Jiawei Sun, Yafang Zhang, Yanhua Lu, Dong Jiang, Tingbo Dai, and Zhongwei Tian. 2025. "Regulatory Effects of Source–Sink Manipulations on Photosynthesis in Wheat with Different Source–Sink Relationships" Plants 14, no. 10: 1456. https://doi.org/10.3390/plants14101456
APA StyleZhang, S., Chai, H., Sun, J., Zhang, Y., Lu, Y., Jiang, D., Dai, T., & Tian, Z. (2025). Regulatory Effects of Source–Sink Manipulations on Photosynthesis in Wheat with Different Source–Sink Relationships. Plants, 14(10), 1456. https://doi.org/10.3390/plants14101456





