Self-Pollinated Types and Ecological Adaptations of the Desert Plant Gymnocarpos przewalskii
Abstract
1. Introduction
2. Results
2.1. Floral Traits, Flowering Progression, and P/O Ratio
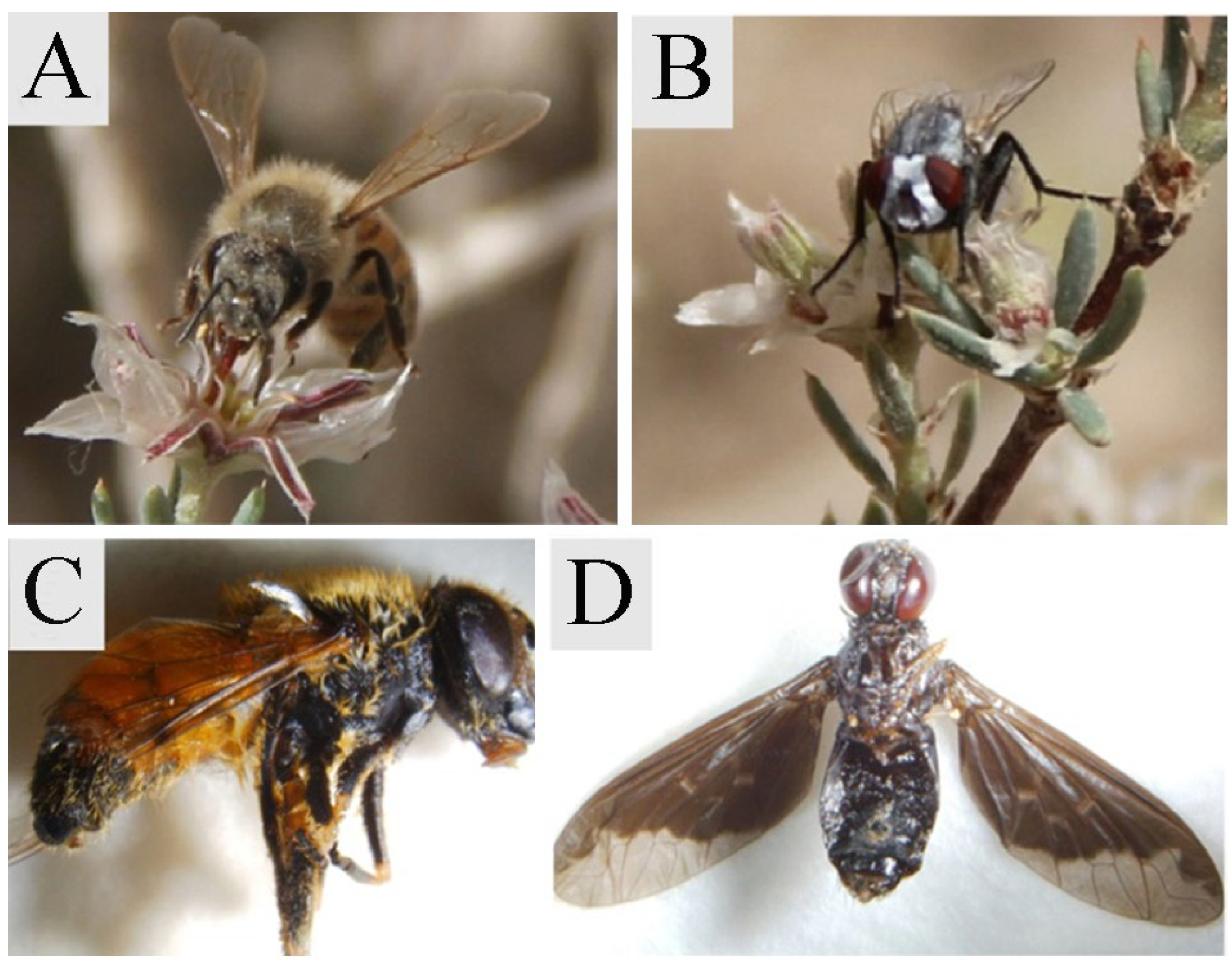
2.2. Bagging Experiment and Pollinator Observation
2.3. Biological Characterization of Bagged Fruiting Seeds and Seedlings
2.3.1. Seed Size and Germination Characteristics
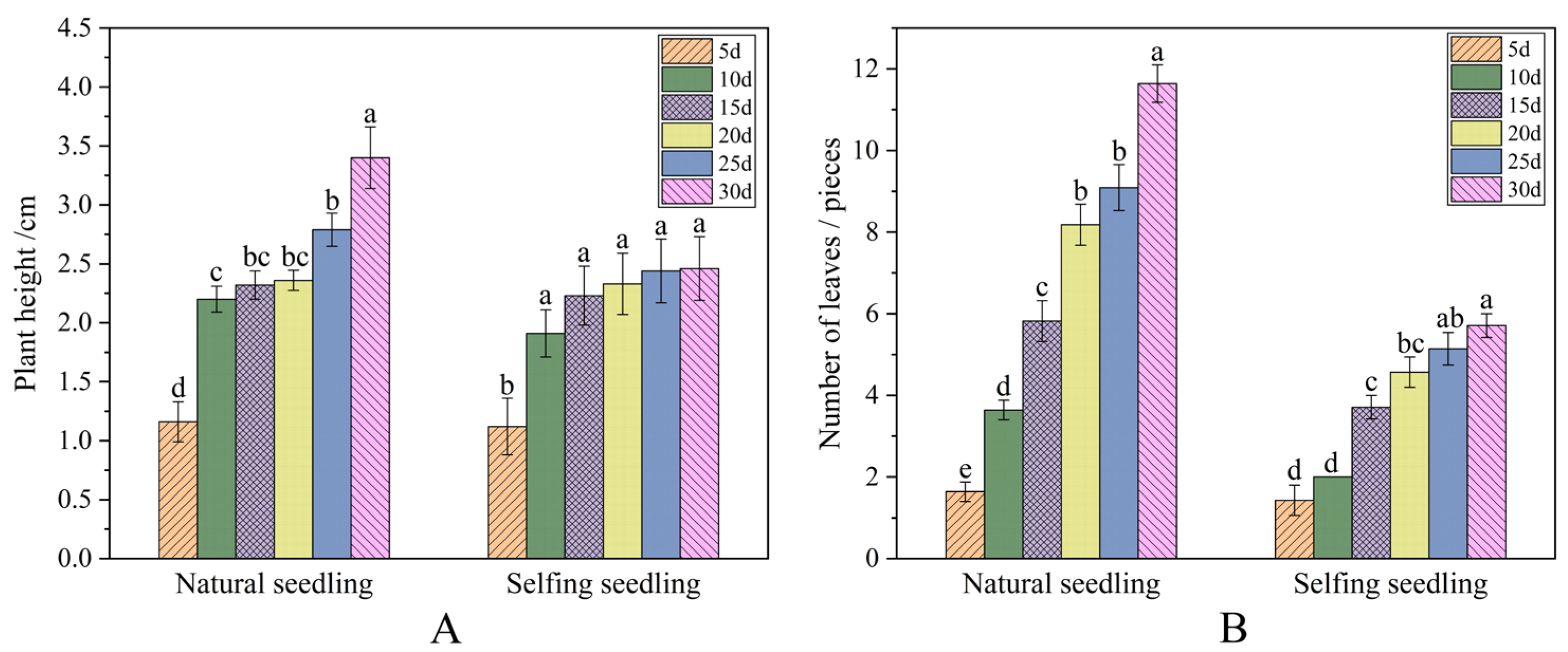
2.3.2. Seedling Growth
3. Discussion
3.1. Floral Traits and Flowering Progression
3.2. Bagging Experiments and Pollinators
3.3. Seed Germination and Seedling Growth
4. Materials and Methods
4.1. Study Site
4.2. Floral Traits, Flowering Progression, and P/O Ratio
4.2.1. Observation of Flower Morphology and Flowering Progression
4.2.2. Determination of P/O Ratio
4.3. Bagging Experiment and Observation of Pollinators
4.4. Seed Size, Germination Characteristics, and Seedling Growth Experiments
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X. Invariables and variables in the evolution of plant reproduction. Biol. Divers. 2024, 1, 75–82. [Google Scholar] [CrossRef]
- Schoen, D.J.; Johnson, M.T.J.; Wright, S.I. The ecology, evolution, and genetics of plant reproductive systems. New Phytol. 2019, 224, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Barrett, S.C. Understanding plant reproductive diversity. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2010, 365, 99–109. [Google Scholar] [CrossRef]
- Theologidis, I.; Chelo, I.M.; Goy, C.; Teotónio, H. Reproductive assurance drives transitions to self-fertilization in experimental Caenorhabditis elegans. BMC Biol. 2014, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Dávila, R.; Martén-Rodríguez, S. A test of the reproductive assurance hypothesis in Ipomoea hederacea: Does inbreeding depression counteract the benefits of self-pollination? Am. J. Bot. 2021, 108, 2162–2173. [Google Scholar] [CrossRef]
- Schoen, D.J.; Morgan, M.T.; Bataillon, T. How does self-pollination evolve? Inferences from floral ecology and molecular genetic variation. Phil. Trans. R. Soc. Lond. B 1996, 351, 1281–1290. [Google Scholar] [CrossRef]
- Gianella, M.; Bradford, K.J.; Guzzon, F. Ecological, (epi)genetic and physiological aspects of bet-hedging in angiosperms. Plant Reprod. 2021, 34, 21–36. [Google Scholar] [CrossRef]
- Falik, O.; Hoffmann, I.; Novoplansky, A. A novel type of neighbour perception elicits reproductive plasticity in an annual plant with a mixed mating system. Plant Biol. 2024, 26, 415–420. [Google Scholar] [CrossRef]
- Lloyd, D.G. Some Reproductive Factors Affecting the Selection of Self-Fertilization in Plants. Am. Nat. 1979, 113, 67–79. [Google Scholar] [CrossRef]
- Brys, R.; Cauwenberghe, J.V.; Jacquemyn, H. The importance of autonomous selfing in preventing hybridization in three closely related plant species. J. Ecol. 2016, 104, 601–610. [Google Scholar] [CrossRef]
- Lloyd, D.G.; Schoen, D.J. Self-and cross-fertilization in plants. I. Functional dimensions. Int. J. Plant Sci. 1992, 153, 358–369. [Google Scholar] [CrossRef]
- Duan, Y.W.; Dafni, A.; Hou, Q.Z.; He, Y.P.; Liu, J.Q. Delayed selfing in an alpine biennial Gentianopsis paludosa (Gentianaceae) in the Qinghai-Tibetan plateau. J. Integr. Plant Biol. 2010, 52, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Goodwillie, C.; Weber, J.J. The best of both worlds? A review of delayed selfing in flowering plants. Am. J. Bot. 2018, 105, 641–655. [Google Scholar] [CrossRef]
- Kalisz, S.; Vogler, D.; Fails, B.; Finer, M.; Shepard, E.; Herman, T.; Gonzales, R. The mechanism of delayed selfing in Collinsia verna (Scrophulariaceae). Am. J. Bot. 1999, 86, 1239–1247. [Google Scholar] [CrossRef]
- Núñez-Hidalgo, S.; Cascante-Marín, A. Selfing in epiphytic bromeliads compensates for the limited pollination services provided by nectarivorous bats in a neotropical montane forest. AoB Plants 2024, 16, plae011. [Google Scholar] [CrossRef]
- Dole, J.A. REPRODUCTIVE ASSURANCE MECHANISMS IN THREE TAXA OF THE MIMULUS GUTTATUS COMPLEX (SCROPHULARIACEAE). Am. J. Bot. 1992, 79, 650–659. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, S.; Su, Y.; Hu, X.; Shen, Y.; Zhao, D. A novel case of autogamy and cleistogamy in Dendrobium wangliangii: A rare orchid distributed in the dry-hot valley. Ecol. Evol. 2019, 9, 12906–12914. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Wang, Q.; Jia, S.-W. Genomic Phylogeography of Gymnocarpos przewalskii (Caryophyllaceae): Insights into Habitat Fragmentation in Arid Northwestern China. Diversity 2020, 12, 335. [Google Scholar] [CrossRef]
- Li, M.; He, C.; Wei, M.; Long, J.; Wang, J.; Yang, X.; Wang, K.; He, X. Temporal and spatial dynamics and functional metabolism of dark septate endophytes of Gymnocarpos przewalskii Maxim. in Northwest Desert, China. Appl. Soil Ecol. 2024, 194, 105194. [Google Scholar] [CrossRef]
- Li, M.; He, C.; Gong, F.; Zhou, X.; Wang, K.; Yang, X.; He, X. Seasonal and soil compartmental responses of soil microbes of Gymnocarpos przewalskii in a hyperarid desert. Appl. Soil Ecol. 2024, 200, 105447. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, K.; Sun, X.; He, X. Dynamics of arbuscular mycorrhizal fungi and glomalin in the rhizosphere of Gymnocarpos przewalskii in Northwest Desert, China. Appl. Soil Ecol. 2022, 170, 104251. [Google Scholar] [CrossRef]
- Jia, S.W.; Zhang, M.L. Pleistocene climate change and phylogeographic structure of the Gymnocarpos przewalskii (Caryophyllaceae) in the northwest China: Evidence from plastid DNA, ITS sequences, and Microsatellite. Ecol. Evol. 2019, 9, 5219–5235. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Cui, P.; Lu, G.; Wang, X.; Jiang, L.; Luo, Y. Photosynthetic and Antioxidant Responses of Gymnocarpos przewalskii to Simulated Rainfall Changes. Forests 2023, 14, 789. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Pan, L.; Fu, C. Characterization of the complete chloroplast genome of Gymnocarpos przewalskii, an endangered species in China and Mongolia. Conserv. Genet. Resour. 2018, 10, 717–721. [Google Scholar] [CrossRef]
- Qi, J.; Luo, Y.; Huang, H.; Lu, S.; Zhao, F.; Deng, Z.; Qiu, Y. Molecular Mechanism of Response and Adaptation of Antioxidant Enzyme System to Salt Stress in Leaves of Gymnocarpos przewalskii. Plants 2023, 12, 3370. [Google Scholar] [CrossRef]
- Fu, R.; Zhu, Y.; Liu, Y.; Yang, Z.; Lu, R.; Qiu, Y.; Lascoux, M.; Li, P.; Chen, J. Shared xerophytic genes and their re-use in local adaptation to aridity in the desert plant Gymnocarpos przewalskii. Mol. Ecol. 2024, 33, e17380. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, Y.; Li, Z. The Leaf blade Anatomical structures and Their Ecological Adaptability of Gymnocarpos przewalskii Maxim. J. Anhui Agric. Sci. 2011, 39, 3929–3931. [Google Scholar]
- Ma, S.M.; Zhang, M.L.; Sanderson, S.C. Phylogeography of the rare Gymnocarpos przewalskii (Caryophyllaceae): Indications of multiple glacial refugia in north-western China. Aust. J. Bot. 2012, 60, 20–31. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, M. Phylogeography and conservation genetics of the relic Gymnocarpos przewalskii (Caryophyllaceae) restricted to northwestern China. Conserv. Genet. 2012, 13, 1531–1541. [Google Scholar] [CrossRef]
- Li, X.R.; Tang, X.; Fu, W.J. Floral syndrome and breeding system of Gymnocarpos przewalskii Maxim. Chin. J. Ecol. 2016, 35, 2592–2598. [Google Scholar] [CrossRef]
- Wang, Z.B.; Gao, Q.X.; Sun, J.Z.; Ma, Q. Study on Biological Characteristics of Rare Endangered Plant Gymnocarpos Przewalskii. Resour. Dev. Mark. 2009, 25, 4. [Google Scholar]
- Mao, J.; Tang, Q.; Wu, H.; Chen, Y. Transcriptome Remodeling in Arabidopsis: A Response to Heterologous Poplar MSL-lncRNAs Overexpression. Plants 2024, 13, 2906. [Google Scholar] [CrossRef]
- Shen, S.; Ma, S.; Liu, Y.; Liao, S.; Li, J.; Wu, L.; Kartika, D.; Mock, H.P.; Ruan, Y.L. Cell Wall Invertase and Sugar Transporters Are Differentially Activated in Tomato Styles and Ovaries During Pollination and Fertilization. Front. Plant Sci. 2019, 10, 506. [Google Scholar] [CrossRef]
- Nepal, S.; Trunschke, J.; Ren, Z.X.; Burgess, K.S.; Wang, H. Community-wide patterns in pollen and ovule production, their ratio (P/O), and other floral traits along an elevation gradient in southwestern China. BMC Plant Biol. 2023, 23, 425. [Google Scholar] [CrossRef]
- Pías, B.; Guitián, P. Flowering phenology and pollen-to-ovule ratio in coastal dune communities near Eurosiberian-Mediterranean border in the NW Iberian peninsula. Flora 2001, 196, 475–482. [Google Scholar] [CrossRef]
- Cruden, R.W. Pollen grains: Why so many? Plant Syst. Evol. 2000, 222, 143–165. [Google Scholar] [CrossRef]
- Etcheverry, A.V.; Alemán, M.M.; Figueroa-Fleming, T.; López-Spahr, D.; Gómez, C.A.; Yáñez, C.; Figueroa-Castro, D.M.; Ortega-Baes, P. Pollen: Ovule ratio and its relationship with other floral traits in Papilionoideae (Leguminosae): An evaluation with Argentine species. Plant Biol. 2012, 14, 171–178. [Google Scholar] [CrossRef]
- Dobeš, C.; Milosevic, A.; Prohaska, D.; Scheffknecht, S.; Sharbel, T.F.; Hülber, K. Reproductive differentiation into sexual and apomictic polyploid cytotypes in Potentilla puberula (Potentilleae, Rosaceae). Ann. Bot. 2013, 112, 1159–1168. [Google Scholar] [CrossRef]
- Surina, B.; Balant, M.; Glasnović, P.; Gogala, A.; Fišer, Ž.; Satovic, Z.; Liber, Z.; Radosavljević, I.; Classen-Bockhoff, R. Lack of pollinators selects for increased selfing, restricted gene flow and resource allocation in the rare Mediterranean sage Salvia brachyodon. Sci. Rep. 2024, 14, 5017. [Google Scholar] [CrossRef]
- Cruden, R.W. Pollen-Ovule Ratios: A Conservative Indicator of Breeding Systems in Flowering Plants. Evol. Int. J. Org. Evol. 1977, 31, 32–46. [Google Scholar] [CrossRef]
- Li, D.F.; Yan, X.C.; Lin, Y.; Wang, L.; Wang, Q. Do flowers removed of either nectar or pollen attract fewer bumblebee pollinators? An experimental test in Impatiens oxyanthera. AoB Plants 2021, 13, plab029. [Google Scholar] [CrossRef]
- Yamasaki, E.; Kawakita, A.; Sakai, S. Modified leaves with disk-shaped nectaries of Macaranga sinensis (Euphorbiaceae) provide reward for pollinators. Am. J. Bot. 2013, 100, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhou, Y.; Zhang, J.; Peng, Y.; Yang, X.; Hao, Z.; Lu, Y.; Wu, W.; Cheng, T.; Shi, J.; et al. Integrative analysis of transcriptome and proteome revealed nectary and nectar traits in the plant-pollinator interaction of Nitraria tangutorum Bobrov. BMC Plant Biol. 2021, 21, 230. [Google Scholar] [CrossRef]
- Zhao, Z.; Lu, N.; Conner, J.K. Adaptive pattern of nectar volume within inflorescences: Bumblebee foraging behavior and pollinator-mediated natural selection. Sci. Rep. 2016, 6, 34499. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, M.L.; Cohen, J.I. Phylogeographic History of Atraphaxis Plants in Arid Northern China and the Origin of A. bracteata in the Loess Plateau. PLoS ONE 2016, 11, e0163243. [Google Scholar] [CrossRef] [PubMed]
- Moody-Weis, J.M.; Heywood, J.S. Pollination limitation to reproductive success in the Missouri evening primrose, Oenothera macrocarpa (Onagraceae). Am. J. Bot. 2001, 88, 1615–1622. [Google Scholar] [CrossRef]
- Fischer, M.; Matthies, D. Mating structure and inbreeding and outbreeding depression in the rare plant Gentianella germanica (Gentianaceae). Am. J. Bot. 1997, 84, 1685. [Google Scholar] [CrossRef]
- Zurita-Silva, A.; Fuentes, F.; Zamora, P.; Jacobsen, S.E.; Schwember, A.R. Breeding quinoa (Chenopodium quinoa Willd.): Potential and perspectives. Mol. Breed. 2014, 34, 13–30. [Google Scholar] [CrossRef]
- del Pozo, A.; Ruf, K.; Alfaro, C.; Zurita, A.; Guerra, F.; Sagredo, B. Traits associated with higher productivity and resilience to drought-prone Mediterranean environments of coastal-lowland quinoa (Chenopodium quinoa Willd.). Field Crops Res. 2023, 299, 108985. [Google Scholar] [CrossRef]
- Paudel, B.R.; Shrestha, M.; Dyer, A.G.; Li, Q.J. Ginger and the beetle: Evidence of primitive pollination system in a Himalayan endemic alpine ginger (Roscoea alpina, Zingiberaceae). PLoS ONE 2017, 12, e0180460. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Li, Q.J. Autonomous selfing provides reproductive assurance in an alpine ginger Roscoea schneideriana (Zingiberaceae). Ann. Bot. 2008, 102, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Schouppe, D.; Brys, R.; Vallejo-Marin, M.; Jacquemyn, H. Geographic variation in floral traits and the capacity of autonomous selfing across allopatric and sympatric populations of two closely related Centaurium species. Sci. Rep. 2017, 7, 46410. [Google Scholar] [CrossRef] [PubMed]
- Brys, R.; Jacquemyn, H. Variation in the functioning of autonomous self-pollination, pollinator services and floral traits in three Centaurium species. Ann. Bot. 2011, 107, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Mamut, J.; Huang, D.H.; Qiu, J.; Tan, D.Y. Stamen curvature and temporal flower closure assure reproductive success in an early-spring-flowering perennial in the cold desert of Middle Asia. J. Plant Res. 2023, 136, 33–45. [Google Scholar] [CrossRef]
- Vickery, R.K. How Does Mimulus verbenaceus (Phrymaceae) Set Seed in the Absence of Pollinators? Evol. Biol. 2008, 35, 199–207. [Google Scholar] [CrossRef]
- Carleial, S.; van Kleunen, M.; Stift, M. Relatively weak inbreeding depression in selfing but also in outcrossing populations of North American Arabidopsis lyrata. J. Evol. Biol. 2017, 30, 1994–2004. [Google Scholar] [CrossRef]
- Tsuchimatsu, T.; Fujii, S. The selfing syndrome and beyond: Diverse evolutionary consequences of mating system transitions in plants. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2022, 377, 20200510. [Google Scholar] [CrossRef]
- Moeller, D.A.; Geber, M.A. Ecological context of the evolution of self-pollination in Clarkia xantiana: Population size, plant communities, and reproductive assurance. Evol. Int. J. Org. Evol. 2005, 59, 786–799. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Deng, C.; Zhu, R. Evidence for enhanced aridity in the Tarim Basin of China since 5.3 Ma. Quat. Sci. Rev. 2008, 27, 1012–1023. [Google Scholar] [CrossRef]
- Yu, S.X.; Jiang, Y.T.; Lin, W.H. Ovule initiation: The essential step controlling offspring number in Arabidopsis. J. Integr. Plant Biol. 2022, 64, 1469–1486. [Google Scholar] [CrossRef]
- Chen, L.N.; Cui, Y.Z.; Wong, K.M.; Li, D.Z.; Yang, H.Q. Breeding system and pollination of two closely related bamboo species. AoB Plants 2017, 9, plx021. [Google Scholar] [CrossRef]
- Hirayama, K.; Ishida, K.; Tomaru, N. Effects of pollen shortage and self-pollination on seed production of an endangered tree, Magnolia stellata. Ann. Bot. 2005, 95, 1009–1015. [Google Scholar] [CrossRef]
- Salas-Arcos, L.; Lara, C.; Ornelas, J.F. Reproductive biology and nectar secretion dynamics of Penstemon gentianoides (Plantaginaceae): A perennial herb with a mixed pollination system? PeerJ 2017, 5, e3636. [Google Scholar] [CrossRef]
- Barrett, S.C. Sexual interference of the floral kind. Heredity 2002, 88, 154–159. [Google Scholar] [CrossRef]
- Katsuhara, K.R.; Ushimaru, A.; Miyazaki, Y. Does a coexisting congener of a mixed mating species affect the genetic structure and selfing rate via reproductive interference? Oecologia 2024, 206, 37–45. [Google Scholar] [CrossRef]
- Cisternas-Fuentes, A.; Jogesh, T.; Broadhead, G.T.; Raguso, R.A.; Skogen, K.A.; Fant, J.B. Evolution of selfing syndrome and its influence on genetic diversity and inbreeding: A range-wide study in Oenothera primiveris. Am. J. Bot. 2022, 109, 789–805. [Google Scholar] [CrossRef]
- Cheptou, P.O. Does the evolution of self-fertilization rescue populations or increase the risk of extinction? Ann. Bot. 2019, 123, 337–345. [Google Scholar] [CrossRef]
- Han, T.; Wang, F.; Song, Q.; Ye, W.; Liu, T.; Wang, L.; Chen, Z.J. An epigenetic basis of inbreeding depression in maize. Sci. Adv. 2021, 7, eabg5442. [Google Scholar] [CrossRef]
- Porcher, E.; Lande, R. Inbreeding depression under mixed outcrossing, self-fertilization and sib-mating. BMC Evol. Biol. 2016, 16, 105. [Google Scholar] [CrossRef] [PubMed]
- Ahlinder, J.; Giles, B.E.; García-Gil, M.R. Life stage-specific inbreeding depression in long-lived Pinaceae species depends on population connectivity. Sci. Rep. 2021, 11, 8834. [Google Scholar] [CrossRef] [PubMed]
- Brandvain, Y.; Thomson, L.; Pyhäjärvi, T. Early-acting inbreeding depression can evolve as an inbreeding avoidance mechanism. Proc. Biol. Sci. 2024, 291, 20232467. [Google Scholar] [CrossRef]
- Cheptou, P.O. The evolutionary ecology of inbreeding depression in wild plant populations and its impact on plant mating systems. Front. Plant Sci. 2024, 15, 1359037. [Google Scholar] [CrossRef]
- Wright, S.I.; Kalisz, S.; Slotte, T. Evolutionary consequences of self-fertilization in plants. Proc. Biol. Sci. 2013, 280, 20130133. [Google Scholar] [CrossRef]
- Lu, J.; Yi, H.; Tan, D.; Baskin, C.C.; Baskin, J.M. Germination of Seeds from Flowers along a Continuum of Long to Short Styles in the Cold Desert Perennial Herb Ixiolirion songaricum. Plants 2022, 11, 1452. [Google Scholar] [CrossRef]
- Xu, Y.W.; Sun, L.; Ma, R.; Gao, Y.Q.; Sun, H.; Song, B. Does pollinator dependence decrease along elevational gradients? Plant Divers. 2023, 45, 446–455. [Google Scholar] [CrossRef]
- Sheridan, M.P.; Karowe, N.D. Inbreeding, outbreeding, and heterosis in the yellow pitcher plant, Sarracenia flava (Sarraceniaceae), in Virginia. Am. J. Bot. 2000, 87, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Donohue, K. Effects of inbreeding on traits that influence dispersal and progeny density in Cakile edentula var. lacustris (Brassicaceae). Am. J. Bot. 1998, 85, 661. [Google Scholar] [CrossRef]
- Snell, R.; Aarssen, L.W. Life history traits in selfing versus outcrossing annuals: Exploring the ‘time-limitation’ hypothesis for the fitness benefit of self-pollination. BMC Ecol. 2005, 5, 2. [Google Scholar] [CrossRef]
- Hanschen, E.R.; Herron, M.D.; Wiens, J.J.; Nozaki, H.; Michod, R.E. Repeated evolution and reversibility of self-fertilization in the volvocine green algae. Evol. Int. J. Org. Evol. 2018, 72, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.D.; Sebbenn, A.M.; Ferreira, R.R.; Lee, T.S.; Figueira, A. Jatropha curcas L. (Euphorbiaceae) exhibits a mixed mating system, high correlated mating and apomixis. Tree Genet. Genomes 2013, 9, 1089–1097. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, L.L.; Yang, Y.P.; Duan, Y.W. Change in floral orientation in Anisodus luridus (Solanaceae) protects pollen grains and facilitates development of fertilized ovules. Am. J. Bot. 2010, 97, 1618–1624. [Google Scholar] [CrossRef]
- He, J.; Dong, T.; Huang, K.; Yang, Y.; Li, D.; Xu, X.; He, X. Sex-specific floral morphology, biomass, and phytohormones associated with altitude in dioecious Populus cathayana populations. Ecol. Evol. 2017, 7, 3976–3986. [Google Scholar] [CrossRef]
- Yang, Z.P.; Liang, J.Y.; Chai, X.P.; Xue, W.T. Influence of Environmental Factors on Seed Germination of Endangered Plant Gymnocarpos przewalskii. J. Southwest For. Univ. Nat. Sci. 2017, 37, 49–53. (In Chinese) [Google Scholar]
- Cheng, X.; Yao, H.; Cheng, Z.; Tian, B.; Gao, C.; Gao, W.; Yan, S.; Cao, J.; Pan, X.; Lu, J.; et al. The Wheat Gene TaVQ14 Confers Salt and Drought Tolerance in Transgenic Arabidopsis thaliana Plants. Front. Plant Sci. 2022, 13, 870586. [Google Scholar] [CrossRef]
- Vandelook, F.; Newton, R.J.; Bobon, N.; Bohley, K.; Kadereit, G. Evolution and ecology of seed internal morphology in relation to germination characteristics in Amaranthaceae. Ann. Bot. 2021, 127, 799–811. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, L.; Wang, L.X. Response of seed germination to sowing depth in Gymnocarpos przewalskii. Rural. Econ. Sci. Technol. 2020, 31, 26–27. (In Chinese) [Google Scholar]
- Guo, Z.; Wang, H.; Yao, J.; Cheng, Y.; Zhang, W.; Xu, Z.; Li, M.; Huang, J.; Zhao, M. Quantitative Trait Loci Mapping Analysis for Cold Tolerance Under Cold Stress and Brassinosteroid-Combined Cold Treatment at Germination and Bud Burst Stages in Rice. Front. Plant Sci. 2022, 13, 938339. [Google Scholar] [CrossRef]





| Pollination Treatment | Fruit Number | Seed Number | Fruit Setting Rate/% |
|---|---|---|---|
| artificial cross-pollination | 192 | 59 | 30.42 |
| natural pollination | 4000 | 276 | 6.90 |
| self-pollination | 433 | 18 | 4.43 |
| parthenogenesis | 188 | 0 | 0.00 |
| Pollination Treatment | Seed Length/mm | Seed Width/mm | Single Seed Weight/g | Mean Germination Time/h | Germination Rate/% |
|---|---|---|---|---|---|
| artificial cross-pollination | 1.77 ± 0.031 a | 1.45 ± 0.038 a | 2.36 × 10−3 ± 0.050 × 10−3 a | 32.14 | 94.64 |
| natural pollination | 1.75 ± 0.018 a | 1.22 ± 0.011 c | 1.72 × 10−3 ± 0.042 × 10−3 b | 47.42 | 92.22 |
| self-pollination | 1.75 ± 0.015 a | 1.32 ± 0.025 b | 1.50 × 10−3 ± 0.052 × 10−3 c | 38.40 | 83.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, J.; Chai, X.; Zhao, X.; Yang, Z. Self-Pollinated Types and Ecological Adaptations of the Desert Plant Gymnocarpos przewalskii. Plants 2025, 14, 1410. https://doi.org/10.3390/plants14101410
Jian J, Chai X, Zhao X, Yang Z. Self-Pollinated Types and Ecological Adaptations of the Desert Plant Gymnocarpos przewalskii. Plants. 2025; 14(10):1410. https://doi.org/10.3390/plants14101410
Chicago/Turabian StyleJian, Jiaxin, Xueping Chai, Xiaonan Zhao, and Zhaoping Yang. 2025. "Self-Pollinated Types and Ecological Adaptations of the Desert Plant Gymnocarpos przewalskii" Plants 14, no. 10: 1410. https://doi.org/10.3390/plants14101410
APA StyleJian, J., Chai, X., Zhao, X., & Yang, Z. (2025). Self-Pollinated Types and Ecological Adaptations of the Desert Plant Gymnocarpos przewalskii. Plants, 14(10), 1410. https://doi.org/10.3390/plants14101410


.png)



