A Novel Approach for Comparing Selected Metabolites in Citrus Leaves and Fruits Across Datasets
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Datasets and Selection Criteria Used
2.2. Leaf Dataset
2.3. Log-Ratios of Two Metabolites Under Analysis
2.4. Statistical Methods
2.4.1. Standard Quantitative Methods
2.4.2. Single Log-Ratio Analysis
2.4.3. Analysis of Multiple Log-Ratios Between Leaves and Fruits
3. Results and Discussion
3.1. Expected Similar Metabolites
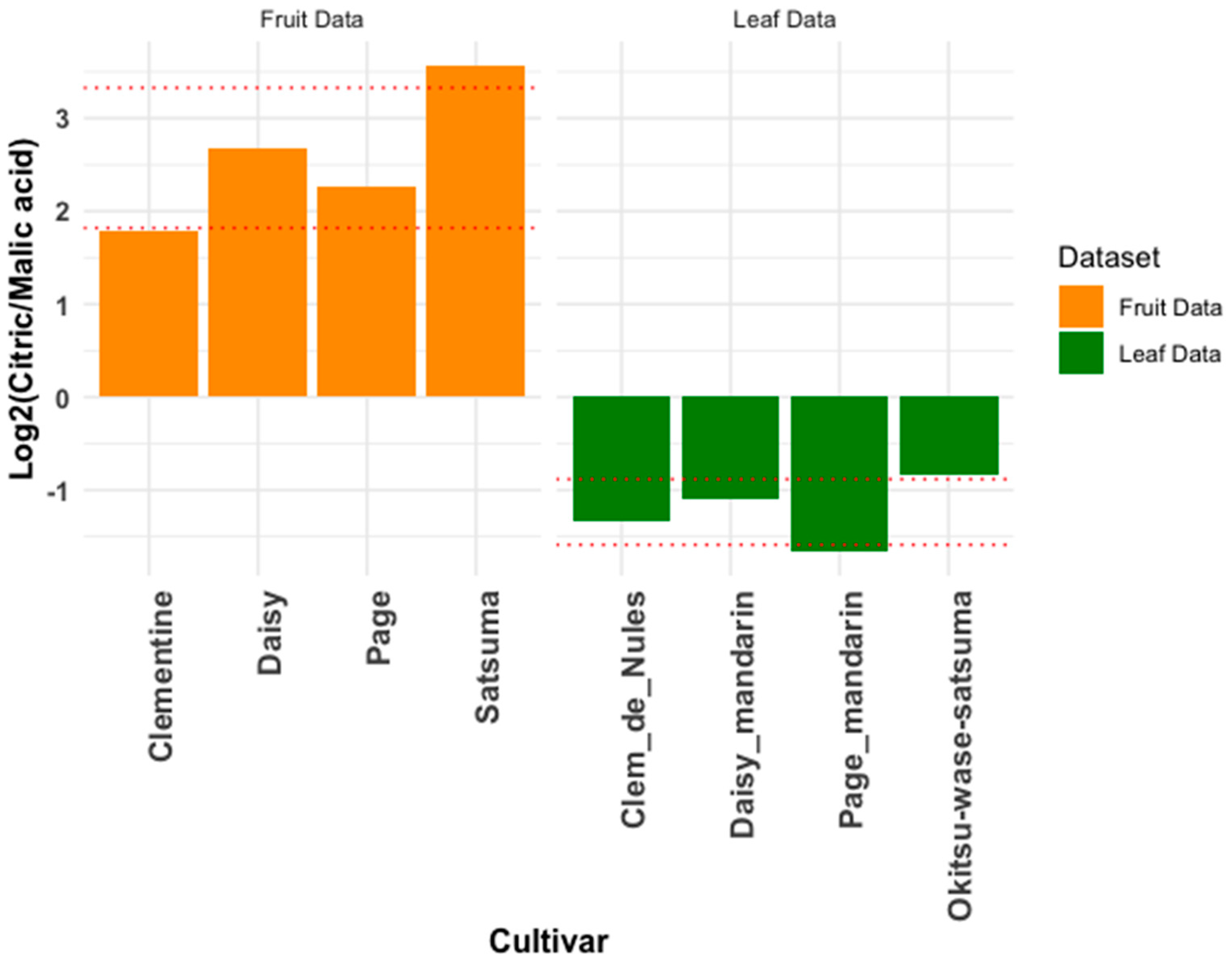
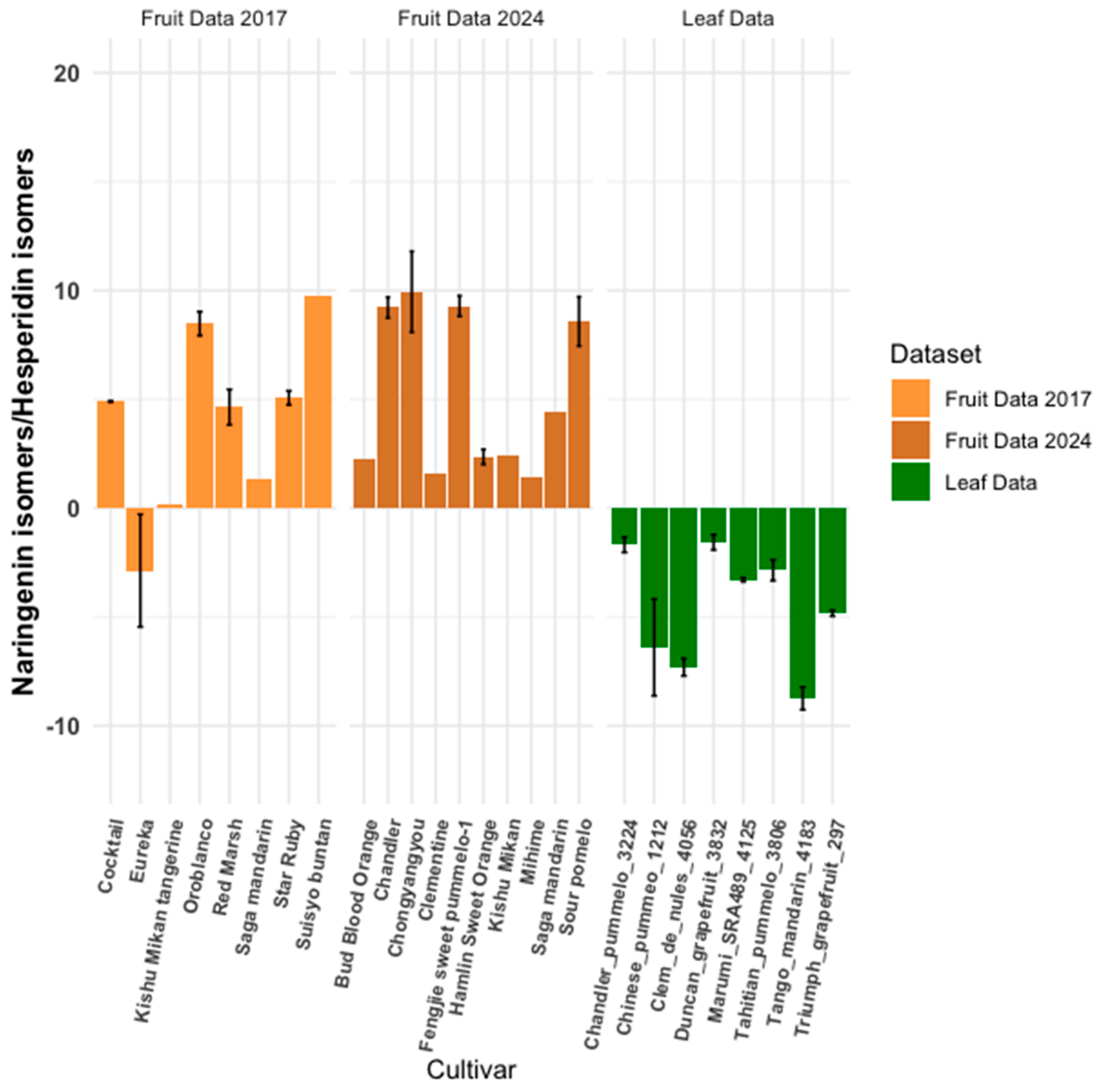
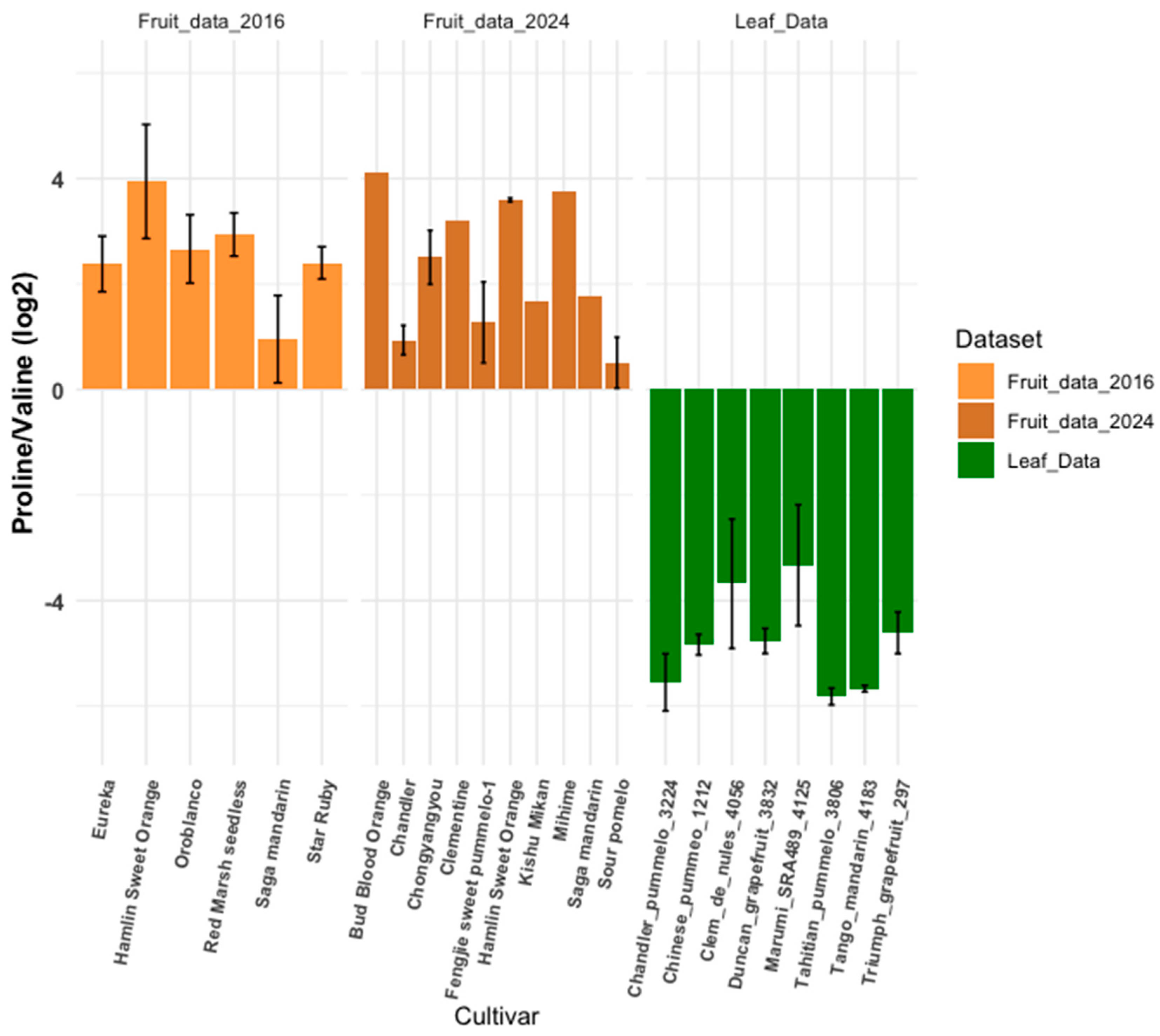
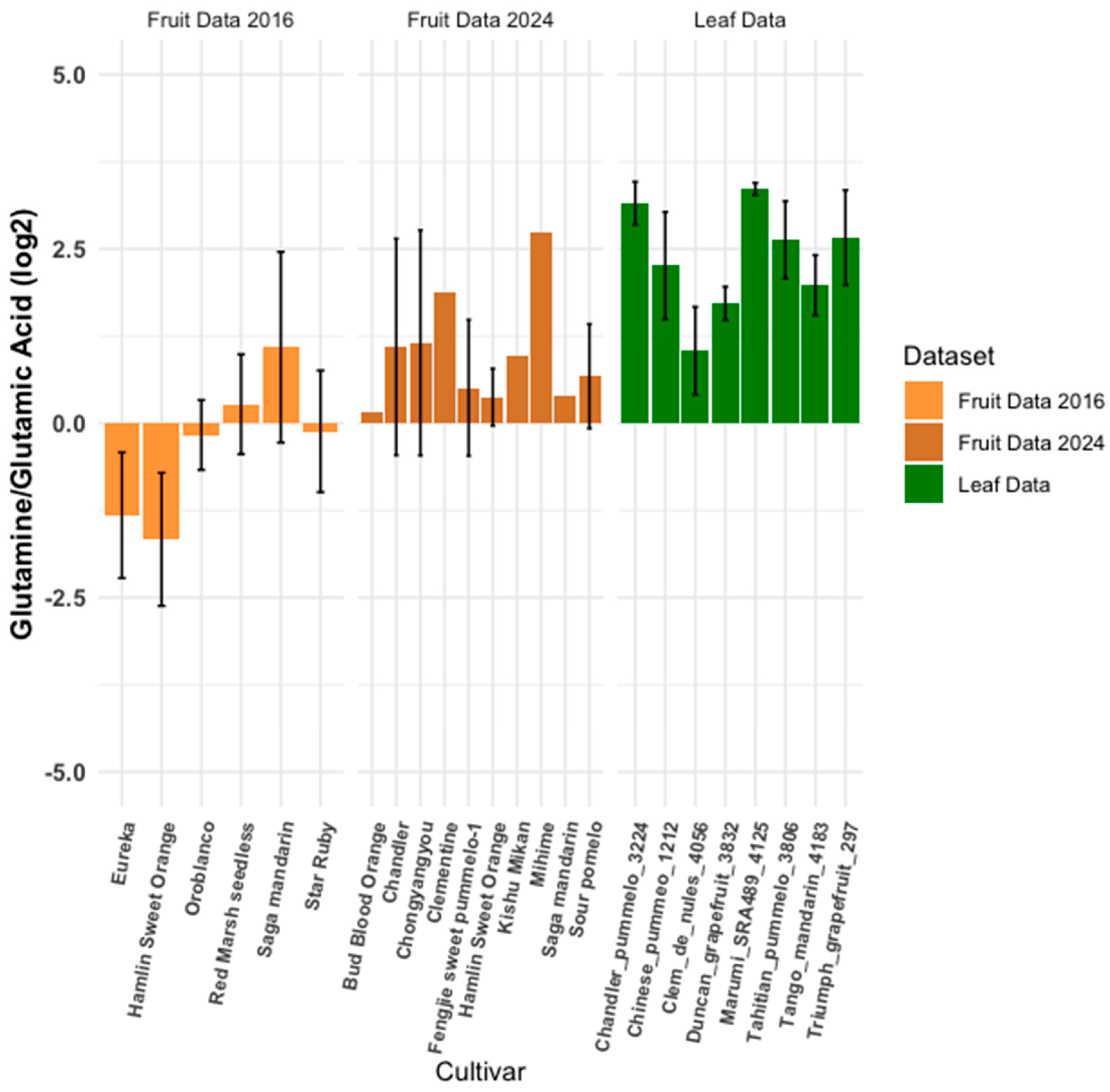
3.2. Expected Similar Metabolite Ratios
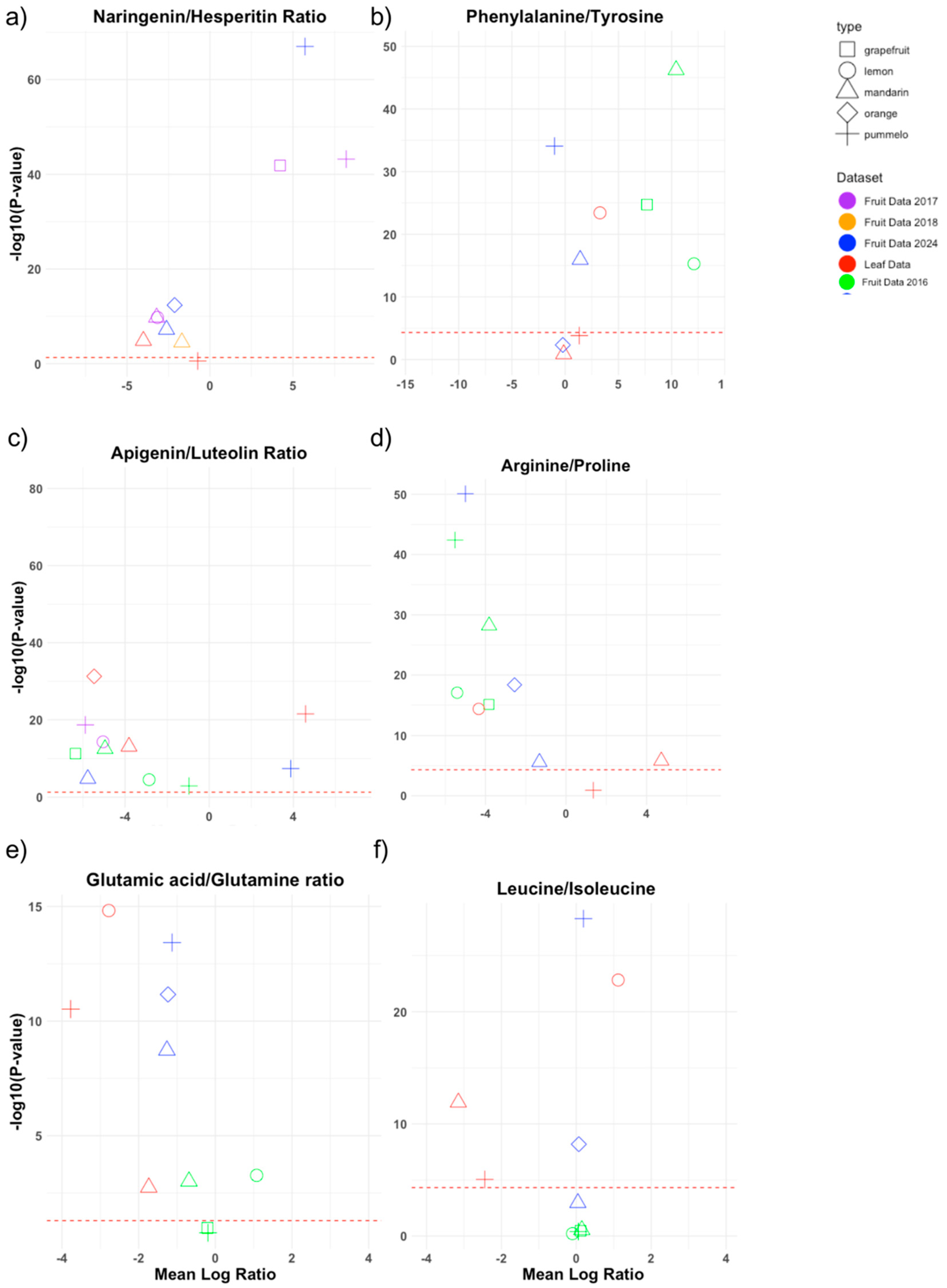
3.3. Metabolite Ratios: Intra-Cultivar-Group Comparison
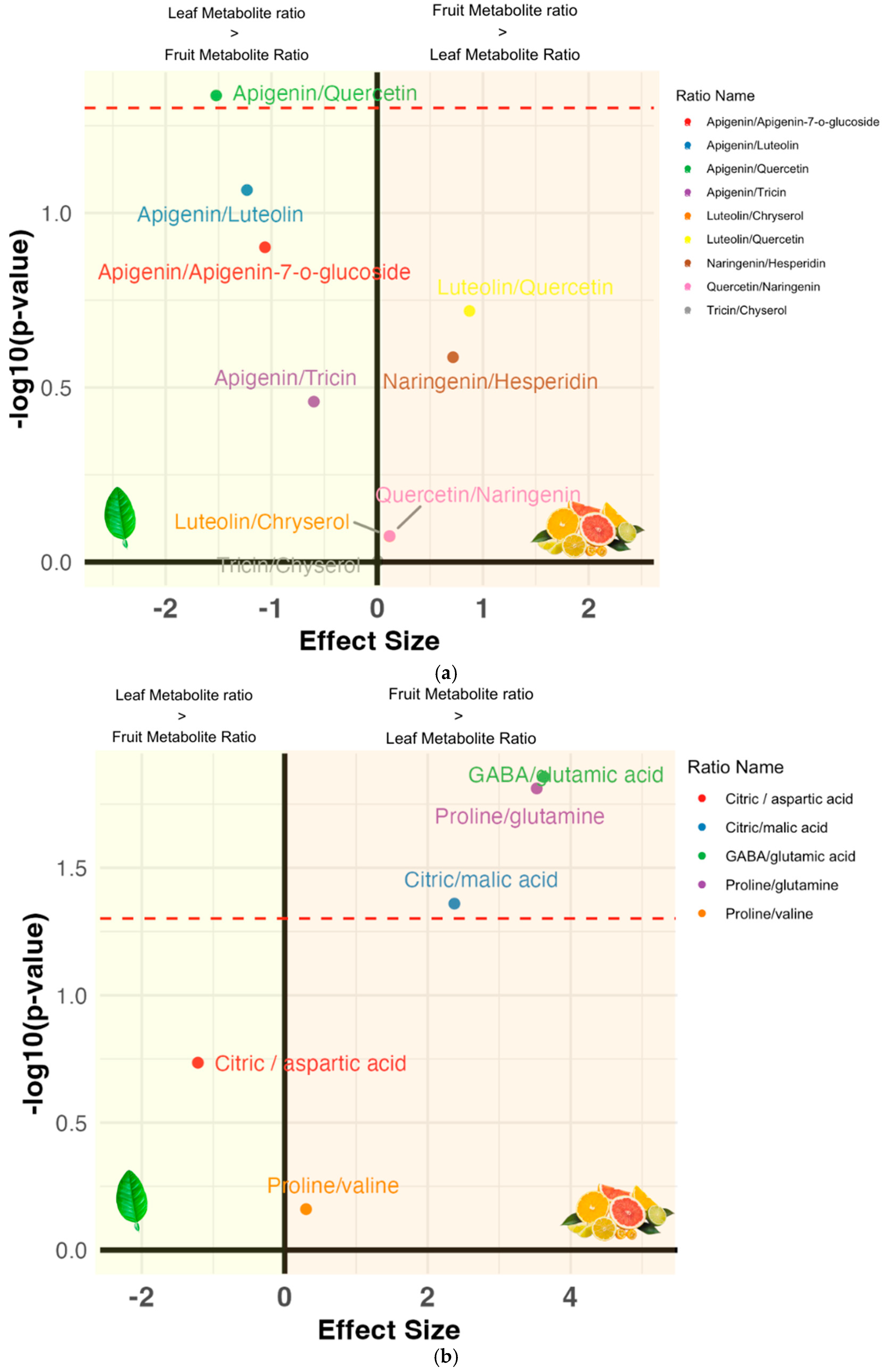
3.4. Metabolite Ratios: Inter-Cultivar Group Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Statistical Bulletin FRESH AND PROCESSED. Available online: https://www.fao.org/3/cb6492en/cb6492en.pdf (accessed on 15 November 2023).
- Maqbool, Z.; Khalid, W.; Atiq, H.T.; Koraqi, H.; Javaid, Z.; Alhag, S.K.; Al-Shuraym, L.A.; Bader, D.M.D.; Almarzuq, M.; Afifi, M.; et al. Citrus Waste as Source of Bioactive Compounds: Extraction and Utilization in Health and Food Industry. Molecules 2023, 28, 1636. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Kalske, A. Plant Secondary Metabolite Diversity and Species Interactions. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 115–138. [Google Scholar] [CrossRef]
- Liang, X.; Wang, Y.; Shen, W.; Liao, B.; Liu, X.; Yang, Z.; Chen, J.; Zhao, C.; Liao, Z.; Cao, J.; et al. Genomic and Metabolomic Insights into the Selection and Differentiation of Bioactive Compounds in Citrus. Mol. Plant 2024, 17, 1753–1772. [Google Scholar] [CrossRef]
- Wang, S.; Tu, H.; Wan, J.; Chen, W.; Liu, X.; Luo, J.; Xu, J.; Zhang, H. Spatio-Temporal Distribution and Natural Variation of Metabolites in Citrus Fruits. Food Chem. 2016, 199, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Yazici, K.; Balijagic, J.; Goksu, B.; Bilgin, O.F.; Ercisli, S. Comparison of Some Fruit Quality Parameters of Selected 12 Mandarin Genotypes from Black Sea Region in Turkey. ACS Omega 2023, 8, 19719–19727. [Google Scholar] [CrossRef]
- Asai, T.; Matsukawa, T.; Kajiyama, S. ichiro Metabolomic Analysis of Primary Metabolites in Citrus Leaf during Defense Responses. J. Biosci. Bioeng. 2017, 123, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Niu, L.; Suh, J.H.; Hung, W.-L.; Wang, Y. Comprehensive Metabolomics Analysis of Mandarins ( Citrus Reticulata) as a Tool for Variety, Rootstock, and Grove Discrimination. J. Agric. Food Chem. 2018, 66, 10317–10326. [Google Scholar] [CrossRef]
- Wang, S.; Shen, S.; Wang, C.; Wang, X.; Yang, C.; Zhou, S.; Zhang, R.; Zhou, Q.; Yu, H.; Guo, H.; et al. A Metabolomics Study in Citrus Provides Insight into Bioactive Phenylpropanoid Metabolism. Hortic. Res. 2024, 11, uhad267. [Google Scholar] [CrossRef]
- Wang, S.; Yang, C.; Tu, H.; Zhou, J.; Liu, X.; Cheng, Y.; Luo, J.; Deng, X.; Zhang, H.; Xu, J. Characterization and Metabolic Diversity of Flavonoids in Citrus Species. Sci. Rep. 2017, 7, 10549. [Google Scholar] [CrossRef]
- Vliet, S.M.F.; Dasgupta, S.; Sparks, N.R.L.; Kirkwood, J.S.; Vollaro, A.; Hur, M.; Zur Nieden, N.I.; Volz, D.C. Maternal-to-Zygotic Transition as a Potential Target for Niclosamide during Early Embryogenesis. Toxicol. Appl. Pharmacol. 2019, 380, 114699. [Google Scholar] [CrossRef]
- Babbar, N.; Oberoi, H.S.; Uppal, D.S.; Patil, R.T. Total Phenolic Content and Antioxidant Capacity of Extracts Obtained from Six Important Fruit Residues. Food Res. Int. 2011, 44, 391–396. [Google Scholar] [CrossRef]
- Romero Rodriguez, M.A.; Vazquez Oderiz, M.L.; Lopez Hernandez, J.; Simal Lozano, J. Determination of Vitamin C and Organic Acids in Various Fruits by HPLC. J. Chromatogr. Sci. 1992, 30, 433–437. [Google Scholar] [CrossRef]
- Brune, A.; Müller, M.; Taiz, L.; Gonzalez, P.; Etxeberria, E. Vacuolar Acidification in Citrus Fruit: Comparison between Acid Lime (Citrus Aurantifolia) and Sweet Lime (Citrus Limmetioides) Juice Cells. J. Am. Soc. Hortic. Sci. 2002, 127, 171–177. [Google Scholar] [CrossRef]
- Müller, M.L.; Jensen, M.; Taiz, L. The Vacuolar H+-ATPase of Lemon Fruits Is Regulated by Variable H+/ATP Coupling and Slip. J. Biol. Chem. 1999, 274, 10706–10716. [Google Scholar] [CrossRef]
- Sun, X.; Han, G.; Meng, Z.; Lin, L.; Sui, N. Roles of Malic Enzymes in Plant Development and Stress Responses. Plant Signal. Behav. 2019, 14, e1644596. [Google Scholar] [CrossRef]
- Zhou, P.; Zheng, M.; Li, X.; Zhou, J.; Shang, Y.; Li, Z.; Qu, L. A Consecutive Extraction of Pectin and Hesperidin from Citrus Aurantium L.:Process Optimization, Extract Mechanism, Characterization and Bio-Activity Analysis. Ind. Crops Prod. 2022, 182, 114849. [Google Scholar] [CrossRef]
- Zhang, M.; Duan, C.; Zang, Y.; Huang, Z.; Liu, G. The Flavonoid Composition of Flavedo and Juice from the Pummelo Cultivar (Citrus Grandis (L.) Osbeck) and the Grapefruit Cultivar (Citrus Paradisi) from China. Food Chem. 2011, 129, 1530–1536. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of Proline under Changing Environments: A Review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Winter, G.; Todd, C.D.; Trovato, M.; Forlani, G.; Funck, D. Physiological Implications of Arginine Metabolism in Plants. Front. Plant Sci. 2015, 6, 534. [Google Scholar] [CrossRef]
- Ingrisano, R.; Tosato, E.; Trost, P.; Gurrieri, L.; Sparla, F. Proline, Cysteine and Branched-Chain Amino Acids in Abiotic Stress Response of Land Plants and Microalgae. Plants 2023, 12, 3410. [Google Scholar] [CrossRef] [PubMed]
- Cánovas, F.M.; Avila, C.; Cantón, F.R.; Cañas, R.A.; de la Torre, F. Ammonium Assimilation and Amino Acid Metabolism in Conifers. J. Exp. Bot. 2007, 58, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Miflin, B.J.; Habash, D.Z. The Role of Glutamine Synthetase and Glutamate Dehydrogenase in Nitrogen Assimilation and Possibilities for Improvement in the Nitrogen Utilization of Crops. J. Exp. Bot. 2002, 53, 979–987. [Google Scholar] [CrossRef]
- Binder, S. Branched-Chain Amino Acid Metabolism in Arabidopsis Thaliana. Arab. Book 2010, 8, e0137. [Google Scholar] [CrossRef]
- Joshi, V.; Joung, J.-G.; Fei, Z.; Jander, G. Interdependence of Threonine, Methionine and Isoleucine Metabolism in Plants: Accumulation and Transcriptional Regulation under Abiotic Stress. Amino Acids 2010, 39, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Shomali, A.; Das, S.; Arif, N.; Sarraf, M.; Zahra, N.; Yadav, V.; Aliniaeifard, S.; Chauhan, D.K.; Hasanuzzaman, M. Diverse Physiological Roles of Flavonoids in Plant Environmental Stress Responses and Tolerance. Plants 2022, 11, 3158. [Google Scholar] [CrossRef]
- Corliss, J. Fruit of the Month: Citrus Fruits. Available online: https://www.health.harvard.edu/heart-health/fruit-of-the-month-citrus-fruits (accessed on 28 February 2025).
- Broadhurst, D.I.; Kell, D.B. Statistical Strategies for Avoiding False Discoveries in Metabolomics and Related Experiments. Metabolomics 2007, 2, 171–196. [Google Scholar] [CrossRef]
- Li, D.; Heiling, S.; Baldwin, I.T.; Gaquerel, E. Illuminating a Plant’s Tissue-Specific Metabolic Diversity Using Computational Metabolomics and Information Theory. Proc. Natl. Acad. Sci. USA 2016, 113, E7610–E7618. [Google Scholar] [CrossRef]
- Ma, X.; Tang, K.; Tang, Z.; Dong, A.; Meng, Y.; Wang, P. Organ-Specific, Integrated Omics Data-Based Study on the Metabolic Pathways of the Medicinal Plant Bletilla Striata (Orchidaceae). BMC Plant Biol. 2021, 21, 504. [Google Scholar] [CrossRef]
- Peters, K.; Blatt-Janmaat, K.L.; Tkach, N.; van Dam, N.V.; Neumann, S. Untargeted Metabolomics for Integrative Taxonomy: Metabolomics, DNA Marker-Based Sequencing, and Phenotype Bioimaging. Plants 2023, 12, 881. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Liu, H.; Liu, J.; Jiao, Z. Profiles of Sugar and Organic Acid of Fruit Juices: A Comparative Study and Implication for Authentication. J. Food Qual. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Bae, H.; Yun, S.; Yoon, I.; Nam, E.; Kwon, J.; Jun, J. Assessment of Organic Acid and Sugar Composition in Apricot, Plumcot, Plum, and Peach during Fruit Development. J. Appl. Bot. Food Qual. 2014, 87, 24–29. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traband, R.C.; Wang, X.; Resendiz, M.; Meng, M.; Hiraoka, Y.; Jia, Q.; Chang, R.; Eurmsirilerd, E.; Kahn, T.; Mauk, P.A.; et al. A Novel Approach for Comparing Selected Metabolites in Citrus Leaves and Fruits Across Datasets. Plants 2025, 14, 1406. https://doi.org/10.3390/plants14101406
Traband RC, Wang X, Resendiz M, Meng M, Hiraoka Y, Jia Q, Chang R, Eurmsirilerd E, Kahn T, Mauk PA, et al. A Novel Approach for Comparing Selected Metabolites in Citrus Leaves and Fruits Across Datasets. Plants. 2025; 14(10):1406. https://doi.org/10.3390/plants14101406
Chicago/Turabian StyleTraband, Ryan C., Xuesong Wang, Mariano Resendiz, Megan Meng, Yoko Hiraoka, Qiong Jia, Rendell Chang, Ethan Eurmsirilerd, Tracy Kahn, Peggy A. Mauk, and et al. 2025. "A Novel Approach for Comparing Selected Metabolites in Citrus Leaves and Fruits Across Datasets" Plants 14, no. 10: 1406. https://doi.org/10.3390/plants14101406
APA StyleTraband, R. C., Wang, X., Resendiz, M., Meng, M., Hiraoka, Y., Jia, Q., Chang, R., Eurmsirilerd, E., Kahn, T., Mauk, P. A., De Souza, A., Bhatia, A., Ke, H., Merhaut, D., Roose, M. L., Jia, Z., & Chater, J. M. (2025). A Novel Approach for Comparing Selected Metabolites in Citrus Leaves and Fruits Across Datasets. Plants, 14(10), 1406. https://doi.org/10.3390/plants14101406









