Search of Reflectance Indices for Estimating Photosynthetic Activity of Wheat Plants Under Drought Stress
Abstract
1. Introduction
2. Results
2.1. Effect of Soil Moisture Deficit on Phenotypic Parameters of Wheat Seedlings
2.1.1. Morphometric Parameters
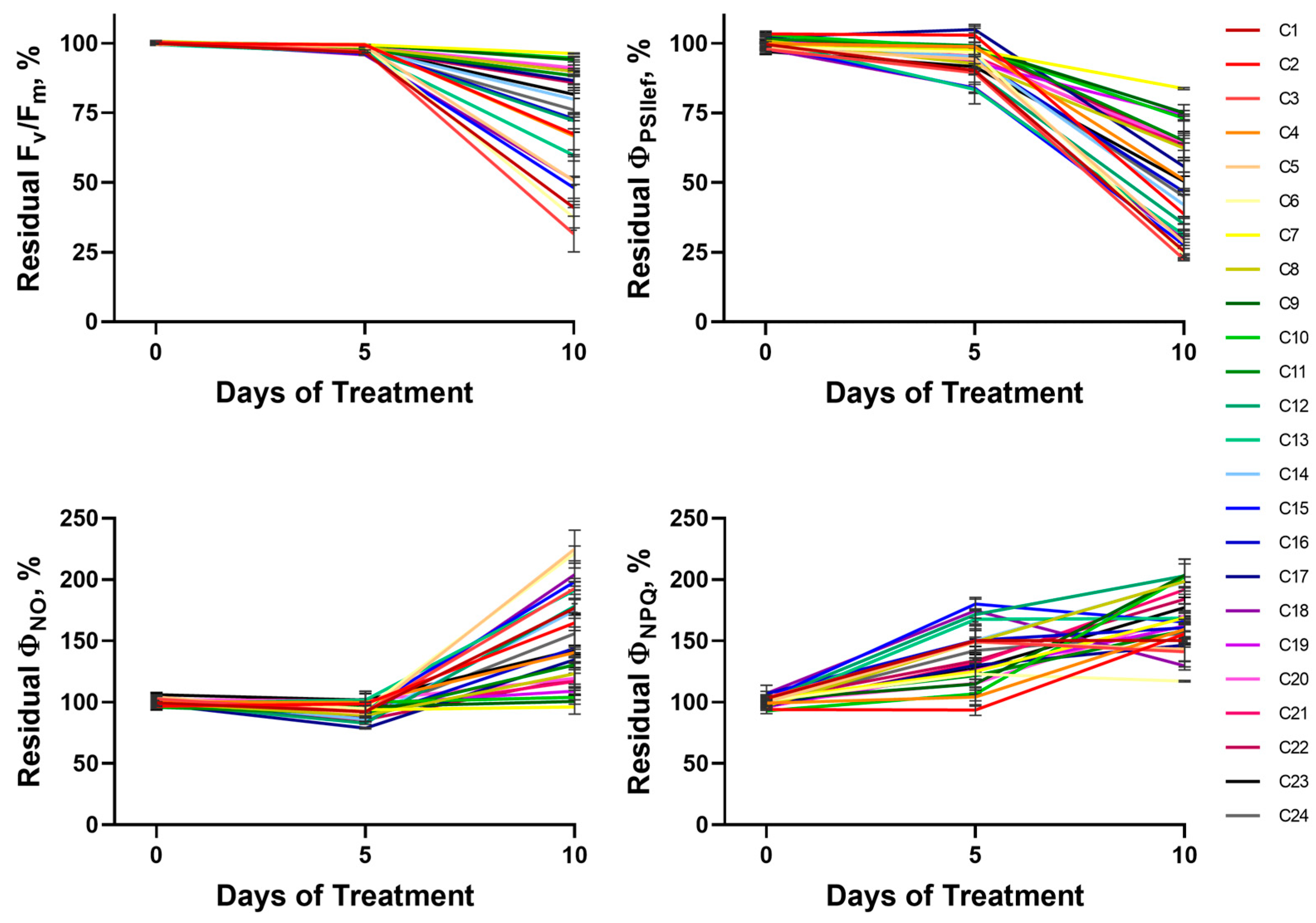
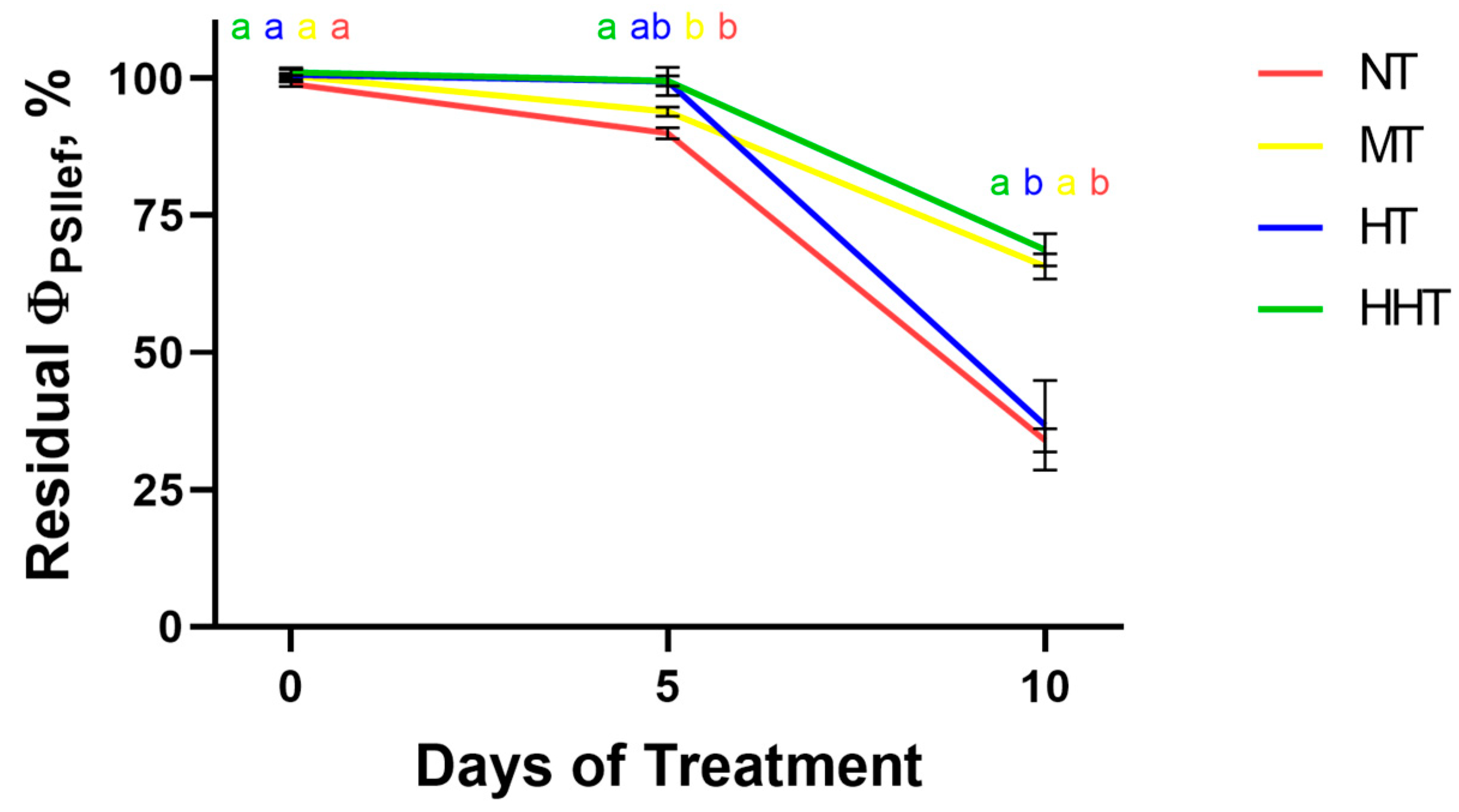
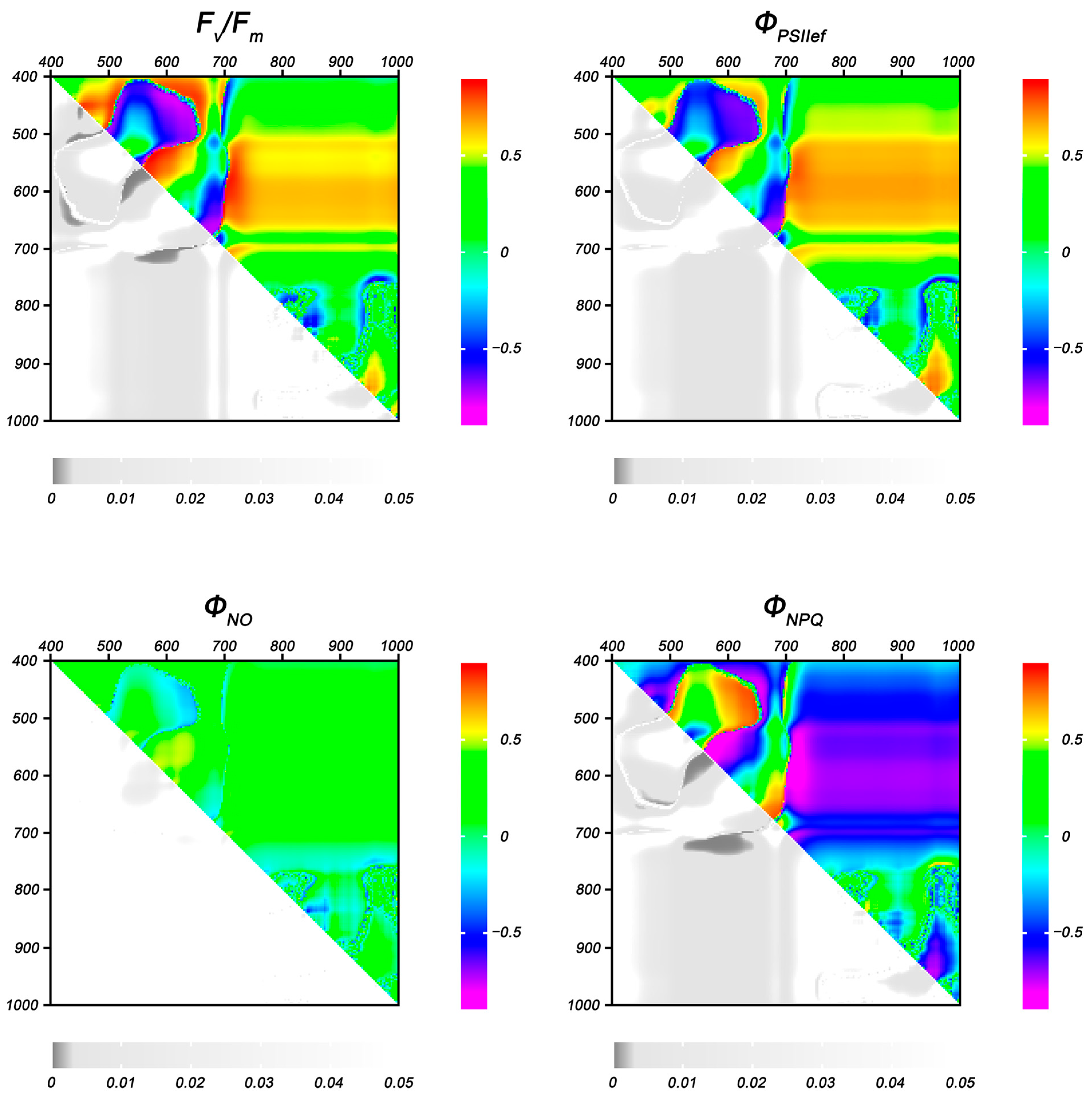
2.1.2. Photosynthetic Activity
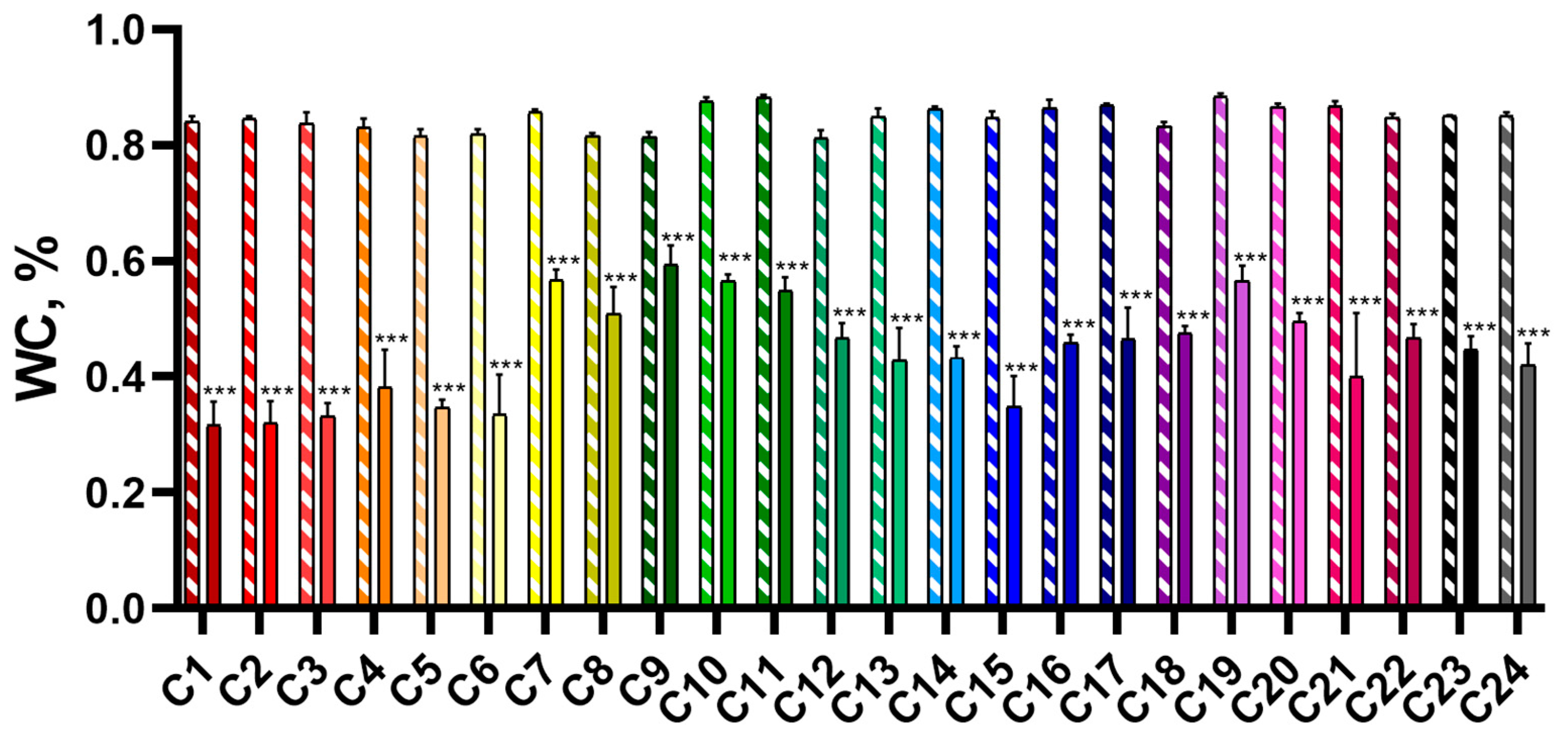
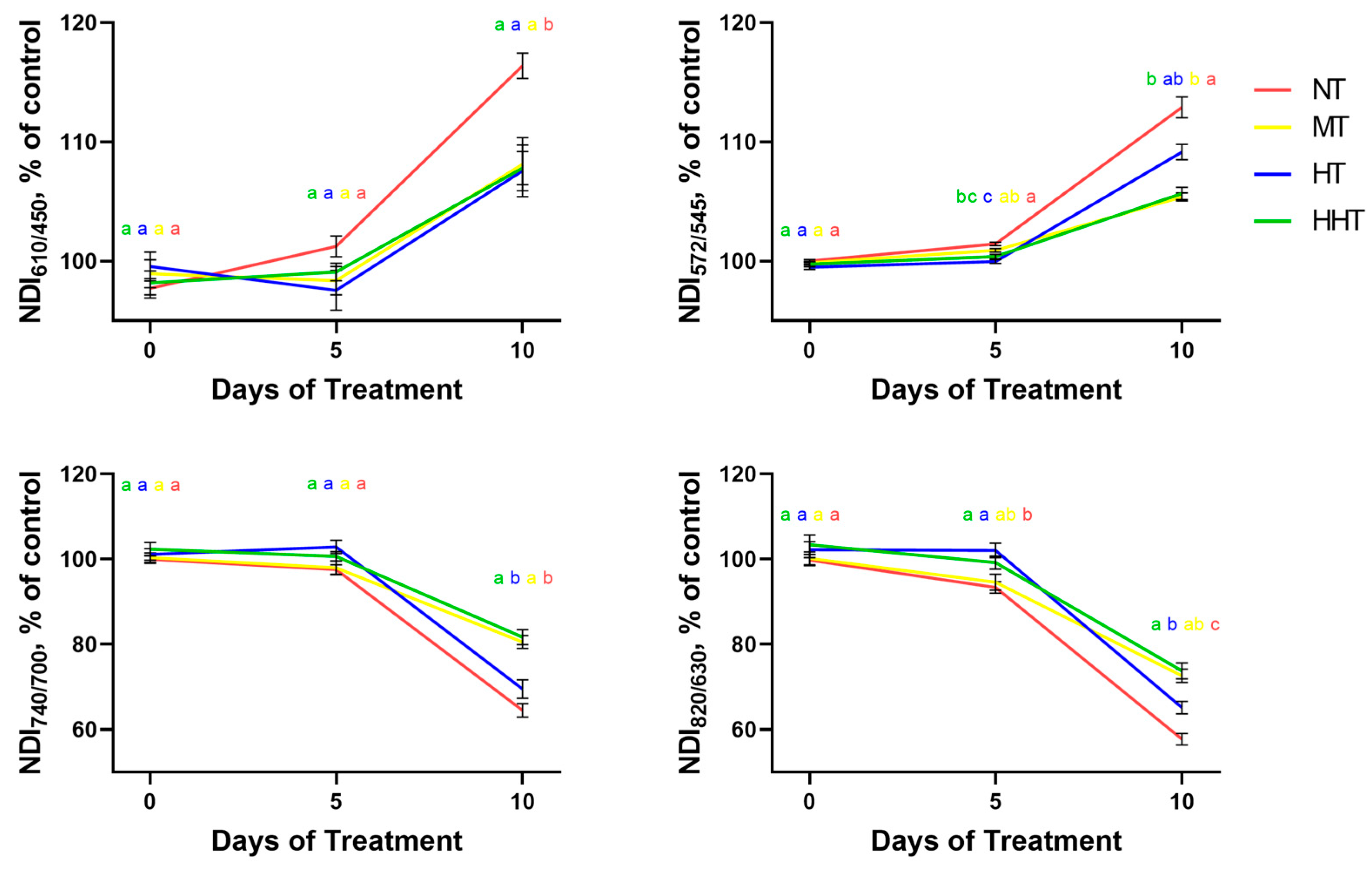
2.1.3. Reflectance Parameters
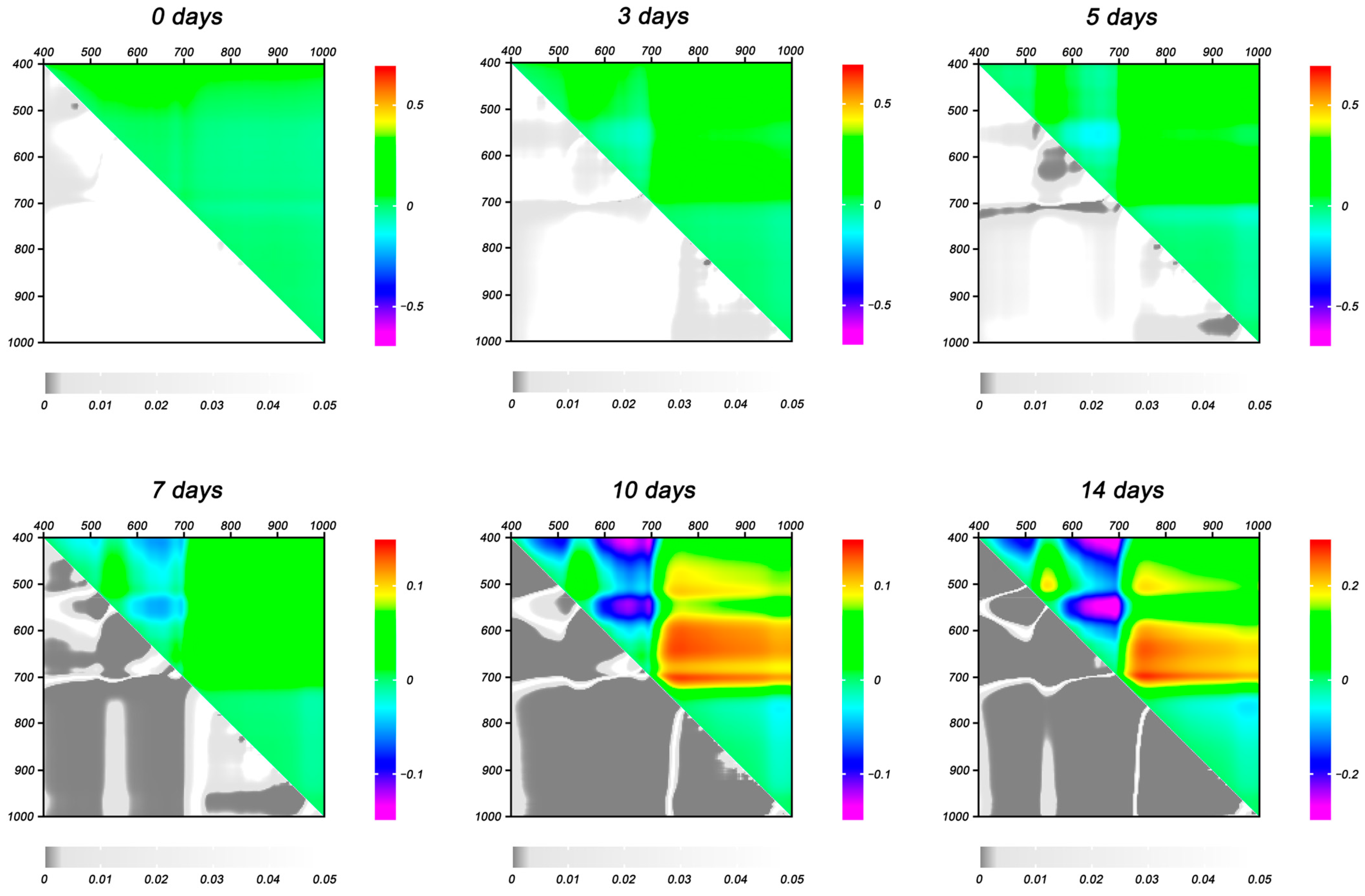
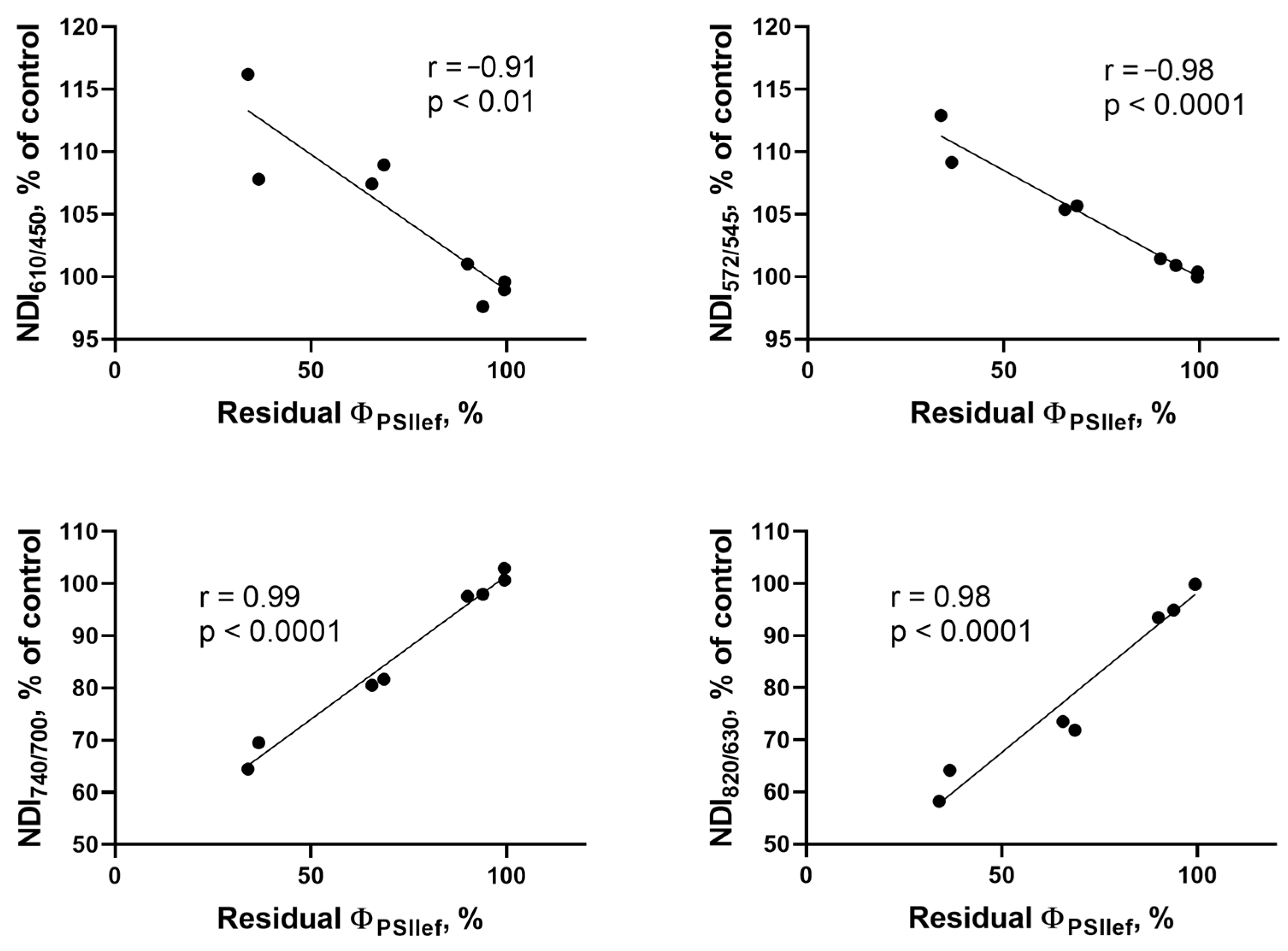
2.2. Search for NDIs Reflecting Photosynthetic Activity Under Drought Stress
3. Discussion
3.1. Effect of Drought Stress on Morphophysiological Characteristics of Wheat
3.2. Relationship Between Photosynthetic Activity Parameters and NDIs
4. Materials and Methods
4.1. Plant Material
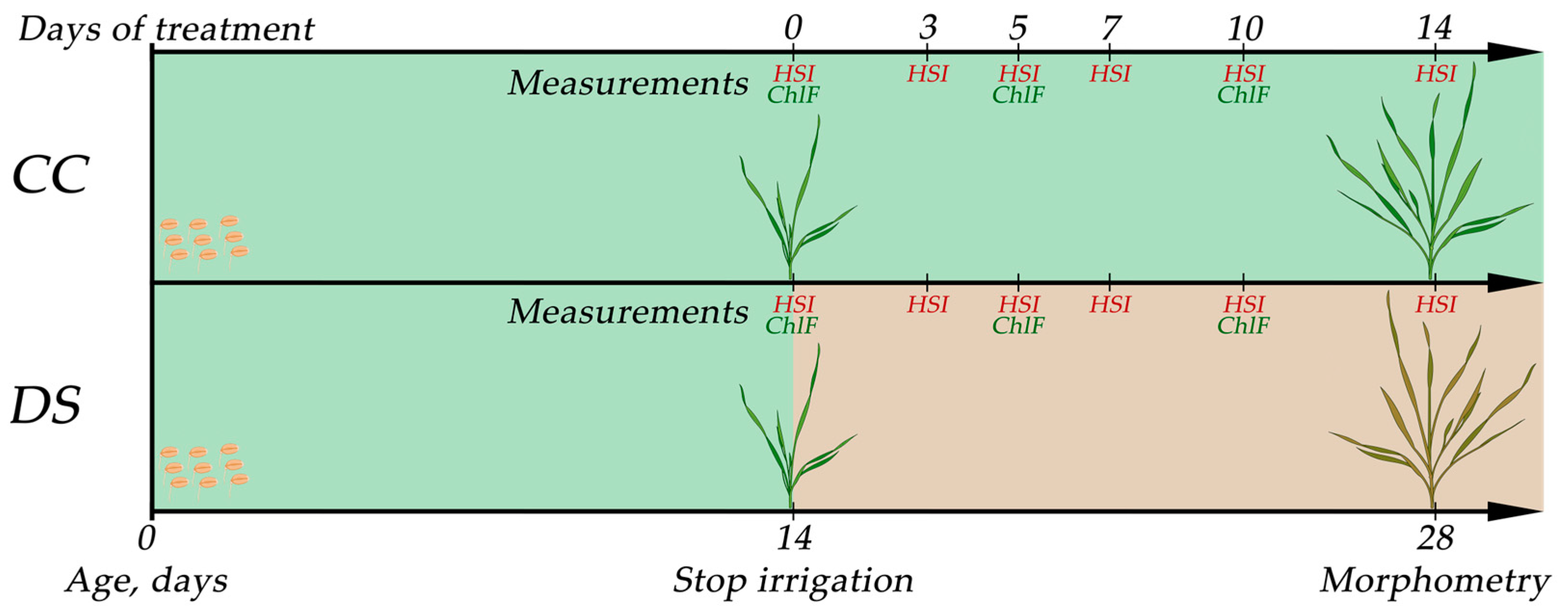
4.2. Experiment Design
4.3. Morphometric Parameters
4.4. ChlF Parameters
4.5. Reflectance Parameters
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops That Feed the World 10. Past Successes and Future Challenges to the Role Played by Wheat in Global Food Security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Mottaleb, K.A.; Sonder, K.; Donovan, J.; Braun, H.-J. Global Trends in Wheat Production, Consumption and Trade. In Wheat Improvement; Springer International Publishing: Cham, Switzerland, 2022; pp. 47–66. ISBN 978-3-030-90672-6. [Google Scholar]
- Bohra, A.; Choudhary, M.; Bennett, D.; Joshi, R.; Mir, R.R.; Varshney, R.K. Drought-Tolerant Wheat for Enhancing Global Food Security. Funct. Integr. Genom. 2024, 24, 212. [Google Scholar] [CrossRef] [PubMed]
- Trnka, M.; Feng, S.; Semenov, M.A.; Olesen, J.E.; Kersebaum, K.C.; Rötter, R.P.; Semerádová, D.; Klem, K.; Huang, W.; Ruiz-Ramos, M.; et al. Mitigation Efforts Will Not Fully Alleviate the Increase in Water Scarcity Occurrence Probability in Wheat-Producing Areas. Sci. Adv. 2019, 5, eaau2406. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil Salinity under Climate Change: Challenges for Sustainable Agriculture and Food Security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef] [PubMed]
- Angon, P.B.; Tahjib-Ul-Arif, M.; Samin, S.I.; Habiba, U.; Hossain, M.A.; Brestic, M. How Do Plants Respond to Combined Drought and Salinity Stress?—A Systematic Review. Plants 2022, 11, 2884. [Google Scholar] [CrossRef] [PubMed]
- Tarolli, P.; Luo, J.; Park, E.; Barcaccia, G.; Masin, R. Soil Salinization in Agriculture: Mitigation and Adaptation Strategies Combining Nature-Based Solutions and Bioengineering. iScience 2024, 27, 108830. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under Drought and Salt Stress: Regulation Mechanisms from Whole Plant to Cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and Drought: Can We Make Metabolic Connections from Available Data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General Mechanisms of Drought Response and Their Application in Drought Resistance Improvement in Plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Hong, C.; Jiao, Y.; Hou, S.; Gao, H. Impacts of Drought on Photosynthesis in Major Food Crops and the Related Mechanisms of Plant Responses to Drought. Plants 2024, 13, 1808. [Google Scholar] [CrossRef]
- Dalal, V.K. Modulation of Photosynthesis and Other Proteins during Water–Stress. Mol. Biol. Rep. 2021, 48, 3681–3693. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.; Han, Z.; Feng, H.; Wang, Y.; Kang, J.; Han, X.; Wang, L.; Wang, C.; Li, H.; et al. Analysis of Physiological Indicators Associated with Drought Tolerance in Wheat under Drought and Re-Watering Conditions. Antioxidants 2022, 11, 2266. [Google Scholar] [CrossRef]
- Abdullaev, F.; Pirogova, P.; Vodeneev, V.; Sherstneva, O. Chlorophyll Fluorescence in Wheat Breeding for Heat and Drought Tolerance. Plants 2024, 13, 2778. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-Induced Responses of Photosynthesis and Antioxidant Metabolism in Higher Plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Bhat, J.A.; Deshmukh, R.; Zhao, T.; Patil, G.; Deokar, A.; Shinde, S.; Chaudhary, J. Harnessing High-Throughput Phenotyping and Genotyping for Enhanced Drought Tolerance in Crop Plants. J. Biotechnol. 2020, 324, 248–260. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Ashraful Alam, M.; Syed, M.A.; Hossain, J.; Sarkar, S.; Saha, S.; Bhadra, P.; et al. Consequences and Mitigation Strategies of Abiotic Stresses in Wheat (Triticum aestivum L.) under the Changing Climate. Agronomy 2021, 11, 241. [Google Scholar] [CrossRef]
- Raj, S.R.G.; Nadarajah, K. QTL and Candidate Genes: Techniques and Advancement in Abiotic Stress Resistance Breeding of Major Cereals. Int. J. Mol. Sci. 2022, 24, 6. [Google Scholar] [CrossRef]
- Song, P.; Wang, J.; Guo, X.; Yang, W.; Zhao, C. High-Throughput Phenotyping: Breaking through the Bottleneck in Future Crop Breeding. Crop J. 2021, 9, 633–645. [Google Scholar] [CrossRef]
- Sarić, R.; Nguyen, V.D.; Burge, T.; Berkowitz, O.; Trtílek, M.; Whelan, J.; Lewsey, M.G.; Čustović, E. Applications of Hyperspectral Imaging in Plant Phenotyping. Trends Plant Sci. 2022, 27, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zeng, A.; Zhu, T.; Fang, S.; Gong, Y.; Tao, Y.; Zhou, Y.; Liu, K. Using Remotely Sensed Spectral Reflectance to Indicate Leaf Photosynthetic Efficiency Derived from Active Fluorescence Measurements. J. Appl. Remote Sens. 2017, 11, 026034. [Google Scholar] [CrossRef]
- El-Hendawy, S.E.; Hassan, W.M.; Al-Suhaibani, N.A.; Schmidhalter, U. Spectral Assessment of Drought Tolerance Indices and Grain Yield in Advanced Spring Wheat Lines Grown under Full and Limited Water Irrigation. Agric. Water Manag. 2017, 182, 1–12. [Google Scholar] [CrossRef]
- Sukhova, E.; Sukhov, V. Relation of Photochemical Reflectance Indices Based on Different Wavelengths to the Parameters of Light Reactions in Photosystems I and II in Pea Plants. Remote Sens. 2020, 12, 1312. [Google Scholar] [CrossRef]
- Sherstneva, O.; Abdullaev, F.; Kior, D.; Yudina, L.; Gromova, E.; Vodeneev, V. Prediction of Biomass Accumulation and Tolerance of Wheat Seedlings to Drought and Elevated Temperatures Using Hyperspectral Imaging. Front. Plant Sci. 2024, 15, 1344826. [Google Scholar] [CrossRef] [PubMed]
- Ihuoma, S.O.; Madramootoo, C.A. Recent Advances in Crop Water Stress Detection. Comput. Electron. Agric. 2017, 141, 267–275. [Google Scholar] [CrossRef]
- Peteinatos, G.; Korsaeth, A.; Berge, T.; Gerhards, R. Using Optical Sensors to Identify Water Deprivation, Nitrogen Shortage, Weed Presence and Fungal Infection in Wheat. Agriculture 2016, 6, 24. [Google Scholar] [CrossRef]
- Li, Q.; Gao, M.; Li, Z.-L. Ground Hyper-Spectral Remote-Sensing Monitoring of Wheat Water Stress during Different Growing Stages. Agronomy 2022, 12, 2267. [Google Scholar] [CrossRef]
- Mukarram, M.; Choudhary, S.; Kurjak, D.; Petek, A.; Khan, M.M.A. Drought: Sensing, Signalling, Effects and Tolerance in Higher Plants. Physiol. Plant. 2021, 172, 1291–1300. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, W.; Ma, J.; Cheng, Z.; Zhang, X.; Liu, X.; Wu, X.; Zhang, J. An Integrated Framework for Drought Stress in Plants. Int. J. Mol. Sci. 2024, 25, 9347. [Google Scholar] [CrossRef] [PubMed]
- Eena, R.; Bharti, R.; Chaudhary, N. Drought Resistance in Wheat (Triticum aestivum L.): A Review. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1780–1792. [Google Scholar] [CrossRef]
- Steduto, P.; Hsiao, T.C.; Fereres, E.; Raes, D. Crop Yield Response to Water; FAO Irrigation and Drainage Paper; FAO: Rome, Italy, 2012; ISBN 978-92-5-107274-5. [Google Scholar]
- Ihsan, M.Z.; El-Nakhlawy, F.S.; Ismail, S.M.; Fahad, S.; Daur, I. Wheat Phenological Development and Growth Studies As Affected by Drought and Late Season High Temperature Stress under Arid Environment. Front. Plant Sci. 2016, 7, 795. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, B.; Borzí, I.; Arosio, M.; Cesarini, L.; Bonaccorso, B.; Martina, M. Modelling the Response of Wheat Yield to Stage-Specific Water Stress in the Po Plain. Agric. Water Manag. 2023, 287, 108444. [Google Scholar] [CrossRef]
- Raines, C.A. Increasing Photosynthetic Carbon Assimilation in C3 Plants to Improve Crop Yield: Current and Future Strategies. Plant Physiol. 2011, 155, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Sherstneva, O.; Khlopkov, A.; Gromova, E.; Yudina, L.; Vetrova, Y.; Pecherina, A.; Kuznetsova, D.; Krutova, E.; Sukhov, V.; Vodeneev, V. Analysis of Chlorophyll Fluorescence Parameters as Predictors of Biomass Accumulation and Tolerance to Heat and Drought Stress of Wheat (Triticum aestivum) plants. Funct. Plant Biol. 2021, 49, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yang, Z. Effects of Drought Stress and Postdrought Rewatering on Winter Wheat: A Meta-Analysis. Agronomy 2024, 14, 298. [Google Scholar] [CrossRef]
- Sukhova, E.; Yudina, L.; Kior, A.; Kior, D.; Popova, A.; Zolin, Y.; Gromova, E.; Sukhov, V. Modified Photochemical Reflectance Indices as New Tool for Revealing Influence of Drought and Heat on Pea and Wheat Plants. Plants 2022, 11, 1308. [Google Scholar] [CrossRef]
- Grieco, M.; Roustan, V.; Dermendjiev, G.; Rantala, S.; Jain, A.; Leonardelli, M.; Neumann, K.; Berger, V.; Engelmeier, D.; Bachmann, G.; et al. Adjustment of Photosynthetic Activity to Drought and Fluctuating Light in Wheat. Plant Cell Environ. 2020, 43, 1484–1500. [Google Scholar] [CrossRef]
- Barboričová, M.; Filaček, A.; Mlynáriková Vysoká, D.; Gašparovič, K.; Živčák, M.; Brestič, M. Sensitivity of Fast Chlorophyll Fluorescence Parameters to Combined Heat and Drought Stress in Wheat Genotypes. Plant Soil Environ. 2022, 68, 309–316. [Google Scholar] [CrossRef]
- Javadipour, Z.; Balouchi, H.; Movahhedi Dehnavi, M.; Yadavi, A. Physiological Responses of Bread Wheat (Triticum aestivum) Cultivars to Drought Stress and Exogenous Methyl Jasmonate. J. Plant Growth Regul. 2022, 41, 3433–3448. [Google Scholar] [CrossRef]
- Yang, Q.; Li, P.; Zhang, D.; Lin, W.; Hao, X.; Zong, Y. Effects of Elevated CO2 on the Photosynthesis, Chlorophyll Fluorescence and Yield of Two Wheat Cultivars (Triticum aestivum L.) under Persistent Drought Stress. Sustainability 2023, 15, 1593. [Google Scholar] [CrossRef]
- Chen, Y.-E.; Cui, J.-M.; Su, Y.-Q.; Zhang, C.-M.; Ma, J.; Zhang, Z.-W.; Yuan, M.; Liu, W.-J.; Zhang, H.-Y.; Yuan, S. Comparison of Phosphorylation and Assembly of Photosystem Complexes and Redox Homeostasis in Two Wheat Cultivars with Different Drought Resistance. Sci. Rep. 2017, 7, 12718. [Google Scholar] [CrossRef]
- Tang, X.; Liu, H.; Zhang, W. Physiological Characteristics, Crop Growth and Grain Yield of Twelve Wheat Varieties Cultivated in the North China Plain. Agronomy 2023, 13, 3041. [Google Scholar] [CrossRef]
- Mshenskaya, N.S.; Grinberg, M.A.; Kalyasova, E.A.; Vodeneev, V.A.; Ilin, N.V.; Slyunyaev, N.N.; Mareev, E.A.; Sinitsyna, Y.V. The Effect of an Extremely Low-Frequency Electromagnetic Field on the Drought Sensitivity of Wheat Plants. Plants 2023, 12, 826. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Nezhadahmadi, A.; Prodhan, Z.H.; Faruq, G. Drought Tolerance in Wheat. Sci. World J. 2013, 2013, 610721. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Hou, X.; Duan, W.; Yin, B.; Ren, J.; Wang, Y.; Liu, X.; Gu, L.; Zhen, W. Screening and Evaluation of Drought Resistance Traits of Winter Wheat in the North China Plain. Front. Plant Sci. 2023, 14, 1194759. [Google Scholar] [CrossRef]
- Kulkarni, M.; Soolanayakanahally, R.; Ogawa, S.; Uga, Y.; Selvaraj, M.G.; Kagale, S. Drought Response in Wheat: Key Genes and Regulatory Mechanisms Controlling Root System Architecture and Transpiration Efficiency. Front. Chem. 2017, 5, 106. [Google Scholar] [CrossRef]
- Czövek, P.; Király, I.; Páldi, E.; Molnár, I.; Gáspár, L. Comparative Analysis of Stress Tolerance in Aegilops Accessions and Triticum Wheat Varieties to Detect Different Drought Tolerance Strategies. Acta Agron. Hung. 2006, 54, 49–60. [Google Scholar] [CrossRef]
- Nenova, V.R.; Kocheva, K.V.; Petrov, P.I.; Georgiev, G.I.; Karceva, T.V.; Börner, A.; Landjeva, S.P. Wheat Rht-B1 Dwarfs Exhibit Better Photosynthetic Response to Water Deficit at Seedling Stage Compared to the Wild Type. J. Agron. Crop Sci. 2014, 200, 434–443. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Shangguan, Z. Leaf Gas Exchange and Fluorescence of Two Winter Wheat Varieties in Response to Drought Stress and Nitrogen Supply. PLoS ONE 2016, 11, e0165733. [Google Scholar] [CrossRef]
- Mendanha, T.; Rosenqvist, E.; Nordentoft Hyldgaard, B.; Doonan, J.H.; Ottosen, C. Drought Priming Effects on Alleviating the Photosynthetic Limitations of Wheat Cultivars (Triticum aestivum L.) with Contrasting Tolerance to Abiotic Stresses. J. Agron. Crop Sci. 2020, 206, 651–664. [Google Scholar] [CrossRef]
- Kartseva, T.; Dobrikova, A.; Kocheva, K.; Alexandrov, V.; Georgiev, G.; Brestič, M.; Misheva, S. Optimal Nitrogen Supply Ameliorates the Performance of Wheat Seedlings under Osmotic Stress in Genotype-Specific Manner. Plants 2021, 10, 493. [Google Scholar] [CrossRef] [PubMed]
- Hickey, K.; Wood, M.; Sexton, T.; Sahin, Y.; Nazarov, T.; Fisher, J.; Sanguinet, K.A.; Cousins, A.; Kirchhoff, H.; Smertenko, A. Drought Tolerance Strategies and Autophagy in Resilient Wheat Genotypes. Cells 2022, 11, 1765. [Google Scholar] [CrossRef]
- Popova, A.V.; Mihailova, G.; Geneva, M.; Peeva, V.; Kirova, E.; Sichanova, M.; Dobrikova, A.; Georgieva, K. Different Responses to Water Deficit of Two Common Winter Wheat Varieties: Physiological and Biochemical Characteristics. Plants 2023, 12, 2239. [Google Scholar] [CrossRef]
- Gallé, Á.; Csiszár, J.; Tari, I.; Erdei, L. Changes in Water and Chlorophyll Fluorescence Parameters under Osmotic Stress in Wheat Cultivars. Acta Biol. Szeged. 2002, 46, 85–86. [Google Scholar]
- Goss, R.; Lepetit, B. Biodiversity of NPQ. J. Plant Physiol. 2015, 172, 13–32. [Google Scholar] [CrossRef]
- Liu, H.; Song, S.; Zhang, H.; Li, Y.; Niu, L.; Zhang, J.; Wang, W. Signaling Transduction of ABA, ROS, and Ca2+ in Plant Stomatal Closure in Response to Drought. Int. J. Mol. Sci. 2022, 23, 14824. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Montes, C.M.; Siebers, M.H.; Gomez-Casanovas, N.; McGrath, J.M.; Ainsworth, E.A.; Bernacchi, C.J. Advances in Field-Based High-Throughput Photosynthetic Phenotyping. J. Exp. Bot. 2022, 73, 3157–3172. [Google Scholar] [CrossRef] [PubMed]
- Perez-Sanz, F.; Navarro, P.J.; Egea-Cortines, M. Plant Phenomics: An Overview of Image Acquisition Technologies and Image Data Analysis Algorithms. GigaScience 2017, 6, gix092. [Google Scholar] [CrossRef] [PubMed]
- Ollinger, S.V. Sources of Variability in Canopy Reflectance and the Convergent Properties of Plants. New Phytol. 2011, 189, 375–394. [Google Scholar] [CrossRef] [PubMed]
- Ustin, S.L.; Jacquemoud, S. How the Optical Properties of Leaves Modify the Absorption and Scattering of Energy and Enhance Leaf Functionality. In Remote Sensing of Plant Biodiversity; Cavender-Bares, J., Gamon, J.A., Townsend, P.A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 349–384. ISBN 978-3-030-33156-6. [Google Scholar]
- Liu, L.; Wang, J.; Huang, W.; Zhao, C.; Zhang, B.; Tong, Q. Estimating Winter Wheat Plant Water Content Using Red Edge Parameters. Int. J. Remote Sens. 2004, 25, 3331–3342. [Google Scholar] [CrossRef]
- Peng, Z.; Lin, S.; Zhang, B.; Wei, Z.; Liu, L.; Han, N.; Cai, J.; Chen, H. Winter Wheat Canopy Water Content Monitoring Based on Spectral Transforms and “Three-Edge” Parameters. Agric. Water Manag. 2020, 240, 106306. [Google Scholar] [CrossRef]
- Klem, K.; Záhora, J.; Zemek, F.; Trunda, P.; Tůma, I.; Novotná, K.; Hodaňová, P.; Rapantová, B.; Hanuš, J.; Vavříková, J.; et al. Interactive Effects of Water Deficit and Nitrogen Nutrition on Winter Wheat. Remote Sensing Methods for Their Detection. Agric. Water Manag. 2018, 210, 171–184. [Google Scholar] [CrossRef]
- Sun, H.; Feng, M.; Xiao, L.; Yang, W.; Wang, C.; Jia, X.; Zhao, Y.; Zhao, C.; Muhammad, S.K.; Li, D. Assessment of Plant Water Status in Winter Wheat (Triticum aestivum L.) Based on Canopy Spectral Indices. PLoS ONE 2019, 14, e0216890. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, S.; Zhang, B. Evaluation of Hyperspectral Indices for Retrieval of Canopy Equivalent Water Thickness and Gravimetric Water Content. Int. J. Remote Sens. 2016, 37, 3384–3399. [Google Scholar] [CrossRef]
- Metternicht, G. Vegetation Indices Derived from High-Resolution Airborne Videography for Precision Crop Management. Int. J. Remote Sens. 2003, 24, 2855–2877. [Google Scholar] [CrossRef]
- Verdebout, J.; Jacquemoud, S.; Schmuck, G. Optical Properties of Leaves: Modelling and Experimental Studies. In Imaging Spectrometry—A Tool for Environmental Observations; Hill, J., Mégier, J., Eds.; Eurocourses: Remote Sensing; Springer Netherlands: Dordrecht, The Netherlands, 1994; Volume 4, pp. 169–191. ISBN 978-0-7923-2965-7. [Google Scholar]
- Gamon, J.A.; Peñuelas, J.; Field, C.B. A Narrow-Waveband Spectral Index That Tracks Diurnal Changes in Photosynthetic Efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Gamon, J.A.; Berry, J.A. Facultative and Constitutive Pigment Effects on the Photochemical Reflectance Index (PRI) in Sun and Shade Conifer Needles. Isr. J. Plant Sci. 2012, 60, 85–95. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, C.; Guo, N.; Li, Y.; Jian, S.; Yu, K. Determining the Canopy Water Stress for Spring Wheat Using Canopy Hyperspectral Reflectance Data in Loess Plateau Semiarid Regions. Spectrosc. Lett. 2015, 48, 492–498. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Lichtenthaler, H.K. Detection of Red Edge Position and Chlorophyll Content by Reflectance Measurements Near 700 Nm. J. Plant Physiol. 1996, 148, 501–508. [Google Scholar] [CrossRef]
- Clevers, J.G.P.W.; Kooistra, L. Using Hyperspectral Remote Sensing Data for Retrieving Canopy Chlorophyll and Nitrogen Content. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2012, 5, 574–583. [Google Scholar] [CrossRef]
- Xiao, L.; Feng, M.; Yang, W.; Ding, G. Estimation of Water Content in Winter Wheat (Triticum aestivum L.) and Soil Based on Remote Sensing Data–Vegetation Index. Commun. Soil Sci. Plant Anal. 2015, 46, 1827–1839. [Google Scholar] [CrossRef]
- Ali, F.; Razzaq, A.; Tariq, W.; Hameed, A.; Rehman, A.; Razzaq, K.; Sarfraz, S.; Rajput, N.A.; Zaki, H.E.M.; Shahid, M.S.; et al. Spectral Intelligence: AI-Driven Hyperspectral Imaging for Agricultural and Ecosystem Applications. Agronomy 2024, 14, 2260. [Google Scholar] [CrossRef]
- Song, Q.; Zhu, X.-G. Techniques for Photosynthesis Phenomics: Gas Exchange, Fluorescence, and Reflectance Spectrums. Crop Environ. 2024, 3, 147–158. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, R.; Wang, M.; Zhang, J.; Yu, F.; Ping, Y.; Qiu, L. Research Progress of Spectral Imaging Techniques in Plant Phenotype Studies. Plants 2024, 13, 3088. [Google Scholar] [CrossRef] [PubMed]
- Klughammer, C.; Schreiber, U. Complementary PS II Quantum Yields Calculated Fromsimple Fluorescence Parameters Measured byPAM Fluorometry and the Saturation Pulse Method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullaev, F.; Churikova, D.; Pirogova, P.; Lysov, M.; Vodeneev, V.; Sherstneva, O. Search of Reflectance Indices for Estimating Photosynthetic Activity of Wheat Plants Under Drought Stress. Plants 2025, 14, 91. https://doi.org/10.3390/plants14010091
Abdullaev F, Churikova D, Pirogova P, Lysov M, Vodeneev V, Sherstneva O. Search of Reflectance Indices for Estimating Photosynthetic Activity of Wheat Plants Under Drought Stress. Plants. 2025; 14(1):91. https://doi.org/10.3390/plants14010091
Chicago/Turabian StyleAbdullaev, Firuz, Daria Churikova, Polina Pirogova, Maxim Lysov, Vladimir Vodeneev, and Oksana Sherstneva. 2025. "Search of Reflectance Indices for Estimating Photosynthetic Activity of Wheat Plants Under Drought Stress" Plants 14, no. 1: 91. https://doi.org/10.3390/plants14010091
APA StyleAbdullaev, F., Churikova, D., Pirogova, P., Lysov, M., Vodeneev, V., & Sherstneva, O. (2025). Search of Reflectance Indices for Estimating Photosynthetic Activity of Wheat Plants Under Drought Stress. Plants, 14(1), 91. https://doi.org/10.3390/plants14010091






