Phenolic Profile and Antioxidant Capacity of Invasive Solidago canadensis L.: Potential Applications in Phytopharmacy
Abstract
:1. Introduction
2. Results and Discussion
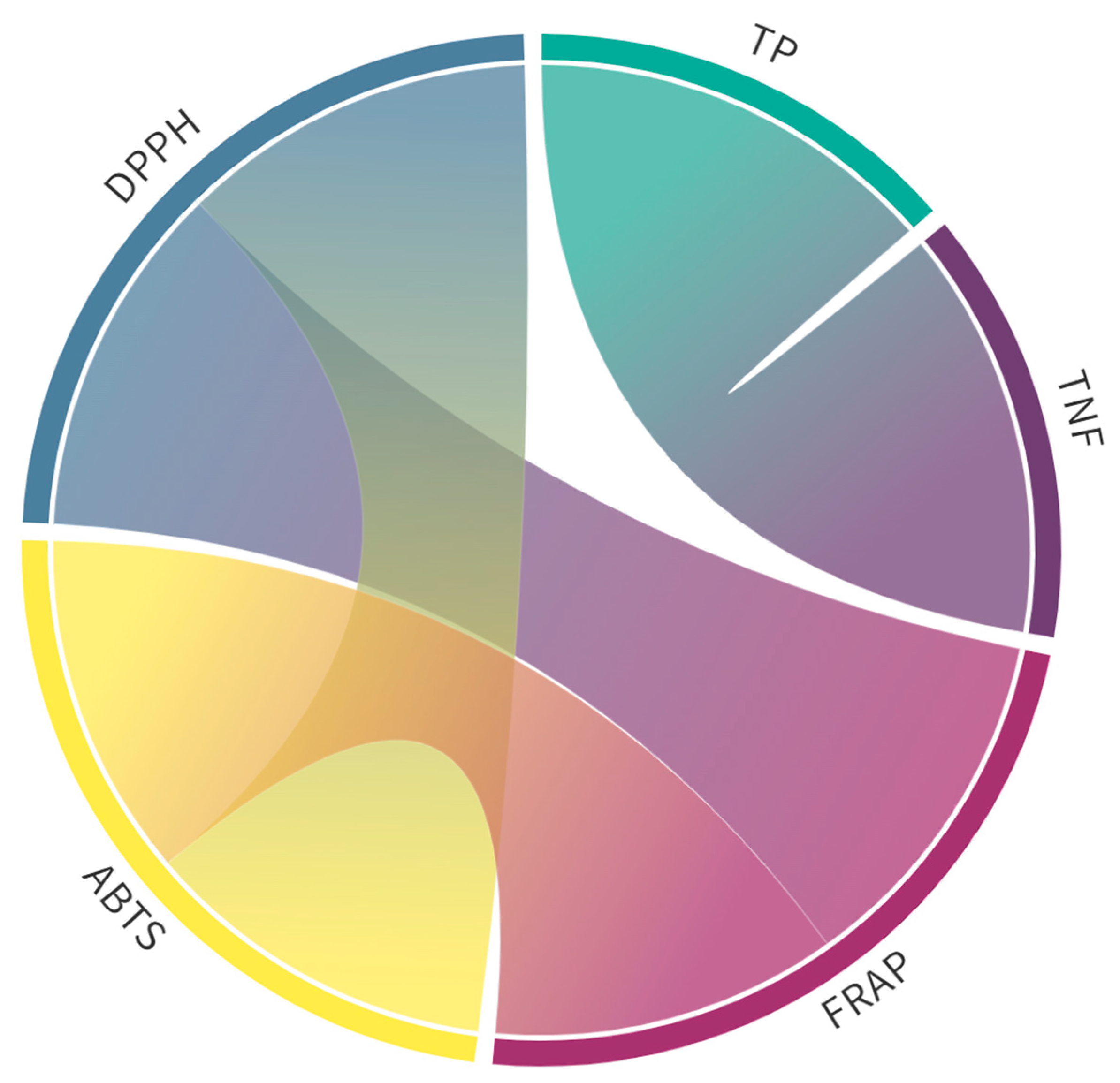
2.1. Phenolic Content and Antioxidant Capacity
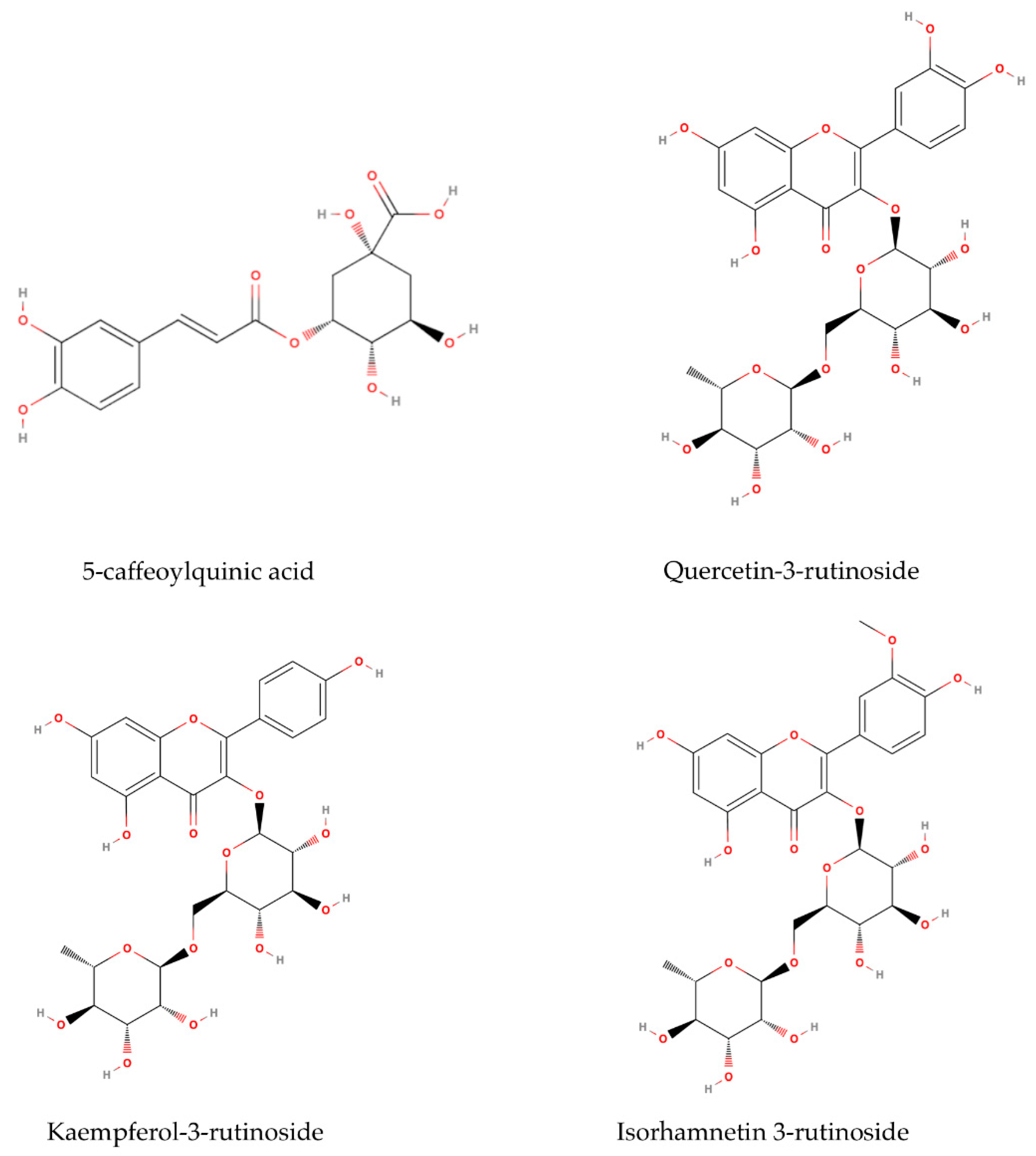
2.2. Identification and Quantification of Phenolic Compounds
3. Materials and Methods
3.1. Plant Material
3.2. Extraction Procedure
3.3. HPLC-DAD-MS Analysis of Phenolic Compounds in Leaf and Flower Extracts
3.4. Total Phenolic, Flavonoid, and Non-Flavonoid Content and Antioxidant Capacity
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fotsing, Y.S.F.; Kezetas, J.J.B.; El-Saber, B.G.; Ali, I.; Ndjakou, B.L. Extraction of Bioactive Compounds from Medicinal Plants and Herbs. In Natural Medicinal Plants; El-Shemy, H., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Corso, M.; Perreau, F.; Mouille, G.; Lepiniec, L. Specialized Phenolic Compounds in Seeds: Structures, Functions, and Regulations. Plant Sci. 2020, 296, 110471. [Google Scholar] [CrossRef] [PubMed]
- Wahle, K.W.; Brown, I.; Rotondo, D.; Heys, S.D. Plant Phenolics in The Prevention and Treatment of Cancer. Adv. Exp. Med. Biol. 2010, 698, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.O.; Lee, E.J. Comparison Of Phenolic Compounds And The Effects of Invasive and Native Species in East Asia: Support for The Novel Weapons Hypothesis. Ecol. Res. 2011, 26, 87–94. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kato, M. Allelopathy and Allelochemicals of Solidago canadensis L. and S. altissima L. for Their Naturalization. Plants 2022, 11, 3235. [Google Scholar] [CrossRef]
- Abhilasha, D.; Quintana, N.; Vivanco, J.; Joshi, J. Do Allelopathic Compounds in Invasive Solidago canadensis s.l. Restrain the Native European Flora? J. Ecol. 2008, 96, 993–1001. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Priyanka, S.K.; Kohli, R.K. Novel Weapon Hypothesis for the Successful Establishment of Invasive Plants in Alien Environments. In Invasive Plant Ecology; Jose, S., Singh, H.P., Batish, D.R., Kohli, R.K., Eds.; Taylor and Francis Group: Abingdon, UK, 2013; pp. 19–28. [Google Scholar]
- Weber, E. The Dynamics of Plant Invasions: A Case Study of Three Exotic Goldenrod Species (Solidago L.) in Europe. J. Biogeogr. 1998, 25, 147–154. [Google Scholar] [CrossRef]
- Semple, J.C.; Beck, J.B. Revised Infrageneric Classification of Solidago (Asteraceae ASTEREAE). Phytoneuron 2021, 10, 1–60. [Google Scholar]
- EPPO Global Database. Solidago canadensis. Available online: https://gd.eppo.int/taxon/SOOCA/distribution (accessed on 7 October 2024).
- Oitsius, L.V.; Volovyk, H.P.; Doletskyi;, S.P.; Lysytsya, A.V. Distribution of Adventive Species Solidago canadensis, Phalacroloma annuum, Ambrosia artemisiifolia, Heracleum sosnowskyi in Phytocenoses of Volyn’ Polissya (Ukraine). Biosyst. Divers. 2020, 28, 343–349. [Google Scholar] [CrossRef]
- Bielecka, A.; Borkowska, L.; Królak, E. Environmental Changes Caused by the Clonal Invasive Plant Solidago canadensis. Ann. Bot. Fenn. 2020, 57, 33. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, Q.; Ruan, X.; Pan, C.D.; Jiang, D.A. Phenolics and Plant Allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Dayan, F.; Nanayakkara, N.; Romagni, J. Ecophysiology and Potential Modes of Action for Selected Lichen Secondary Metabolites, In Allelopathy—Chemistry and Mode of Action of Allelochemicals; Macias, F.A., Galindo, J.C.G., Molinillo, J.M.G., Eds.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar] [CrossRef]
- Apáti, P.; Szentmihályi, K.; Kristó, S.T.; Papp, I.; Vinkler, P.; Szoke, É.; Kéry, Á. Herbal Remedies of Solidago—Correlation of Phytochemical Characteristics and Antioxidative Properties. J. Pharm. Biomed. Anal. 2003, 32, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Shelepova, O.; Vinogradova, Y.; Vergun, O.; Grygorieva, O.; Brindza, J. Assessment of Flavonoids and Phenolic Compound Accumulation in Invasive Solidago canadensis L. in Slovakia. Potravin. Slovak J. Food Sci. 2020, 14, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Marksa, M.; Zymone, K.; Ivanauskas, L.; Radušienė, J.; Pukalskas, A.; Raudone, L. Antioxidant Profiles of Leaves and Inflorescences of Native, Invasive and Hybrid Solidago Species. Ind. Crops Prod. 2020, 145, 112123. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, Y.; Padilla-Zakour, O.; Yang, G. Polyphenols, Antioxidant and Antimicrobial Activities of Leaf and Bark Extracts of Solidago canadensis L. Ind. Crop Prod. 2015, 74, 803–809. [Google Scholar] [CrossRef]
- Quinty, V.; Nasreddine, R.; Colas, C.; Launay, A.; Nehmé, R.; El-Khiraoui, A.; Piot, C.; Draye, M.; Destandau, E.; Da Silva, D.; et al. Antioxidant and Anti-Lipase Capacities from the Extracts Obtained from Two Invasive Plants: Ambrosia artemisiifolia and Solidago canadensis. Food Biosci. 2023, 55, 103069. [Google Scholar] [CrossRef]
- Poljuha, D.; Sladonja, B.; Uzelac Božac, M.; Šola, I.; Damijanić, D.; Weber, T. The Invasive Alien Plant Solidago canadensis: Phytochemical Composition, Ecosystem Service Potential, and Application in Bioeconomy. Plants 2024, 13, 1745. [Google Scholar] [CrossRef]
- Pietta, P.; Minoggio, M.; Bramati, L. Plant Polyphenols: Structure, Occurrence and Bioactivity. Stud. Nat. Prod. Chem. 2003, 28, 257–312. [Google Scholar] [CrossRef]
- Rhazi, N.; Oumam, M.; Hannache, H.; Sesbou, A.; Charrier, B.; Pizzi, A.; Charrier—El Bouhtoury, F. Comparison of the impact of different extraction methods on polyphenols yields and tannins extracted from Moroccan Acacia mollissima barks. Ind. Crops Prod. 2015, 70, 245–252. [Google Scholar] [CrossRef]
- Du, L.; Liu, H.; Yan, M.; Li, J.; Li, J. Individual Plasticity of the Shade Response of the Invasive Solidago canadensis in China. PLoS ONE 2017, 12, e0170049. [Google Scholar] [CrossRef]
- Shelepova, O.; Vinogradova, Y. Phytochemistry and Inflorescences Morphometry of Invasive Solidago L. (Goldenrods) Species—Valuable Late Autumn Mellifers. Agrobiodiver. Improv. Nutr. Health Life Qual. 2021, 5, 209–214. [Google Scholar] [CrossRef]
- Rosłon, W.; Osińska, E.; Mazur, K.; Geszprych, A. Chemical Characteristics of European Goldenrod (Solidago virgaurea L. subsp. virgaurea) From Natural Sites In Central And Eastern Poland. Acta Sci. Pol. Hortorum Cultus 2014, 13, 55–65. [Google Scholar]
- Karlová, K. Accumulation of Flavonoid Compounds in Flowering Shoots of Achillea collina Becker ex. Rchb. Alba During Flower Development. Hortic. Sci. 2006, 33, 158–162. [Google Scholar] [CrossRef]
- Stevenson, F.J.; Nommik, H.; Vahtras, K. Retention and Fixation of Ammonium and Ammonia in Soils. Agron. Monogr. 1982, 22, 123–171. [Google Scholar] [CrossRef]
- Suleymanova, F.; Nesterova, O.; Matyushin, A. HPLC Quantification of Hydroxycinnamic and Organic Acids of Canadian Goldenrod (Solidago canadensis L.). Pharmacogn. J. 2019, 11, 400–404. [Google Scholar] [CrossRef]
- Vinogradova, Y.; Vergun, O.; Grygorieva, O.; Brindza, J. Antioxidant Activity of Solidago L. Complex. Acta Hortic. 2021, 1324, 373–380. [Google Scholar] [CrossRef]
- Woźniak, D.; Ślusarczyk, S.; Domaradzki, K.; Dryś, A.; Matkowski, A. Comparison of Polyphenol Profile and Antimutagenic and Antioxidant Activities in Two Species Used as Source of Solidaginis herba—Goldenrod. Chem. Biodivers. 2018, 15, e1800023. [Google Scholar] [CrossRef]
- Radušienė, J.; Karpavičienė, B.; Vilkickytė, G.; Marksa, M.; Raudonė, L. Comparative Analysis of Root Phenolic Profiles and Antioxidant Activity of Five Native and Invasive Solidago L. Species. Plants 2024, 13, 132. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Avertseva, I.N.; Suleymanova, F.S.; Nesterova, O.V.; Reshetnyak, V.Y.; Matveenko, V.N.; Zhukov, P.A. Study of Polyphenolic Compounds in Extracts from Flowers and Leaves of Canadian Goldenrod and Dwarf Goldenrod (Solidago canadensis L. and Solidago nana Nitt.). Mosc. Univ. Chem. Bull. 2020, 75, 47–51. [Google Scholar] [CrossRef]
- Zekič, J.; Vovk, I.; Glavnik, V. Extraction and Analysis of Flavonoids and Phenolic Acids from Canadian Goldenrod and Giant Goldenrod. Forests 2021, 12, 40. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, J.; Ma, Z.; Xiao, M.; Yang, L.; Tian, B.; Yu, Y.; Bi, C.; Fang, A.; Yang, Y. The Role of Hydroxycinnamic Acid Amide Pathway in Plant Immunity. Front. Plant Sci. 2022, 13, 922119. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant Phenolics: Recent Advances on Their Biosynthesis, Genetics, and Ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Grace, S.C.; Logan, B.A. Energy Dissipation And Radical Scavenging By The Plant Phenylpropanoid Pathway. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1499–1510. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary Phenolics: Chemistry, Bioavailability And Effects on Health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources And Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants in Plants: Location and Functional Significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Treutter, D. Significance of Flavonoids in Plant Resistance and Enhancement of Their Biosynthesis. Plant Biol. 2005, 7, 581–591. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Biosynthesis of Flavonoids and Effects of Stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Agati, G.; Tattini, M. Multiple Functional Roles of Flavonoids in Photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.K.; Weaver, M.L. The Shikimate Pathway. Annu. Rev. Physiol. Plant Mol. Biol. 1999, 50, 473–503. [Google Scholar] [CrossRef] [PubMed]
- Bajko, E.; Kalinowska, M.; Borowski, P.; Siergiejczyk, L.; Lewandowski, W. 5-O-Caffeoylquinic Acid: A Spectroscopic Study And Biological Screening For Antimicrobial Activity. LWT—Food Sci. Technol. 2015, 65, 471–479. [Google Scholar] [CrossRef]
- Ruano González, A.; Pinto, A.; Chinchilla, N.; Palma, M.; Barbero, G.; Carrera, C.; Vázquez Espinosa, M. Determination of Caffeoylquinic Acids Content by UHPLC in Scolymus hispanicus Extracts Obtained through Ultrasound-Assisted Extraction. Plants 2023, 12, 2340. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ye, Y.; Huang, H.; Dong, L. Kaempferol-3-O-β-D-glucoside, a Potential Allelochemical Isolated From Solidago canadensis. Allelopath. J. 2011, 28, 259–266. [Google Scholar]
- Apati, P.; Szentmihályi, K.; Balázs, A.; Baumann, D.; Hamburger, M.; Kristó, T.S.; Szőke, É.; Kéry, Á. HPLC Analysis of the Flavonoids in Pharmaceutical Preparations from Canadian Goldenrod (Solidago canadensis). Chromatographia 2002, 56, S65–S68. [Google Scholar] [CrossRef]
- Al-Shabibi, M.H.S.; Al-Touby, S.S.J.; Hossain, M.A. Isolation, Characterization and Prediction of Biologically Active Glycoside Compounds Quercetin-3-rutinoside From the Fruits of Ficus sycomorus. Carbohydr. Res. 2022, 511, 108483. [Google Scholar] [CrossRef]
- Likhanov, A.; Oliinyk, M.; Pashkevych, N.; Churilov, A.; Kozyr, M. The Role of Flavonoids in Invasion Strategy of Solidago canadensis L. Plants 2021, 10, 1748. [Google Scholar] [CrossRef]
- Zhang, C.B.; Wang, J.; Qian, B.Y.; Li, W.H. Effects of the Invader Solidago canadensis on Soil Properties. Appl. Soil Ecol. 2009, 43, 163–169. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Trepiana, J.; Laskibar, I.; Macarulla, M.; Eseberri, I.; Laura, A.G.I.; Merino-Valdeolmillos, R.; McGeoch, I.; Fernández-Quintela, A.; Portillo, M. Isorhamnetin: Current Knowledge And Potential Benefits For Disease Management. In Handbook of Dietary Flavonoids; Xiao, J., Ed.; Springer: Cham, Switzerland, 2023; pp. 1–61. [Google Scholar]
- Zhao, Z.; Liu, Y. Cardiovascular Protective Effect of Isorhamnetin. Med. Recapitul. 2008, 14, 2321–2323. [Google Scholar]
- Li, J.; Wang, G.; Du, S. Research progress on antitumor effect and mechanism of isorhamnetin. Shanxi Med. J. 2011, 12, 1215–1217. [Google Scholar]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A Review of Pharmacological Effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Yang, J.; Bao, C.; Lu, F.; Wu, Q.; Wu, Z.; Lv, H.; Zhou, Y.; Liu, Y.; Zhu, N.; et al. Isorhamnetin: What is the in Vitro Evidence for its Antitumor Potential and Beyond? Front. Pharmacol. 2024, 15, 1309178. [Google Scholar] [CrossRef] [PubMed]
- Havsteen, B.H. The Biochemistry and Medical Significance of the Flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef] [PubMed]
- Li, R.P.; Guo, M.L.; Zhang, G.; Xu, X.F. Neuroprotection of Nicotiflorin in Permanent Focal Cerebral Ischemia and in Neuronal Cultures. Biol. Pharm. Bull. 2006, 29, 1868–1872. [Google Scholar] [CrossRef]
- Huang, J.L.; Fu, S.T.; Jiang, Y.Y.; Cao, Y.B. Protective Effects of Nicotiflorin on Reducing Memory Dysfunction, Energy Metabolism Failure and Oxidative Stress in Multi-Infarct Dementia Model Rats. Pharmacol. Biochem. Behav. 2007, 86, 741–748. [Google Scholar] [CrossRef]
- Abreu, P.M.; Braham, H.; Ben Jannet, H.; Mighri, Z.; Matthew, S. Antioxidant Compounds from Ebenus pinnata. Fitoterapia 2007, 78, 32–34. [Google Scholar] [CrossRef]
- Patel, D.K. Medicinal Importance, Pharmacological Activities and Analytical Aspects of a Flavonoid Glycoside ‘Nicotiflorin’ in the Medicine. Drug Metab. Bioanal. Lett. 2022, 15, 2–11. [Google Scholar] [CrossRef]
- Xu, S.; Chen, S.; Xia, W.; Sui, H.; Fu, X. Hyperoside: A Review of Its Structure, Synthesis, Pharmacology, Pharmacokinetics and Toxicity. Molecules 2022, 27, 3009. [Google Scholar] [CrossRef]
- Dobjanschi, L.; Zdrinca, M.; Mureşan, M.; Vicas, S.; Antonescu, A. The Thin Layer Chromatography Analysis of Saponins Belonging to Solidago Species. Ann. Univ. Oradea 2014, 11, 56–60. [Google Scholar]
- Takahashi, Y.; Shiojiri, K.; Yamawo, A. Aboveground Plant-to-Plant Communication Reduces Root Nodule Symbiosis and Soil Nutrient Concentrations. Sci. Rep. 2021, 11, 12675. [Google Scholar] [CrossRef] [PubMed]
- Szakiel, A.; Pączkowski, C.; Henry, M. Influence of Environmental Biotic Factors on the Content of Saponins in Plants. Phytochem. Rev. 2011, 10, 493–502. [Google Scholar] [CrossRef]
- Global Biodiversity Information Facility. Solidago canadensis. Available online: https://www.gbif.org/species/5389029 (accessed on 7 October 2024).
- Huang, Y.; Bai, Y.; Wang, Y.; Kong, H. Allelopathic Effects of the Extracts From an Invasive Species Solidago canadensis L. on Microcystis aeruginosa. Lett. Appl. Microbiol. 2013, 57, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Gruľová, D.; Baranová, B.; Caputo, L.; De Martino, L.; Sedlák, V.; Camele, I.; De Feo, V. Antimicrobial Activity and Chemical Composition of Essential Oil Extracted from Solidago canadensis L. Growing Wild in Slovakia. Molecules 2018, 24, 1206. [Google Scholar] [CrossRef]
- Mariychuk, R.; Gruľová, D.; Grishchenko, L.M.; Linni, R.P.; Lisnyak, V.V. Green Synthesis of Non-spherical Gold Nanoparticles Using Solidago canadensis L. Extract. Appl. Nanosci. 2020, 10, 4817–4826. [Google Scholar] [CrossRef]
- Bilić, J.; Svorcina, M.; Poljuha, D. Antioxidant Capacity of Fruit Species Characteristic for Gardens in Istria (Croatia). In Proceedings of the 9th International Congress of Food Technologists, Biotechnologists and Nutritionists, Zagreb, Croatia, 3–5 October 2018. [Google Scholar]
- Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Sircelj, H. Wild Prunus Fruit Species as a Rich Source of Bioactive Compounds. J. Food Sci. 2016, 81, C1928–C1937. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Koron, D.; Rusjan, D. The Impact of Food Processing on the Phenolic Content in Products Made from Juneberry (Amelanchier lamarckii) Fruits. J. Food Sci. 2020, 85, 386–393. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Amerine, A.; Ough, C.S. Methods for Analysis of Musts and Wines. J. Inst. Brew. 1981, 87, 223–224. [Google Scholar] [CrossRef]
- Martins, D.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Nutritional and in Vitro Antioxidant Properties of Edible Wild Greens in Iberian Peninsula Traditional Diet. Food Chem. 2011, 125, 488–494. [Google Scholar] [CrossRef]
- Poljuha, D.; Šola, I.; Bilić, J.; Dudaš, S.; Bilušić, T.; Markić, J.; Rusak, G. Phenolic Composition, Antioxidant Capacity, Energy Content and Gastrointestinal Stability of Croatian Wild Edible Plants. Eur. Food Res. Technol. 2015, 241, 573–585. [Google Scholar] [CrossRef]


| Phenolic Compounds | Solidago canadensis Leaf | Solidago canadensis Flower | ||
|---|---|---|---|---|
| 70% EtOH | 80% MeOH | 70% EtOH | 80% MeOH | |
| 3-caffeoylquinic acid 1 | 1.393 ± 0.092 a | 1.270 ± 0.124 a | 0.433 ± 0.161 b | 0.617 ± 0.071 b |
| 4-caffeoylquinic acid 1 | 0.542 ± 0.098 a | 0.653 ± 0.123 a | 0.383 ± 0.096 a | 0.477 ± 0.121 a |
| 5-caffeoylquinic acid 1 | 17.748 ±1.558 a | 20.423 ± 0.737 a | 9.837 ± 0.685 c | 13.600 ± 0.667 b |
| 5-caffeoylquinic acid 2 | 0.920 ± 0.053 a | 0.797 ± 0.078 a | 0.732 ±0.200 ab | 0.413 ± 0.088 b |
| Caffeic acid | 0.008 ± 0.000 c | 0.007 ± 0.000 c | 0.172 ± 0.047 a | 0.097 ± 0.021 b |
| Caffeic acid hexoside 1 | 0.652 ± 0.013 a | 0.293 ± 0.017 b | 0.766 ± 0.128 a | 0.532 ± 0.088 ab |
| Caffeic acid hexoside 2 | 0.006 ± 0.001 a | 0.006 ± 0.001 a | 0.008 ± 0.001 a | 0.009 ± 0.002 a |
| Dicaffeoylquinic acid 1 | 0.349 ± 0.023 c | 0.225 ± 0.014 c | 0.930 ± 0.337 ab | 1.538 ± 0.244 a |
| Dicaffeoylquinic acid 2 | 2.412 ± 0.058 b | 2.868 ± 0.018 a | n.d. | n.d. |
| Dicaffeoylquinic acid 3 | 0.149 ± 0.014 a | 0.130 ± 0.013 a | 0.237 ± 0.117 a | 0.275 ± 0.015 a |
| Dicaffeoylquinic acid 4 | 0.344 ± 0.042 c | 0.463 ± 0.024 c | 0.965 ± 0.124 b | 1.247 ± 0.088 a |
| p-coumaric acid | 0.486 ± 0.007 a | 0.169 ± 0.006 b | 0.124 ± 0.040 bc | 0.069 ± 0.011 c |
| p-coumaric acid hexoside 1 | 0.261± 0.026 a | 0.114 ± 0.013 b | 0.069 ± 0.011 c | 0.048 ± 0.008 c |
| p-coumaric acid hexoside 2 | 0.173 ± 0.010 a | 0.188 ± 0.012 a | 0.001 ± 0.000 b | 0.001 ± 0.000 b |
| 3-p-coumaroylquinic acid | 1.694 ± 0.554 a | 0.468 ± 0.080 b | 0.752 ± 0.071 b | 0.119 ± 0.024 b |
| 4-p-coumaroylquinic acid 1 | 0.293 ± 0.018 a | 0.088 ± 0014 b | n.d. | n.d. |
| 5-p-coumaroylquinic acid 1 | 0.556 ± 0.036 a | 0.572 ± 0.046 a | 0.264 ± 0.062 b | 0.200 ± 0.051 b |
| 5-p-coumaroylquinic acid 2 | 0.290 ± 0.033 a | 0.381 ± 0.012 a | 0.273 ±0.138 a | 0.234 ± 0.039 a |
| 3-feruloylquinic acid | 0.081 ± 0.014 ab | 0.092 ± 0.019 a | 0.034 ± 0.009 c | 0.043 ± 0.011 bc |
| 5-feruloylquinic acid 1 | 1.013 ± 0.095 ab | 1.225 ± 0.060 a | 0.805 ± 0.056 bc | 0.750 ± 0.130 c |
| Ferulic acid | 0.002 ± 0.000 b | 0.002 ± 0.001 b | 0.191 ± 0.045 a | 0.145 ± 0.037 a |
| Hydroxycinnamic acid derivatives | 27.749 ± 1.081 a | 28.594 ± 3.694 a | 17.272 ± 1.480 b | 20.498 ± 0.944 b |
| p-hydroxybenzoic acid | 0.591 ± 0.023 a | 0.213 ± 0.027 b | n.d. | n.d. |
| Syringic acid | 0.713 ± 0.021 b | 0.417 ± 0.009 c | 0.901 ± 0.002 a | 0.224 ± 0.033 d |
| Protocatechuic acid | 0.023 ± 0.002 b | 0.021 ± 0.002 b | 0.526 ± 0.024 a | 0.535 ± 0.008 a |
| Hydroxybenzoic acid derivatives | 1.328 ± 0.020 a | 0.645 ± 0.020 c | 1.427 ± 0.023 b | 0.759 ± 0.023 d |
| Quercetin pentoside 1 | 0.045 ± 0.005 a | 0.036 ± 0.003 a | 0.041 ± 0.014 a | 0.037 ± 0.001 a |
| Quercetin pentoside 2 | 0.324 ± 0.021 a | 0.210 ± 0.013 b | 0.056 ± 0.020 c | 0.092 ± 0.015 c |
| Quercetin-3-rutinoside | 14.465 ± 0.265 bc | 11.317 ± 0.559 c | 30.702 ± 4.158 a | 19.653 ± 2.434 b |
| Quercetin-3-galactoside | 0.816 ± 0.106 a | 0.454 ± 0.072 b | 0.734 ± 0.160 ab | 0.699 ± 0.056 ab |
| Quercetin-3-glucoside | 0.710 ± 0.018 c | 0.684 ± 0.058 c | 4.881 ± 0.442 a | 4.020 ± 0.223 b |
| Quercetin-3-rhamnoside | 0.127 ± 0.005 b | 0.157 ± 0.016 a | n.d. | n.d. |
| Quercetin acetylhexoside 1 | 3.210 ± 0.258 b | 3.957 ± 0.017 a | n.d. | n.d. |
| Quercetin pentosylhexoside | 0.578 ± 0.090 b | 0.252 ± 0.069 b | 1.064 ± 0.171 a | 1.242 ± 0.162 a |
| Isorhamnetin hexoside | 0.827 ± 0.000 a | 1.226 ± 0.677 a | 0.382 ± 0.144 b | 0.159 ± 0.018 b |
| Isorhamnetin pentosylhexoside | 0.395 ± 0.044 b | 0.438 ± 0.046 b | 0.537 ± 0.137 b | 1.210 ± 0.062 a |
| Isorhamnetin-3-rutinoside | 3.765 ± 0.327 b | 3.781 ± 0.502 b | 6.034 ± 0.452 a | 6.548 ± 0.393 a |
| Isorhamnetin acetylhexoside | 1.489 ± 0.151 b | 1.590 ± 0.128 b | 2.436 ± 0.332 a | 2.123 ± 0256 a |
| Kaempferol rhamnosylhexoside 1 | 2.275 ± 0.058 a | 2.100 ± 0.058 a | 1.514 ± 0.137 b | 1.248 ± 0.069 c |
| Kaempferol-3-galactoside | 0.749 ± 0.089 a | 0.601 ± 0.051 ab | 0.450 ± 0.157 b | 0.408 ± 0.016 b |
| Kaempferol-3-rutinoside | 7.921 ± 0.340 a | 6.792 ± 0.465 a | 5.411 ± 0.555 b | 4.189 ± 0.254 c |
| Kaempferol-3-glucoside | 2.120 ± 0.203 a | 1.430 ± 0.207 b | 0.937 ± 0.354 bc | 0.391 ± 0.044 c |
| Kaempferol acetylhexoside 1 | 4.595 ± 0.306 a | 4.277 ± 0.450 a | 1.851 ± 0.102 b | 1.982 ± 0.154 b |
| Flavonols | 43.580 ± 1.392 b | 38.686 ± 1.699 b | 57.028 ± 4.649 a | 44.000 ± 3.774 b |
| TOTAL | 73.063 ± 1.553 ab* | 67.924 ± 3.701 ab* | 75.723 ± 5.836 a* | 65.257 ± 4.702 b* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzelac Božac, M.; Poljuha, D.; Dudaš, S.; Bilić, J.; Šola, I.; Mikulič-Petkovšek, M.; Sladonja, B. Phenolic Profile and Antioxidant Capacity of Invasive Solidago canadensis L.: Potential Applications in Phytopharmacy. Plants 2025, 14, 44. https://doi.org/10.3390/plants14010044
Uzelac Božac M, Poljuha D, Dudaš S, Bilić J, Šola I, Mikulič-Petkovšek M, Sladonja B. Phenolic Profile and Antioxidant Capacity of Invasive Solidago canadensis L.: Potential Applications in Phytopharmacy. Plants. 2025; 14(1):44. https://doi.org/10.3390/plants14010044
Chicago/Turabian StyleUzelac Božac, Mirela, Danijela Poljuha, Slavica Dudaš, Josipa Bilić, Ivana Šola, Maja Mikulič-Petkovšek, and Barbara Sladonja. 2025. "Phenolic Profile and Antioxidant Capacity of Invasive Solidago canadensis L.: Potential Applications in Phytopharmacy" Plants 14, no. 1: 44. https://doi.org/10.3390/plants14010044
APA StyleUzelac Božac, M., Poljuha, D., Dudaš, S., Bilić, J., Šola, I., Mikulič-Petkovšek, M., & Sladonja, B. (2025). Phenolic Profile and Antioxidant Capacity of Invasive Solidago canadensis L.: Potential Applications in Phytopharmacy. Plants, 14(1), 44. https://doi.org/10.3390/plants14010044












