Phenolic Profile and Antioxidant Capacity of Invasive Solidago canadensis L.: Potential Applications in Phytopharmacy
Abstract
1. Introduction
2. Results and Discussion
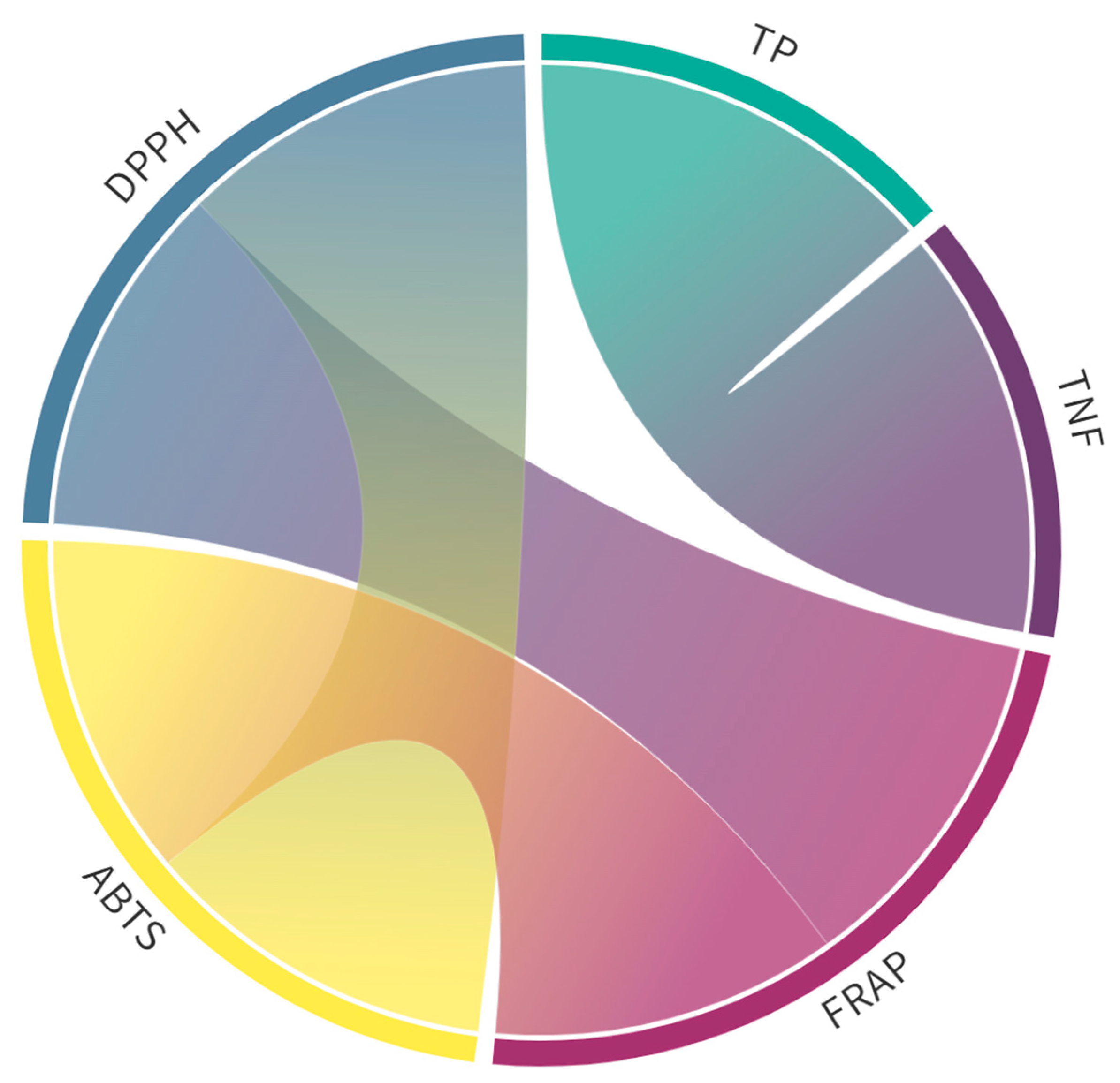
2.1. Phenolic Content and Antioxidant Capacity
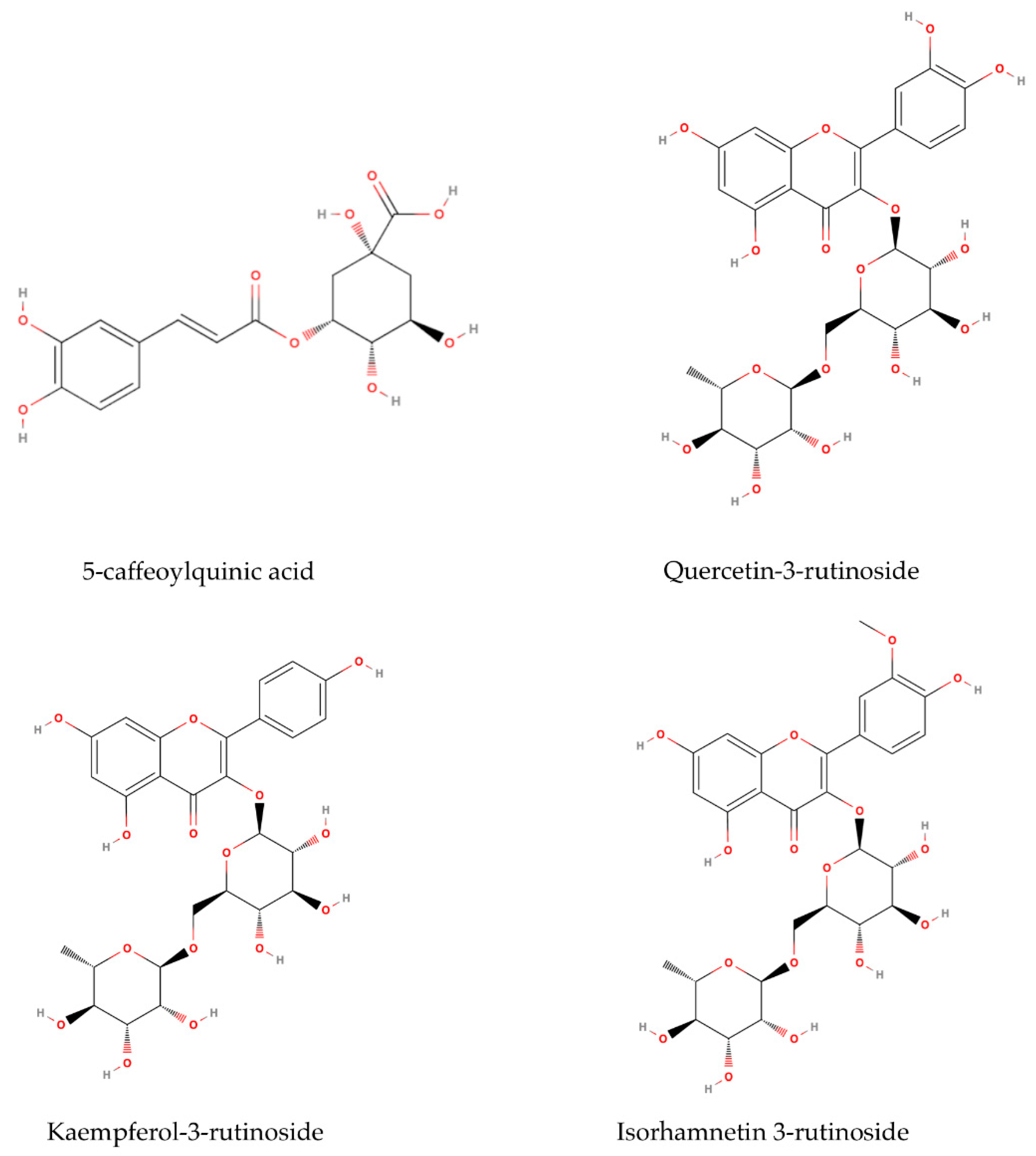
2.2. Identification and Quantification of Phenolic Compounds
3. Materials and Methods
3.1. Plant Material
3.2. Extraction Procedure
3.3. HPLC-DAD-MS Analysis of Phenolic Compounds in Leaf and Flower Extracts
3.4. Total Phenolic, Flavonoid, and Non-Flavonoid Content and Antioxidant Capacity
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fotsing, Y.S.F.; Kezetas, J.J.B.; El-Saber, B.G.; Ali, I.; Ndjakou, B.L. Extraction of Bioactive Compounds from Medicinal Plants and Herbs. In Natural Medicinal Plants; El-Shemy, H., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Corso, M.; Perreau, F.; Mouille, G.; Lepiniec, L. Specialized Phenolic Compounds in Seeds: Structures, Functions, and Regulations. Plant Sci. 2020, 296, 110471. [Google Scholar] [CrossRef] [PubMed]
- Wahle, K.W.; Brown, I.; Rotondo, D.; Heys, S.D. Plant Phenolics in The Prevention and Treatment of Cancer. Adv. Exp. Med. Biol. 2010, 698, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.O.; Lee, E.J. Comparison Of Phenolic Compounds And The Effects of Invasive and Native Species in East Asia: Support for The Novel Weapons Hypothesis. Ecol. Res. 2011, 26, 87–94. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kato, M. Allelopathy and Allelochemicals of Solidago canadensis L. and S. altissima L. for Their Naturalization. Plants 2022, 11, 3235. [Google Scholar] [CrossRef]
- Abhilasha, D.; Quintana, N.; Vivanco, J.; Joshi, J. Do Allelopathic Compounds in Invasive Solidago canadensis s.l. Restrain the Native European Flora? J. Ecol. 2008, 96, 993–1001. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Priyanka, S.K.; Kohli, R.K. Novel Weapon Hypothesis for the Successful Establishment of Invasive Plants in Alien Environments. In Invasive Plant Ecology; Jose, S., Singh, H.P., Batish, D.R., Kohli, R.K., Eds.; Taylor and Francis Group: Abingdon, UK, 2013; pp. 19–28. [Google Scholar]
- Weber, E. The Dynamics of Plant Invasions: A Case Study of Three Exotic Goldenrod Species (Solidago L.) in Europe. J. Biogeogr. 1998, 25, 147–154. [Google Scholar] [CrossRef]
- Semple, J.C.; Beck, J.B. Revised Infrageneric Classification of Solidago (Asteraceae ASTEREAE). Phytoneuron 2021, 10, 1–60. [Google Scholar]
- EPPO Global Database. Solidago canadensis. Available online: https://gd.eppo.int/taxon/SOOCA/distribution (accessed on 7 October 2024).
- Oitsius, L.V.; Volovyk, H.P.; Doletskyi;, S.P.; Lysytsya, A.V. Distribution of Adventive Species Solidago canadensis, Phalacroloma annuum, Ambrosia artemisiifolia, Heracleum sosnowskyi in Phytocenoses of Volyn’ Polissya (Ukraine). Biosyst. Divers. 2020, 28, 343–349. [Google Scholar] [CrossRef]
- Bielecka, A.; Borkowska, L.; Królak, E. Environmental Changes Caused by the Clonal Invasive Plant Solidago canadensis. Ann. Bot. Fenn. 2020, 57, 33. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, Q.; Ruan, X.; Pan, C.D.; Jiang, D.A. Phenolics and Plant Allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Dayan, F.; Nanayakkara, N.; Romagni, J. Ecophysiology and Potential Modes of Action for Selected Lichen Secondary Metabolites, In Allelopathy—Chemistry and Mode of Action of Allelochemicals; Macias, F.A., Galindo, J.C.G., Molinillo, J.M.G., Eds.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar] [CrossRef]
- Apáti, P.; Szentmihályi, K.; Kristó, S.T.; Papp, I.; Vinkler, P.; Szoke, É.; Kéry, Á. Herbal Remedies of Solidago—Correlation of Phytochemical Characteristics and Antioxidative Properties. J. Pharm. Biomed. Anal. 2003, 32, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Shelepova, O.; Vinogradova, Y.; Vergun, O.; Grygorieva, O.; Brindza, J. Assessment of Flavonoids and Phenolic Compound Accumulation in Invasive Solidago canadensis L. in Slovakia. Potravin. Slovak J. Food Sci. 2020, 14, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Marksa, M.; Zymone, K.; Ivanauskas, L.; Radušienė, J.; Pukalskas, A.; Raudone, L. Antioxidant Profiles of Leaves and Inflorescences of Native, Invasive and Hybrid Solidago Species. Ind. Crops Prod. 2020, 145, 112123. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, Y.; Padilla-Zakour, O.; Yang, G. Polyphenols, Antioxidant and Antimicrobial Activities of Leaf and Bark Extracts of Solidago canadensis L. Ind. Crop Prod. 2015, 74, 803–809. [Google Scholar] [CrossRef]
- Quinty, V.; Nasreddine, R.; Colas, C.; Launay, A.; Nehmé, R.; El-Khiraoui, A.; Piot, C.; Draye, M.; Destandau, E.; Da Silva, D.; et al. Antioxidant and Anti-Lipase Capacities from the Extracts Obtained from Two Invasive Plants: Ambrosia artemisiifolia and Solidago canadensis. Food Biosci. 2023, 55, 103069. [Google Scholar] [CrossRef]
- Poljuha, D.; Sladonja, B.; Uzelac Božac, M.; Šola, I.; Damijanić, D.; Weber, T. The Invasive Alien Plant Solidago canadensis: Phytochemical Composition, Ecosystem Service Potential, and Application in Bioeconomy. Plants 2024, 13, 1745. [Google Scholar] [CrossRef]
- Pietta, P.; Minoggio, M.; Bramati, L. Plant Polyphenols: Structure, Occurrence and Bioactivity. Stud. Nat. Prod. Chem. 2003, 28, 257–312. [Google Scholar] [CrossRef]
- Rhazi, N.; Oumam, M.; Hannache, H.; Sesbou, A.; Charrier, B.; Pizzi, A.; Charrier—El Bouhtoury, F. Comparison of the impact of different extraction methods on polyphenols yields and tannins extracted from Moroccan Acacia mollissima barks. Ind. Crops Prod. 2015, 70, 245–252. [Google Scholar] [CrossRef]
- Du, L.; Liu, H.; Yan, M.; Li, J.; Li, J. Individual Plasticity of the Shade Response of the Invasive Solidago canadensis in China. PLoS ONE 2017, 12, e0170049. [Google Scholar] [CrossRef]
- Shelepova, O.; Vinogradova, Y. Phytochemistry and Inflorescences Morphometry of Invasive Solidago L. (Goldenrods) Species—Valuable Late Autumn Mellifers. Agrobiodiver. Improv. Nutr. Health Life Qual. 2021, 5, 209–214. [Google Scholar] [CrossRef]
- Rosłon, W.; Osińska, E.; Mazur, K.; Geszprych, A. Chemical Characteristics of European Goldenrod (Solidago virgaurea L. subsp. virgaurea) From Natural Sites In Central And Eastern Poland. Acta Sci. Pol. Hortorum Cultus 2014, 13, 55–65. [Google Scholar]
- Karlová, K. Accumulation of Flavonoid Compounds in Flowering Shoots of Achillea collina Becker ex. Rchb. Alba During Flower Development. Hortic. Sci. 2006, 33, 158–162. [Google Scholar] [CrossRef]
- Stevenson, F.J.; Nommik, H.; Vahtras, K. Retention and Fixation of Ammonium and Ammonia in Soils. Agron. Monogr. 1982, 22, 123–171. [Google Scholar] [CrossRef]
- Suleymanova, F.; Nesterova, O.; Matyushin, A. HPLC Quantification of Hydroxycinnamic and Organic Acids of Canadian Goldenrod (Solidago canadensis L.). Pharmacogn. J. 2019, 11, 400–404. [Google Scholar] [CrossRef]
- Vinogradova, Y.; Vergun, O.; Grygorieva, O.; Brindza, J. Antioxidant Activity of Solidago L. Complex. Acta Hortic. 2021, 1324, 373–380. [Google Scholar] [CrossRef]
- Woźniak, D.; Ślusarczyk, S.; Domaradzki, K.; Dryś, A.; Matkowski, A. Comparison of Polyphenol Profile and Antimutagenic and Antioxidant Activities in Two Species Used as Source of Solidaginis herba—Goldenrod. Chem. Biodivers. 2018, 15, e1800023. [Google Scholar] [CrossRef]
- Radušienė, J.; Karpavičienė, B.; Vilkickytė, G.; Marksa, M.; Raudonė, L. Comparative Analysis of Root Phenolic Profiles and Antioxidant Activity of Five Native and Invasive Solidago L. Species. Plants 2024, 13, 132. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Avertseva, I.N.; Suleymanova, F.S.; Nesterova, O.V.; Reshetnyak, V.Y.; Matveenko, V.N.; Zhukov, P.A. Study of Polyphenolic Compounds in Extracts from Flowers and Leaves of Canadian Goldenrod and Dwarf Goldenrod (Solidago canadensis L. and Solidago nana Nitt.). Mosc. Univ. Chem. Bull. 2020, 75, 47–51. [Google Scholar] [CrossRef]
- Zekič, J.; Vovk, I.; Glavnik, V. Extraction and Analysis of Flavonoids and Phenolic Acids from Canadian Goldenrod and Giant Goldenrod. Forests 2021, 12, 40. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, J.; Ma, Z.; Xiao, M.; Yang, L.; Tian, B.; Yu, Y.; Bi, C.; Fang, A.; Yang, Y. The Role of Hydroxycinnamic Acid Amide Pathway in Plant Immunity. Front. Plant Sci. 2022, 13, 922119. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant Phenolics: Recent Advances on Their Biosynthesis, Genetics, and Ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Grace, S.C.; Logan, B.A. Energy Dissipation And Radical Scavenging By The Plant Phenylpropanoid Pathway. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1499–1510. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary Phenolics: Chemistry, Bioavailability And Effects on Health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources And Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants in Plants: Location and Functional Significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Treutter, D. Significance of Flavonoids in Plant Resistance and Enhancement of Their Biosynthesis. Plant Biol. 2005, 7, 581–591. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Biosynthesis of Flavonoids and Effects of Stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Agati, G.; Tattini, M. Multiple Functional Roles of Flavonoids in Photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.K.; Weaver, M.L. The Shikimate Pathway. Annu. Rev. Physiol. Plant Mol. Biol. 1999, 50, 473–503. [Google Scholar] [CrossRef] [PubMed]
- Bajko, E.; Kalinowska, M.; Borowski, P.; Siergiejczyk, L.; Lewandowski, W. 5-O-Caffeoylquinic Acid: A Spectroscopic Study And Biological Screening For Antimicrobial Activity. LWT—Food Sci. Technol. 2015, 65, 471–479. [Google Scholar] [CrossRef]
- Ruano González, A.; Pinto, A.; Chinchilla, N.; Palma, M.; Barbero, G.; Carrera, C.; Vázquez Espinosa, M. Determination of Caffeoylquinic Acids Content by UHPLC in Scolymus hispanicus Extracts Obtained through Ultrasound-Assisted Extraction. Plants 2023, 12, 2340. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ye, Y.; Huang, H.; Dong, L. Kaempferol-3-O-β-D-glucoside, a Potential Allelochemical Isolated From Solidago canadensis. Allelopath. J. 2011, 28, 259–266. [Google Scholar]
- Apati, P.; Szentmihályi, K.; Balázs, A.; Baumann, D.; Hamburger, M.; Kristó, T.S.; Szőke, É.; Kéry, Á. HPLC Analysis of the Flavonoids in Pharmaceutical Preparations from Canadian Goldenrod (Solidago canadensis). Chromatographia 2002, 56, S65–S68. [Google Scholar] [CrossRef]
- Al-Shabibi, M.H.S.; Al-Touby, S.S.J.; Hossain, M.A. Isolation, Characterization and Prediction of Biologically Active Glycoside Compounds Quercetin-3-rutinoside From the Fruits of Ficus sycomorus. Carbohydr. Res. 2022, 511, 108483. [Google Scholar] [CrossRef]
- Likhanov, A.; Oliinyk, M.; Pashkevych, N.; Churilov, A.; Kozyr, M. The Role of Flavonoids in Invasion Strategy of Solidago canadensis L. Plants 2021, 10, 1748. [Google Scholar] [CrossRef]
- Zhang, C.B.; Wang, J.; Qian, B.Y.; Li, W.H. Effects of the Invader Solidago canadensis on Soil Properties. Appl. Soil Ecol. 2009, 43, 163–169. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Trepiana, J.; Laskibar, I.; Macarulla, M.; Eseberri, I.; Laura, A.G.I.; Merino-Valdeolmillos, R.; McGeoch, I.; Fernández-Quintela, A.; Portillo, M. Isorhamnetin: Current Knowledge And Potential Benefits For Disease Management. In Handbook of Dietary Flavonoids; Xiao, J., Ed.; Springer: Cham, Switzerland, 2023; pp. 1–61. [Google Scholar]
- Zhao, Z.; Liu, Y. Cardiovascular Protective Effect of Isorhamnetin. Med. Recapitul. 2008, 14, 2321–2323. [Google Scholar]
- Li, J.; Wang, G.; Du, S. Research progress on antitumor effect and mechanism of isorhamnetin. Shanxi Med. J. 2011, 12, 1215–1217. [Google Scholar]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A Review of Pharmacological Effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Yang, J.; Bao, C.; Lu, F.; Wu, Q.; Wu, Z.; Lv, H.; Zhou, Y.; Liu, Y.; Zhu, N.; et al. Isorhamnetin: What is the in Vitro Evidence for its Antitumor Potential and Beyond? Front. Pharmacol. 2024, 15, 1309178. [Google Scholar] [CrossRef] [PubMed]
- Havsteen, B.H. The Biochemistry and Medical Significance of the Flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef] [PubMed]
- Li, R.P.; Guo, M.L.; Zhang, G.; Xu, X.F. Neuroprotection of Nicotiflorin in Permanent Focal Cerebral Ischemia and in Neuronal Cultures. Biol. Pharm. Bull. 2006, 29, 1868–1872. [Google Scholar] [CrossRef]
- Huang, J.L.; Fu, S.T.; Jiang, Y.Y.; Cao, Y.B. Protective Effects of Nicotiflorin on Reducing Memory Dysfunction, Energy Metabolism Failure and Oxidative Stress in Multi-Infarct Dementia Model Rats. Pharmacol. Biochem. Behav. 2007, 86, 741–748. [Google Scholar] [CrossRef]
- Abreu, P.M.; Braham, H.; Ben Jannet, H.; Mighri, Z.; Matthew, S. Antioxidant Compounds from Ebenus pinnata. Fitoterapia 2007, 78, 32–34. [Google Scholar] [CrossRef]
- Patel, D.K. Medicinal Importance, Pharmacological Activities and Analytical Aspects of a Flavonoid Glycoside ‘Nicotiflorin’ in the Medicine. Drug Metab. Bioanal. Lett. 2022, 15, 2–11. [Google Scholar] [CrossRef]
- Xu, S.; Chen, S.; Xia, W.; Sui, H.; Fu, X. Hyperoside: A Review of Its Structure, Synthesis, Pharmacology, Pharmacokinetics and Toxicity. Molecules 2022, 27, 3009. [Google Scholar] [CrossRef]
- Dobjanschi, L.; Zdrinca, M.; Mureşan, M.; Vicas, S.; Antonescu, A. The Thin Layer Chromatography Analysis of Saponins Belonging to Solidago Species. Ann. Univ. Oradea 2014, 11, 56–60. [Google Scholar]
- Takahashi, Y.; Shiojiri, K.; Yamawo, A. Aboveground Plant-to-Plant Communication Reduces Root Nodule Symbiosis and Soil Nutrient Concentrations. Sci. Rep. 2021, 11, 12675. [Google Scholar] [CrossRef] [PubMed]
- Szakiel, A.; Pączkowski, C.; Henry, M. Influence of Environmental Biotic Factors on the Content of Saponins in Plants. Phytochem. Rev. 2011, 10, 493–502. [Google Scholar] [CrossRef]
- Global Biodiversity Information Facility. Solidago canadensis. Available online: https://www.gbif.org/species/5389029 (accessed on 7 October 2024).
- Huang, Y.; Bai, Y.; Wang, Y.; Kong, H. Allelopathic Effects of the Extracts From an Invasive Species Solidago canadensis L. on Microcystis aeruginosa. Lett. Appl. Microbiol. 2013, 57, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Gruľová, D.; Baranová, B.; Caputo, L.; De Martino, L.; Sedlák, V.; Camele, I.; De Feo, V. Antimicrobial Activity and Chemical Composition of Essential Oil Extracted from Solidago canadensis L. Growing Wild in Slovakia. Molecules 2018, 24, 1206. [Google Scholar] [CrossRef]
- Mariychuk, R.; Gruľová, D.; Grishchenko, L.M.; Linni, R.P.; Lisnyak, V.V. Green Synthesis of Non-spherical Gold Nanoparticles Using Solidago canadensis L. Extract. Appl. Nanosci. 2020, 10, 4817–4826. [Google Scholar] [CrossRef]
- Bilić, J.; Svorcina, M.; Poljuha, D. Antioxidant Capacity of Fruit Species Characteristic for Gardens in Istria (Croatia). In Proceedings of the 9th International Congress of Food Technologists, Biotechnologists and Nutritionists, Zagreb, Croatia, 3–5 October 2018. [Google Scholar]
- Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Sircelj, H. Wild Prunus Fruit Species as a Rich Source of Bioactive Compounds. J. Food Sci. 2016, 81, C1928–C1937. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Koron, D.; Rusjan, D. The Impact of Food Processing on the Phenolic Content in Products Made from Juneberry (Amelanchier lamarckii) Fruits. J. Food Sci. 2020, 85, 386–393. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Amerine, A.; Ough, C.S. Methods for Analysis of Musts and Wines. J. Inst. Brew. 1981, 87, 223–224. [Google Scholar] [CrossRef]
- Martins, D.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Nutritional and in Vitro Antioxidant Properties of Edible Wild Greens in Iberian Peninsula Traditional Diet. Food Chem. 2011, 125, 488–494. [Google Scholar] [CrossRef]
- Poljuha, D.; Šola, I.; Bilić, J.; Dudaš, S.; Bilušić, T.; Markić, J.; Rusak, G. Phenolic Composition, Antioxidant Capacity, Energy Content and Gastrointestinal Stability of Croatian Wild Edible Plants. Eur. Food Res. Technol. 2015, 241, 573–585. [Google Scholar] [CrossRef]


| Phenolic Compounds | Solidago canadensis Leaf | Solidago canadensis Flower | ||
|---|---|---|---|---|
| 70% EtOH | 80% MeOH | 70% EtOH | 80% MeOH | |
| 3-caffeoylquinic acid 1 | 1.393 ± 0.092 a | 1.270 ± 0.124 a | 0.433 ± 0.161 b | 0.617 ± 0.071 b |
| 4-caffeoylquinic acid 1 | 0.542 ± 0.098 a | 0.653 ± 0.123 a | 0.383 ± 0.096 a | 0.477 ± 0.121 a |
| 5-caffeoylquinic acid 1 | 17.748 ±1.558 a | 20.423 ± 0.737 a | 9.837 ± 0.685 c | 13.600 ± 0.667 b |
| 5-caffeoylquinic acid 2 | 0.920 ± 0.053 a | 0.797 ± 0.078 a | 0.732 ±0.200 ab | 0.413 ± 0.088 b |
| Caffeic acid | 0.008 ± 0.000 c | 0.007 ± 0.000 c | 0.172 ± 0.047 a | 0.097 ± 0.021 b |
| Caffeic acid hexoside 1 | 0.652 ± 0.013 a | 0.293 ± 0.017 b | 0.766 ± 0.128 a | 0.532 ± 0.088 ab |
| Caffeic acid hexoside 2 | 0.006 ± 0.001 a | 0.006 ± 0.001 a | 0.008 ± 0.001 a | 0.009 ± 0.002 a |
| Dicaffeoylquinic acid 1 | 0.349 ± 0.023 c | 0.225 ± 0.014 c | 0.930 ± 0.337 ab | 1.538 ± 0.244 a |
| Dicaffeoylquinic acid 2 | 2.412 ± 0.058 b | 2.868 ± 0.018 a | n.d. | n.d. |
| Dicaffeoylquinic acid 3 | 0.149 ± 0.014 a | 0.130 ± 0.013 a | 0.237 ± 0.117 a | 0.275 ± 0.015 a |
| Dicaffeoylquinic acid 4 | 0.344 ± 0.042 c | 0.463 ± 0.024 c | 0.965 ± 0.124 b | 1.247 ± 0.088 a |
| p-coumaric acid | 0.486 ± 0.007 a | 0.169 ± 0.006 b | 0.124 ± 0.040 bc | 0.069 ± 0.011 c |
| p-coumaric acid hexoside 1 | 0.261± 0.026 a | 0.114 ± 0.013 b | 0.069 ± 0.011 c | 0.048 ± 0.008 c |
| p-coumaric acid hexoside 2 | 0.173 ± 0.010 a | 0.188 ± 0.012 a | 0.001 ± 0.000 b | 0.001 ± 0.000 b |
| 3-p-coumaroylquinic acid | 1.694 ± 0.554 a | 0.468 ± 0.080 b | 0.752 ± 0.071 b | 0.119 ± 0.024 b |
| 4-p-coumaroylquinic acid 1 | 0.293 ± 0.018 a | 0.088 ± 0014 b | n.d. | n.d. |
| 5-p-coumaroylquinic acid 1 | 0.556 ± 0.036 a | 0.572 ± 0.046 a | 0.264 ± 0.062 b | 0.200 ± 0.051 b |
| 5-p-coumaroylquinic acid 2 | 0.290 ± 0.033 a | 0.381 ± 0.012 a | 0.273 ±0.138 a | 0.234 ± 0.039 a |
| 3-feruloylquinic acid | 0.081 ± 0.014 ab | 0.092 ± 0.019 a | 0.034 ± 0.009 c | 0.043 ± 0.011 bc |
| 5-feruloylquinic acid 1 | 1.013 ± 0.095 ab | 1.225 ± 0.060 a | 0.805 ± 0.056 bc | 0.750 ± 0.130 c |
| Ferulic acid | 0.002 ± 0.000 b | 0.002 ± 0.001 b | 0.191 ± 0.045 a | 0.145 ± 0.037 a |
| Hydroxycinnamic acid derivatives | 27.749 ± 1.081 a | 28.594 ± 3.694 a | 17.272 ± 1.480 b | 20.498 ± 0.944 b |
| p-hydroxybenzoic acid | 0.591 ± 0.023 a | 0.213 ± 0.027 b | n.d. | n.d. |
| Syringic acid | 0.713 ± 0.021 b | 0.417 ± 0.009 c | 0.901 ± 0.002 a | 0.224 ± 0.033 d |
| Protocatechuic acid | 0.023 ± 0.002 b | 0.021 ± 0.002 b | 0.526 ± 0.024 a | 0.535 ± 0.008 a |
| Hydroxybenzoic acid derivatives | 1.328 ± 0.020 a | 0.645 ± 0.020 c | 1.427 ± 0.023 b | 0.759 ± 0.023 d |
| Quercetin pentoside 1 | 0.045 ± 0.005 a | 0.036 ± 0.003 a | 0.041 ± 0.014 a | 0.037 ± 0.001 a |
| Quercetin pentoside 2 | 0.324 ± 0.021 a | 0.210 ± 0.013 b | 0.056 ± 0.020 c | 0.092 ± 0.015 c |
| Quercetin-3-rutinoside | 14.465 ± 0.265 bc | 11.317 ± 0.559 c | 30.702 ± 4.158 a | 19.653 ± 2.434 b |
| Quercetin-3-galactoside | 0.816 ± 0.106 a | 0.454 ± 0.072 b | 0.734 ± 0.160 ab | 0.699 ± 0.056 ab |
| Quercetin-3-glucoside | 0.710 ± 0.018 c | 0.684 ± 0.058 c | 4.881 ± 0.442 a | 4.020 ± 0.223 b |
| Quercetin-3-rhamnoside | 0.127 ± 0.005 b | 0.157 ± 0.016 a | n.d. | n.d. |
| Quercetin acetylhexoside 1 | 3.210 ± 0.258 b | 3.957 ± 0.017 a | n.d. | n.d. |
| Quercetin pentosylhexoside | 0.578 ± 0.090 b | 0.252 ± 0.069 b | 1.064 ± 0.171 a | 1.242 ± 0.162 a |
| Isorhamnetin hexoside | 0.827 ± 0.000 a | 1.226 ± 0.677 a | 0.382 ± 0.144 b | 0.159 ± 0.018 b |
| Isorhamnetin pentosylhexoside | 0.395 ± 0.044 b | 0.438 ± 0.046 b | 0.537 ± 0.137 b | 1.210 ± 0.062 a |
| Isorhamnetin-3-rutinoside | 3.765 ± 0.327 b | 3.781 ± 0.502 b | 6.034 ± 0.452 a | 6.548 ± 0.393 a |
| Isorhamnetin acetylhexoside | 1.489 ± 0.151 b | 1.590 ± 0.128 b | 2.436 ± 0.332 a | 2.123 ± 0256 a |
| Kaempferol rhamnosylhexoside 1 | 2.275 ± 0.058 a | 2.100 ± 0.058 a | 1.514 ± 0.137 b | 1.248 ± 0.069 c |
| Kaempferol-3-galactoside | 0.749 ± 0.089 a | 0.601 ± 0.051 ab | 0.450 ± 0.157 b | 0.408 ± 0.016 b |
| Kaempferol-3-rutinoside | 7.921 ± 0.340 a | 6.792 ± 0.465 a | 5.411 ± 0.555 b | 4.189 ± 0.254 c |
| Kaempferol-3-glucoside | 2.120 ± 0.203 a | 1.430 ± 0.207 b | 0.937 ± 0.354 bc | 0.391 ± 0.044 c |
| Kaempferol acetylhexoside 1 | 4.595 ± 0.306 a | 4.277 ± 0.450 a | 1.851 ± 0.102 b | 1.982 ± 0.154 b |
| Flavonols | 43.580 ± 1.392 b | 38.686 ± 1.699 b | 57.028 ± 4.649 a | 44.000 ± 3.774 b |
| TOTAL | 73.063 ± 1.553 ab* | 67.924 ± 3.701 ab* | 75.723 ± 5.836 a* | 65.257 ± 4.702 b* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzelac Božac, M.; Poljuha, D.; Dudaš, S.; Bilić, J.; Šola, I.; Mikulič-Petkovšek, M.; Sladonja, B. Phenolic Profile and Antioxidant Capacity of Invasive Solidago canadensis L.: Potential Applications in Phytopharmacy. Plants 2025, 14, 44. https://doi.org/10.3390/plants14010044
Uzelac Božac M, Poljuha D, Dudaš S, Bilić J, Šola I, Mikulič-Petkovšek M, Sladonja B. Phenolic Profile and Antioxidant Capacity of Invasive Solidago canadensis L.: Potential Applications in Phytopharmacy. Plants. 2025; 14(1):44. https://doi.org/10.3390/plants14010044
Chicago/Turabian StyleUzelac Božac, Mirela, Danijela Poljuha, Slavica Dudaš, Josipa Bilić, Ivana Šola, Maja Mikulič-Petkovšek, and Barbara Sladonja. 2025. "Phenolic Profile and Antioxidant Capacity of Invasive Solidago canadensis L.: Potential Applications in Phytopharmacy" Plants 14, no. 1: 44. https://doi.org/10.3390/plants14010044
APA StyleUzelac Božac, M., Poljuha, D., Dudaš, S., Bilić, J., Šola, I., Mikulič-Petkovšek, M., & Sladonja, B. (2025). Phenolic Profile and Antioxidant Capacity of Invasive Solidago canadensis L.: Potential Applications in Phytopharmacy. Plants, 14(1), 44. https://doi.org/10.3390/plants14010044












