Leaf Phosphorus Fractions Are Coordinated with Leaf Functional Traits in Four Juvenile Tree Species from the Chinese Subtropics
Abstract
1. Introduction
2. Results
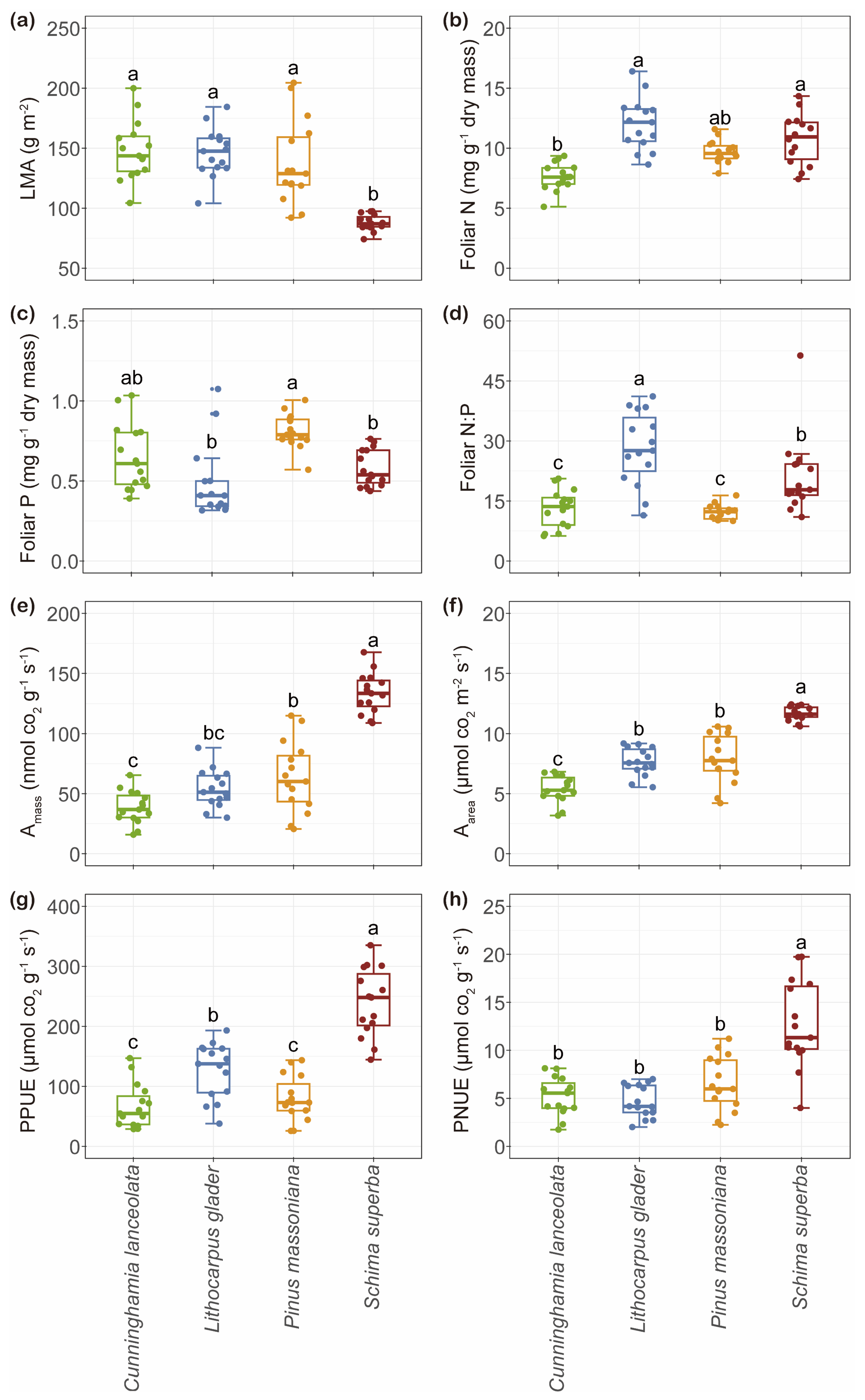
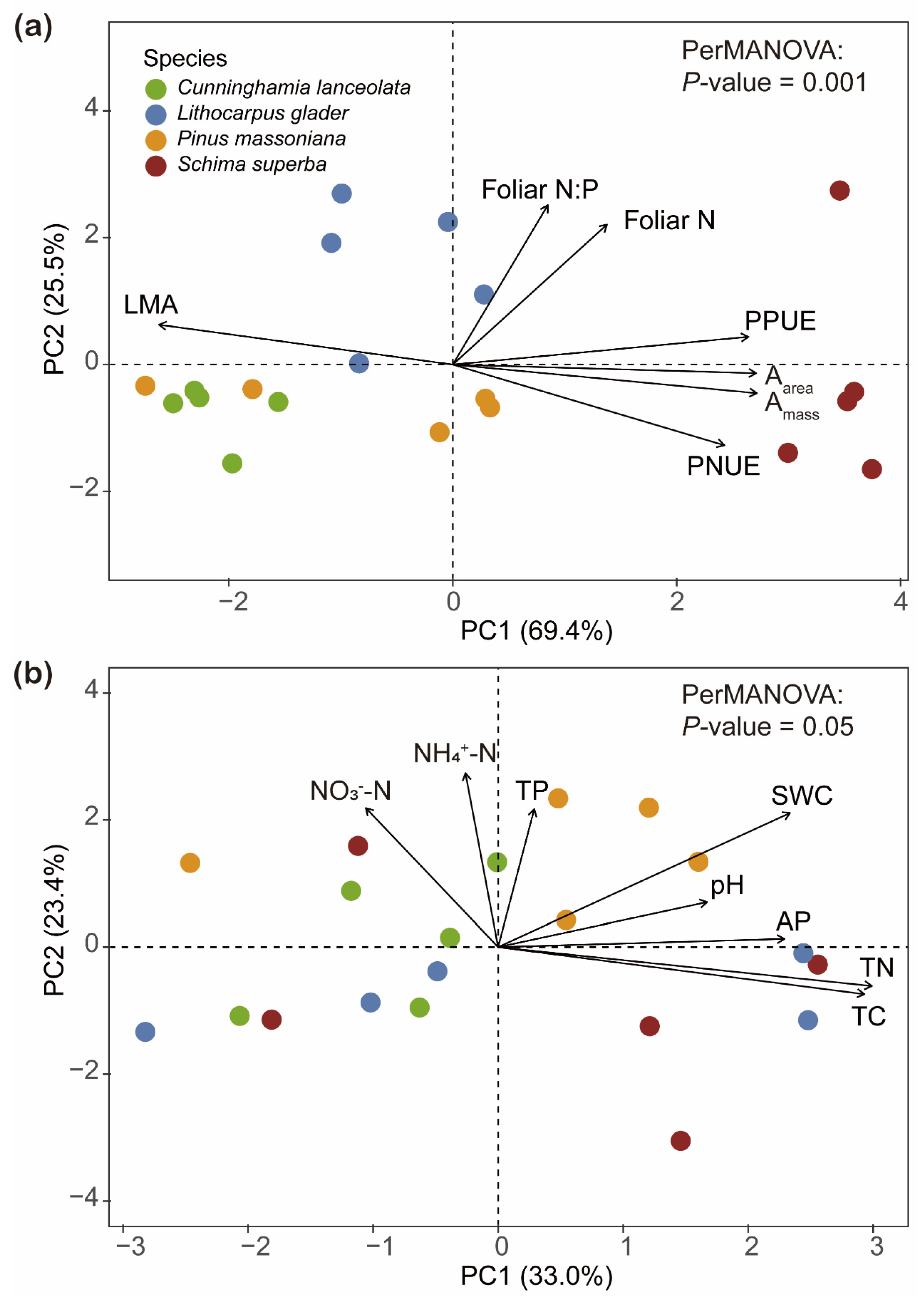
2.1. Variation in Leaf Functional Traits and Soil Properties
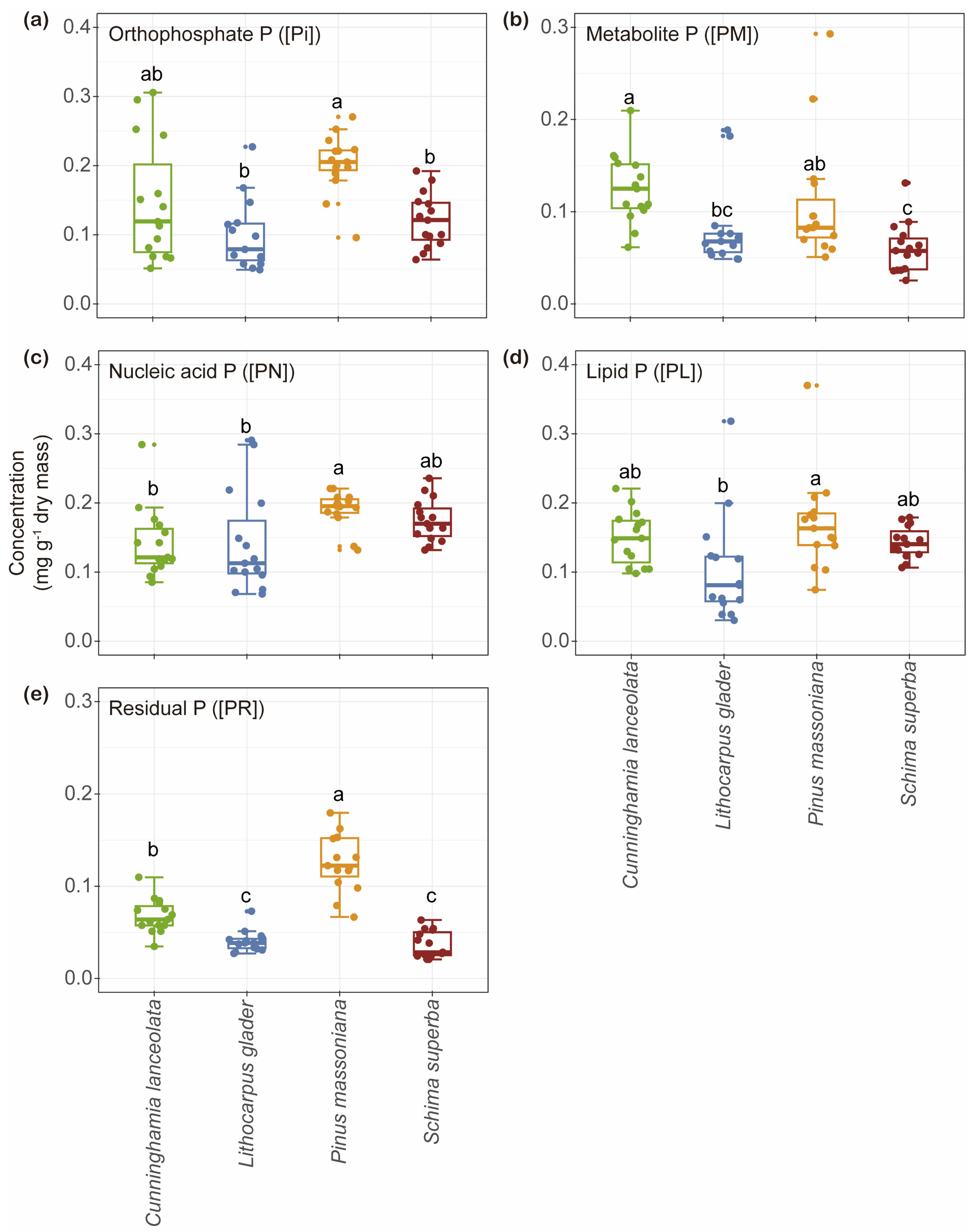
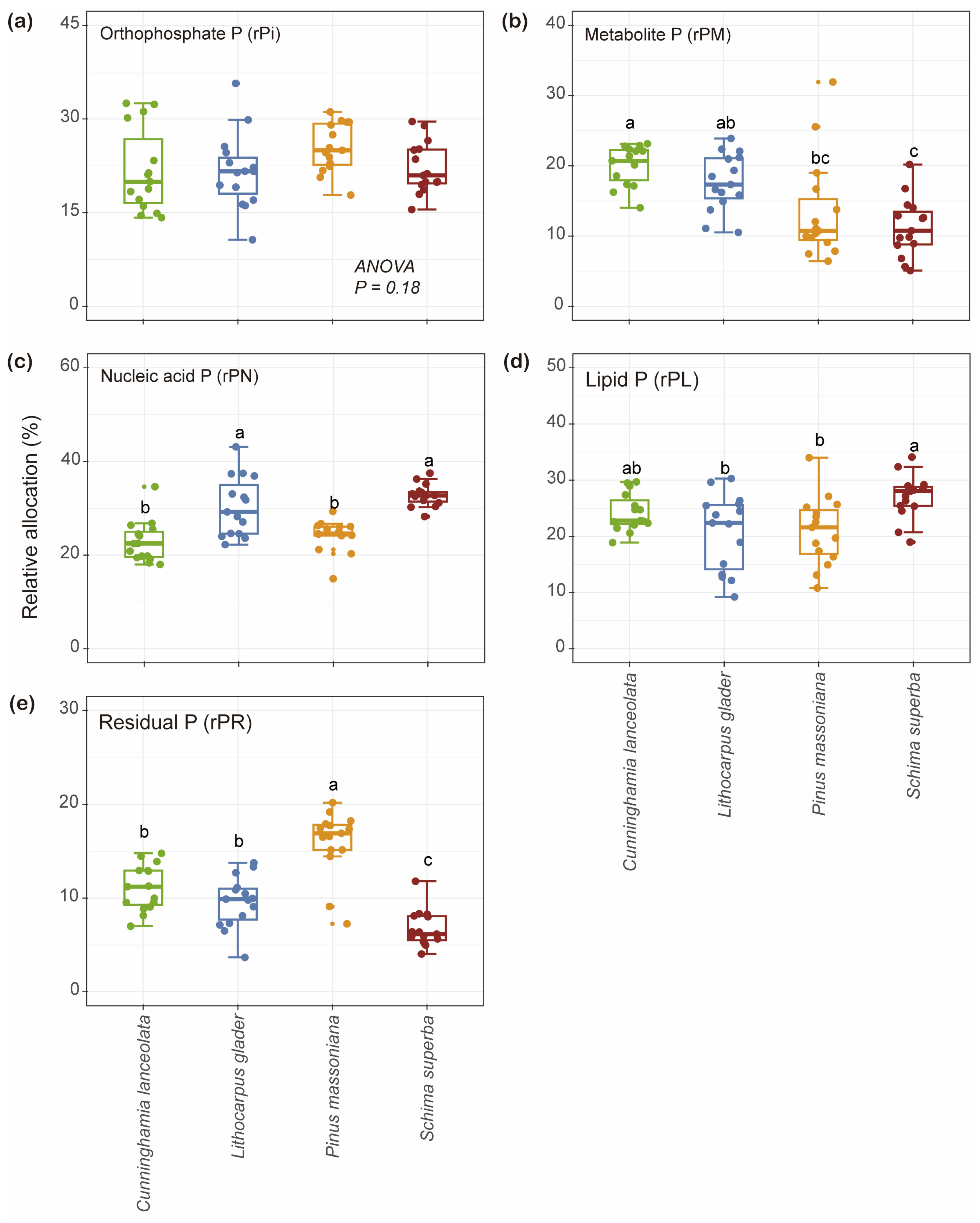
2.2. Variation in the Concentration of Foliar P Fractions and Their Relative Allocations
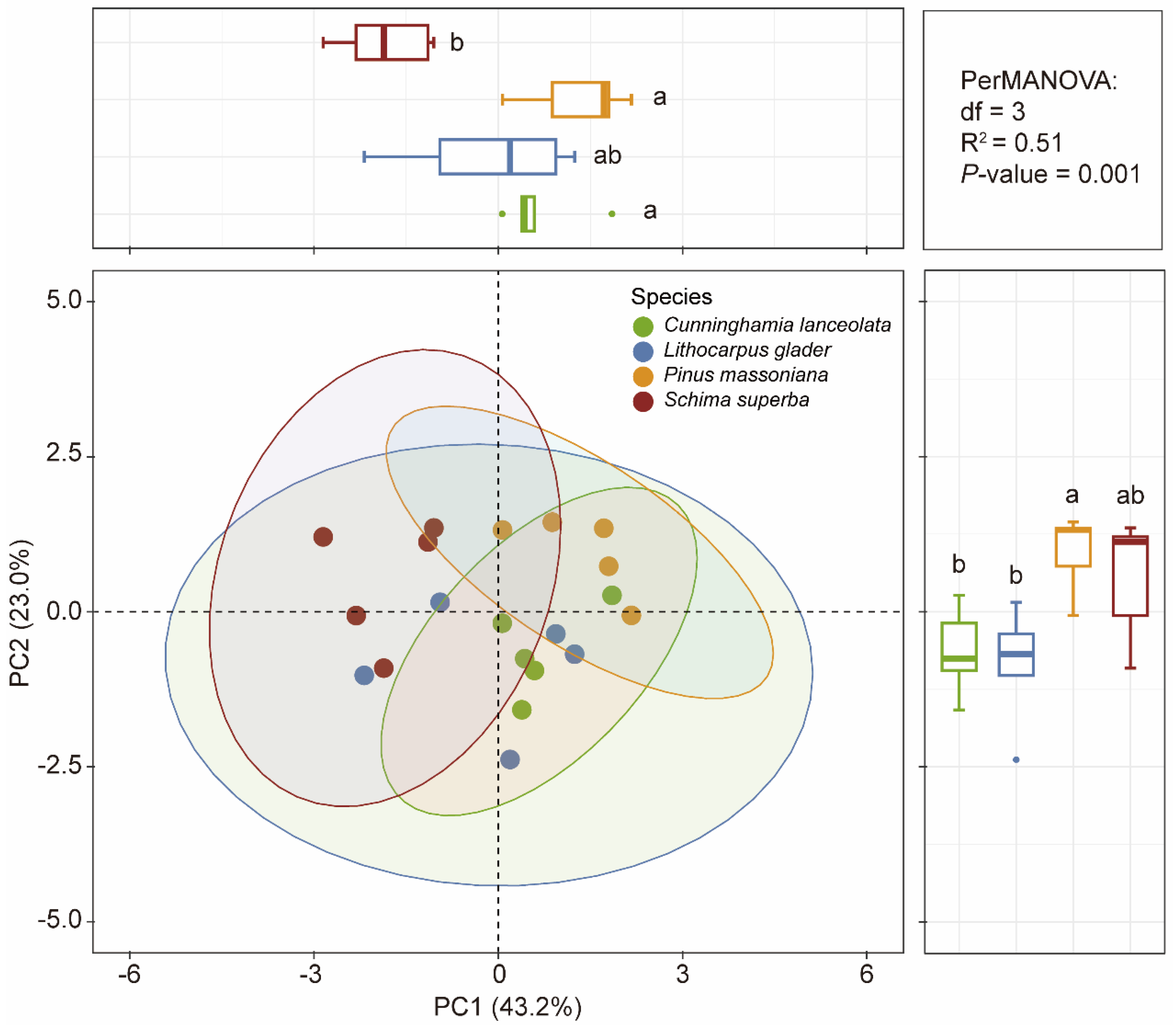
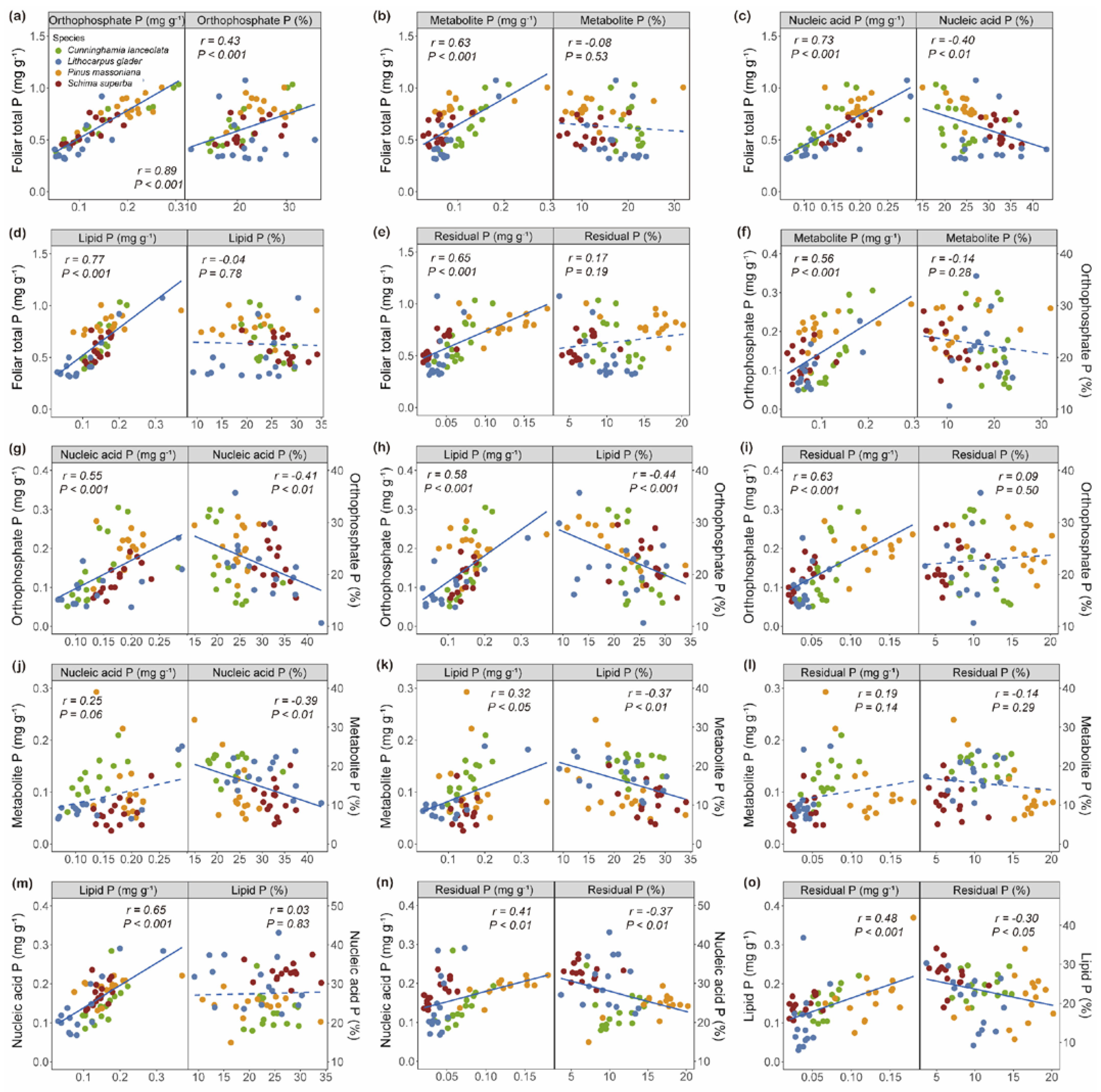
2.3. Correlations Between Leaf P Fractions
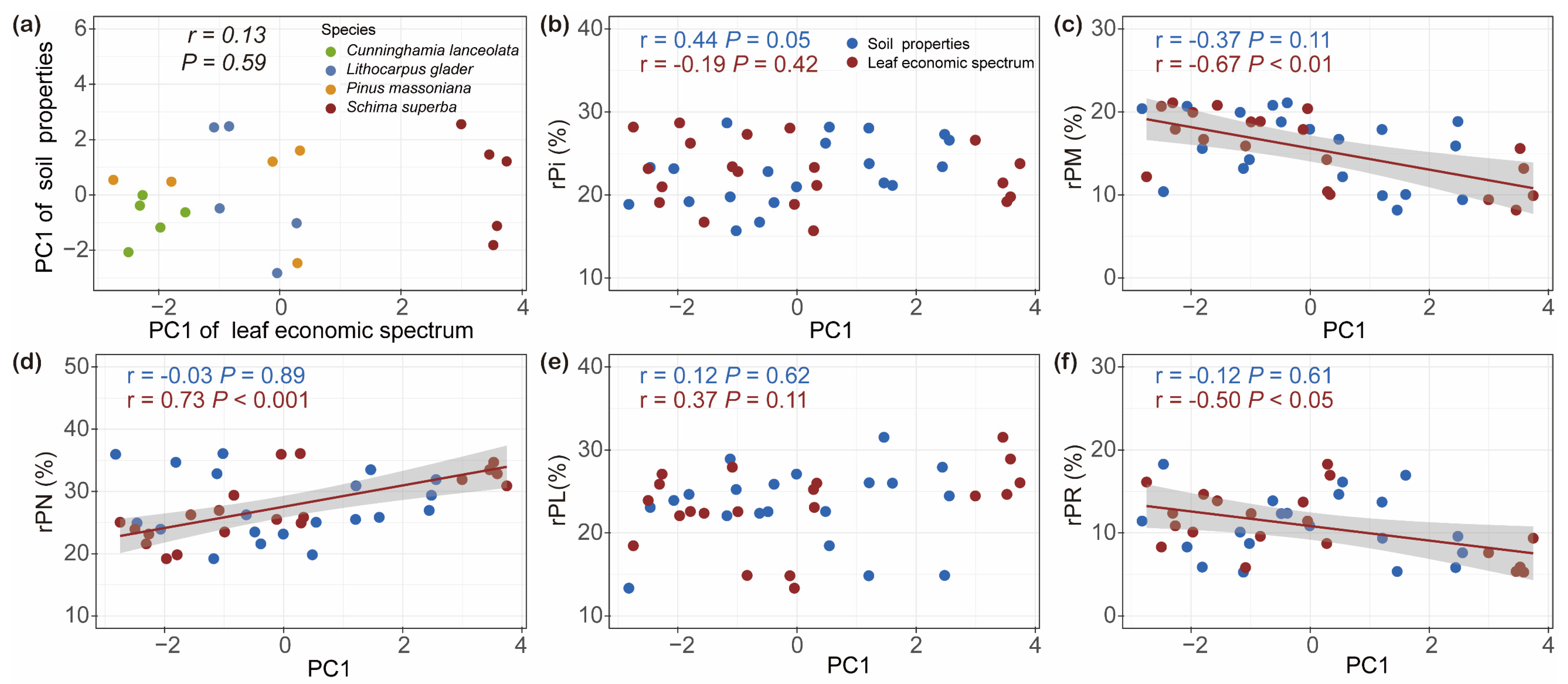
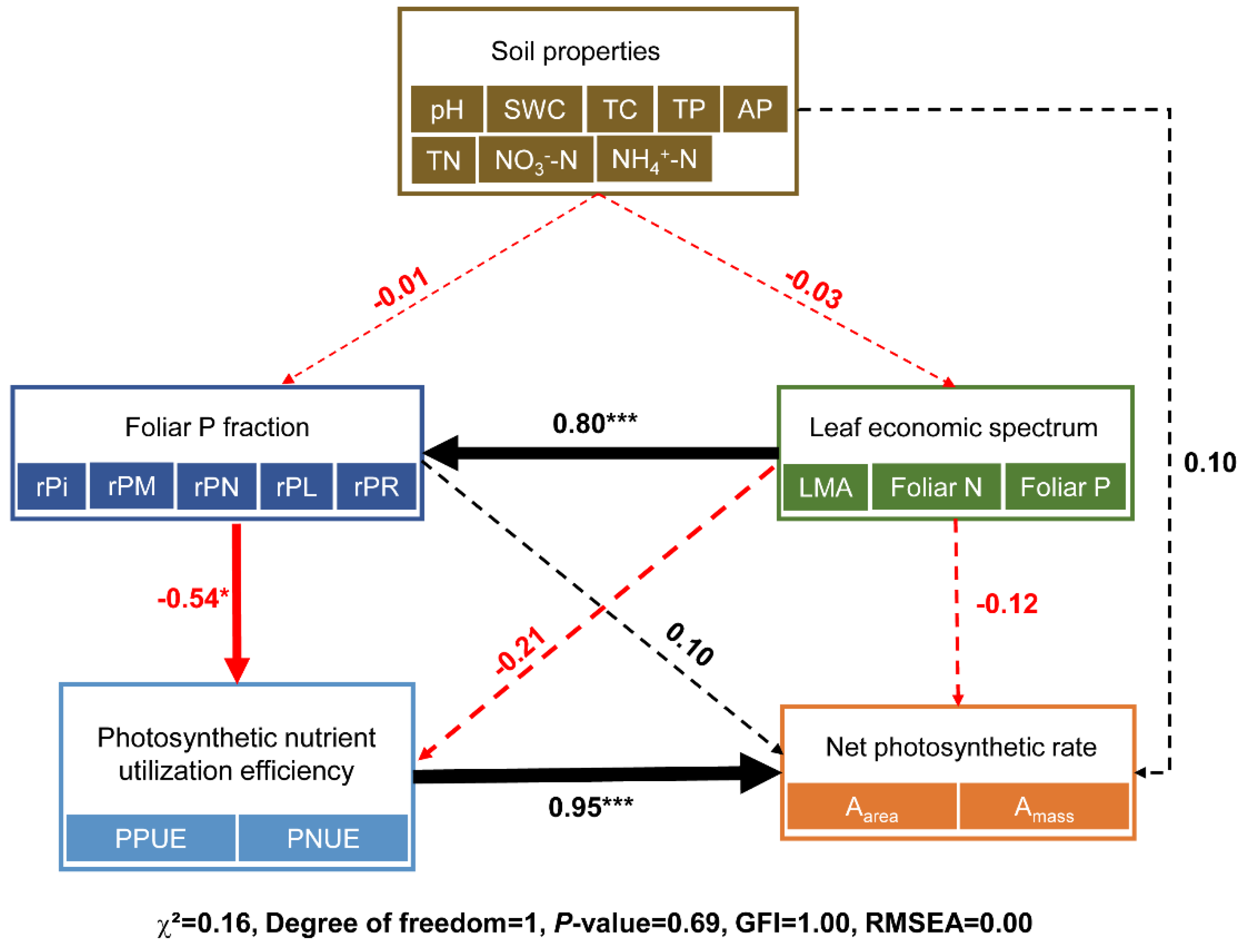
2.4. Linking Leaf Functional Traits and Soil Properties with Relative Allocations of Leaf P Fractions
3. Discussion
3.1. Species-Specific Foliar P Allocation Patterns and Covariations Among P Fractions
3.2. Linkages Between Leaf Functional Traits and Foliar P Fractions
3.3. Dominant Influence of Leaf Functional Traits over Soil Properties on Leaf Foliar P Fractions
4. Materials and Methods
4.1. Experimental Design
4.2. Photosynthesis Measurement
4.3. Leaf and Soil Sampling
4.4. Leaf Analyses
4.5. Soil Analyses
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LMA | leaf mass per unit leaf area |
| Amass | mass-based maximum photosynthetic carbon assimilation rate |
| Aarea | area-based maximum photosynthetic carbon assimilation rate |
| PPUE | photosynthetic phosphorus utilization efficiency |
| PNUE | photosynthetic nitrogen utilization efficiency |
| [Pi] | concentration of orthophosphate P in leaf |
| [PM] | concentration of metabolic P in leaf |
| [PL] | concentration of lipid P in leaf |
| [PN] | concentration of nucleic P in leaf |
| [PR] | concentration of residual P in leaf |
| rPi | relative allocation of inorganic P in leaf |
| rPM | relative allocation of metabolic P in leaf |
| rPL | relative allocation of lipid P in leaf |
| rPN | relative allocation of nucleic P in leaf |
| rPR | relative allocation of residual P in leaf |
| Leaf [N] | leaf nitrogen concentration |
| LES | Leaf economic spectrum |
References
- Lambers, H. Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef]
- Suriyagoda, L.D.; Ryan, M.H.; Gille, C.E.; Dayrell, R.L.; Finnegan, P.M.; Ranathunge, K.; Nicol, D.; Lambers, H. Phosphorus fractions in leaves. New Phytol. 2022, 237, 1122–1135. [Google Scholar] [CrossRef] [PubMed]
- Tsujii, Y.; Fan, B.; Atwell, B.J.; Lambers, H.; Lei, Z.; Wright, I.J. A survey of leaf phosphorus fractions and leaf economic traits among 12 co-occurring woody species on phosphorus-impoverished soils. Plant Soil 2023, 489, 107–124. [Google Scholar] [CrossRef]
- Hooda, N.; Weston, C.J. Influence of site and fertilizer addition on nutrient cycling in Eucalyptus globulus plantations in Gippsland, south-eastern Australia. I. Foliage and litter quality. Aust. J. Bot. 1999, 47, 189–206. [Google Scholar] [CrossRef]
- Ostertag, R. Foliar nitrogen and phosphorus accumulation responses after fertilization: An example from nutrient-limited Hawaiian forests. Plant Soil 2010, 334, 85–98. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.M.; Raven, J.A. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Fang, X.; Wang, W.; Tang, D.; Lambers, H. More than colorful: Phosphorus allocation to major chemical fractions shifts during leaf development in species exhibiting delayed greening. Curr. Plant Biol. 2024, 37, 100323. [Google Scholar] [CrossRef]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Onoda, Y.; Wright, I.J.; Evans, J.R.; Hikosaka, K.; Kitajima, K.; Niinemets, Ü.; Poorter, H.; Tosens, T.; Westoby, M. Physiological and structural trade-offs underlying the leaf economics spectrum. New Phytol. 2017, 214, 1447–1463. [Google Scholar] [CrossRef]
- Shipley, B.; Lechowicz, M.J.; Wright, I.; Reich, P.B. Fundamental trade-offs generating the worldwide leaf economics spectrum. Ecology 2006, 87, 535–541. [Google Scholar] [CrossRef]
- Tsujii, Y.; Atwell, B.J.; Lambers, H.; Wright, I.J. Leaf phosphorus fractions vary with leaf economic traits among 35 Australian woody species. New Phytol. 2024, 241, 1985–1997. [Google Scholar] [CrossRef] [PubMed]
- Gille, C.E.; Hayes, P.E.; Ranathunge, K.; Liu, S.T.; Newman, R.P.G.; de Tombeur, F.; Lambers, H.; Finnegan, P.M. Life at the conservative end of the leaf economics spectrum: Intergeneric variation in the allocation of phosphorus to biochemical fractions in species of Banksia (Proteaceae) and Hakea (Proteaceae). New Phytol. 2024, 244, 74–90. [Google Scholar] [CrossRef]
- Hidaka, A.; Kitayama, K. Allocation of foliar phosphorus fractions and leaf traits of tropical tree species in response to decreased soil phosphorus availability on Mount Kinabalu, Borneo. J. Ecol. 2011, 99, 849–857. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, X.; Han, Z.; Pang, J.; Lambers, H.; Finnegan, P.M. Responses of foliar phosphorus fractions to soil age are diverse along a 2 Myr dune chronosequence. New Phytol. 2019, 223, 1621–1633. [Google Scholar] [CrossRef]
- Gao, Y.; Tariq, A.; Zeng, F.; Graciano, C.; Zhang, Z.; Sardans, J.; Peñuelas, J. Allocation of foliar-P fractions of Alhagi sparsifolia and its relationship with soil-P fractions and soil properties in a hyperarid desert ecosystem. Geoderma 2022, 407, 115546. [Google Scholar] [CrossRef]
- Si, S.; He, Y.; Li, Z.; Xia, Z. Sex-specific phosphorus (P)-use and-acquisition in dioecious Populus euphratica under different soil moisture levels. J. Plant Ecol. 2024, 17, rtae064. [Google Scholar] [CrossRef]
- Liu, S.T.; Gille, C.E.; Bird, T.; Ranathunge, K.; Finnegan, P.M.; Lambers, H. Leaf phosphorus allocation to chemical fractions and its seasonal variation in south-western Australia is a species-dependent trait. Sci. Total Environ. 2023, 901, 166395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Luo, X.; Lambers, H.; Zhang, G.; Liu, N.; Zang, X.; Wen, D. Effects of elevated CO2 concentration and nitrogen addition on foliar phosphorus fractions of Mikania micranatha and Chromolaena odorata under low phosphorus availability. Physiol. Plant. 2021, 173, 2068–2080. [Google Scholar] [CrossRef] [PubMed]
- Mo, Q.F.; Li, Z.A.; Sayer, E.J.; Lambers, H.; Li, Y.W.; Zou, B.; Tang, J.W.; Heskel, M.; Ding, Y.Z.; Wang, F.M. Foliar phosphorus fractions reveal how tropical plants maintain photosynthetic rates despite low soil phosphorus availability. Funct. Ecol. 2019, 33, 503–513. [Google Scholar] [CrossRef]
- Wang, F.C.; Fang, X.M.; Wang, G.G.; Mao, R.; Lin, X.F.; Wang, H.; Chen, F.S. Effects of nutrient addition on foliar phosphorus fractions and their resorption in different-aged leaves of Chinese fir in subtropical China. Plant Soil 2019, 443, 41–54. [Google Scholar] [CrossRef]
- Su, Y.; Tang, Y.; Hu, Y.; Liu, M.; Lu, X.; Duan, B. Exploring plant adaptation strategies to phosphorus limitation induced by nitrogen addition: Foliar phosphorus allocation and root functional traits analysis in two dominant subalpine tree species. J. Plant Ecol. 2024, 17, rtae060. [Google Scholar] [CrossRef]
- Cui, E.; Lu, R.; Xu, X.; Sun, H.; Qiao, Y.; Ping, J.; Qiu, S.; Lin, Y.; Bao, J.; Yong, Y.; et al. Soil phosphorus drives plant trait variations in a mature subtropical forest. Glob. Chang. Biol. 2022, 28, 3310–3320. [Google Scholar] [CrossRef] [PubMed]
- Marenco, R.A.; Antezana-Vera, S.A.; Dias, D.P.; Candido, L.A. Litter production and leaf nutrient concentration and remobilization in response to climate seasonality in the central Amazon. J. For. Res. 2024, 35, 54. [Google Scholar] [CrossRef]
- Wen, Z.; Pang, J.; Wang, X.; Gille, C.E.; De Borda, A.; Hayes, P.E.; Clode, P.L.; Ryan, M.H.; Siddique, K.H.; Shen, J. Differences in foliar phosphorus fractions, rather than in cell-specific phosphorus allocation, underlie contrasting photosynthetic phosphorus-use efficiency among chickpea genotypes. J. Exp. Bot. 2023, 74, 1974–1989. [Google Scholar] [CrossRef]
- Poirier, Y.; Jaskolowski, A.; Clua, J. Phosphate acquisition and metabolism in plants. Curr. Biol. 2022, 32, R623–R629. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhu, S.; Chen, Q.; Lin, Y.; Tian, J.; Liang, C. Cell wall proteins play critical roles in plant adaptation to phosphorus deficiency. Int. J. Mol. Sci. 2019, 20, 5259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, L.; Wen, D. Photosynthesis of subtropical forest species from different successional status in relation to foliar nutrients and phosphorus fractions. Sci. Rep. 2018, 8, 10455. [Google Scholar] [CrossRef]
- Dissanayaka, D.M.; Ghahremani, M.; Siebers, M.; Wasaki, J.; Plaxton, W.C. Recent insights into the metabolic adaptations of phosphorus-deprived plants. J. Exp. Bot. 2021, 72, 199–223. [Google Scholar] [CrossRef] [PubMed]
- Hayes, P.E.; Adem, G.D.; Pariasca-Tanaka, J.; Wissuwa, M. Leaf phosphorus fractionation in rice to understand internal phosphorus-use efficiency. Ann. Bot. 2022, 129, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, A.; Kitayama, K. Relationship between photosynthetic phosphorus-use efficiency and foliar phosphorus fractions in tropical tree species. Ecol. Evol. 2013, 3, 4872–4880. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Cawthray, G.R.; Giavalisco, P.; Kuo, J.; Laliberté, E.; Pearse, S.J.; Scheible, W.R.; Stitt, M.; Testé, F.; Turner, B.L. Proteaceae from severely phosphorus-impoverished soils extensively replace phospholipids with galactolipids and sulfolipids during leaf development to achieve a high photosynthetic phosphorus-use-efficiency. New Phytol. 2012, 196, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Hong, W.; Xiong, C.; Lambers, H.; Sun, Y.; Xu, Z.; Schulze, W.X.; Cheng, L. Combining analyses of metabolite profiles and phosphorus fractions to explore high phosphorus-utilization efficiency in maize. J. Exp. Bot. 2022, 73, 4184–4203. [Google Scholar] [CrossRef] [PubMed]
- Hawes, C. Cell biology of the plant Golgi apparatus. New Phytol. 2005, 165, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, J.; Wang, M.; Song, Z.; Liu, J. Protein quality control in plant organelles: Current progress and future perspectives. Mol. Plant 2021, 14, 95–114. [Google Scholar] [CrossRef]
- Evans, J.R.; Clarke, V.C. The nitrogen cost of photosynthesis. J. Exp. Bot. 2019, 70, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Shi, J.; Pei, J.; Li, Y.; Finnegan, P.M.; Lambers, H. Foliar nutrient allocation patterns in Banksia attenuata and Banksia sessilis differing in growth rate and adaptation to low-phosphorus habitats. Ann. Bot. 2021, 128, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.J.; Millar, A.H. Protein turnover in plant biology. Nat. Plants 2015, 1, 15017. [Google Scholar] [CrossRef]
- Raven, J.A. The evolution of autotrophy in relation to phosphorus requirement. J. Exp. Bot. 2013, 64, 4023–4046. [Google Scholar] [CrossRef] [PubMed]
- Reichert, T.; Rammig, A.; Fuchslueger, L.; Lugli, L.F.; Quesada, C.A.; Fleischer, K. Plant phosphorus-use and -acquisition strategies in Amazonia. New Phytol. 2022, 234, 1126–1143. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Eisenhauer, N.; Peñuelas, J.; Fu, Y.; Wang, J.; Chen, Y.; Liu, S.; He, L.; Lucas-Borja, M.E.; Wang, L.; et al. Trophic interactions in soil micro-food webs drive ecosystem multifunctionality along tree species richness. Glob. Chang. Biol. 2024, 30, e17234. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S., III; Kedrowski, R.A. Seasonal changes in nitrogen and phosphorus fractions and autumn retranslocation in evergreen and deciduous taiga trees. Ecology 1983, 64, 376–391. [Google Scholar] [CrossRef]
- Hurley, B.A.; Tran, H.T.; Marty, N.J.; Park, J.; Snedden, W.A.; Mullen, R.T.; Plaxton, W.C. The dual-targeted purple acid phosphatase isozyme AtPAP26 is essential for efficient acclimation of Arabidopsis to nutritional phosphate deprivation. Plant Physiol. 2010, 153, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Methods of Soil Analysis, 2nd ed.; Agronomy Series No. 9, Part 2; Page, A., Miller, R., Keeney, D., Eds.; Soil Science Society of America: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Kedrowski, R.A. Extraction and analysis of nitrogen, phosphorus, and carbon fractions in plant material. J. Plant Nutr. 1983, 6, 989–1011. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Guan, J.; Li, Z.; Chen, Z.; Yu, Z.; Xia, Z. Leaf Phosphorus Fractions Are Coordinated with Leaf Functional Traits in Four Juvenile Tree Species from the Chinese Subtropics. Plants 2025, 14, 4. https://doi.org/10.3390/plants14010004
Wang L, Guan J, Li Z, Chen Z, Yu Z, Xia Z. Leaf Phosphorus Fractions Are Coordinated with Leaf Functional Traits in Four Juvenile Tree Species from the Chinese Subtropics. Plants. 2025; 14(1):4. https://doi.org/10.3390/plants14010004
Chicago/Turabian StyleWang, Lei, Jinhong Guan, Zongpei Li, Zhijie Chen, Zaipeng Yu, and Zhichao Xia. 2025. "Leaf Phosphorus Fractions Are Coordinated with Leaf Functional Traits in Four Juvenile Tree Species from the Chinese Subtropics" Plants 14, no. 1: 4. https://doi.org/10.3390/plants14010004
APA StyleWang, L., Guan, J., Li, Z., Chen, Z., Yu, Z., & Xia, Z. (2025). Leaf Phosphorus Fractions Are Coordinated with Leaf Functional Traits in Four Juvenile Tree Species from the Chinese Subtropics. Plants, 14(1), 4. https://doi.org/10.3390/plants14010004





