Optimum Nitrogen and Phosphorus Combination Improved Yield and Nutrient Use Efficiency of Sorghum in Saline Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Cultivar and Site
2.2. Experimental Design
2.3. Sampling and Measurement
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Basak, N.; Barman, A.; Sundha, P.; Rai, A.K. Recent trends in soil salinity appraisal and management. In Soil Analysis: Recent Trends and Applications; Rakshit, A., Ghosh, S., Chakraborty, S., Philip, V., Datta, A., Eds.; Springer: Singapore, 2020; pp. 143–161. [Google Scholar]
- Shi, P.B.; Gu, M.F. Transcriptome analysis and differential gene expression profiling of two contrasting quinoa genotypes in response to salt stress. BMC Plant Biol. 2020, 20, 568. [Google Scholar] [CrossRef]
- Shabala, S.; Bose, J.; Hedrich, R. Salt bladders: Do they matter? Trends Plant Sci. 2014, 19, 687–691. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.A.; Khan, M.J.; Ijaz, M.; Hassan, S.S. Combined effect of organic amendments and seed placement techniques on sorghum yield under salt-stressed conditions. J. Soil Sci. Plant Nutr. 2022, 22, 4752–4767. [Google Scholar] [CrossRef]
- Mrubata, K.; Nciizah, A.D.; Muchaonyerwa, P. Planting date and tillage effects on yield and nutrient uptake of two sorghum cultivars grown in sub-humid and semi-arid regions in South Africa. Front. Agron. 2024, 6, 1388823. [Google Scholar] [CrossRef]
- Mahmoud, N.E.; Abdel-Gawad, H.; Abdelhameed, R.M. Post-synthetic modification of nano-chitosan using gibberellic acid: Foliar application on sorghum under salt stress conditions and estimation of biochemical parameters. Plant Physiol. Biochem. 2024, 211, 108655. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.H.; Ren, L.T.; Spiertz, H.; Zhu, Y.B.; Xie, G.H. An economic analysis of sweet sorghum cultivation for ethanol production in North China. GCB Bioenergy 2015, 7, 1176–1184. [Google Scholar] [CrossRef]
- Malherbe, S.J.M.; Cripwell, R.A.; Favaro, L.; van Zyl, W.H.; Viljoen-Bloom, M. Triticale and sorghum as feedstock for bioethanol production via consolidated bioprocessing. Renew. Energy 2023, 206, 498–505. [Google Scholar] [CrossRef]
- Fu, H.M.; Chen, Y.H.; Yang, X.M.; Di, J.Y.; Xu, M.G.; Zhang, B.G. Water resource potential for large-scale sweet sorghum production as bioenergy feedstock in Northern China. Sci. Total Environ. 2019, 653, 758–764. [Google Scholar] [CrossRef]
- Rakgotho, T.; Ndou, N.; Mulaudzi, T.; Iwuoha, E.; Mayedwa, N.; Ajayi, R.F. Green-synthesized zinc oxide nanoparticles mitigate salt stress in Sorghum bicolor. Agric. 2022, 12, 597. [Google Scholar] [CrossRef]
- Cámara-Zapata, J.M.; García-Sánchez, F.; Martinez, V.; Nieves, M.; Cerdá, A. Effect of NaCl on citrus cultivars. Agronomie 2004, 24, 155–160. [Google Scholar] [CrossRef]
- Soare, T.M.; Coelho, F.S.; Oliverira, V.B.; Pontes, O.; Pavinato, P.S. Soil nitrogen dynamics under tobacco with different fertilizer management in southern Brazil. Geoderma Reg. 2020, 21, e00282. [Google Scholar] [CrossRef]
- Farhan, M.; Sathish, M.; Kiran, R.; Mushtaq, A.; Baazeem, A.; Hasnain, A.; Hakim, F.; Naqvi, S.A.H.; Mubeen, M.; Iftikhar, Y.; et al. Plant nitrogen metabolism: Balancing resilience to nutritional stress and abiotic challenges. Phyton-Int. J. Exp. Bot. 2024, 93, 581–609. [Google Scholar] [CrossRef]
- Zahedifar, M.; Ronaghi, A.; Moosavi, A.A.; Shirazi, S.S. Influence of nitrogen and salinity levels on the fruit yield and chemical composition of tomato in a hydroponic culture. J. Plant Nutr. 2012, 35, 2211–2221. [Google Scholar] [CrossRef]
- Zamani, A.; Emam, Y.; Pessarakli, M.; Shakeri, E. Growth and biochemical responses of sorghum genotypes to nitrogen fertilizer under salinity stress conditions. J. Plant Nutr. 2021, 44, 569–579. [Google Scholar] [CrossRef]
- Soliman, M.S.; Shalabi, H.G.; Campbell, W.F. Interaction of salinity, nitrogen, and phosphorus fertilization on wheat. J. Plant Nutr. 1994, 17, 1163–1173. [Google Scholar] [CrossRef]
- Sima, N.A.K.K.; Ahmad, S.T.; Alitabar, R.A.; Mottaghi, A.; Pessarakli, M. Interactive effects of salinity and phosphorus nutrition on physiological responses of two barley species. J. Plant Nutr. 2012, 35, 1411–1428. [Google Scholar] [CrossRef]
- Bouras, H.; Choukr-Allah, R.; Mosseddaq, F.; Bouaziz, A.; Devkota, K.P.; Mouttaqi, A.E.; Bouazzama, B.; Hirich, A. Does phosphorus fertilization increase biomass production and salinity tolerance of blue panicum (Panicum antidotale Retz.) in the salt-affected soils of arid regions? Agronomy 2022, 12, 791. [Google Scholar] [CrossRef]
- Belouchrani, A.S.; Latati, M.; Ounane, S.M.; Drouiche, N.; Lounici, H. Study of the interaction salinity: Phosphorus fertilization on sorghum. J. Plant Growth Regul. 2020, 39, 1205–1210. [Google Scholar] [CrossRef]
- Liang, T.B.; Wang, Z.L.; Liu, L.L.; Wang, R.J.; Chen, X.G.; Zhang, X.D.; Shi, C.Y. Effects of humic acid urea on yield and nitrogen absorption, assimilation and quality of ginger. J. Plant Nutr. Fertil. 2007, 13, 903–909. [Google Scholar]
- Gao, J.F. Plant Physiology Experiment Instruction; Higher Education Press: Beijing, China, 2006. (In Chinese) [Google Scholar]
- Ozer, H. The effect of plant population densities on growth, yield and yield components of two spring rapeseed cultivars. Plant Soil Environ. 2003, 49, 422–426. [Google Scholar] [CrossRef]
- Hao, X.N. Application of atomic absorption spectrometry in agrochemical analysis of soil. Qinghai Sci. Technol. 2006, 6, 51–52. (In Chinese) [Google Scholar]
- Kuai, J.; Sun, Y.Y.; Zhou, M.; Zhang, P.P.; Zuo, Q.S.; Wu, J.S.; Zhou, G. The effect of nitrogen application and planting density on the radiation use efficiency and the stem lignin metabolism in rapeseed (Brassica napus L.). Field Crop. Res. 2016, 199, 89–98. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, J.; You, J.; Li, J.; Qian, C.; Leng, S.; Yang, G.; Zuo, Q. Effects of phosphorus supply on the leaf photosynthesis, and biomass and phosphorus accumulation and partitioning of canola (Brassica napus L.) in saline environment. Agronomy 2021, 11, 1918. [Google Scholar] [CrossRef]
- Bouras, H.; Choukr-Allah, R.; Amouaouch, Y.; Bouaziz, A.; Devkota, K.P.; El Mouttaqi, A.; Bouazzama, B.; Hirich, A. How does quinoa (Chenopodium quinoa Wild.) respond to phosphorus fertilization and irrigation water salinity? Plants 2022, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Wang, Z.H.; Li, H.Q.; Wang, T.Y.; Chen, R. Effects of nitrogen application and brackish water irrigation on yield and quality of cotton. Agric. Water Manag. 2022, 264, 107512. [Google Scholar] [CrossRef]
- Mussarat, M.; Shair, M.; Muhammad, D.; Mian, I.A.; Khan, S.; Adnan, M.; Fahad, S.; Dessoky, E.S.; EL Sabagh, A.; Zia, A.; et al. Accentuating the role of nitrogen to phosphorus ratio on the growth and yield of wheat crop. Sustainability 2021, 13, 2253. [Google Scholar] [CrossRef]
- Song, X.; Zhou, G.; Ma, B.-L.; Wu, W.; Ahmad, I.; Zhu, G.; Yan, W.; Jiao, X. Nitrogen application improved photosynthetic productivity, chlorophyll fluorescence, yield and yield components of two oat genotypes under saline conditions. Agronomy 2019, 9, 115. [Google Scholar] [CrossRef]
- Bouras, H.; Bouaziz, A.; Choukr-Allah, R.; Hirich, A.; Devkota, K.P.; Bouazzama, B. Phosphorus fertilization enhances productivity of forage corn (Zea mays L.) irrigated with saline water. Plants 2021, 10, 2608. [Google Scholar] [CrossRef]
- Tewari, R.K.; Kumar, P.; Sharma, P.N. Oxidative stress and antioxidant responses in young leaves of mulberry plants grown under nitrogen, phosphorus or potassium deficiency. J. Integr. Plant Biol. 2010, 49, 313–322. [Google Scholar] [CrossRef]
- Lin, J.X.; Wang, Y.N.; Sun, S.G.; Mu, C.S.; Yan, X.F. Effects of arbuscular mycorrhizal fungi on the growth, photosynthesis and photosynthetic pigments of Leymus chinensis seedlings under salt-alkali stress and nitrogen deposition. Sci. Total Environ. 2017, 576, 234–241. [Google Scholar] [CrossRef]
- Raven, J.A.; Ball, L.A.; Beardall, J.; Giordano, M.; Maberly, S.C. Algae lacking carbon-concentrating mechanisms. Can. J. Bot. 2005, 83, 879–890. [Google Scholar] [CrossRef]
- Yan, B.; Wu, B.; Gao, Y.H.; Wu, J.M.; Niu, J.Y.; Xie, Y.P.; Cui, Z.; Zhang, Z. Effects of nitrogen and phosphorus on the regulation of nonstructural carbohydrate accumulation, translocation and the yield formation of oilseed flax. Field Crop. Res. 2018, 219, 229–241. [Google Scholar] [CrossRef]
- Al-Hamdani, S.H.; Sirna, C.B. Physiological responses of Salvinia minima to different phosphorus and nitrogen concentrations. Am. Fern J. 2008, 98, 71–82. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: London, UK, 1995; p. 889. [Google Scholar]
- Huang, L.H.; Liu, X.; Wang, Z.C.; Liang, Z.W.; Wang, M.M.; Liu, M.; Suarez, D.L. Interactive effects of pH, EC and nitrogen on yields and nutrient absorption of rice (Oryza sativa L.). Agric. Water Manag. 2017, 194, 48–57. [Google Scholar] [CrossRef]
- Zhang, D.M.; Li, W.J.; Xin, C.S.; Tang, W.; Eneji, A.E.; Dong, H.Z. Lint yield and nitrogen use efficiency of field-grown cotton vary with soil salinity and nitrogen application rate. Field Crop. Res. 2012, 138, 63–70. [Google Scholar] [CrossRef]
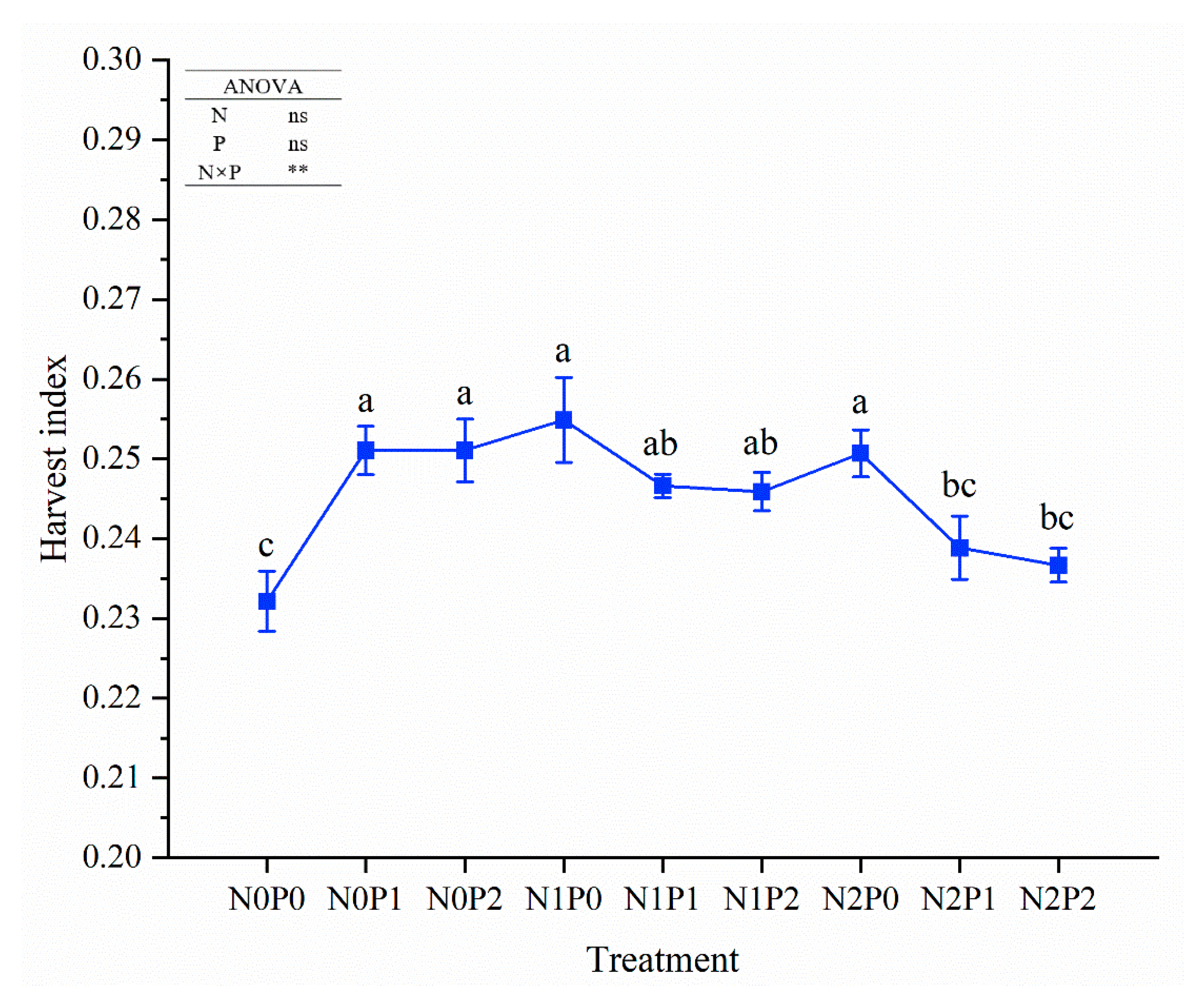
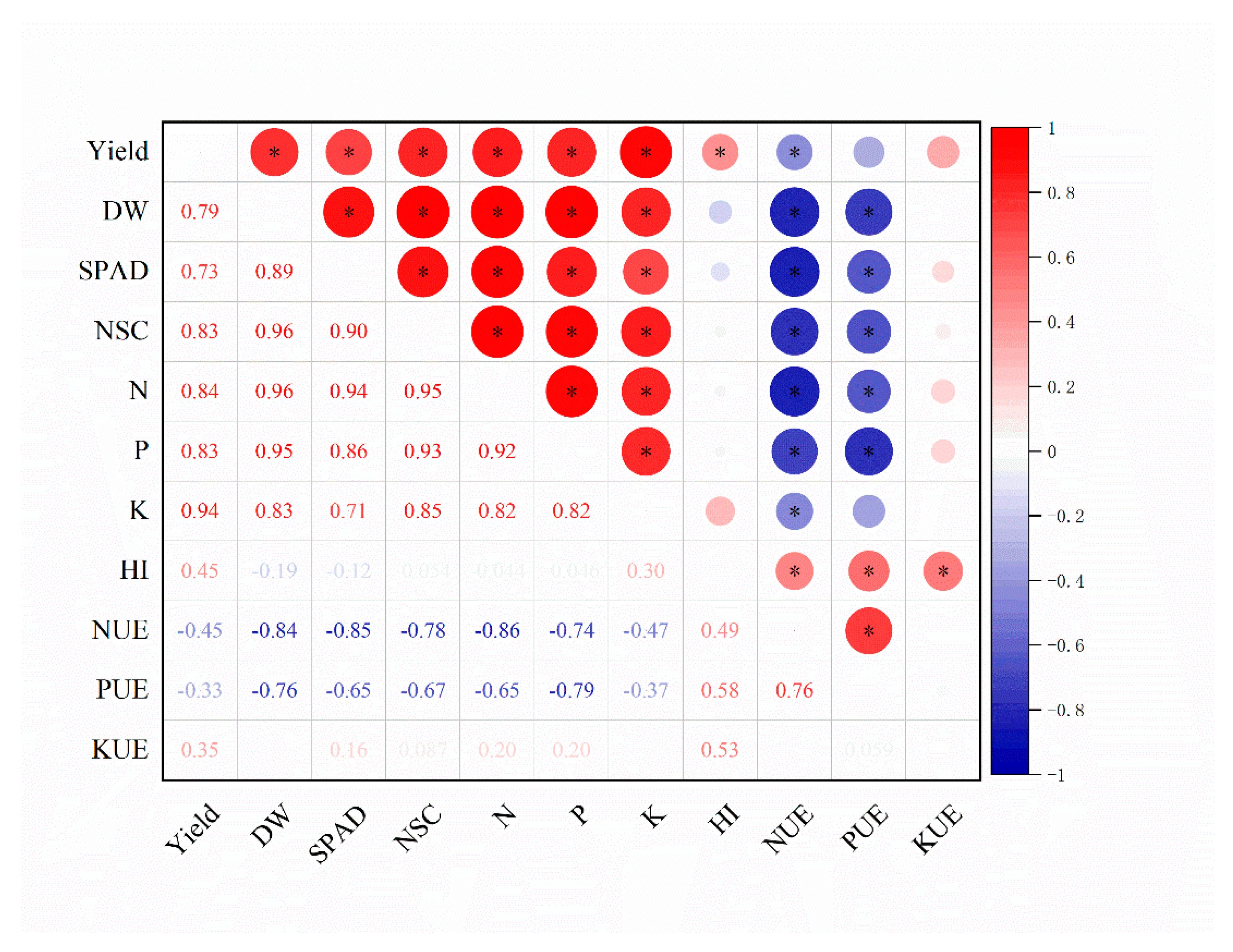
| Nitrogen | Phosphorus | Aerial Fresh Weight (kg ha−1) | Aerial Dry Weight (kg ha−1) | ||||
|---|---|---|---|---|---|---|---|
| Seedling | Jointing | Maturity | Seedling | Jointing | Maturity | ||
| N0 | P0 | 744.0 ± 7.3 e | 4488 ± 96 f | 40,372 ± 530 f | 169.7 ± 2.5 f | 945 ± 10 g | 14,990 ± 159 g |
| P1 | 848.4 ± 3.4 c | 5160 ± 26 d | 47,409 ± 902 d | 190.9 ± 2.7 cd | 1057 ± 7 d | 16,638 ± 111 d | |
| P2 | 847.4 ± 6.4 c | 5001 ± 58 de | 44,740 ± 259 e | 179.1 ± 1.4 e | 982 ± 13 f | 15,745 ± 96 f | |
| N1 | P0 | 829.2 ± 10.3 cd | 5132 ± 43 de | 44,974 ± 220 e | 184.4 ± 1.9 de | 1021 ± 7 e | 15,884 ± 211 ef |
| P1 | 961.4 ± 12.8 a | 5523 ± 49 b | 51,045 ± 374 ab | 212.8 ± 4.1 a | 1143 ± 12 b | 18,082 ± 21 ab | |
| P2 | 902.4 ± 12.9 b | 5327 ± 32 c | 48,477 ± 641 cd | 198.7 ± 2.6 bc | 1101 ± 3 c | 17,505 ± 111 c | |
| N2 | P0 | 810.6 ± 8.4 d | 4988 ± 29 e | 45,005 ± 1009 e | 181.2 ± 1.8 e | 1030 ± 3 e | 16,266 ± 145 de |
| P1 | 924.1 ± 9.5 b | 5534 ± 74 b | 49,680 ± 611 bc | 203.7 ± 2.1 b | 1142 ± 13 b | 17,889 ± 174 bc | |
| P2 | 979.3 ± 6.0 a | 5814 ± 24 a | 52,203 ± 578 a | 215.3 ± 4.2 a | 1176 ± 2 a | 18,401 ± 157 a | |
| Nitrogen | Phosphorus | Seed Weight per Spike (g) | 1000-Seed Weight (g) | Seed Number per Spike | Seed Yield (kg ha−1) |
|---|---|---|---|---|---|
| N0 | P0 | 46.9 ± 0.5 d | 25.6 ± 0.9 ab | 1838 ± 68 d | 4530 ± 47 d |
| P1 | 57.7 ± 0.7 bc | 25.4 ± 0.4 ab | 2276 ± 45 bc | 5575 ± 68 bc | |
| P2 | 54.6 ± 1.5 c | 23.8 ± 0.8 b | 2300 ± 20 bc | 5280 ± 143 c | |
| N1 | P0 | 56.2 ± 1.3 bc | 26.0 ± 0.3 a | 2160 ± 45 c | 5432 ± 124 bc |
| P1 | 61.2 ± 0.5 a | 23.8 ± 0.8 b | 2578 ± 66 a | 5919 ± 49 a | |
| P2 | 59.1 ± 1.1 ab | 24.9 ± 0.8 ab | 2382 ± 66 b | 5711 ± 111 ab | |
| N2 | P0 | 56.3 ± 1.2 bc | 25.5 ± 0.4 ab | 2217 ± 11 c | 5443 ± 114 bc |
| P1 | 58.1 ± 0.8 b | 26.0 ± 0.3 a | 2236 ± 33 bc | 5612 ± 75 b | |
| P2 | 59.0 ± 0.2 ab | 26.8 ± 0.3 a | 2211 ± 32 c | 5705 ± 18 ab |
| Nitrogen | Phosphorus | SPAD Reading | Aerial NSC Content (mg g−1) | ||||
|---|---|---|---|---|---|---|---|
| Seedling | Jointing | Maturity | Seedling | Jointing | Maturity | ||
| N0 | P0 | 30.2 ± 0.4 g | 37.2 ± 0.5 e | 28.2 ± 0.4 g | 165.6 ± 0.8 h | 77.5 ± 0.4 e | 265.0 ± 5.8 f |
| P1 | 32.5 ± 0.4 ef | 41.4 ± 0.5 c | 30.4 ± 0.3 ef | 195.9 ± 0.8 e | 86.7 ± 0.3 d | 325.4 ± 4.7 c | |
| P2 | 31.8 ± 0.4 f | 38.7 ± 0.7 d | 29.6 ± 0.6 fg | 200.9 ± 1.4 d | 93.1 ± 0.7 c | 308.3 ± 2.6 de | |
| N1 | P0 | 33.5 ± 0.4 de | 39.7 ± 0.3 d | 30.5 ± 0.7 ef | 184.9 ± 1.6 g | 87.7 ± 1.0 d | 302.1 ± 3.3 e |
| P1 | 37.2 ± 0.1 bc | 45.6 ± 0.3 a | 34.3 ± 0.4 b | 226.6 ± 0.8 a | 103.9 ± 0.5 a | 366.4 ± 3.4 a | |
| P2 | 36.0 ± 0.4 c | 43.2 ± 0.6 b | 32.4 ± 0.6 cd | 211.1 ± 1.8 c | 98.0 ± 1.3 b | 344.5 ± 2.5 b | |
| N2 | P0 | 34.3 ± 0.2 d | 42.3 ± 0.1 bc | 31.4 ± 0.3 de | 190.0 ± 2.0 f | 87.7 ± 1.5 d | 317.9 ± 3.8 cd |
| P1 | 38.0 ± 0.6 b | 46.1 ± 0.6 a | 33.1 ± 0.3 bc | 220.6 ± 2.6 b | 98.8 ± 0.4 b | 348.2 ± 3.7 b | |
| P2 | 39.1 ± 0.3 a | 47.1 ± 0.4 a | 36.4 ± 0.4 a | 231.1 ± 2.2 a | 106.5 ± 1.2 a | 374.3 ± 4.0 a | |
| Nitrogen | Phosphorus | Aerial N Accumulation (kg ha−1) | NUE (kg kg−1) | ||
|---|---|---|---|---|---|
| Seedling | Jointing | Maturity | |||
| N0 | P0 | 1.932 ± 0.050 f | 11.38 ± 0.16 g | 123.3 ± 0.2 g | 36.8 ± 0.4 b |
| P1 | 2.234 ± 0.025 de | 13.27 ± 0.11 e | 146.8 ± 0.6 e | 38.0 ± 0.3 a | |
| P2 | 2.133 ± 0.018 e | 12.23 ± 0.30 f | 140.7 ± 3.3 f | 37.5 ± 0.3 ab | |
| N1 | P0 | 2.292 ± 0.011 cd | 13.61 ± 0.02 de | 148.5 ± 0.7 e | 36.6 ± 0.7 b |
| P1 | 2.966 ± 0.068 a | 16.63 ± 0.13 b | 180.0 ± 0.6 ab | 32.9 ± 0.4 d | |
| P2 | 2.662 ± 0.032 b | 15.47 ± 0.23 c | 166.8 ± 3.4 c | 34.3 ± 0.1 c | |
| N2 | P0 | 2.374 ± 0.025 c | 14.00 ± 0.13 d | 154.8 ± 1.7 d | 35.2 ± 0.4 c |
| P1 | 2.995 ± 0.048 a | 16.68 ± 0.20 b | 176.0 ± 1.2 b | 31.9 ± 0.3 de | |
| P2 | 3.085 ± 0.057 a | 17.96 ± 0.08 a | 184.9 ± 1.7 a | 30.9 ± 0.4 e | |
| Nitrogen | Phosphorus | Aerial P Accumulation (kg ha−1) | PUE (kg kg−1) | ||
|---|---|---|---|---|---|
| Seedling | Jointing | Maturity | |||
| N0 | P0 | 0.301 ± 0.001 f | 1.923 ± 0.036 e | 32.7 ± 0.1 e | 138.8 ± 1.4 b |
| P1 | 0.376 ± 0.004 c | 2.259 ± 0.034 c | 39.4 ± 0.2 c | 142.0 ± 2.1 b | |
| P2 | 0.356 ± 0.005 d | 2.130 ± 0.027 d | 37.9 ± 0.9 cd | 139.9 ± 0.8 b | |
| N1 | P0 | 0.338 ± 0.002 de | 2.108 ± 0.004 d | 36.6 ± 0.7 d | 148.9 ± 0.6 a |
| P1 | 0.470 ± 0.011 a | 2.652 ± 0.030 b | 45.0 ± 0.2 ab | 131.9 ± 0.8 c | |
| P2 | 0.463 ± 0.003 a | 2.618 ± 0.021 b | 45.8 ± 0.4 a | 125.1 ± 1.4 d | |
| N2 | P0 | 0.331 ± 0.003 e | 2.120 ± 0.021 d | 37.2 ± 0.8 d | 146.7 ± 1.9 a |
| P1 | 0.436 ± 0.006 b | 2.593 ± 0.044 b | 43.6 ± 0.3 b | 129.2 ± 1.7 c | |
| P2 | 0.481 ± 0.011 a | 2.779 ± 0.017 a | 46.3 ± 0.1 a | 123.6 ± 0.5 d | |
| Nitrogen | Phosphorus | Aerial K Accumulation (kg ha−1) | KUE (kg kg−1) | ||
|---|---|---|---|---|---|
| Seedling | Jointing | Maturity | |||
| N0 | P0 | 1.585 ± 0.023 d | 7.58 ± 0.05 e | 100.4 ± 0.2 e | 45.2 ± 0.4 a |
| P1 | 2.047 ± 0.027 a | 9.52 ± 0.07 a | 125.6 ± 0.9 ab | 44.5 ± 0.4 a | |
| P2 | 1.780 ± 0.026 c | 8.39 ± 0.18 d | 114.2 ± 0.5 d | 46.3 ± 1.3 a | |
| N1 | P0 | 1.910 ± 0.049 b | 9.04 ± 0.16 bc | 118.2 ± 1.7 c | 46.1 ± 0.6 a |
| P1 | 2.083 ± 0.054 a | 9.61 ± 0.22 a | 128.8 ± 0.9 a | 46.1 ± 0.7 a | |
| P2 | 1.922 ± 0.020 b | 9.31 ± 0.08 ab | 123.5 ± 1.1 b | 46.3 ± 0.7 a | |
| N2 | P0 | 1.885 ± 0.024 bc | 8.74 ± 0.04 cd | 119.4 ± 1.8 c | 45.7 ± 0.5 a |
| P1 | 2.048 ± 0.026 a | 9.48 ± 0.09 a | 123.5 ± 1.0 b | 45.5 ± 0.9 a | |
| P2 | 2.051 ± 0.064 a | 9.60 ± 0.07 a | 125.4 ± 1.2 ab | 45.6 ± 0.5 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Wu, Q.; Wang, L.; Zhou, G.; Zhu, G.; Suliman, M.S.E.; Nimir, N.E.A. Optimum Nitrogen and Phosphorus Combination Improved Yield and Nutrient Use Efficiency of Sorghum in Saline Soil. Plants 2025, 14, 102. https://doi.org/10.3390/plants14010102
Guo X, Wu Q, Wang L, Zhou G, Zhu G, Suliman MSE, Nimir NEA. Optimum Nitrogen and Phosphorus Combination Improved Yield and Nutrient Use Efficiency of Sorghum in Saline Soil. Plants. 2025; 14(1):102. https://doi.org/10.3390/plants14010102
Chicago/Turabian StyleGuo, Xiaoqian, Qidi Wu, Luqi Wang, Guisheng Zhou, Guanglong Zhu, Mohamed Suliman Eltyb Suliman, and Nimir Eltyb Ahmed Nimir. 2025. "Optimum Nitrogen and Phosphorus Combination Improved Yield and Nutrient Use Efficiency of Sorghum in Saline Soil" Plants 14, no. 1: 102. https://doi.org/10.3390/plants14010102
APA StyleGuo, X., Wu, Q., Wang, L., Zhou, G., Zhu, G., Suliman, M. S. E., & Nimir, N. E. A. (2025). Optimum Nitrogen and Phosphorus Combination Improved Yield and Nutrient Use Efficiency of Sorghum in Saline Soil. Plants, 14(1), 102. https://doi.org/10.3390/plants14010102







