Phenotypic Variability in Resistance to Anthracnose, White, Brown, and Blight Leaf Spot in Cassava Germplasm
Abstract
1. Introduction
2. Results
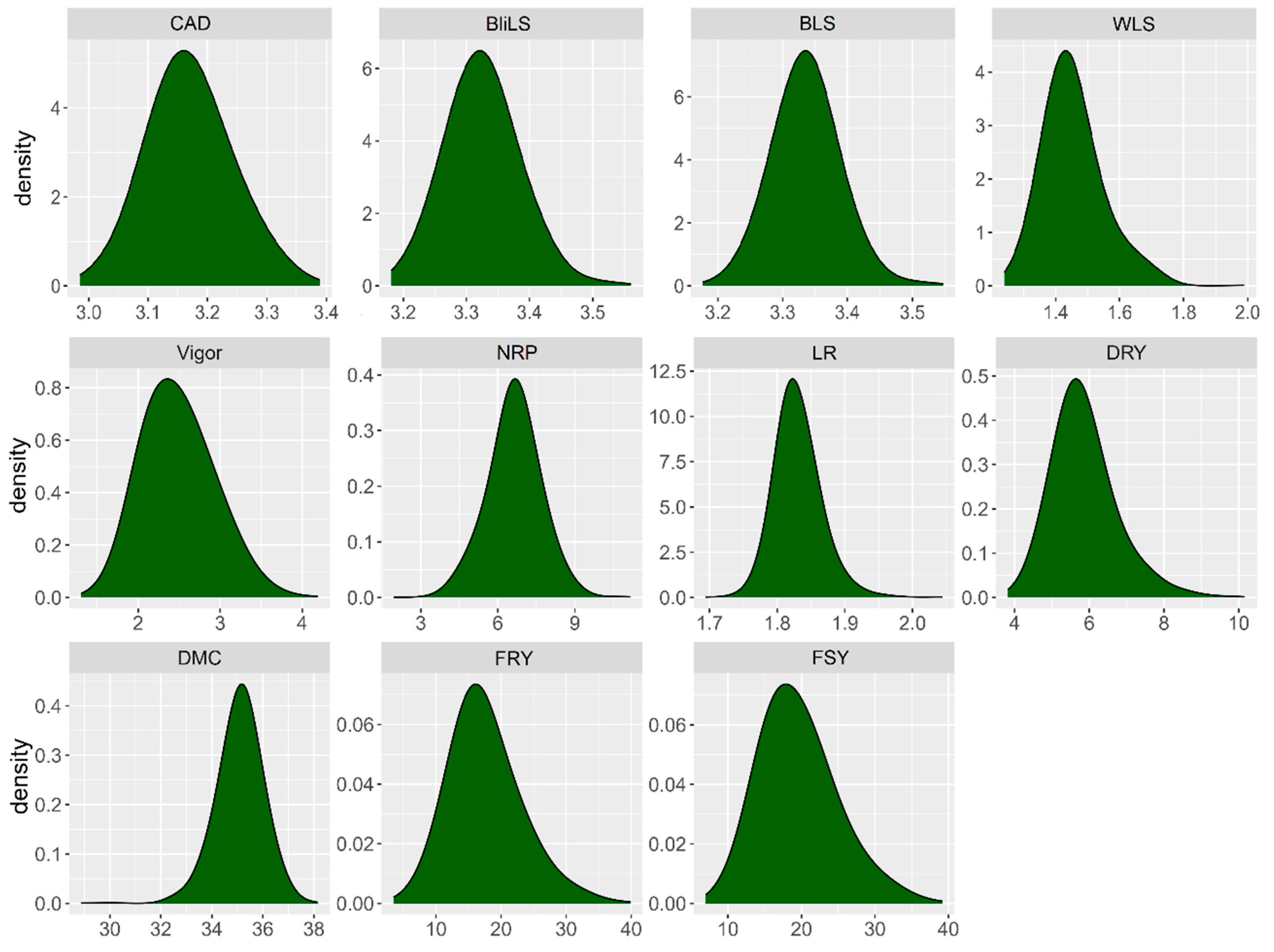
2.1. Diversity, Variances, and Genetic Parameters of Foliar Part Diseases and Yield Traits
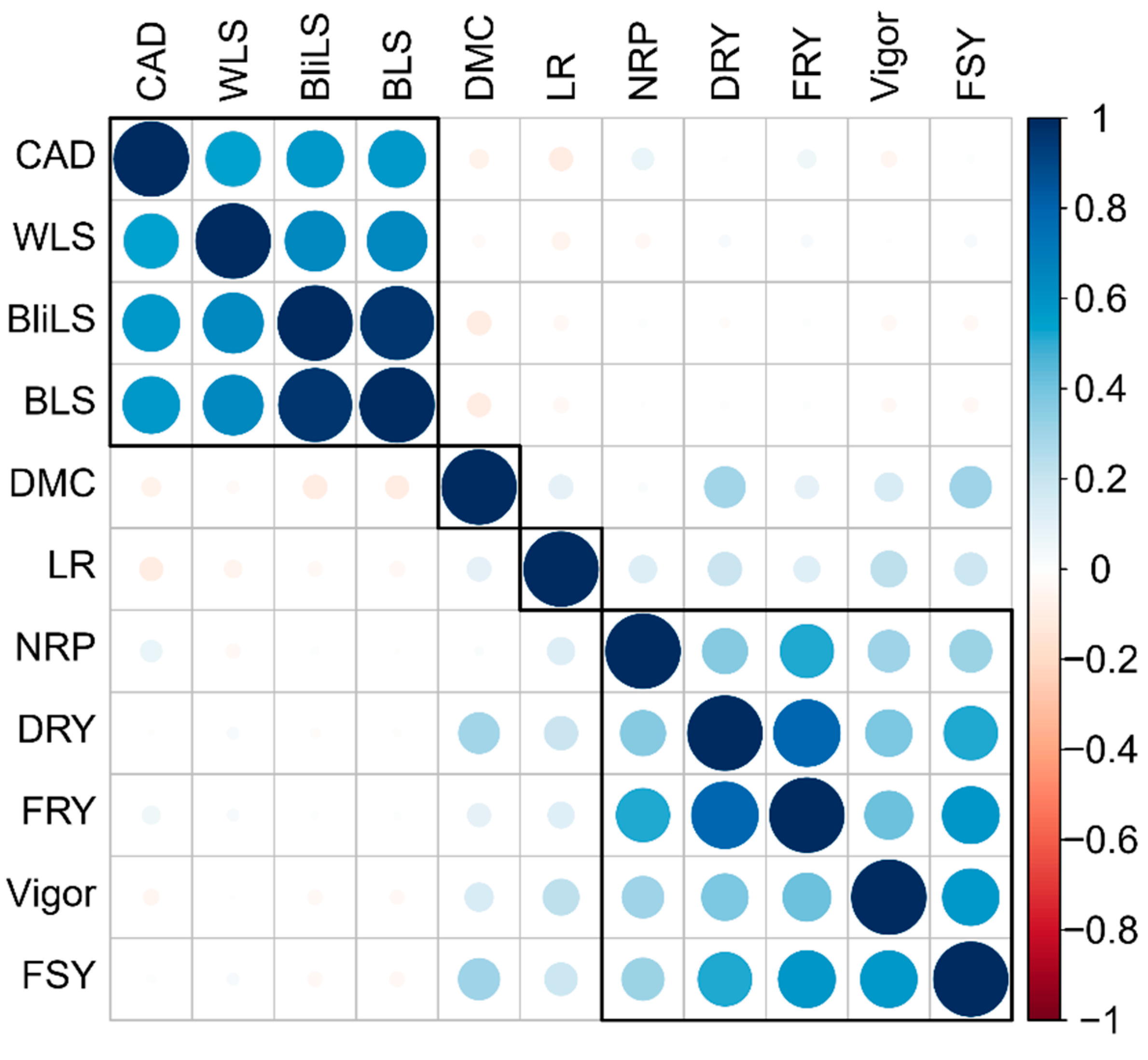
2.2. Phenotypic Correlations
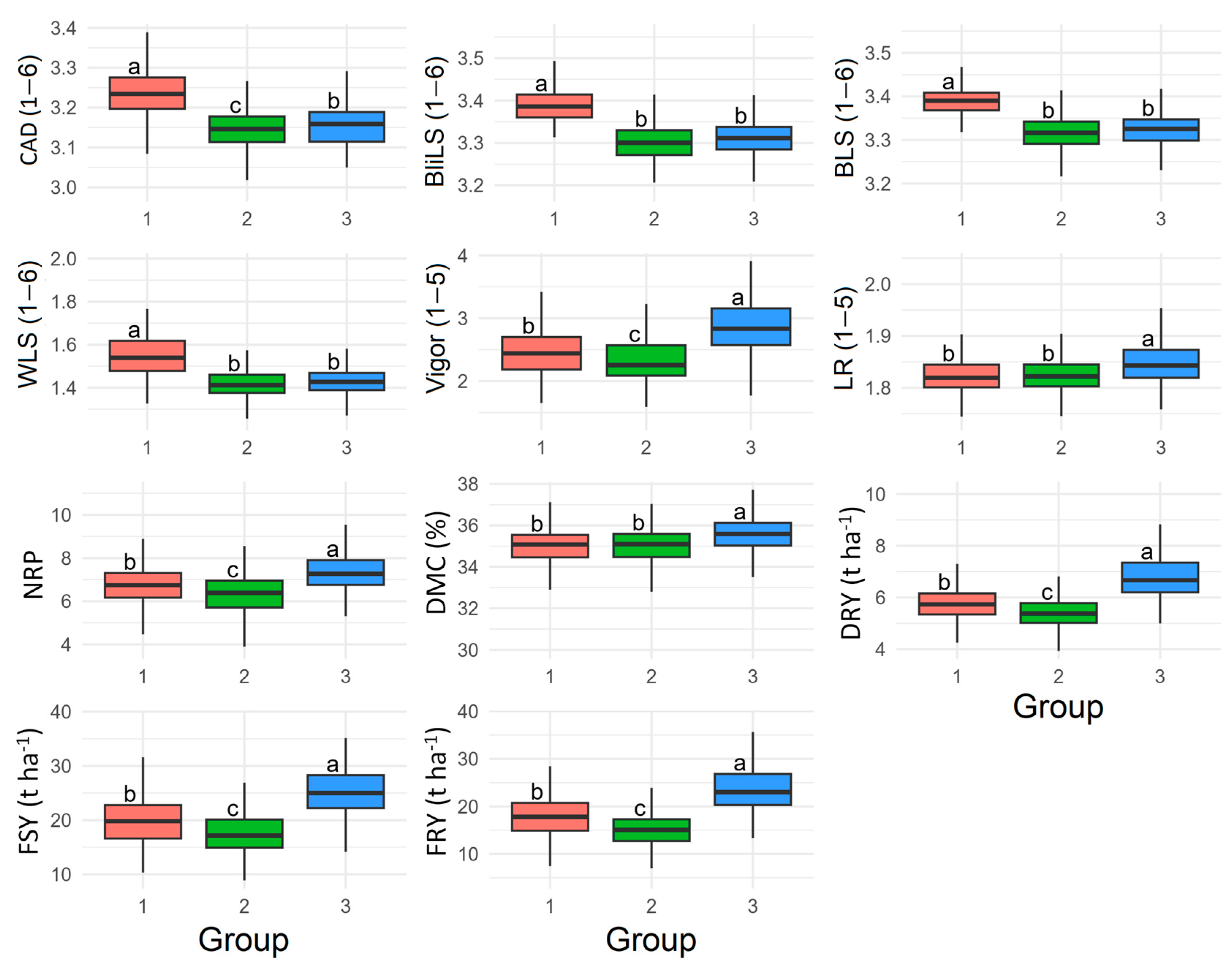
2.3. Analysis of Phenotypic Diversity
2.4. Genotype Selection for Recombination
3. Discussion
3.1. Components of Variation for Resistance to Aerial Part Diseases in Cassava Germplasm
3.2. Association of Resistance to Aerial Part Disease with Yield Attributes
3.3. Clustering of Phenotypic Diversity for Aerial Part Disease Resistance and Yield Attributes
3.4. Genotype Selection for Population Improvement
4. Materials and Methods
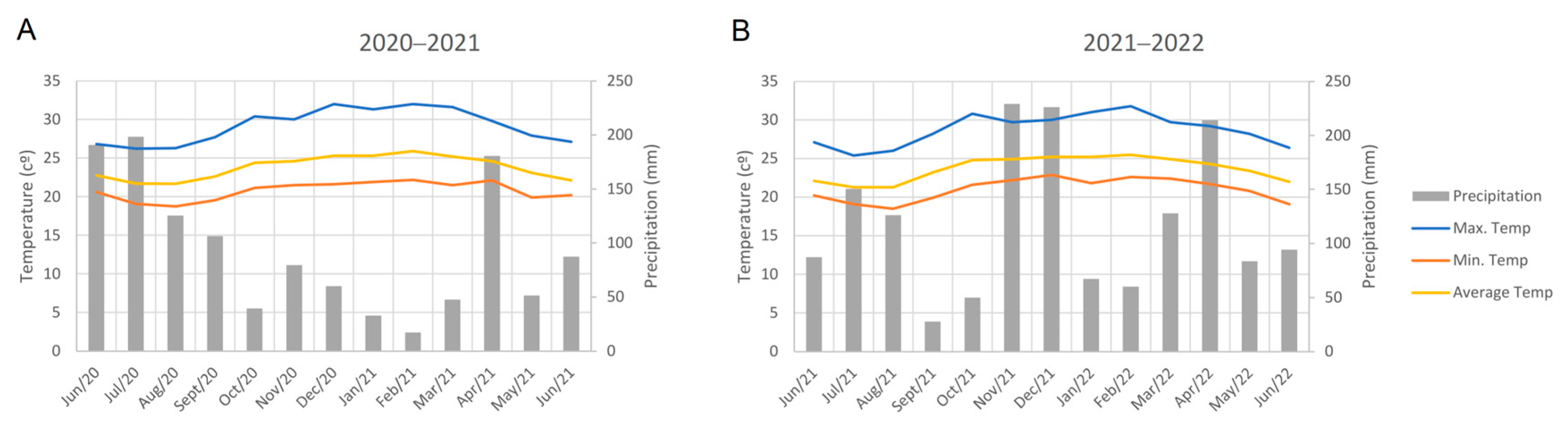
4.1. Plant Material and Experiment Conduct
4.2. Collection of Diseased Leaves and Isolation of Pathogens
4.3. Preparation of Inoculum and Artificial Inoculations
4.4. Evaluated Traits
- (i)
- Anthracnose (CAD): (0) = no infection; (1) = small lesions on leaves and stems; (2) = few shallow cankers on the stem or leaves in the lower half of the plant; (3) = many stem cankers followed by distortion and/or leaf lesions in the upper half of the plant; (4) = many hard and woody lesions on the stem and/or leaves; (5) = highly severe attack with many lesions on hard and woody stems and severe necrosis in leaf axils followed by wilting and severe defoliation of the plant; and (6) = drying of all branches and/or plant death.
- (ii)
- White leaf spot (WLS): (0) = no symptoms; (1) = presence of some affected leaves in the lower third of the plant; (2) = >50% of leaves affected in the lower third of the plant; (3) = affected leaves in the middle and lower third; (4) = mild incidence distributed throughout the plant; (5) = moderate incidence distributed throughout the plant, along with yellowing and/or defoliation of the lower third; (6) = complete defoliation of the plant.
- (iii)
- Brown (BLS) and blight leaf spot (BliLS): (0) = no symptoms; (1) = presence of some affected leaves in the lower third of the plant; (2) = mild incidence in the lower third of the plant; (3) = moderate incidence in the middle and lower thirds, along with yellowing of affected leaves; (4) = severe incidence distributed throughout the plant, along with yellowing and/or defoliation; (5) = partial defoliation of the plant; (6) = complete defoliation of the plant.
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bredeson, J.V.; Lyons, J.B.; Prochnik, S.E.; Wu, G.A.; Ha, C.M.; Edsinger-Gonzales, E.; Grimwood, J.; Schmutz, J.; Rabbi, I.Y.; Egesi, C.; et al. Sequencing wild and cultivated cassava and related species reveals extensive interspecific hybridization and genetic diversity. Nat. Biotechnol. 2016, 34, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Hohenfeld, C.S.; Passos, A.R.; de Carvalho, H.W.L.; de Oliveira, S.A.S.; de Oliveira, E.J. Genome-wide association study and selection for field resistance to cassava root rot disease and productive traits. PLoS ONE 2022, 17, e0270020. [Google Scholar] [CrossRef] [PubMed]
- FAO, Foundation Agricultural Organization. FAOSTAT Database. 2022. Available online: https://www.fao.org/faostat/en/#home (accessed on 7 March 2022).
- Souza, L.D.; Silva, A.F. Sistema de Produção de Mandioca no Semiárido. CNPMF. 2020. Available online: https://www.bdpa.cnptia.embrapa.br/consulta/busca?b=ad&id=1131107&biblioteca=vazio&busca=1131107&qFacets=1131107&sort=&paginacao=t&paginaAtual=1 (accessed on 7 March 2024).
- IBGE, Instituto Brasileiro de Geografia e Estatística. Sistema IBGE de Recuperação Automática-SIDRA. 2022. Available online: https://sidra.ibge.gov.br/home/pimpfbr/brasil (accessed on 7 July 2022).
- Liu, Z.; Braun, U.; Crous, P.W.; Si, J.; Zhang, Y. Taxonomy and phylogeny of cercosporoid fungi (Mycosphaerellaceae) from China. Phytotaxa 2016, 278, 212–224. [Google Scholar] [CrossRef]
- Oliveira, S.A.S.; da Silva, L.L.; de Souza Nascimento, D.; Diamantino, M.S.A.S.; Ferreira, C.F.; de Oliveira, T.A.S. Colletotrichum species causing cassava (Manihot esculenta Crantz) anthracnose in different eco-zones within the Recôncavo Region of Bahia, Brazil. J. Plant Dis. Prot. 2020, 127, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Kunkeaw, S.; Worapong, J.; Smith, D.R.; Triwitayakorn, K. An in vitro detached leaf assay for pre-screening resistance to anthracnose disease in cassava (Manihot esculenta Crantz). Australas. Plant Pathol. 2010, 39, 547. [Google Scholar] [CrossRef]
- Aisyah, S.N.; Hasbiyallah, M.S.; Huzaeni, A.; Trisnawati, D.W.; Nurkomar, I.; Jamsari, J. Survey of cassava anthracnose disease occurrence in various local cultivars of cassava cultivated in Regency of Gunungkidul, special region of Yogyakarta. IOP Conf. Ser. Earth Environ. Sci. 2020, 752, 012051. [Google Scholar] [CrossRef]
- Sangpueak, R.; Phansak, P.; Buensanteai, N. Morphological and molecular identification of Colletotrichum species associated with cassava anthracnose in Thailand. J. Phytopathol. 2018, 166, 129–142. [Google Scholar] [CrossRef]
- Pei, Y.L.; Shi, T.; Li, C.P.; Liu, X.B.; Cai, J.M.; Huang, G.X. Distribution and pathogen identification of cassava brown leaf spot in China. Genet. Mol. Res. 2014, 13, 3461–3473. [Google Scholar] [CrossRef]
- Massola, N.S.; Bedendo, I.P.; Oliveira, S.A.S. Doenças da mandioca. In Manual de Fitopatologia; Amorim, L., Rezende, J.A.M., Camargo, L.F.A., Eds.; Agronômica Ceres: Ouro Fino, Brazil, 2016; p. 810. [Google Scholar]
- Leite, I.C.H.L.; Lima Filho, F.A.S.; Freitas-Lopes, R.L.; Michereff, S.J.; Capucho, A.S.; Lopes, U.P. Standard area diagrams to aid assessments of the severity of blight leaf spot symptoms on cassava leaves. J. Plant Pathol. 2019, 101, 155–160. [Google Scholar] [CrossRef]
- Silva, H.S.A.; de Andrade, E.C. Impacto potencial das mudanças climáticas sobre as doenças da mandioca no Brasil. In Impactos das Mudanças Climáticas Sobre Doenças de Importantes Culturas no Brasil; Ghini, R., Hamada, E., Bettiol, W., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2011; pp. 263–272. [Google Scholar]
- Costa, G.; Freitas-Lopes, R.; Lino, J.; Julião, E.; Michereff, S.; Lopes, U. An efficient method for inducing sporulation of cercosporoid fungal species causing leaf spots in cassava. J. Plant Pathol. 2019, 102, 201–204. [Google Scholar] [CrossRef]
- McCallum, E.J.; Anjanappa, R.B.; Gruissem, W. Tackling agriculturally relevant diseases in the staple crop cassava (Manihot esculenta). Curr. Opin. Plant Biol. 2017, 38, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Julião, E.C.; Santana, M.D.; do Livramento Freitas-Lopes, R.; dos Passos Vieira, A.; de Carvalho, J.S.B.; Lopes, U.P. Reduction of brown leaf spot and changes in the chlorophyll a content induced by fungicides in cassava plants. Eur. J. Plant Pathol. 2020, 157, 433–439. [Google Scholar] [CrossRef]
- Venturini, M.T.; da Silva Araújo, T.; Abreu, E.F.M.; de Andrade, E.C.; da Silva Santos, V.; da Silva, M.R.; Oliveira, E.J. Crop losses in Brazilian cassava varieties induced by the Cassava common mosaic virus. Sci. Agric. 2016, 73, 520–524. [Google Scholar] [CrossRef]
- Boas, S.A.V.; Hohenfeld, C.S.; de Oliveira, S.A.S.; da Silva Santos, V.; de Oliveira, E.J. Sources of resistance to cassava root rot caused by Fusarium spp.: A genotypic approach. Euphytica 2016, 209, 237–251. [Google Scholar] [CrossRef]
- Freitas, J.P.X.; Diniz, R.P.; Oliveira, S.A.S.; da Silva Santos, V.; de Oliveira, E.J. Inbreeding depression for severity caused by leaf diseases in cassava. Euphytica 2017, 213, 205. [Google Scholar] [CrossRef]
- de Albuquerque, H.Y.G.; do Carmo, C.D.; Brito, A.C.; de Oliveira, E.J. Genetic diversity of Manihot esculenta Crantz germplasm based on single-nucleotide polymorphism markers. Ann. Appl. Biol. 2018, 173, 271–284. [Google Scholar] [CrossRef]
- Ogbonna, A.C.; Braatz de Andrade, L.R.; Mueller, L.A.; de Oliveira, E.J.; Bauchet, G.J. Comprehensive genotyping of a Brazilian cassava (Manihot esculenta Crantz) germplasm bank: Insights into diversification and domestication. Theor. Appl. Genet. 2021, 134, 1343–1362. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.E.; Hearne, S.J.; Close, T.J.; Wanamaker, S.; Moskal, W.A.; Town, C.D.; de Young, J.; Marri, P.R.; Rabbi, I.Y.; de Villiers, E.P. Identification, validation and high-throughput genotyping of transcribed gene SNPs in cassava. Theor. Appl. Genet. 2012, 124, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.G.F.; dos Santos, T.P.; Ferreira, A.F.A.; Monteiro, L.N.H.; Nakanishi, E.S.; Boliani, A.C. Morphological characterization of active germplasm bank fig tree accessions. Rev. Bras. Frutic. 2019, 41, e-074. [Google Scholar] [CrossRef]
- Meyer, K. Factor-analytic models for genotype x environment type problems and structured covariance matrices. Genet. Sel. Evol. 2009, 41, 21. [Google Scholar] [CrossRef]
- Bakare, M.A.; Kayondo, S.I.; Aghogho, C.I.; Wolfe, M.D.; Parkes, E.Y.; Kulakow, P.; Egesi, C.; Jannink, J.L.; Rabbi, I.Y. Parsimonious genotype by environment interaction covariance models for cassava (Manihot esculenta). Front. Plant Sci. 2022, 13, 978248. [Google Scholar] [CrossRef] [PubMed]
- Dalarosa, L.E.; Davide, L.M.C.; Gonçalves, M.C.; Bacchi, L.M.A.; dos Santos, A.; Guimarães, A.G.; da Silva, J.L.; da Costa, R.C.L.; de Almeida Amorim, G.A. Parameters and genetic divergence to identify resistance to anthracnose and bacteriosis in cassava accessions. Pesqui. Agropecuária Bras. 2022, 57, e02790. [Google Scholar] [CrossRef]
- Oliveira, E.J.; Oliveira, S.A.S.; Boas, S.A.V.; Hohenfeld, C.S.; da Silva Santos, V. Selection of cassava accessions with multiple resistance to pathogens associated with root rot disease. Euphytica 2017, 213, 185. [Google Scholar] [CrossRef]
- Valentor, A.O.; Ochwo-Ssemakula, M.; Kaweesi, T.; Ozimati, A.; Mrema, E.; Mwale, E.S.; Gibson, P.; Achola, E.; Edema, R.; Baguma, Y.; et al. Plot based heritability estimates and categorization of cassava genotype response to cassava brown streak disease. Crop Prot. 2018, 108, 39–46. [Google Scholar] [CrossRef]
- Reynolds, M.; Chapman, S.; Crespo-Herrera, L.; Molero, G.; Mondal, S.; Pequeno, D.N.L.; Pinto, F.; Pinera-Chavez, F.J.; Poland, J.; Rivera-Amado, C.; et al. Breeder friendly phenotyping. Plant Sci. Int. J. Exp. Plant Biol. 2020, 295, 110396. [Google Scholar] [CrossRef] [PubMed]
- Ano, C.U.; Ochwo-Ssemakula, M.; Ibanda, A.; Ozimati, A.; Gibson, P.; Onyeka, J.; Njoku, D.; Egesi, C.; Kawuki, R.S. Cassava Brown Streak Disease response and association with agronomic traits in elite Nigerian cassava cultivars. Front. Plant Sci. 2021, 12, 720532. [Google Scholar] [CrossRef]
- Obilo, O.P.; Ikotun, B.; Ihejirika, G.O.; Ibeawuchi, I.I.; Oben, T.T. The effect of the incidence of cassava anthracnose disease (CAD) on the performance and yield of cassava cultivars. Crop Prot. 2010, 29, 482–486. [Google Scholar] [CrossRef]
- Teixeira, J.H.; Guimarães, M.A.; Cardoso, S.C.; Brito, A.D.; Diniz, R.P.; de Oliveira, E.J.; de Oliveira, S.A. Evaluation of resistance to bacterial blight in Brazilian cassava germplasm and disease-yield relationships. Trop. Plant Pathol. 2021, 46, 324–335. [Google Scholar] [CrossRef]
- Conceicão, L.V.; Cortes, D.F.M.; Klauser, D.; Robinson, M.; de Oliveira, E.J. New protocol for rapid cassava multiplication in field conditions: A perspective on speed breeding. Front. Plant Sci. 2023, 14, 1258101. [Google Scholar] [CrossRef]
- Mycobank. Mycobank Database. International Mycological Association. 2019. Available online: https://www.mycobank.org/page/Home (accessed on 5 April 2022).
- Peprah, B.B.; Parkes, E.; Manu-Aduening, J.; Kulakow, P.; van Biljon, A.; Labuschagne, M. Genetic variability, stability and heritability for quality and yield characteristics in provitamin A cassava varieties. Euphytica 2020, 216, 31. [Google Scholar] [CrossRef]
- Sampaio Filho, J.S.; Olivoto, T.; de Souza Campos, M.; de Oliveira, E.J. Multi-trait selection in multi-environments for performance and stability in cassava genotypes. Front. Plant Sci. 2023, 14, 1282221. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.J.; Aud, F.F.; Morales, C.F.G.; Oliveira, S.A.S.; Santos, V.S. Non-hierarchical clustering of Manihot esculenta Crantz germplasm based on quantitative traits. Rev. Ciênc. Agronômica 2016, 47, 548–555. [Google Scholar] [CrossRef][Green Version]
- Carvalho, R.R.B.; Bandeira e Sousa, M.; de Oliveira, L.A.; Oliveira, E.J. Phenotypic diversity and selection in biofortified cassava germplasm for yield and quality root traits. Euphytica 2022, 218, 173. [Google Scholar] [CrossRef] [PubMed]
- Rabbi, I.Y.; Udoh, L.I.; Wolfe, M.; Parkes, E.Y.; Gedil, M.A.; Dixon, A.; Ramu, P.; Jannink, J.; Kulakow, P. Genome-wide association mapping of correlated traits in cassava: Dry matter and total carotenoid content. Plant Genome 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.G.; Vilela de Resende, M.D.; Azevedo, C.F.; Fonseca e Silva, F.; Oliveira, E.J. Genomic selection for productive traits in biparental cassava breeding populations. PLoS ONE 2019, 14, e0220245. [Google Scholar] [CrossRef] [PubMed]
- Crevelari, J.A.; Pereira, M.G.; Azevedo, F.H.V.; Vieira, R.A.M. Genetic improvement of silage maize: Predicting genetic gain using selection indexes and best linear unbiased prediction. Rev. Ciênc. Agronômica 2019, 50, 197–204. [Google Scholar] [CrossRef]
- León, R.; Rosero, A.; García, J.-L.; Morelo, J.; Orozco, A.; Silva, G.; De la Ossa, V.; Correa, E.; Cordero, C.; Villalba, L.; et al. Multi-trait selection indices for identifying new cassava varieties adapted to the Caribbean region of Colombia. Agronomy 2021, 11, 1694. [Google Scholar] [CrossRef]
- Oliveira, S.A.S.; Diamantino MS, A.S.; Diniz, R.P.; Oliveira, E.J. Escala de Notas Inespecíficas Para Seleção de Plantas de Mandioca Resistentes a Doenças Foliares. Embrapa Mandioca e Fruticultura. Boletim de Pesquisa e Desenvolvimento, 112. 2020. Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1124859/escala-de-notas-inespecificas-para-selecao-de-plantas-de-mandioca-resistentes-a-doencas-foliares (accessed on 12 December 2023).
- Kawano, K.; Fukuda, W.; Cenpukdee, U. Genetic and environmental effects on dry matter content of cassava root. Crop Sci. 1987, 27, 69–74. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Jombart, T.; Devillard, S.; Balloux, F. Discriminant analysis of principal components: A new method for the analysis of genetically structured populations. BMC Genet. 2010, 11, 94. [Google Scholar] [CrossRef]
- Jombart, T.; Ahmed, I. adegenet 1.3-1: New tools for the analysis of genome-wide SNP data. Bioinformatics 2011, 27, 3070–3071. [Google Scholar] [CrossRef] [PubMed]
- Grenier, C.; Bramel-Cox, P.J.; Noirot, M.; Prasada Rao, K.E.; Hamon, P. Assessment of genetic diversity in three subsets constituted from the ICRISAT sorghum collection using random vs non-random sampling procedures. Using morpho-agronomical and passport data. Theor. Appl. Genet. 2000, 101, 190–196. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2022. Available online: https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing (accessed on 28 August 2023).
- Mulamba, N.N.; Mock, J. Improvement of yield potential of the ETO blanco maize (Zea mays L.) population by breeding for plant traits. Egypt. J. Genet. Cytol. 1978, 1, 40–51. [Google Scholar]

| Disease | Factor | DF | SS | MS | F |
|---|---|---|---|---|---|
| CAD | Year | 1 | 14.23 | 14.23 | 48.88 * |
| Block | 28 | 308.68 | 11.02 | 33.22 ns | |
| WLS | Year | 1 | 111.25 | 111.25 | 276.94 * |
| Block | 28 | 264.52 | 9.45 | 23.52 ns | |
| BLS | Year | 1 | 76.57 | 76.57 | 179.61 * |
| Block | 28 | 336.92 | 12.00 | 28.23 ns | |
| BliLS | Year | 1 | 310.44 | 310.44 | 590.33 * |
| Block | 28 | 269.55 | 9.56 | 18.17 ns |
| Disease | Factor | Deviance | DF | p-Valor |
|---|---|---|---|---|
| CAD | Genotype (G) | 1165.85 | 1 | <0.01 ** |
| G × Year | 2185.42 | 1 | ||
| WLS | Genotype (G) | 865.05 | 1 | <0.01 ** |
| G × Year | 1298.36 | 1 | ||
| BLS | Genotype (G) | 807.06 | 1 | <0.01 ** |
| G × Year | 1521.75 | 1 | ||
| BliLS | Genotype (G) | 835.09 | 1 | <0.01 ** |
| G × Year | 1550.09 | 1 |
| Disease | (%) | (%) | ||||
|---|---|---|---|---|---|---|
| CAD | 0.49 | 0.80 | 0.33 | 0.41 | 6.95 | 18.17 |
| WLS | 0.39 | 0.68 | 0.40 | 0.34 | 5.92 | 19.06 |
| BLS | 0.34 | 0.71 | 0.43 | 0.29 | 5.59 | 19.57 |
| BliLS | 0.70 | 0.77 | 0.53 | 0.51 | 18.16 | 49.84 |
| Group | CAD | BliLS | BLS | WLS | Vigor | NRP | LR | DRY | DMC | FRY | FSY |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.79 | 0.82 | 0.76 | 0.80 | 0.74 | 0.86 | 0.71 | 0.79 | 0.79 | 0.78 | 0.78 |
| 2 | 0.84 | 0.77 | 0.75 | 0.70 | 0.89 | 0.88 | 0.83 | 0.85 | 0.67 | 0.75 | 0.85 |
| 3 | 0.79 | 0.79 | 0.79 | 0.82 | 0.88 | 0.73 | 0.69 | 0.76 | 0.77 | 0.87 | 0.86 |
| Average | 0.81 | 0.79 | 0.76 | 0.77 | 0.84 | 0.82 | 0.74 | 0.80 | 0.74 | 0.80 | 0.83 |
| Genotype | CAD | BliLS | BLS | WLS | Vigor | NRP | LR | DRY | DMC | FRY | FSY | SelInd |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BR-11-34-41 | 3.18 | 3.32 | 3.31 | 1.33 | 3.85 | 11.16 | 1.94 | 8.83 | 32.15 | 38.41 | 32.35 | 719.03 |
| BRS Caipira | 3.07 | 3.17 | 3.34 | 1.39 | 3.58 | 10.82 | 1.82 | 8.44 | 35.66 | 35.32 | 32.30 | 718.32 |
| BGM-0442 | 3.10 | 3.20 | 3.21 | 1.60 | 3.01 | 8.44 | 1.84 | 7.78 | 34.87 | 37.69 | 33.24 | 706.39 |
| BGM-0847 | 3.03 | 3.24 | 3.23 | 1.32 | 3.25 | 7.72 | 1.82 | 9.19 | 35.95 | 34.02 | 27.83 | 691.10 |
| BGM-2082 | 3.11 | 3.21 | 3.17 | 1.38 | 3.61 | 8.30 | 1.88 | 7.41 | 35.67 | 35.30 | 25.48 | 690.48 |
| BRS Novo Horizonte | 3.04 | 3.23 | 3.27 | 1.42 | 3.50 | 9.23 | 1.89 | 8.50 | 36.56 | 32.68 | 34.04 | 689.79 |
| BGM-0049 | 2.99 | 3.26 | 3.24 | 1.36 | 3.22 | 6.94 | 1.85 | 8.58 | 36.83 | 33.56 | 38.69 | 683.06 |
| BGM-0093 | 3.21 | 3.34 | 3.35 | 1.51 | 2.79 | 7.49 | 1.85 | 8.78 | 35.27 | 35.33 | 32.56 | 680.62 |
| BGM-2042 | 3.03 | 3.27 | 3.28 | 1.35 | 3.66 | 8.46 | 1.86 | 7.70 | 36.55 | 33.18 | 31.26 | 680.42 |
| BRS Tapioqueira | 3.10 | 3.24 | 3.36 | 1.47 | 3.32 | 9.85 | 1.90 | 7.78 | 35.40 | 33.08 | 27.65 | 675.46 |
| BGM-0287 | 3.14 | 3.26 | 3.23 | 1.28 | 2.79 | 7.52 | 1.83 | 7.34 | 35.05 | 35.63 | 28.77 | 673.81 |
| BRS Poti Branca | 3.01 | 3.22 | 3.30 | 1.37 | 3.58 | 9.41 | 1.84 | 7.85 | 35.28 | 32.48 | 33.97 | 670.47 |
| BGM-1626 | 3.06 | 3.19 | 3.21 | 1.31 | 2.73 | 7.97 | 1.82 | 7.95 | 35.25 | 33.80 | 28.19 | 670.04 |
| BRS Poti-Branca | 3.00 | 3.14 | 3.32 | 1.46 | 3.05 | 9.54 | 1.94 | 8.69 | 35.84 | 30.92 | 31.63 | 668.79 |
| BGM-0901 | 3.08 | 3.08 | 3.18 | 1.33 | 3.33 | 8.52 | 1.80 | 8.37 | 35.64 | 31.76 | 30.78 | 667.14 |
| BR-11-34-45 | 3.12 | 3.32 | 3.20 | 1.40 | 3.91 | 8.24 | 1.92 | 9.23 | 35.55 | 31.32 | 26.71 | 665.77 |
| BGM-0279 | 3.15 | 3.17 | 3.22 | 1.50 | 3.26 | 8.18 | 1.81 | 8.57 | 34.86 | 32.51 | 29.98 | 660.80 |
| BGM-0058 | 3.07 | 3.19 | 3.17 | 1.36 | 3.37 | 8.32 | 1.83 | 7.31 | 34.12 | 33.26 | 30.68 | 653.24 |
| BGM-1282 | 3.02 | 3.19 | 3.13 | 1.32 | 3.02 | 6.35 | 1.81 | 9.15 | 36.85 | 30.16 | 34.83 | 652.11 |
| BGM-0394 | 3.05 | 3.37 | 3.21 | 1.49 | 2.83 | 7.75 | 1.83 | 7.82 | 35.15 | 32.76 | 27.89 | 651.76 |
| BGM-0693 | 3.09 | 3.22 | 3.22 | 1.35 | 2.78 | 7.79 | 1.83 | 7.86 | 35.30 | 32.25 | 26.48 | 651.19 |
| BGM-0120 | 3.16 | 3.25 | 3.24 | 1.37 | 2.79 | 7.52 | 1.90 | 8.52 | 33.66 | 33.14 | 33.58 | 646.37 |
| BGM-0714 | 3.05 | 3.22 | 3.25 | 1.26 | 3.03 | 8.06 | 1.87 | 7.21 | 35.51 | 31.76 | 29.38 | 646.22 |
| BRS Amansa Burro | 3.16 | 3.18 | 3.19 | 1.40 | 3.30 | 8.64 | 1.88 | 8.42 | 36.94 | 28.53 | 28.66 | 645.61 |
| BGM-0323 | 3.15 | 3.17 | 3.28 | 1.37 | 2.90 | 7.76 | 1.83 | 8.28 | 35.98 | 30.43 | 23.71 | 642.82 |
| Média selecionados | 3.09 | 3.23 | 3.24 | 1.39 | 3.22 | 8.40 | 1.86 | 8.22 | 35.43 | 33.17 | 30.43 | 672.03 |
| Média população | 3.17 | 3.32 | 3.33 | 1.45 | 2.50 | 6.71 | 1.83 | 5.90 | 35.15 | 18.22 | 20.31 | 471.90 |
| Diferencial de seleção (%) | −2.59 | −2.90 | −2.68 | −4.55 | 28.49 | 25.20 | 1.29 | 39.47 | 0.81 | 82.06 | 49.82 | 42.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, J.H.B.; Andrade, L.R.B.d.; Oliveira, S.A.S.d.; Oliveira, E.J.d. Phenotypic Variability in Resistance to Anthracnose, White, Brown, and Blight Leaf Spot in Cassava Germplasm. Plants 2024, 13, 1187. https://doi.org/10.3390/plants13091187
Nascimento JHB, Andrade LRBd, Oliveira SASd, Oliveira EJd. Phenotypic Variability in Resistance to Anthracnose, White, Brown, and Blight Leaf Spot in Cassava Germplasm. Plants. 2024; 13(9):1187. https://doi.org/10.3390/plants13091187
Chicago/Turabian StyleNascimento, José Henrique Bernardino, Luciano Rogerio Braatz de Andrade, Saulo Alves Santos de Oliveira, and Eder Jorge de Oliveira. 2024. "Phenotypic Variability in Resistance to Anthracnose, White, Brown, and Blight Leaf Spot in Cassava Germplasm" Plants 13, no. 9: 1187. https://doi.org/10.3390/plants13091187
APA StyleNascimento, J. H. B., Andrade, L. R. B. d., Oliveira, S. A. S. d., & Oliveira, E. J. d. (2024). Phenotypic Variability in Resistance to Anthracnose, White, Brown, and Blight Leaf Spot in Cassava Germplasm. Plants, 13(9), 1187. https://doi.org/10.3390/plants13091187







