Distribution and Mechanism of Japanese Brome (Bromus japonicus) Resistance to ALS-Inhibiting Herbicides in China
Abstract
1. Introduction
2. Results
2.1. Resistant Population Screening and Distribution
2.2. Amplification and Sequencing of the ALS Gene Fragment
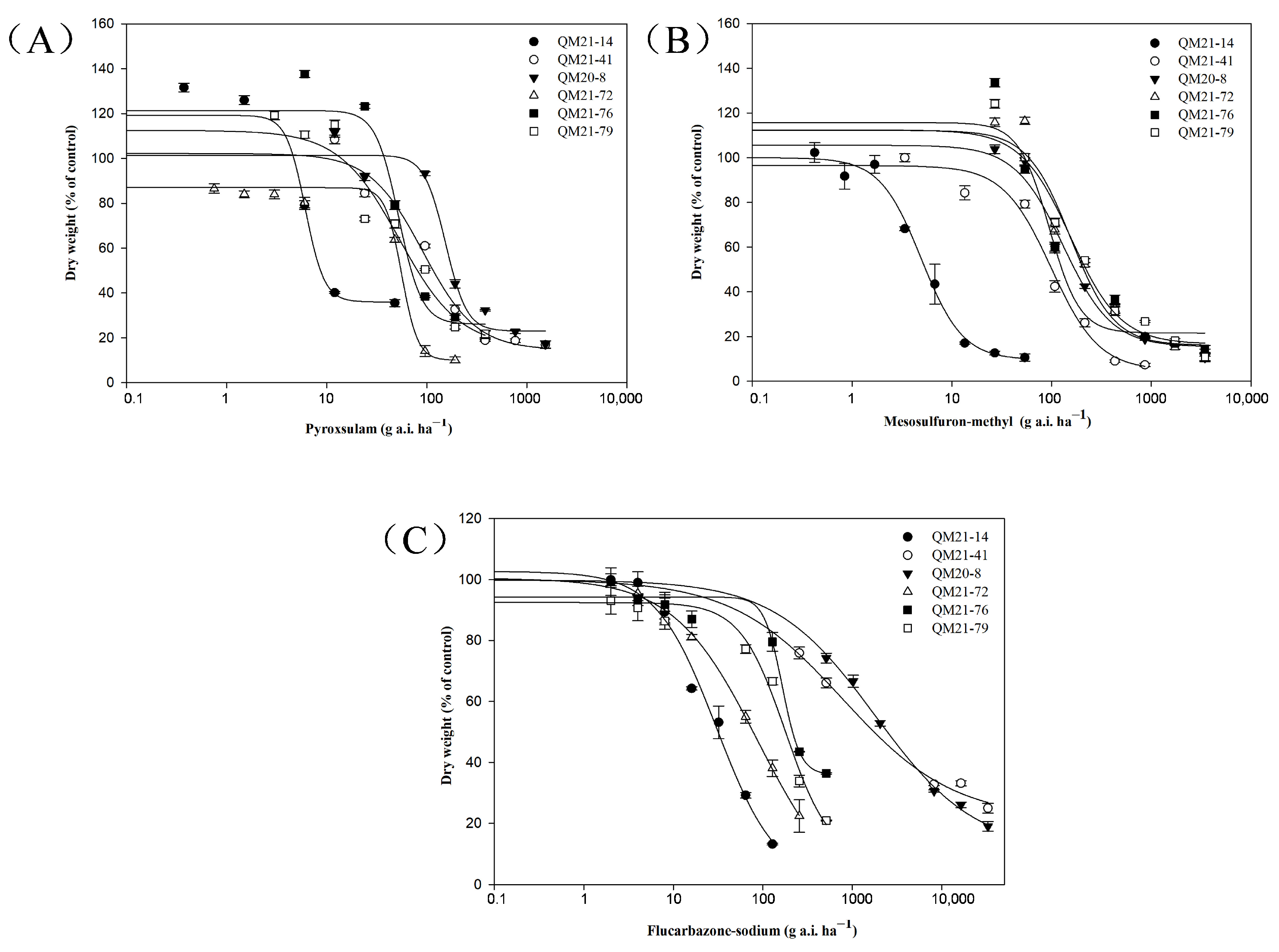
2.3. Cross-Resistance to Other ALS-Inhibiting Herbicides
3. Discussion
4. Materials and Methods
4.1. Resistant Populations Screening
4.2. Amplification and Sequencing of the ALS Gene Fragment
4.3. Cross-Resistance Patterns to ALS-Inhibiting Herbicides
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, M. Biological characteristics of Japanese brome observed in the wheat of Zhuanglang country. Gansu Agric. Sci. Technol 2010, 8, 30. [Google Scholar]
- Flora of China. Available online: www.iplant.cn/foc (accessed on 1 April 2023).
- Lan, Y.; Zhou, X.; Lin, S.; Cao, Y.; Wei, S.; Huang, H.; Li, W.; Huang, Z. Pro-197-Ser Mutation and Cytochrome P450-Mediated Metabolism Conferring Resistance to Flucarbazone-Sodium in Bromus japonicus. Plants 2022, 11, 1641. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, J.; Guo, W.; Du, L.; Bai, P.; Liu, Y. Target-site basis for resistance to flucarbazone-sodium in Japanese brome (Bromus japonicus Houtt.) in China. Chil. J. Agric. Res. 2022, 82, 493–501. [Google Scholar] [CrossRef]
- Liu, L.; Wu, L.; Li, Z.; Fang, Y.; Ju, B.; Zhang, S.; Bai, L.; Pan, L. The Pro-197-Thr mutation in the ALS gene confers novel resistance patterns to ALS-inhibiting herbicides in Bromus japonicus in China. Front. Plant Sci. 2024, 15, 1348815. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, B.; Li, B.; Shen, B.; Qi, Z.; Wang, J.; Cui, H.; Chen, S.; Wang, G.; Liu, X. Diverse ALS mutations and cross-and multiple-resistance to ALS and EPSPS inhibitors in flucarbazone-sodium-resistant Bromus japonicus populations from Hebei province, China. Pest. Biochem. Physiol. 2024, 199, 105794. [Google Scholar] [CrossRef]
- Tan, S.; Evans, R.R.; Dahmer, M.L.; Singh, B.K.; Shaner, D.L. Imidazolinone-tolerant crops: History, current status and future. Pest Manag. Sci. 2004, 61, 246–257. [Google Scholar] [CrossRef]
- Fischer, A.J.; Bayer, D.E.; Carriere, M.D.; Ateh, C.M.; Yim, K.-O. Mechanisms of Resistance to Bispyribac-Sodium in an Echinochloa phyllopogon Accession. Pest. Biochem. Physiol. 2000, 68, 156–165. [Google Scholar] [CrossRef]
- Bai, S. Excavation and Functional Verification of Tribenuron-Methyl Metabolic Resistance Genes in Myosoton aquaticum L. Ph. D. Thesis, Shandong Agricultural University, Tai’an, China, 2019.
- Heap, I. International Survey of Herbicide Resistant Weeds. Available online: www.weedscience.org (accessed on 1 March 2023).
- Délye, C. Weed resistance to acetyl coenzyme A carboxylase inhibitors: An update. Weed Sci. 2017, 53, 728–746. [Google Scholar] [CrossRef]
- Fang, J.; Yang, D.; Zhao, Z.; Chen, J.; Dong, L. A novel Phe-206-Leu mutation in acetolactate synthase confers resistance to penoxsulam in barnyardgrass (Echinochloa crus-galli (L.) P. Beauv). Pest. Manag. Sci. 2022, 78, 2560–2570. [Google Scholar] [CrossRef]
- Kumar, V.; Jha, P. First report of Ser653Asn mutation endowing high-level resistance to imazamox in downy brome (Bromus tectorum L.). Pest Manag. Sci. 2017, 73, 2585–2591. [Google Scholar] [CrossRef]
- Deng, W.; Di, Y.; Cai, J.; Chen, Y.; Yuan, S. Target-Site Resistance Mechanisms to Tribenuron-methyl and Cross-resistance Patterns to ALS-inhibiting Herbicides of Catchweed Bedstraw (Galium aparine) with Different ALS Mutations. Weed Sci. 2018, 67, 183–188. [Google Scholar] [CrossRef]
- Kaya Altop, E.; Erken Meral, S.; Zandstra, B.H.; Mennan, H. Target-Site Point Mutation Conferring Resistance to ALS Herbicides in Italian Ryegrass (Lolium multiflorum L.). Phytoparasitica 2022, 50, 1133–1142. [Google Scholar] [CrossRef]
- Délye, C. Unravelling the genetic bases of non-target-site-based resistance (NTSR) to herbicides: A major challenge for weed science in the forthcoming decade. Pest Manag. Sci. 2012, 69, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Nakka, S.; Thompson, C.R.; Peterson, D.E.; Jugulam, M. Target Site–Based and Non–Target Site Based Resistance to ALS Inhibitors in Palmer Amaranth (Amaranthus palmeri). Weed Sci. 2017, 65, 681–689. [Google Scholar] [CrossRef]
- Huang, Z.; Lu, X.; Wei, S.; Zhang, C.; Jiang, C.; Li, W.; Saeed, M.; Huang, H. The target-site based resistance mechanism of Alopecurus myosuroides Huds. to pyroxsulam. Crop Prot. 2021, 147, 105707. [Google Scholar] [CrossRef]
- Feng, Y.; Gao, Y.; Zhang, Y.; Dong, L.; Li, J. Mechanisms of Resistance to Pyroxsulam and ACCase Inhibitors in Japanese Foxtail (Alopecurus japonicus). Weed Sci. 2017, 64, 695–704. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, H.; Gao, H.; Liu, Y.; Li, J.; Feng, Z.; Dong, L. Multiple resistance to three modes of action of herbicides in a single italian ryegrass (Lolium multiflorum L.) population in China. Agronomy 2023, 13, 216. [Google Scholar] [CrossRef]
- McCourt, J.A.; Pang, S.S.; King-Scott, J.; Guddat, L.W.; Duggleby, R.G. Herbicide-binding sites revealed in the structure of plant acetohydroxyacid synthase. Proc. Natl. Acad. Sci. USA 2006, 103, 569–573. [Google Scholar] [CrossRef]
- Beckie, H.J.; Tardif, F.J. Herbicide cross resistance in weeds. Crop Prot. 2012, 35, 15–28. [Google Scholar] [CrossRef]
- Deng, W.; Yang, Q.; Zhang, Y.; Jiao, H.; Mei, Y.; Li, X.; Zheng, M. Cross-resistance patterns to acetolactate synthase (ALS)-inhibiting herbicides of flixweed (Descurainia sophia L.) conferred by different combinations of ALS isozymes with a Pro-197-Thr mutation or a novel Trp-574-Leu mutation. Pest. Biochem. Physiol. 2017, 136, 41–45. [Google Scholar] [CrossRef]
- Deng, W.; Yang, M.; Duan, Z.; Peng, C.; Xia, Z.; Yuan, S. Molecular basis of resistance to bensulfuron-methyl and cross-resistance patterns to ALS-inhibiting herbicides in Ludwigia prostrata. Weed Technol. 2021, 35, 656–661. [Google Scholar] [CrossRef]
- Yu, Q.; Han, H.; Li, M.; Purba, E.; Walsh, M.J.; Powles, S.B. Resistance evaluation for herbicide resistance-endowing acetolactate synthase (ALS) gene mutations using Raphanus raphanistrum populations homozygous for specific ALS mutations. Weed Res. 2012, 52, 178–186. [Google Scholar] [CrossRef]
- Zhao, B.; Fu, D.; Yu, Y.; Huang, C.; Yan, K.; Li, P.; Shafi, J.; Zhu, H.; Wei, S.; Ji, M. Non-target-site resistance to ALS-inhibiting herbicides in a Sagittaria trifolia L. population. Pest. Biochem. Physiol. 2017, 140, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zhang, F.; Li, Z.; Wang, H.; Wang, Q.; Wang, J.; Liu, W.; Bai, L. Target-site and non-target-site-based resistance to tribenuron-methyl in multiply-resistant Myosoton aquaticum L. Pest. Biochem Physiol. 2019, 155, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.L.; Ge, M.; Zhang, Q.; Gao, J.; Zhang, Z.; Li, Y. Biological activity of eight herbicides to eight species of major grasses in wheat fields. Acta. Phytophylacica Sin. 2011, 38, 557–562. [Google Scholar]
- Yu, Q.; Han, H.; Vila-Aiub, M.M.; Powles, S.B. AHAS herbicide resistance endowing mutations: Effect on AHAS functionality and plant growth. J. Exp. Bot. 2010, 61, 3925–3934. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, L.; Li, X.; Zheng, M. Investigation of resistant level to tribenuron-methyl, diversity and regional difference of the resistant mutations on acetolactate synthase (ALS) isozymes in Descurainia sophia L. from China. Pest. Biochem. Physiol. 2020, 169, 104653. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, X.; Chen, J.; Peng, L.; Wang, J.; Cui, H. Variation in mutations providing resistance to acetohydroxyacid synthase inhibitors in Cyperus difformis in China. Pest. Biochem. Physiol. 2020, 166, 104571. [Google Scholar] [CrossRef] [PubMed]
- Tanigaki, S.; Uchino, A.; Okawa, S.; Miura, C.; Hamamura, K.; Matsuo, M.; Yoshino, N.; Ueno, N.; Toyama, Y.; Fukumi, N. Gene expression shapes the patterns of parallel evolution of herbicide resistance in the agricultural weed Monochoria vaginalis. New Phytol. 2021, 232, 928–940. [Google Scholar] [CrossRef]
- Long, W.; Malone, J.; Boutsalis, P.; Preston, C. Diversity and extent of mutations endowing resistance to the acetolactate synthase (AHAS)-inhibiting herbicides in Indian hedge mustard (Sisymbrium orientale) populations in Australia. Pest. Biochem. Physiol. 2019, 157, 53–59. [Google Scholar] [CrossRef]
- Merriam, A.B. Herbicide Resistant Common Sowthistle (Sonchus oleraceus L.) and Prickly Lettuce (Lactuca serriola L.): Management Considerations in Lentils. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 2022. [Google Scholar]
- Yanniccari, M.; Vázquez-García, J.G.; Gómez-Lobato, M.E.; Rojano-Delgado, A.M.; Alves, P.L.d.C.A.; De Prado, R. First Case of Glyphosate Resistance in Bromus catharticus Vahl.: Examination of Endowing Resistance Mechanisms. Front. Plant Sci. 2021, 12, 617945. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.K.; Hamouzová, K.; Mikulka, J.; Bharati, R.; Košnarová, P.; Hamouz, P.; Roy, A.; Soukup, J. Enhanced metabolism and target gene overexpression confer resistance against acetolactate synthase-inhibiting herbicides in Bromus sterilis. Pest Manag. Sci. 2021, 77, 2122–2128. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.J.; Goggin, D.E.; Powles, S.B. Non-target-site-based resistance to ALS-inhibiting herbicides in six Bromus rigidus populations from Western Australian cropping fields. Pest Manag. Sci. 2012, 68, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Cao, Y.; Yang, Q.; Liu, M.J.; Mei, Y.; Zheng, M.Q. Different cross-resistance patterns to AHAS herbicides of two tribenuron-methyl resistant flixweed (Descurainia sophia L.) biotypes in China. Pest. Biochem. Physiol. 2014, 112, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Shafi, J.; Zhao, B.; Li, X.; Zhu, H.; Wei, S.; Ji, M. Bensulfuron-methyl resistant Sagittaria trifolia L.: Multiple resistance, cross-resistance and molecular basis of resistance to acetolactate synthase-inhibiting herbicides. Arch. Biol. Sci. 2017, 69, 649–658. [Google Scholar] [CrossRef]
- Huang, Z.; Lu, Z.; Huang, H.; Li, W.; Cao, Y.; Wei, S. Target site mutations and cytochrome P450s-involved metabolism confer resistance to nicosulfuron in green foxtail (Setaria viridis). Pest. Biochem. Physiol. 2021, 179, 104956. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Li, D.; Han, Y.; Li, Z.; Yu, H.; Cui, H. Non-target-site and target-site resistance to AHAS inhibitors in American sloughgrass (Beckmannia syzigachne). J. Integr Agric. 2018, 17, 2714–2723. [Google Scholar] [CrossRef]
- Zhao, N.; Bi, Y.; Wu, C.; Wang, D.; You, L.; Liu, W.; Wang, J. Cross-resistance to acetolactate synthase (ALS) inhibitors associated with different mutations in Japanese foxtail (Alopecurus japonicus). Weed Sci. 2019, 67, 389–396. [Google Scholar] [CrossRef]
- Saari, L.L.; Cotterman, J.C.; Primiani, M.M. Mechanism of sulfonylurea herbicide resistance in the broadleaf weed, Kochia scoparia. Plant Physiol. 1990, 93, 55–61. [Google Scholar] [CrossRef]
- Yu, Q.; Powles, S.B. Resistance to AHAS inhibitor herbicides: Current understanding. Pest Manag. Sci. 2014, 70, 1340–1350. [Google Scholar] [CrossRef]
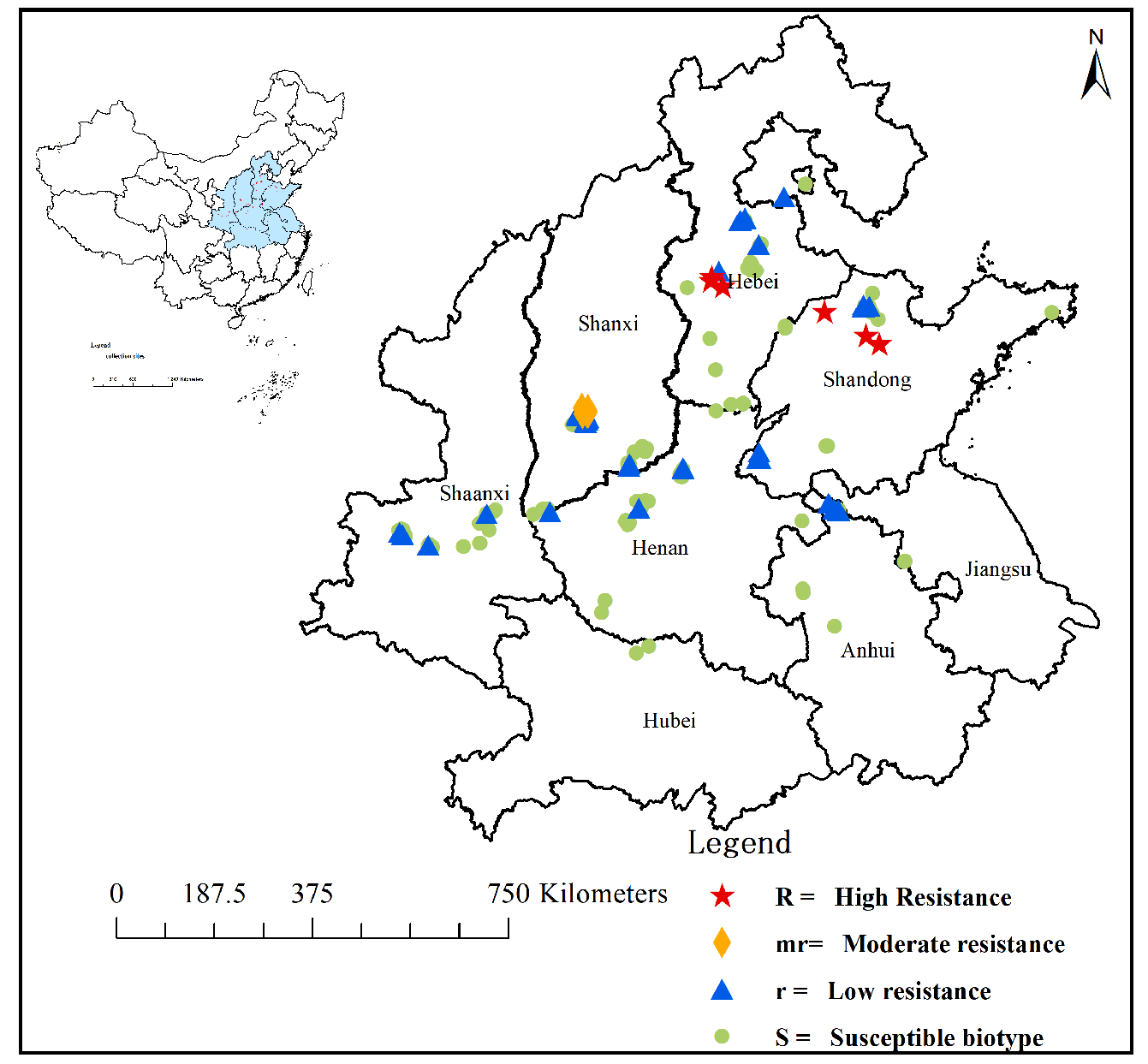
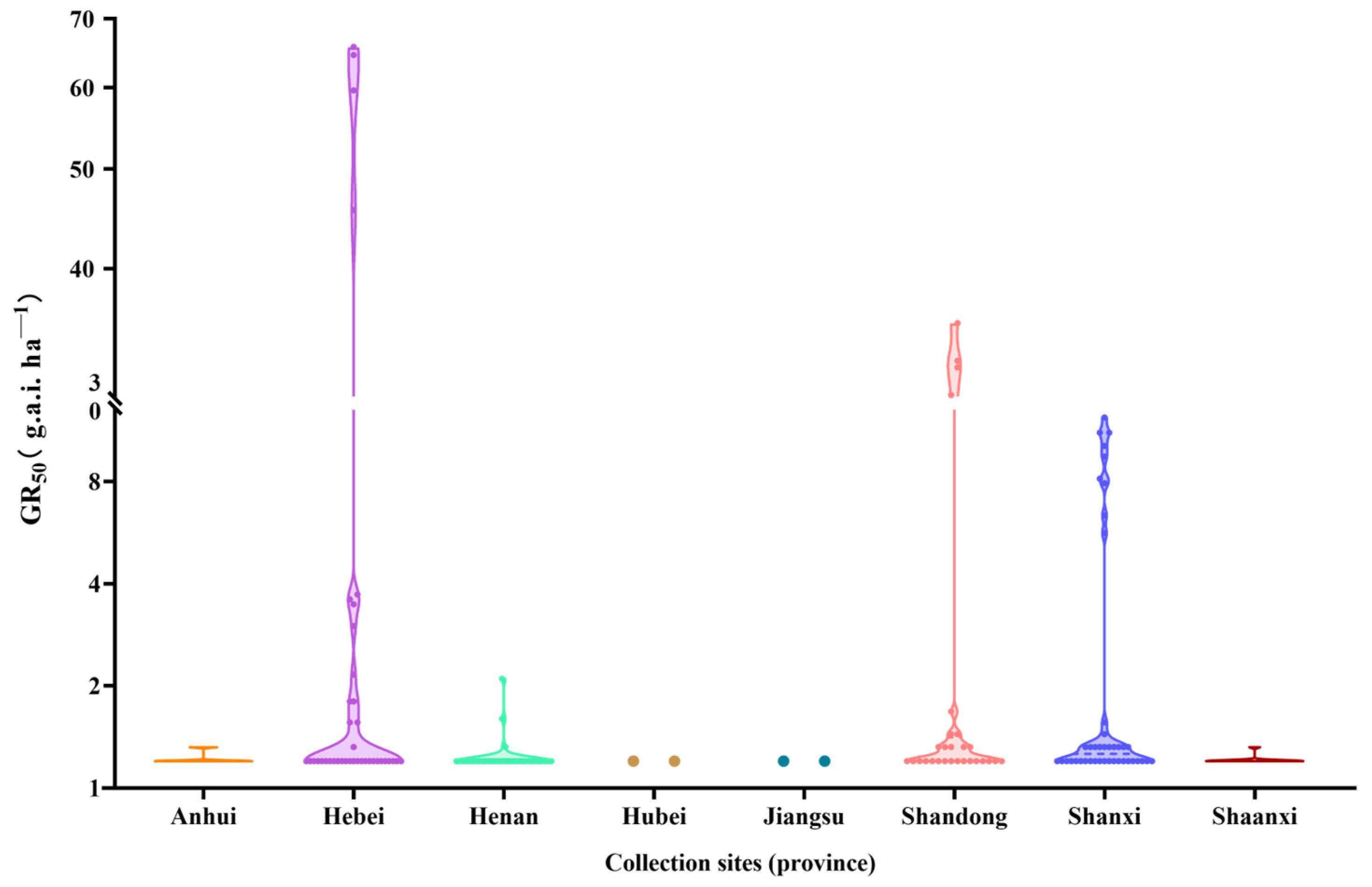
| Populations | Collection Sites | RI a | Mutation Types of ALS Gene b | ||
|---|---|---|---|---|---|
| 197 (Pro) | 376 (Asp) | ||||
| QM21-14 | Dongleitou, Anxin, Baoding, Hebei | - | - | - | 15/15 |
| QM21-41 | Liguizi, Wuji, Shijiazhuang, Hebei | 38 | Ser | - | 15/15 |
| QM22-18 | Zhongtong, Xinle, Shijiazhuang, Hebei | 54.8 | Ser | - | 15/15 |
| QM22-19 | Tongyizhuang, Xinle, Shijiazhuang, Hebei | 53.8 | Ser | - | 15/15 |
| QM22-20 | Hejiazhuang, Xinle, Shijiazhuang, Hebei | 49.7 | Ser | - | 15/15 |
| QM20-7 | Beiguo, Gaoqing, Zibo, Shandong | 25.1 | Thr | - | 15/15 |
| QM20-8 | Xiaohexi1, Gaoqing, Zibo, Shandong | 26.7 | Thr Phe | - | 11/15 4/15 |
| QM20-9 | Xiaohexi2, Gaoqing, Zibo, Shandong | 29.5 | Thr | - | 15/15 |
| QM22-49 | Jijiang, Zhanhua, Binzhou, Shandong | 27.1 | - | Glu | 15/15 |
| QM21-71 | Nanqin, Hongdong, Linfen, Shanxi | 6.6 | - | Glu | 15/15 |
| QM21-72 | Shiqiao, Hongdong, Linfen, Shanxi | 9.3 | - | Glu | 15/15 |
| QM21-73 | Yanjiazhuang, Hongdong, Linfen, Shanxi | 8.5 | Thr - | - Glu | 1/15 14/15 |
| QM21-74 | Beizhuang, Hongdong, Linfen, Shanxi | 7.9 | - | Glu | 15/15 |
| QM21-75 | Houhetou, Hongdong, Linfen, Shanxi | 10.0 | - | Glu | 15/15 |
| QM21-76 | Shitun, Hongdong, Linfen, Shanxi | 9.3 | - | Glu | 15/15 |
| QM21-77 | Gaochi, Hongdong, Linfen, Shanxi | 6.8 | - | Glu | 15/15 |
| QM21-78 | Masan, Hongdong, Linfen, Shanxi | 4.7 | - | Glu | 15/15 |
| QM21-79 | Nanduan, Hongdong, Linfen, Shanxi | 5.3 | - | Glu | 15/15 |
| Herbicides | Populations | ALS Mutation | GR50 a (SE) b (g a.i. ha−1) | RI c |
|---|---|---|---|---|
| Pyroxsulam | QM21-14 | Wild type | 6.11 (1.30) | - |
| QM21-41 | Pro-197-Ser | 91.67 (20.30) | 15.0 | |
| QM20-8 | Pro-197-Thr/Phe | 154.24 (21.03) | 25.2 | |
| QM21-72 | Asp-376-Glu | 56.13 (8.62) | 9.2 | |
| QM21-76 | Asp-376-Glu | 52.54 (11.37) | 8.6 | |
| QM21-79 | Asp-376-Glu | 56.40 (7.44) | 9.2 | |
| Mesosulfuron–methyl | QM21-14 | Wild type | 4.94 (0.49) | - |
| QM21-41 | Pro-197-Ser | 99.33 (17.56) | 20.1 | |
| QM20-8 | Pro-197-Thr/Phe | 131.33 (27.63) | 26.6 | |
| QM21-72 | Asp-376-Glu | 155.27 (36.08) | 31.4 | |
| QM21-76 | Asp-376-Glu | 94.95 (23.97) | 19.2 | |
| QM21-79 | Asp-376-Glu | 155.05 (38.69) | 31.4 | |
| Flucarbazone–sodium | QM21-14 | Wild type | 30.22 (8.18) | - |
| QM21-41 | Pro-197-Ser | 782.07 (350.75) | 25.9 | |
| QM20-8 | Pro-197-Thr/Phe | 1797.84 (312.61) | 59.5 | |
| QM21-72 | Asp-376-Glu | 87.71 (11.42) | 2.9 | |
| QM21-76 | Asp-376-Glu | 164.65 (23.30) | 5.4 | |
| QM21-79 | Asp-376-Glu | 179.10 (62.98) | 5.9 |
| Primer | Sequence (5′→3′) | Product Size (bp) | Containing the Confirmed Mutation Sites |
|---|---|---|---|
| ALS-F1 | CTCCCCAATTCCAACCCTCT | 593 | Ala-122, Pro-197, Ala-205 |
| ALS-R1 | GGCTTCCTGAATGACACGGG | ||
| ALS-F2 | CGTCATCACCAACCACCT | 1440 | Pro-197, Ala-205, Asp-376, Arg-377, Trp-574 |
| ALS-R2 | TCTTTGTCACACGAACTGC | ||
| ALS-F3 | AGCCACCACAGCCGCCGTCG | 598 | Trp-574, Ser-653, Gly-654 |
| ALS-R3 | GTCGAACCCCTAGTAGTTGATA |
| Classes | Herbicides | Formulation | Supplier | Recommended Field Dose (g a.i. ha−1) | Populations | Doses (g a.i. ha−1) |
|---|---|---|---|---|---|---|
| TP | pyroxsulam | 4% OD | Corteva Agriscience, Beijing, China | 12 | QM21-14 | 0, 0.38, 0.75, 1.5, 3, 6, 12, 24, 48 |
| QM21-41, QM20-8 | 0, 6, 12, 24, 48, 96, 192, 384, 768 | |||||
| QM21-72, QM21-76, QM21-79 | 0, 0.75, 1.5, 3, 6, 12, 24, 48, 96 | |||||
| SU | mesosulfuron–methyl | 30 g/L OD | Bayer Limited, Hangzhou, China | 13.5 | QM21-14 | 0, 0.42, 0.84, 1.68, 3.38, 6.75, 13.5, 27, 54 |
| QM21-41 | 0, 6.75, 13.5, 27, 54, 108, 216, 432, 864 | |||||
| QM20-8 | 0, 27, 54, 108, 216, 432, 864, 1728, 3456 | |||||
| QM21-72, QM21-76, QM21-79 | 0, 27, 54, 108, 216, 432, 864, 1728, 3456 | |||||
| SCT | flucarbazone–sodium | 70% WDG | Arysta LifeScience Corporation, Shanghai, China | 32 | QM21-14 | 0, 1, 2, 4, 8, 16, 32, 64, 128 |
| QM21-41, QM20-8 | 0, 256, 512, 1024, 2048, 4096, 8192, 16, 384, 32, 768 | |||||
| QM21-72, QM21-76, QM21-79 | 0, 2, 4, 8, 16, 32, 64, 128, 256 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, L.; Li, X.; Guo, X.; Chen, J.; Yu, H.; Cui, H. Distribution and Mechanism of Japanese Brome (Bromus japonicus) Resistance to ALS-Inhibiting Herbicides in China. Plants 2024, 13, 1139. https://doi.org/10.3390/plants13081139
Bai L, Li X, Guo X, Chen J, Yu H, Cui H. Distribution and Mechanism of Japanese Brome (Bromus japonicus) Resistance to ALS-Inhibiting Herbicides in China. Plants. 2024; 13(8):1139. https://doi.org/10.3390/plants13081139
Chicago/Turabian StyleBai, Linzhi, Xiangju Li, Xiaotong Guo, Jingchao Chen, Haiyan Yu, and Hailan Cui. 2024. "Distribution and Mechanism of Japanese Brome (Bromus japonicus) Resistance to ALS-Inhibiting Herbicides in China" Plants 13, no. 8: 1139. https://doi.org/10.3390/plants13081139
APA StyleBai, L., Li, X., Guo, X., Chen, J., Yu, H., & Cui, H. (2024). Distribution and Mechanism of Japanese Brome (Bromus japonicus) Resistance to ALS-Inhibiting Herbicides in China. Plants, 13(8), 1139. https://doi.org/10.3390/plants13081139






