Jasmonates Promote β-Amylase-Mediated Starch Degradation to Confer Cold Tolerance in Tomato Plants
Abstract
1. Introduction
2. Results
2.1. Cold Stress Enhances SlBAM3 Expression, Accelerates Starch Degradation, and Promotes Soluble Sugar Accumulation in Tomato Leaves
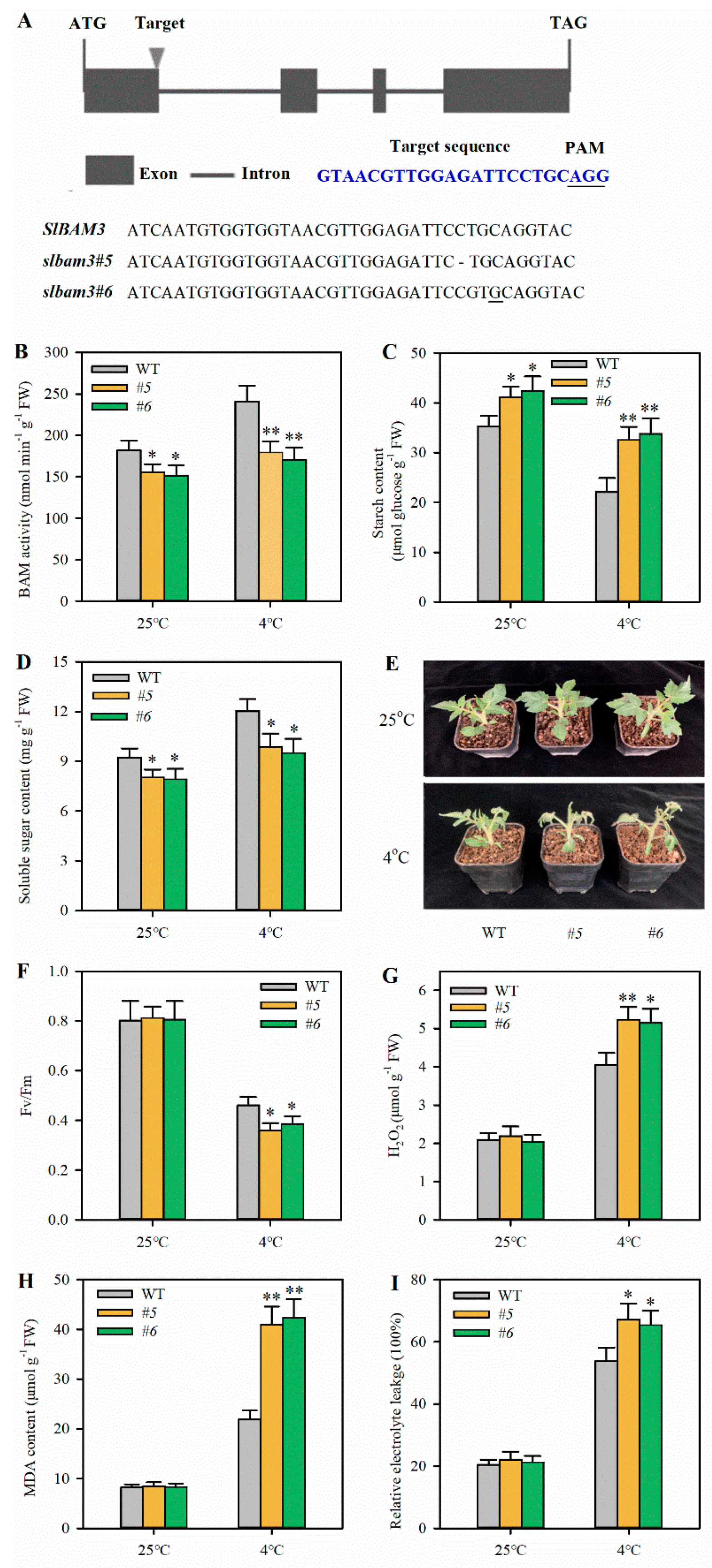
2.2. Knockout of SlBAM3 Impairs Cold Tolerance in Tomato Leaves
2.3. JA Induces SlBAM3 Expression and Enhances BAM Activity under Cold Stress
2.4. MYC2 Directly Activates the Expression of SlBAM3
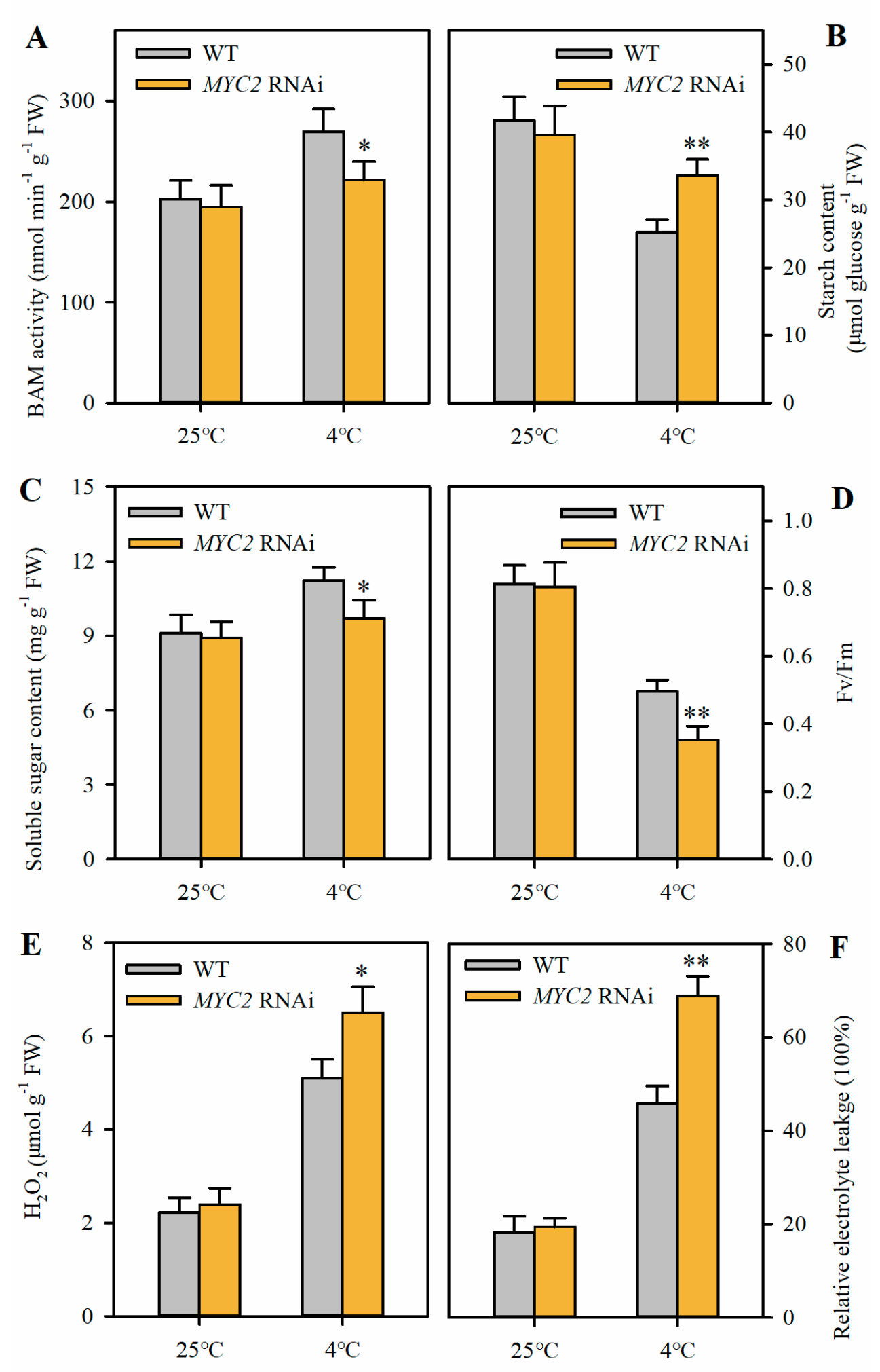
2.5. Suppression of MYC2 Reduces Soluble Sugar Accumulation and Attenuates Cold Tolerance
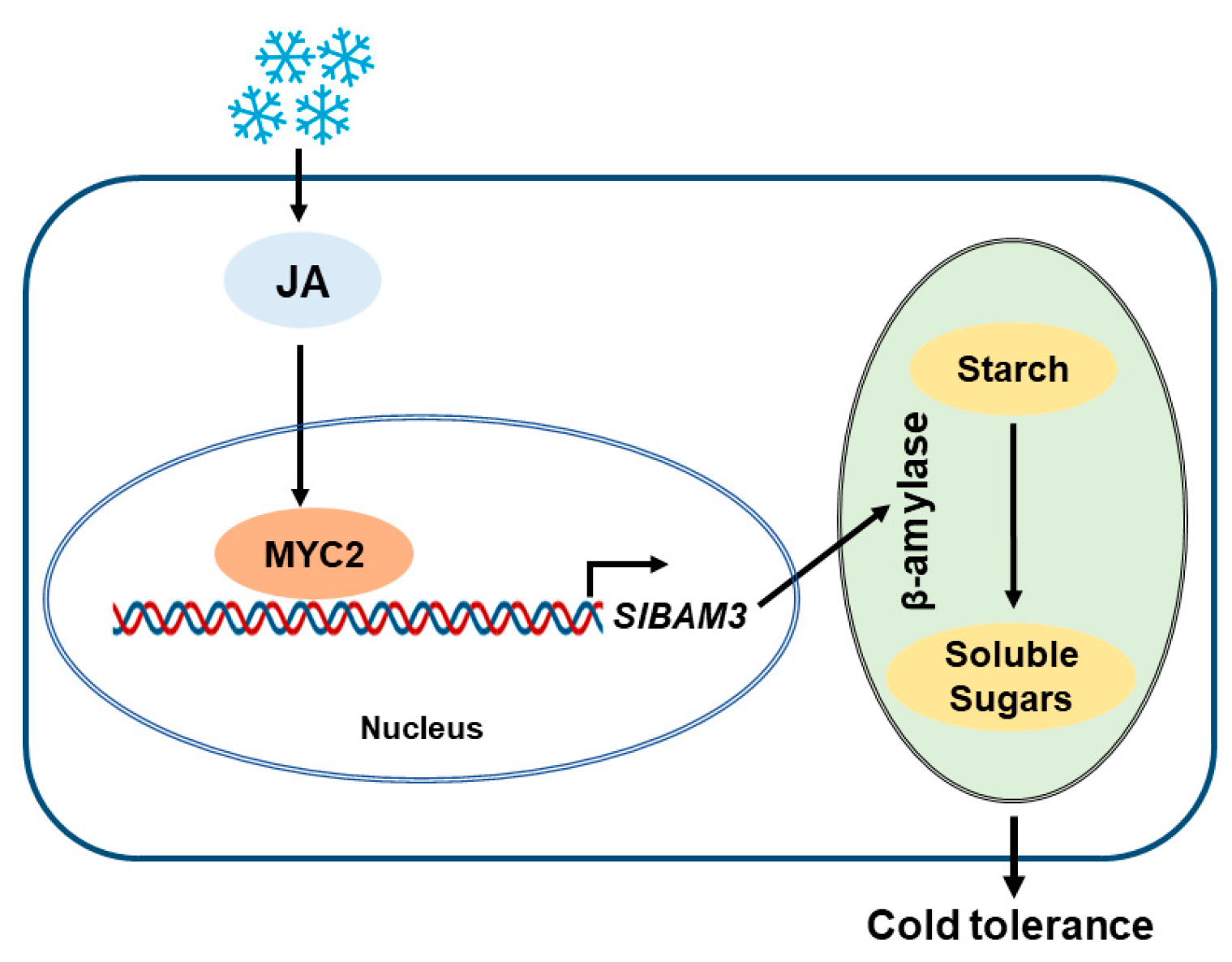
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Treatments
4.3. Cold Tolerance Assay
4.4. CRISPR/Cas9-Mediated Mutation of SlBAM3
4.5. Determination of Carbohydrate Accumulation and Enzyme Activity
4.6. Yeast One-Hybrid (Y1H) Assay
4.7. Gene Expression Analysis
4.8. Dual Luciferase Assay
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jung, J.H.; Seo, P.J.; Oh, E.; Kim, J. Temperature Perception by Plants. Trends Plant Sci. 2023, 28, 924–940. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A. Plant Abiotic Stress Challenges from the Changing Environment. Front. Plant Sci. 2016, 7, 1123. [Google Scholar] [CrossRef]
- Ding, F.; Wang, C.; Xu, N.; Wang, M.; Zhang, S. Jasmonic Acid-Regulated Putrescine Biosynthesis Attenuates Cold-Induced Oxidative Stress in Tomato Plants. Sci. Hortic. 2021, 288, 110373. [Google Scholar] [CrossRef]
- Ding, F.; Wang, M.; Zhang, S. Overexpression of a Calvin Cycle Enzyme SBPase Improves Tolerance to Chilling-Induced Oxidative Stress in Tomato Plants. Sci. Hortic. 2017, 214, 27–33. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, S.; Ding, F. Melatonin Mitigates Chilling-Induced Oxidative Stress and Photosynthesis Inhibition in Tomato Plants. Antioxidants 2020, 9, 218. [Google Scholar] [CrossRef]
- Karabudak, T.; Bor, M.; Özdemir, F.; Türkan, I. Glycine Betaine Protects Tomato (Solanum lycopersicum) Plants at Low Temperature by Inducing Fatty Acid Desaturase7 and Lipoxygenase Gene Expression. Mol. Biol. Rep. 2014, 41, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Ming, R.; Zhang, Y.; Wang, Y.; Khan, M.; Dahro, B.; Liu, J. The JA-responsive MYC2- BADH-like Transcriptional Regulatory Module in Poncirus trifoliata Contributes to Cold Tolerance by Modulation of Glycine Betaine Biosynthesis. New Phytol. 2020, 229, 2730–2750. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline Accumulation in Plants: A Review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of Soluble Sugars in Reactive Oxygen Species Balance and Responses to Oxidative Stress in Plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, Y.; Lu, J.; Shao, H. Roles of Plant Soluble Sugars and Their Responses to Plant Cold Stress. Afr. J. Biotechnol. 2009, 8, 2004–2010. [Google Scholar]
- Zhao, L.; Yang, T.; Xing, C.; Dong, H.; Qi, K.; Gao, J.; Tao, S.; Wu, J.; Wu, J.; Zhang, S.; et al. The β-Amylase PbrBAM3 from Pear (Pyrus betulaefolia) Regulates Soluble Sugar Accumulation and ROS Homeostasis in Response to Cold Stress. Plant Sci. 2019, 287, 110184. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hawkins, E.; Seung, D. Towards Targeted Starch Modification in Plants. Curr. Opin. Plant Biol. 2021, 60, 102013. [Google Scholar] [CrossRef] [PubMed]
- Thalmann, M.; Santelia, D. Starch as a Determinant of Plant Fitness under Abiotic Stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.; Sung, D.Y.; Guy, C.L. Roles of β-Amylase and Starch Breakdown during Temperatures Stress. Physiol. Plant 2006, 126, 120–128. [Google Scholar] [CrossRef]
- Monroe, J.D.; Storm, A.R. Review: The Arabidopsis β-Amylase (BAM) Gene Family: Diversity of Form and Function. Plant Sci. 2018, 276, 163–170. [Google Scholar] [CrossRef]
- Thalmann, M.; Coiro, M.; Meier, T.; Wicker, T.; Zeeman, S.C.; Santelia, D. The Evolution of Functional Complexity within the β-Amylase Gene Family in Land Plants. BMC Evol. Biol. 2019, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, J.; Khan, M.; Wang, Y.; Xiao, W.; Fang, T.; Qu, J.; Xiao, P.; Li, C.; Liu, J.H. Transcription Factors ABF4 and ABR1 Synergistically Regulate Amylase-Mediated Starch Catabolism in Drought Tolerance. Plant Physiol. 2023, 191, 591–609. [Google Scholar] [CrossRef]
- Ma, Q.J.; Sun, M.H.; Lu, J.; Liu, Y.J.; Hu, D.G.; Hao, Y.J. Transcription Factor AREB2 Is Involved in Soluble Sugar Accumulation by Activating Sugar Transporter and Amylase Genes. Plant Physiol. 2017, 174, 2348–2362. [Google Scholar] [CrossRef]
- Tsamir-Rimon, M.; Ben-Dor, S.; Feldmesser, E.; Oppenhimer-Shaanan, Y.; David-Schwartz, R.; Samach, A.; Klein, T. Rapid Starch Degradation in the Wood of Olive Trees under Heat and Drought Is Permitted by Three Stress-Specific Beta Amylases. New Phytol. 2021, 229, 1398–1414. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yang, X.; Wang, X.; Li, Q.; Guo, J.; Ma, T.; Zhao, C.; Tang, Y.; Qiao, L.; Wang, J.; et al. The Sweetpotato β-Amylase Gene IbBAM1.1 Enhances Drought and Salt Stress Resistance by Regulating ROS Homeostasis and Osmotic Balance. Plant Physiol. Biochem. 2021, 168, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Hou, Y.; Wang, H.; Wang, P.; Mao, J.; Chen, B. VaBAM1 Weakens Cold Tolerance by Interacting with the Negative Regulator VaSR1 to Suppress β-Amylase Expression. Int. J. Biol. Macromol. 2023, 225, 1394–1404. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, M.; Hu, D.; Yang, Z.; Ma, S.; Li, X.; Xiong, L. The OsMYb30 Transcription Factor Suppresses Cold Tolerance by Interacting with a JAZ Protein and Suppressing β-Amylase Expression1[OPEN]. Plant Physiol. 2017, 173, 1475–1491. [Google Scholar] [CrossRef]
- Peng, T.; Zhu, X.; Duan, N.; Liu, J.H. PtrBAM1, a β-Amylase-Coding Gene of Poncirus trifoliata, Is a CBF Regulon Member with Function in Cold Tolerance by Modulating Soluble Sugar Levels. Plant Cell Environ. 2014, 37, 2754–2767. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Yang, H.; Gao, H.; Yan, J.; Xie, D. Control of Seed Size by Jasmonate. Sci. China Life Sci. 2021, 64, 1215–1226. [Google Scholar] [CrossRef]
- Han, X.; Zhang, M.; Yang, M.; Hu, Y. Arabidopsis JAZ Proteins Interact with and Suppress RHD6 Transcription Factor to Regulate Jasmonate-Stimulated Root Hair Development. Plant Cell 2020, 32, 1049–1062. [Google Scholar] [CrossRef]
- Ding, F.; Wang, C.; Xu, N.; Zhang, S.; Wang, M. SlMYC2 Mediates Jasmonate-Induced Tomato Leaf Senescence by Promoting Chlorophyll Degradation and Repressing Carbon Fixation. Plant Physiol. Biochem. 2022, 180, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Rasool, S.; Gul, A.; Sheikh, S.A.; Akram, N.A.; Ashraf, M.; Kazi, A.M.; Gucel, S. Jasmonates: Multifunctional Roles in Stress Tolerance. Front. Plant Sci. 2016, 7, 813. [Google Scholar] [CrossRef]
- Sharma, M.; Laxmi, A. Jasmonates: Emerging Players in Controlling Temperature Stress Tolerance. Front. Plant Sci. 2016, 6, 1129. [Google Scholar] [CrossRef]
- Wang, M.; Fan, X.; Ding, F. Jasmonate: A Hormone of Primary Importance for Temperature Stress Response in Plants. Plants 2023, 12, 4080. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, H.; Xiong, L. Endogenous Auxin and Jasmonic Acid Levels Are Differentially Modulated by Abiotic Stresses in Rice. Front. Plant Sci. 2013, 4, 397. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jiang, L.; Wang, F.; Yu, D. Jasmonate regulates the inducer of CBF expression–c-repeat binding factor/DRE binding factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell 2013, 25, 2907–2924. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, H.; Chen, Y.; Zhu, S.; Chen, M.; Lan, X.; Chen, G.; Liao, Z. Cold Stress Improves the Production of Artemisinin Depending on the Increase in Endogenous Jasmonate. Biotechnol. Appl. Biochem. 2017, 64, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Wang, X.; Li, Z.; Wang, M. Jasmonate Positively Regulates Cold Tolerance by Promoting ABA Biosynthesis in Tomato. Plants 2023, 12, 60. [Google Scholar] [CrossRef]
- Zhao, M.L.; Wang, J.N.; Shan, W.; Fan, J.G.; Kuang, J.F.; Wu, K.Q.; Li, X.P.; Chen, W.X.; He, F.Y.; Chen, J.Y.; et al. Induction of Jasmonate Signalling Regulators MaMYC2s and Their Physical Interactions with MaICE1 in Methyl Jasmonate-Induced Chilling Tolerance in Banana Fruit. Plant Cell Environ. 2013, 36, 30–51. [Google Scholar] [CrossRef] [PubMed]
- Habibi, F.; Ramezanian, A.; Rahemi, M.; Eshghi, S.; Guillén, F.; Serrano, M.; Valero, D. Postharvest Treatments with γ-Aminobutyric Acid, Methyl Jasmonate, or Methyl Salicylate Enhance Chilling Tolerance of Blood Orange Fruit at Prolonged Cold Storage. J. Sci. Food Agric. 2019, 99, 6408–6417. [Google Scholar] [CrossRef]
- Ding, F.; Wang, C.; Zhang, S.; Wang, M. A Jasmonate-Responsive Glutathione S-Transferase Gene SlGSTU24 Mitigates Cold-Induced Oxidative Stress in Tomato Plants. Sci. Hortic. 2022, 303, 111231. [Google Scholar] [CrossRef]
- Ding, F.; Ren, L.; Xie, F.; Wang, M.; Zhang, S. Jasmonate and Melatonin Act Synergistically to Potentiate Cold Tolerance in Tomato Plants. Front. Plant Sci. 2022, 12, 763284. [Google Scholar] [CrossRef]
- Beecher, G.R. Nutrient Content of Tomatoes and Tomato Products. Exp. Biol. Med. 1998, 218, 98–100. [Google Scholar] [CrossRef]
- Dorais, M.; Ehret, D.L.; Papadopoulos, A.P. Tomato (Solanum lycopersicum) Health Components: From the Seed to the Consumer. Phytochem. Rev. 2008, 7, 231–250. [Google Scholar] [CrossRef]
- Park, E.J.; Jeknić, Z.; Sakamoto, A.; DeNoma, J.; Yuwansiri, R.; Murata, N.; Chen, T.H.H. Genetic Engineering of Glycinebetaine Synthesis in Tomato Protects Seeds, Plants, and Flowers from Chilling Damage. Plant J. 2004, 40, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Wang, M.; Zhang, S.; Ai, X. Changes in SBPase Activity Influence Photosynthetic Capacity, Growth, and Tolerance to Chilling Stress in Transgenic Tomato Plants. Sci. Rep. 2016, 6, 32741. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Hu, C.; Liu, C.; Wang, J.; Zhou, Y.; Yu, J. ELONGATED HYPOCOTYL 5 Mediates Blue Light-Induced Starch Degradation in Tomato. J. Exp. Bot. 2021, 72, 2627–2641. [Google Scholar] [CrossRef] [PubMed]
- Villadsen, D.; Rung, J.H.; Nielsen, T.H. Osmotic Stress Changes Carbohydrate Partitioning and Fructose-2,6- Bisphosphate Metabolism in Barley Leaves. Funct. Plant Biol. 2005, 32, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Damour, G.; Vandame, M.; Urban, L. Long-Term Drought Modifies the Fundamental Relationships between Light Exposure, Leaf Nitrogen Content and Photosynthetic Capacity in Leaves of the Lychee Tree (Litchi chinensis). J. Plant Physiol. 2008, 165, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chang, L.; Wang, B.; Wang, D.; Li, P.; Wang, L.; Yi, X.; Huang, Q.; Peng, M.; Guo, A. Comparative Proteomics of Thellungiella Halophila Leaves from Plants Subjected to Salinity Reveals the Importance of Chloroplastic Starch and Soluble Sugars in Halophyte Salt Tolerance. Mol. Cell. Proteom. 2013, 12, 2174–2195. [Google Scholar] [CrossRef] [PubMed]
- Siaut, M.; Cuiné, S.; Cagnon, C.; Fessler, B.; Nguyen, M.; Carrier, P.; Beyly, A.; Beisson, F.; Triantaphylidès, C.; Li-Beisson, Y.; et al. Oil Accumulation in the Model Green Alga Chlamydomonas reinhardtii: Characterization, Variability between Common Laboratory Strains and Relationship with Starch Reserves. BMC Biotechnol. 2011, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.; Guy, C.L. β-Amylase Induction and the Protective Role of Maltose during Temperature Shock. Plant Physiol. 2004, 135, 1674–1684. [Google Scholar] [CrossRef]
- Zhuang, K.; Kong, F.; Zhang, S.; Meng, C.; Yang, M.; Liu, Z.; Wang, Y.; Ma, N.; Meng, Q. Whirly1 Enhances Tolerance to Chilling Stress in Tomato via Protection of Photosystem II and Regulation of Starch Degradation. New Phytol. 2019, 221, 1998–2012. [Google Scholar] [CrossRef]
- Monroe, J.D. Involvement of Five Catalytically Active Arabidopsis β-Amylases in Leaf Starch Metabolism and Plant Growth. Plant Direct 2020, 4, e00199. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.A.R.; Baldwin, I.T.; Erb, M. Herbivory-Induced Jasmonates Constrain Plant Sugar Accumulation and Growth by Antagonizing Gibberellin Signaling and Not by Promoting Secondary Metabolite Production. New Phytol. 2017, 215, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Lu, L.; Liu, H.Y.; Li, S.; Xing, F.; Chen, L.L. CRISPR-P: A Web Tool for Synthetic Single-Guide RNA Design of CRISPR-System in Plants. Mol. Plant 2014, 7, 1494–1496. [Google Scholar] [CrossRef] [PubMed]
- Scheidig, A.; Fröhlich, A.; Schulze, S.; Lloyd, J.R.; Kossmann, J. Downregulation of a Chloroplast-Targeted β-Amylase Leads to a Starch-Excess Phenotype in Leaves. Plant J. 2002, 30, 581–591. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Lin, H.; Ding, F.; Wang, M. Jasmonates Promote β-Amylase-Mediated Starch Degradation to Confer Cold Tolerance in Tomato Plants. Plants 2024, 13, 1055. https://doi.org/10.3390/plants13081055
Fan X, Lin H, Ding F, Wang M. Jasmonates Promote β-Amylase-Mediated Starch Degradation to Confer Cold Tolerance in Tomato Plants. Plants. 2024; 13(8):1055. https://doi.org/10.3390/plants13081055
Chicago/Turabian StyleFan, Xiulan, Huanru Lin, Fei Ding, and Meiling Wang. 2024. "Jasmonates Promote β-Amylase-Mediated Starch Degradation to Confer Cold Tolerance in Tomato Plants" Plants 13, no. 8: 1055. https://doi.org/10.3390/plants13081055
APA StyleFan, X., Lin, H., Ding, F., & Wang, M. (2024). Jasmonates Promote β-Amylase-Mediated Starch Degradation to Confer Cold Tolerance in Tomato Plants. Plants, 13(8), 1055. https://doi.org/10.3390/plants13081055




