Variations in Fruit Ploidy Level and Cell Size between Small- and Large-Fruited Olive Cultivars during Fruit Ontogeny
Abstract
1. Introduction
2. Results
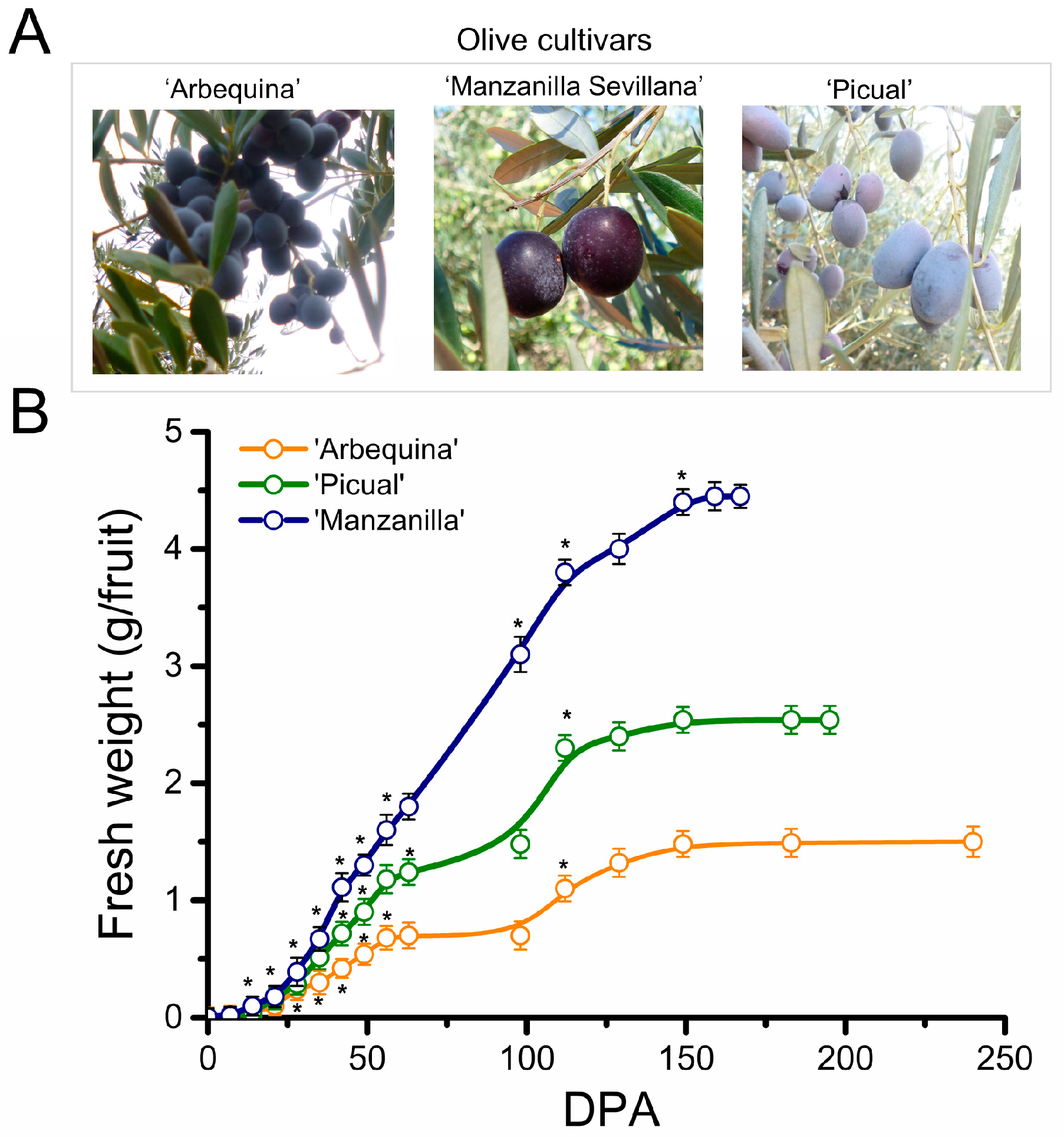
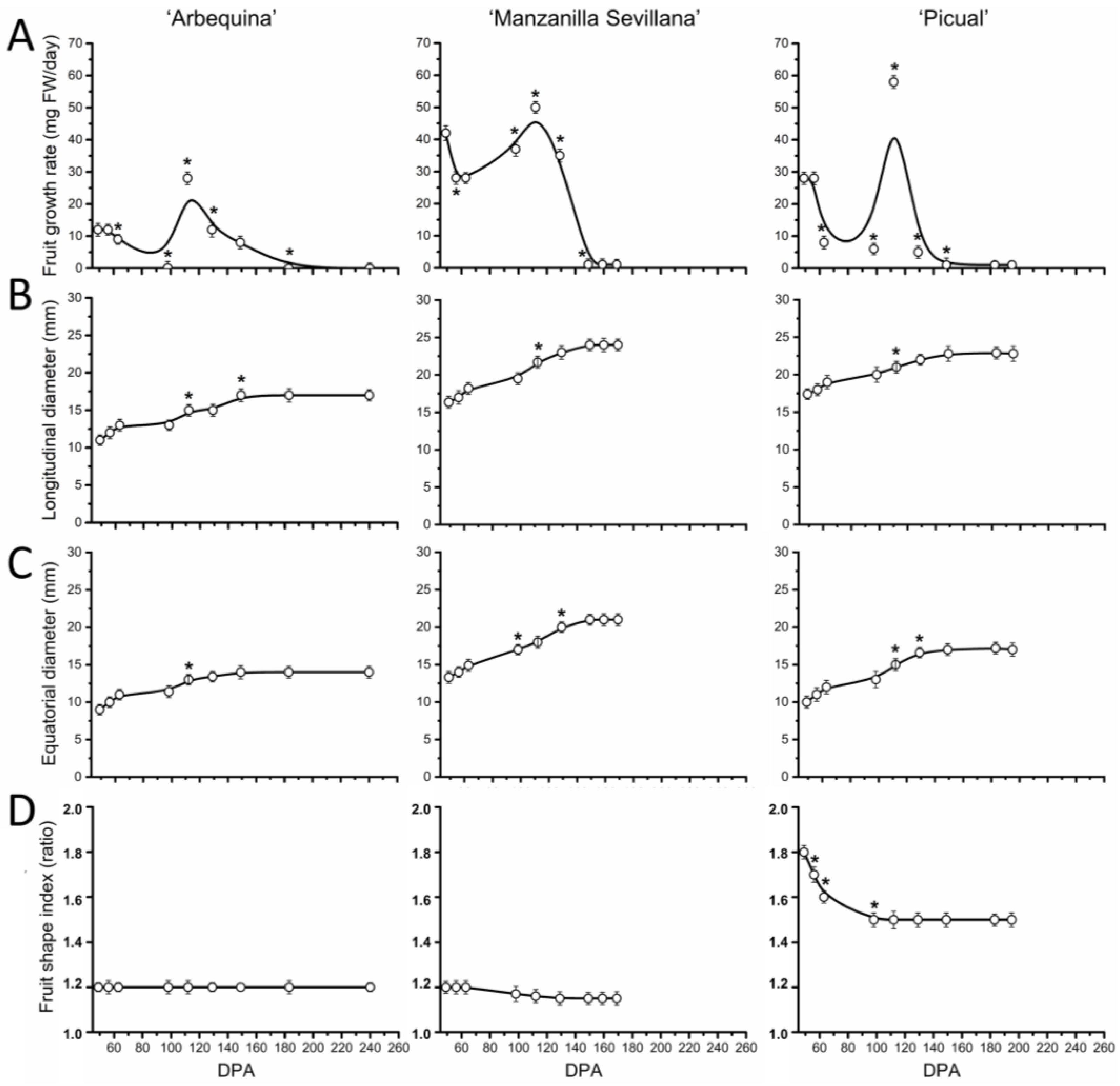
2.1. Morphological Changes during Fruit Development in Olive Cultivars
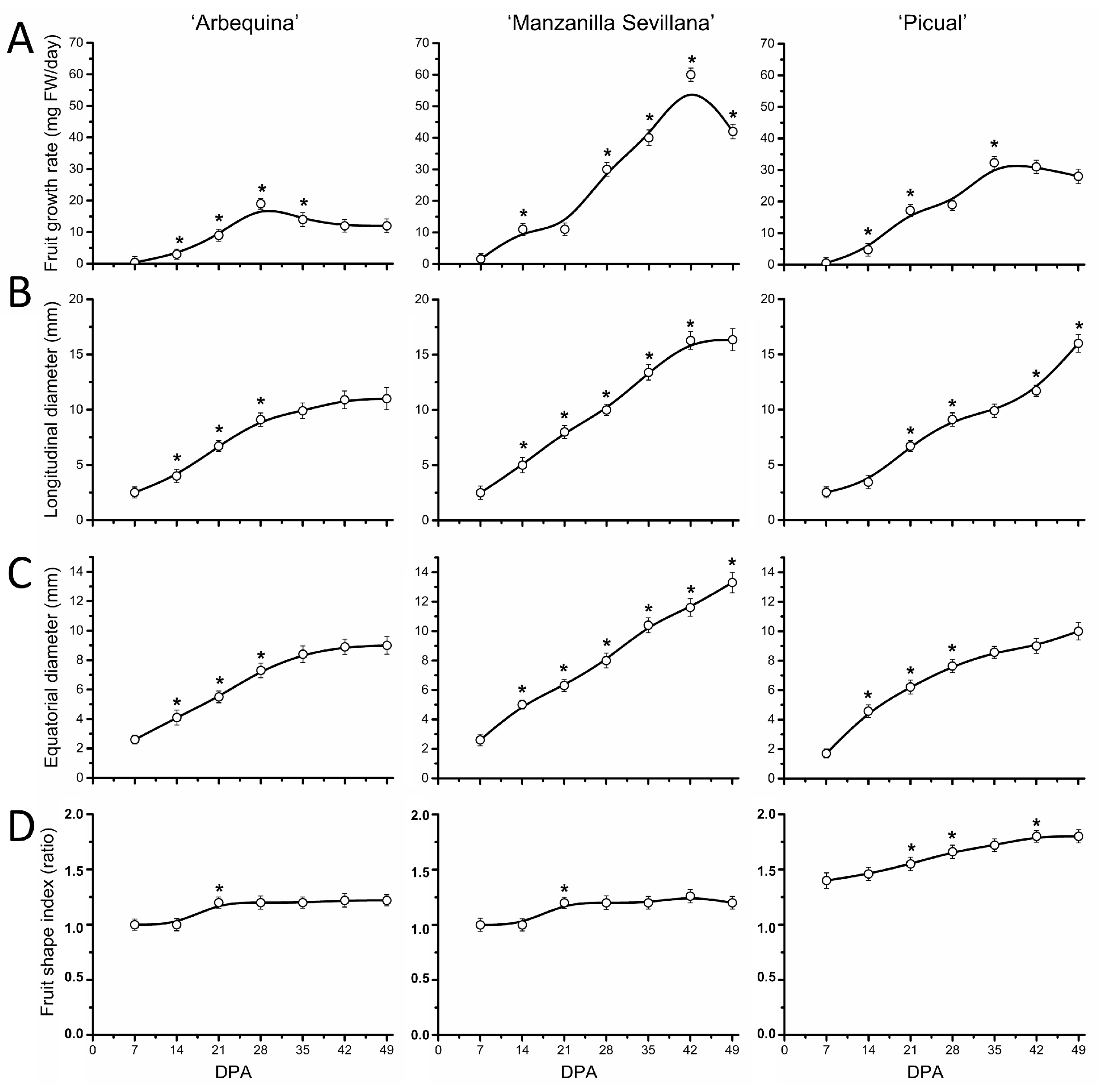
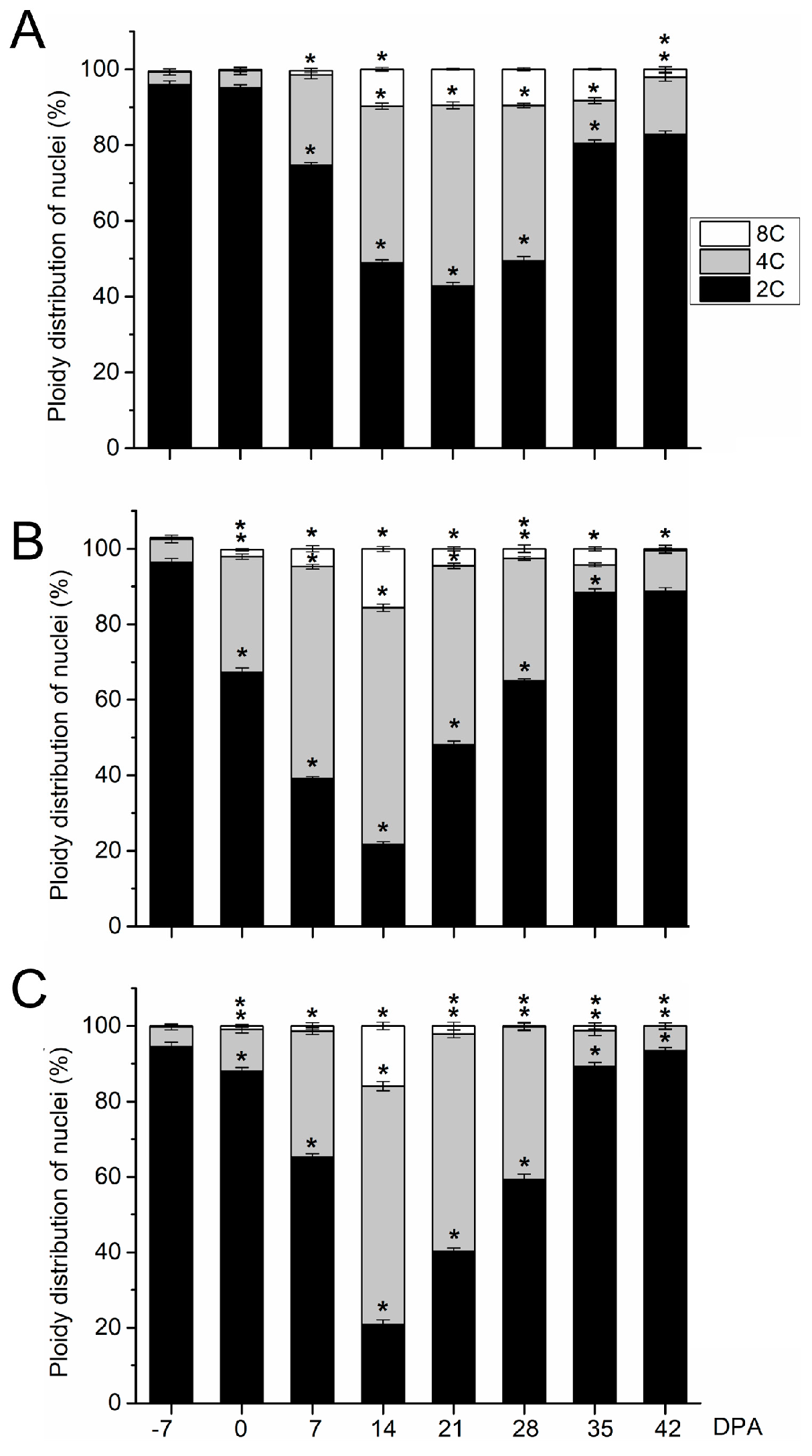
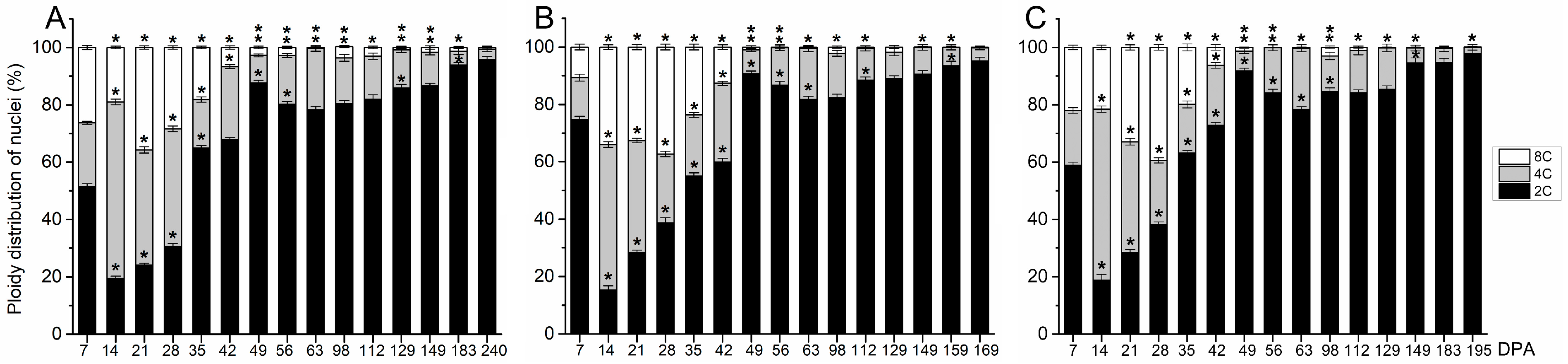
2.2. Early Fruit Development in Olive Cultivars
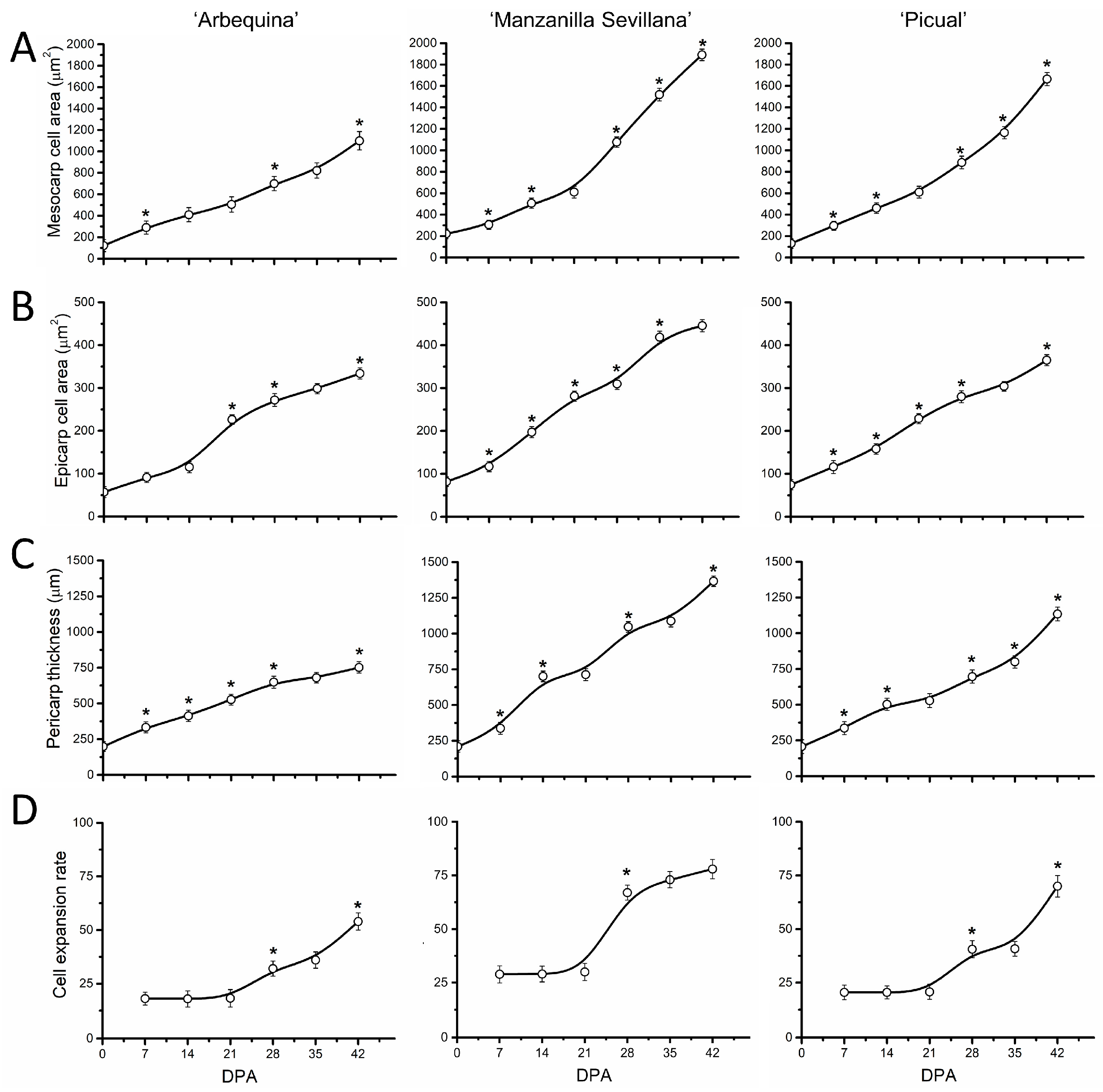
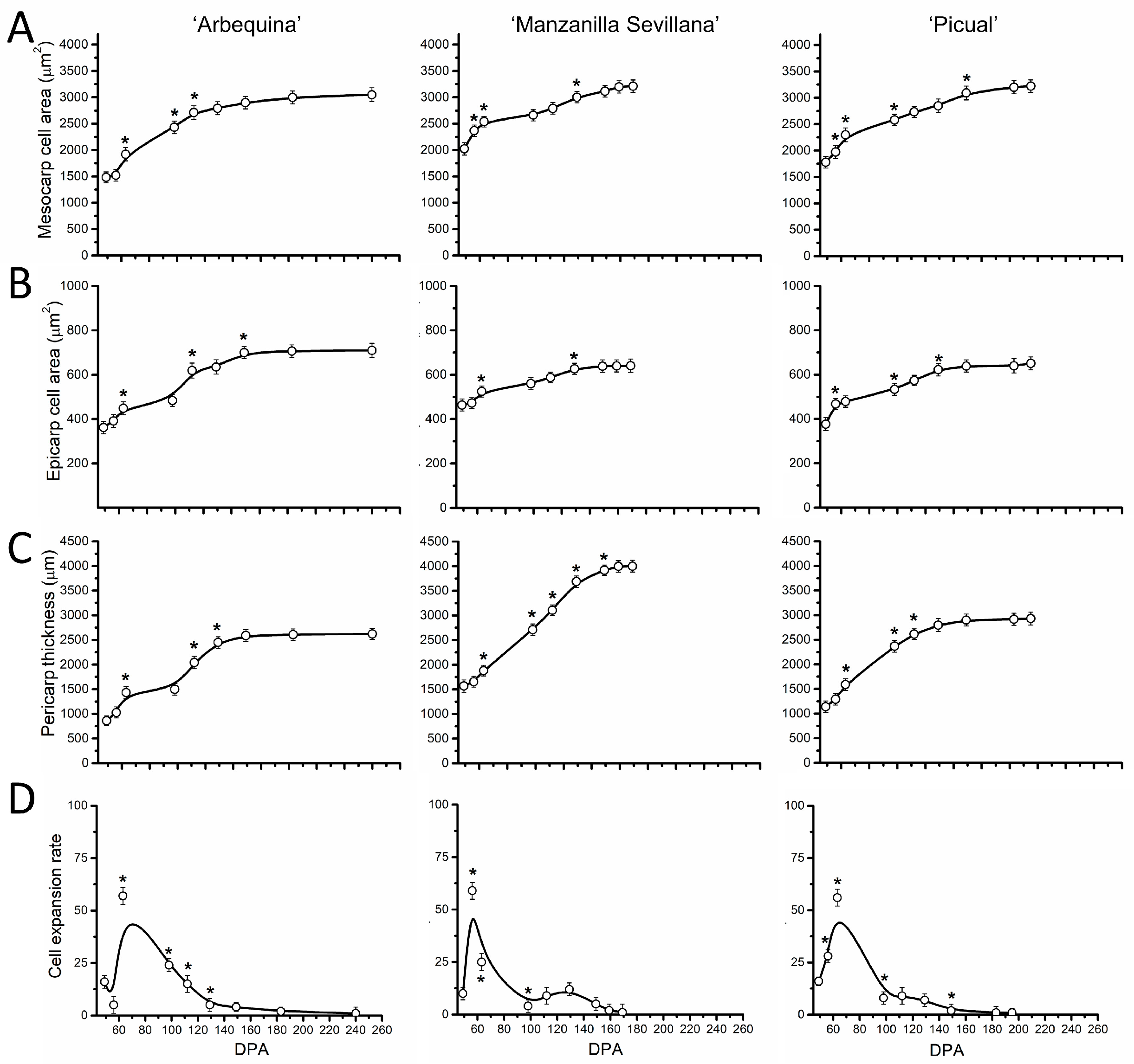
2.3. Late Fruit Development in Olive Cultivars
3. Discussion
4. Materials and Methods
4.1. Plant Material and Cytological Analysis
4.2. Cytological Analysis
4.3. Flow Cytometry Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giovannoni, J.; Nguyen, C.; Ampofo, B.; Zhong, S.; Fei, Z. The epigenome and transcriptional dynamics of fruit ripening. Annu. Rev. Plant Biol. 2017, 68, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.P.; Lutts, S. Tomato fruit development and metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Gallusci, P.; Lang, Z. Fruit development and epigenetic modifications. New Phytol. 2020, 228, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Ezura, K.; Nomura, Y.; Ariizumi, T. Molecular, hormonal, and metabolic mechanisms of fruit set, the ovary-to-fruit transition, in horticultural crops. J. Exp. Bot. 2023, 74, 6254–6268. [Google Scholar] [CrossRef] [PubMed]
- Mauxion, J.P.; Chevalier, C.; Gonzalez, N. Complex cellular and molecular events determining fruit size. Trends Plant Sci. 2021, 26, 1023–1038. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Muhammad, N.; Zhao, Z.; Yin, K.; Liu, Z.; Wang, L.; Luo, Z.; Wang, L.; Liu, M. Molecular regulation of fruit size in horticultural plants: A review. Sci. Hortic. 2021, 288, 110353. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, G.; Bouzayen, M.; Li, Z. Advances in ripening regulation, quality formation, pre- and post-harvest applications of horticultural products. Front. Plant Sci. 2023, 14, 1285104. [Google Scholar] [CrossRef] [PubMed]
- Cheniclet, C.; Rong, W.Y.; Causse, M.; Frangne, N.; Bolling, L.; Carde, J.P. Cell expansion and endoreduplication show a large genetic variability in pericarp and contribute strongly to tomato fruit growth. Plant Physiol. 2005, 139, 1984–1994. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, C.; Nafati, M.; Mathieu-Rivet, E.; Bourdon, M.; Frangne, N.; Cheniclet, C.; Renaudin, J.P.; Gvaudant, F.; Hernould, M. Elucidating the functional role of endoreduplication in tomato fruit development. Ann. Bot. 2011, 107, 1159–1169. [Google Scholar] [CrossRef]
- Chevalier, C.; Bourdon, M.; Pirrello, J.; Cheniclet, C.; Gévaudant, F.; Frangne, N. Endoreduplication and fruit growth in tomato: Evidence in favour of the karyoplasmic ratio theory. J. Exp. Bot. 2014, 65, 2731–2746. [Google Scholar] [CrossRef]
- Tourdot, E.; Mauxion, J.-P.; Gonzalez, N.; Chevalier, C. Endoreduplication in plant organogenesis: A means to boost fruit growth. J. Exp. Bot. 2023, 74, 6269–6284. [Google Scholar] [CrossRef] [PubMed]
- Renaudin, J.P.; Deluche, C.; Cheniclet, C.; Chevalier, C.; Frangne, N. Cell layer-specific patterns of cell division and cell expansion during fruit set and fruit growth in tomato pericarp. J. Exp. Bot. 2017, 68, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, M.; Popłonska, K.; Kazmierczak, A.; Stepinski, D.; Rogala, K.; Polewczyk, K. Role of DNA endoreduplication, lipotubuloids, and gibberellic acid in epidermal cell growth during fruit development of Ornithogalum umbellatum. J. Exp. Bot. 2007, 58, 2023–2031. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lukaszewska, E.; Sliwinska, E. Most organs of sugar-beet (Beta vulgaris L.) plants at the vegetative and reproductive stages of development are polysomatic. Sexual Plant Reprod. 2007, 20, 99–107. [Google Scholar] [CrossRef]
- Fu, F.Q.; Mao, W.H.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Spatio-temporal changes in cell division, endoreduplication and expression of cell cycle-related genes in pollinated and plant growth substances-treated ovaries of cucumber. Plant Biol. 2010, 12, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Farinati, S.; Forestan, C.; Canton, M.; Galla, G.; Bonghi, C.; Varotto, S. Regulation of fruit growth in a peach slow ripening phenotype. Genes 2021, 12, 482. [Google Scholar] [CrossRef] [PubMed]
- Barranco, D.; Caballero, J.M.; Martín, A.; Rallo, L.; Del Río, C.; Tous, J.; Trujillo, I. Variedades de olivo en España; Mundiprensa: Madrid, Spain, 2005. [Google Scholar]
- Rallo, L.; Díez, C.M.; Morales-Sillero, A.; Mihoa, H.; Priego-Capote, F.; Rallo, P. Quality of olives: A focus on agricultural preharvest factors. Sci. Hortic. 2018, 233, 491–509. [Google Scholar] [CrossRef]
- Kaya, H.B.; Akdemir, D.; Lozano, R.; Cetin, O.; Kaya, H.S.; Sahin, M.; Smith, J.L.; Tanyolac, B.; Jannink, J.-L. Genome wide association study of 5 agronomic traits in olive (Olea europaea L.). Sci. Rep. 2019, 9, 18764. [Google Scholar] [CrossRef] [PubMed]
- Moret, M.; Ramírez-Tejero, J.A.; Serrano, A.; Ramírez-Yera, E.; Cueva-López, M.D.; Belaj, A.; Luque, F. Identification of genetic markers and genes putatively involved in determining olive fruit weight. Plants 2023, 12, 155. [Google Scholar] [CrossRef]
- Bazakos, C.; Alexiou, K.G.; Ramos-Onsins, S.; Koubouris, G.; Tourvas, N.; Xanthopoulou, A.; Mellidou, I.; Moysiadis, T.; Vourlaki, I.T.; Metzidakis, I.; et al. Whole genome scanning of a Mediterranean basin hotspot collection provides new insights into olive tree biodiversity and biology. Plant J. 2023, 116, 303–319. [Google Scholar] [CrossRef]
- Camarero, M.C.; Briegas, B.; Corbacho, J.; Labrador, J.; Gallardo, M.; Gomez-Jimenez, M.C. Characterization of transcriptome dynamics during early fruit development in olive (Olea europaea L.). Int. J. Mol. Sci. 2023, 24, 961. [Google Scholar] [CrossRef] [PubMed]
- Hammami, S.B.; Manrique, T.; Rapoport, H.F. Cultivar-based fruit size in olive depends on different tissue and cellular processes throughout growth. Sci. Hortic. 2011, 130, 445–451. [Google Scholar] [CrossRef]
- Rosati, A.; Caporali, S.; Hammami, S.B.M.; Moreno-Alìas, I.; Paoletti, A.; Rapoport, H.F. Differences in ovary size among olive (Olea europaea L.) cultivars are mainly related to cell number, not to cell size. Sci. Hortic. 2011, 130, 185–190. [Google Scholar] [CrossRef]
- Rosati, A.; Caporali, S.; Hammami, S.B.M.; Moreno-Alìas, I.; Paoletti, A.; Rapoport, H.F. Tissue size and cell number in the olive (Olea europaea) ovary determine tissue growth and partitioning in the fruit. Funct. Plant Biol. 2012, 39, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Hammami, S.B.; Costagli, G.; Rapoport, H.F. Cell and tissue dynamics of olive endocarp sclerification vary according to water availability. Physiol. Plant. 2013, 149, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, H.F.; Hammami, S.B.M.; Rosati, A.; Gucci, R. Advances in olive fruit cell and tissue development. Acta Hortic. 2017, 1177, 209–214. [Google Scholar] [CrossRef]
- Rosati, A.; Caporali, S.; Hammami, S.B.; Moreno-Alías, I.; Rapoport, H. Fruit growth and sink strength in olive (Olea europaea) are related to cell number, not to tissue size. Funct. Plant Biol. 2020, 47, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Rosati, A.; Lodolini, E.M.; Famiani, F. From flower to fruit: Fruit growth and development in olive (Olea europaea L.)—A review. Front Plant Sci. 2023, 14, 1276178. [Google Scholar]
- Hernández-Santana, V.; Perez-Arcoiza, A.; Gomez-Jimenez, M.C.; Diaz-Espejo, A. Disentangling the link between leaf photosynthesis and turgor in fruit growth. Plant J. 2021, 107, 1788–1801. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.Q.; Mao, W.H.; Shi, K.; Zhou, Y.H.; Asami, T.; Yu, J.Q. A role of brassinosteroids in early fruit development in cucumber. J. Exp. Bot. 2008, 59, 2299–2308. [Google Scholar] [CrossRef]
- Agulló-Antón, M.A.; Olmos, E.; Pérez-Pérez, J.M.; Acosta, M. Evaluation of ploidy level and endoreduplication in carnation (Dianthus spp.). Plant Sci. 2013, 202, 1–11. [Google Scholar] [CrossRef]
- Coombe, B.G. The development of fleshy fruits. Ann. Rev. Plant Physiol. 1976, 27, 207–228. [Google Scholar] [CrossRef]
- Bertin, N. Analysis of the tomato fruit growth response to temperature and plant fruit load in relation to cell division, cell expansion and DNA endoreduplication. Ann. Bot. 2005, 295, 439–447. [Google Scholar] [CrossRef]
- Harada, T.; Kurahashi, W.; Yanai, M.; Wakasa, Y.; Satoh, T. Involvement of cell proliferation and cell enlargement in increasing the fruit size of Malus species. Sci. Hortic. 2005, 105, 447–456. [Google Scholar] [CrossRef]
- Li, Y.-H.; Zhang, Z.; Sun, G.-M. Changes in cell number and cell size during pineapple (Ananas comosus L.) fruit development and their relationship with fruit size. Aust. J. Bot. 2010, 58, 673–678. [Google Scholar] [CrossRef]
- Malladi, A.; Hirst, P.M. Increase in fruit size of a spontaneous mutant of ‘Gala’apple (Malus × domestica Borkh.) is facilitated by altered cell production and enhanced cell size. J. Exp. Bot. 2010, 61, 3003–3013. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pan, Y.; Liu, C.; Ding, Y.; Wang, X.; Cheng, Z.; Meng, H. Cucumber fruit size and shape variations explored from the aspects of morphology, histology, and endogenous hormones. Plants 2020, 9, 772. [Google Scholar] [CrossRef]
- Barlow, P.W. Endopolyploidy: Towards an understanding of its biological significance. Acta Biotheor. 1978, 27, 1–18. [Google Scholar] [CrossRef]
- Traas, J.; Hülskamp, M.; Gendreau, E.; Höfte, H. Endoreduplication and development: Rule without dividing? Curr. Opin. Plant Biol. 1998, 1, 498–503. [Google Scholar] [CrossRef]
- Scholes, D.R.; Paige, K.N. Plasticity in ploidy: A generalized response to stress. Trends Plant Sci. 2015, 20, 165–175. [Google Scholar] [CrossRef]
- Lang, L.; Schnittger, A. Endoreplication—A means to an end in cell growth and stress response. Curr. Opin. Plant Biol. 2020, 54, 85–92. [Google Scholar] [CrossRef]
- Gomez-Jimenez, M.C.; Paredes, M.A.; Gallardo, M.; Fernandez-Garcia, N.; Olmos, E.; Sanchez-Calle, I.M. Tissue-specific expression of olive S-adenosyl methionine decarboxylase and spermidine synthase genes and polyamine metabolism during flower opening and early fruit development. Planta 2010, 232, 629–647. [Google Scholar] [CrossRef] [PubMed]
- Corbacho, J.; Inês, C.; Paredes, M.A.; Labrador, J.; Cordeiro, A.M.; Gallardo, M.; Gomez-Jimenez, M.C. Modulation of sphingolipid long-chain base composition and gene expression during early olive-fruit development, and putative role of brassinosteroid. J. Plant Physiol. 2018, 231, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Inês, C.; Corbacho, J.; Paredes, M.A.; Labrador, J.; Cordeiro, A.M.; Gomez-Jimenez, M.C. Regulation of sterol content and biosynthetic gene expression during flower opening and early fruit development in olive. Physiol. Plant. 2019, 167, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Inês, C.; Parra-Lobato, M.C.; Paredes, M.A.; Labrador, J.; Gallardo, M.; Saucedo-García, M.; Gavilanes-Ruiz, M.; Gomez-Jimenez, M.C. Sphingolipid distribution, content and gene expression during olive-fruit development and ripening. Front. Plant Sci. 2018, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Briegas, B.; Corbacho, J.; Parra-Lobato, M.C.; Paredes, M.A.; Labrador, J.; Gallardo, M.; Gomez-Jimenez, M.C. Transcriptome and hormone analyses revealed insights into hormonal and vesicle trafficking regulation among Olea europaea fruit tissues in late development. Int. J. Mol. Sci. 2020, 21, 4819. [Google Scholar] [CrossRef] [PubMed]
- Camarero, M.C.; Briegas, B.; Corbacho, J.; Labrador, J.; Gomez-Jimenez, M.C. Hormonal content and gene expression during olive fruit growth and ripening. Plants 2023, 12, 3832. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, M.R.; Sabatini, D.M.; Carpenter, A.E. CellProfiler: Free, versatile software for automated biological image analysis. Biotechniques 2007, 42, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J. Flow cytometric approaches to study plant genomes. Ecosistemas 2009, 18, 103–108. [Google Scholar]
- Dolezel, J.; Greilhuber, J.; Suda, J. Estimation of nuclear DNA content in plants using flow cytometry. Nat. Protoc. 2007, 2, 2233–2244. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camarero, M.C.; Briegas, B.; Corbacho, J.; Labrador, J.; Román, Á.-C.; Verde, A.; Gallardo, M.; Gomez-Jimenez, M.C. Variations in Fruit Ploidy Level and Cell Size between Small- and Large-Fruited Olive Cultivars during Fruit Ontogeny. Plants 2024, 13, 990. https://doi.org/10.3390/plants13070990
Camarero MC, Briegas B, Corbacho J, Labrador J, Román Á-C, Verde A, Gallardo M, Gomez-Jimenez MC. Variations in Fruit Ploidy Level and Cell Size between Small- and Large-Fruited Olive Cultivars during Fruit Ontogeny. Plants. 2024; 13(7):990. https://doi.org/10.3390/plants13070990
Chicago/Turabian StyleCamarero, Maria C., Beatriz Briegas, Jorge Corbacho, Juana Labrador, Ángel-Carlos Román, Antía Verde, Mercedes Gallardo, and Maria C. Gomez-Jimenez. 2024. "Variations in Fruit Ploidy Level and Cell Size between Small- and Large-Fruited Olive Cultivars during Fruit Ontogeny" Plants 13, no. 7: 990. https://doi.org/10.3390/plants13070990
APA StyleCamarero, M. C., Briegas, B., Corbacho, J., Labrador, J., Román, Á.-C., Verde, A., Gallardo, M., & Gomez-Jimenez, M. C. (2024). Variations in Fruit Ploidy Level and Cell Size between Small- and Large-Fruited Olive Cultivars during Fruit Ontogeny. Plants, 13(7), 990. https://doi.org/10.3390/plants13070990






