Straw Addition Enhances Crop Yield, Soil Aggregation, and Soil Microorganisms in a 14-Year Wheat–Rice Rotation System in Central China
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Field Fertilization Experiment Design
2.3. Sample Collection and Determination
2.3.1. Soil Samples
2.3.2. Plant Samples
2.4. Data Analysis
3. Results
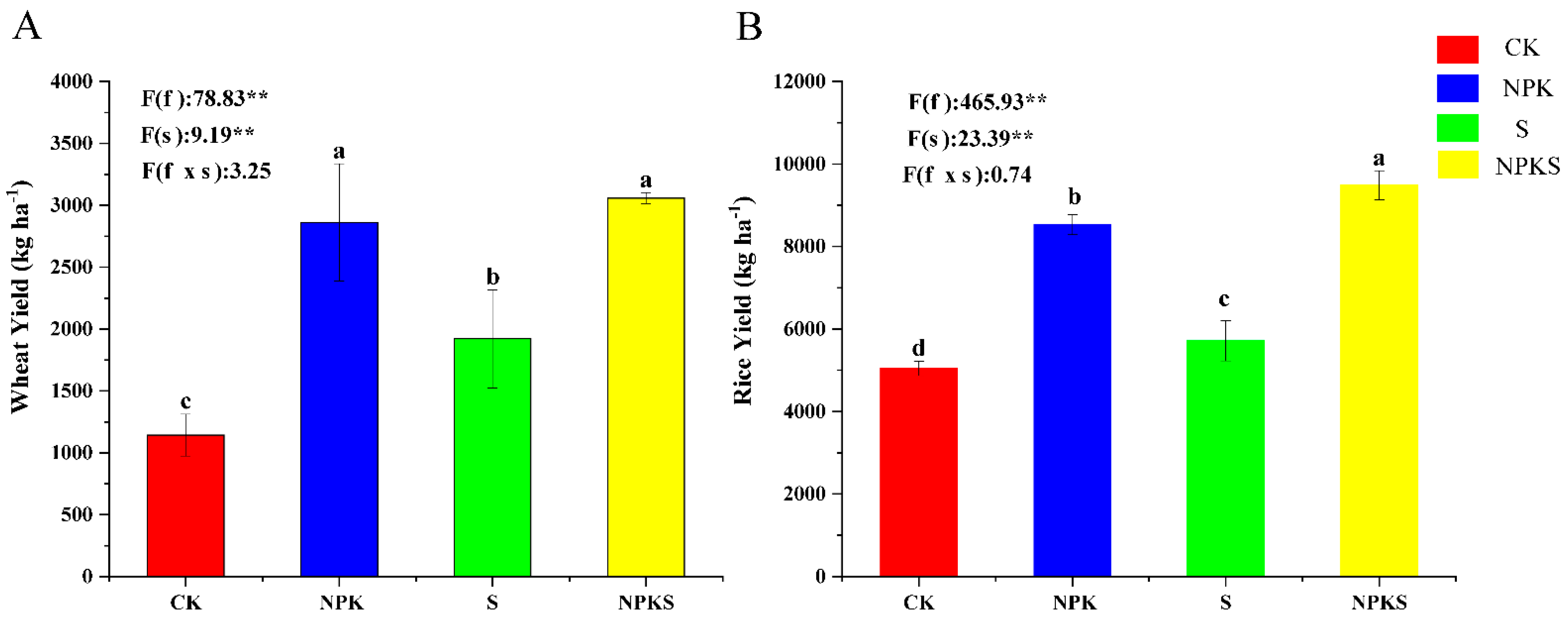
3.1. Effects of Straw Return and Fertilization on Crop Yield and Nutrient Absorption
3.2. Effects of Straw Return and Fertilization on Soil Fertility
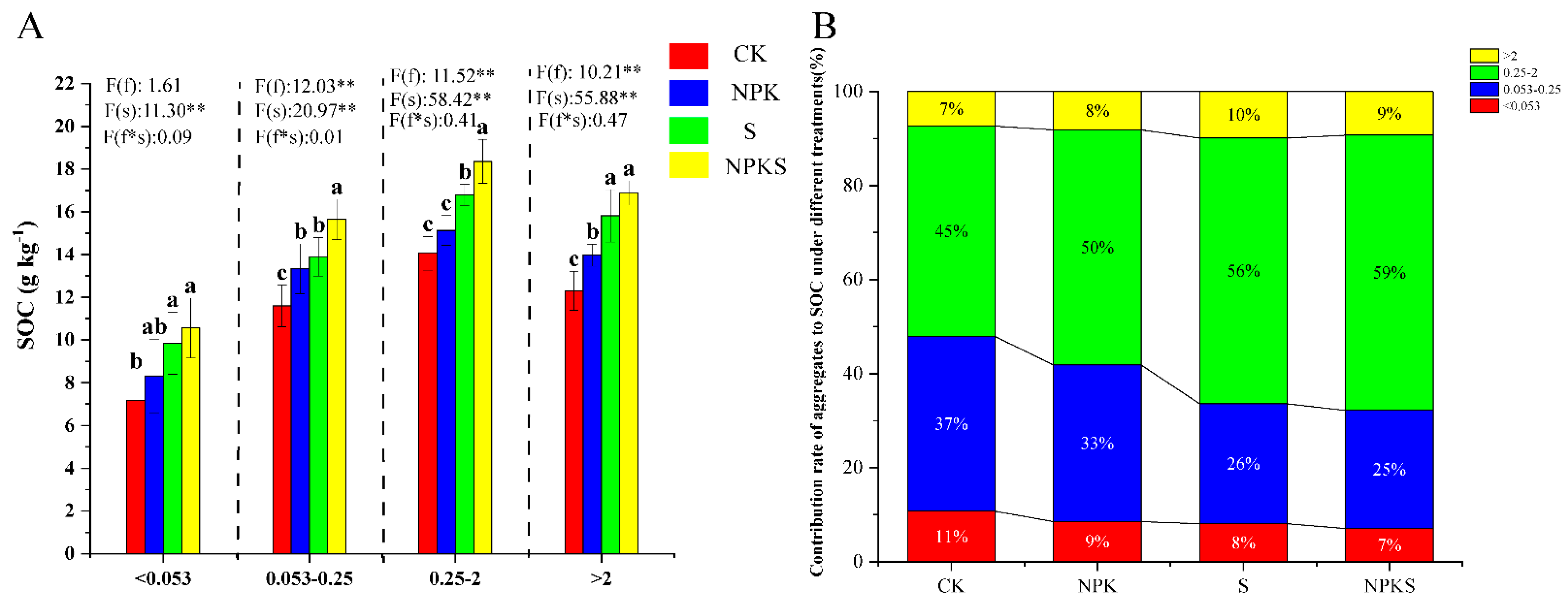
3.3. Effects of Straw Return and Fertilization on Soil Aggregates
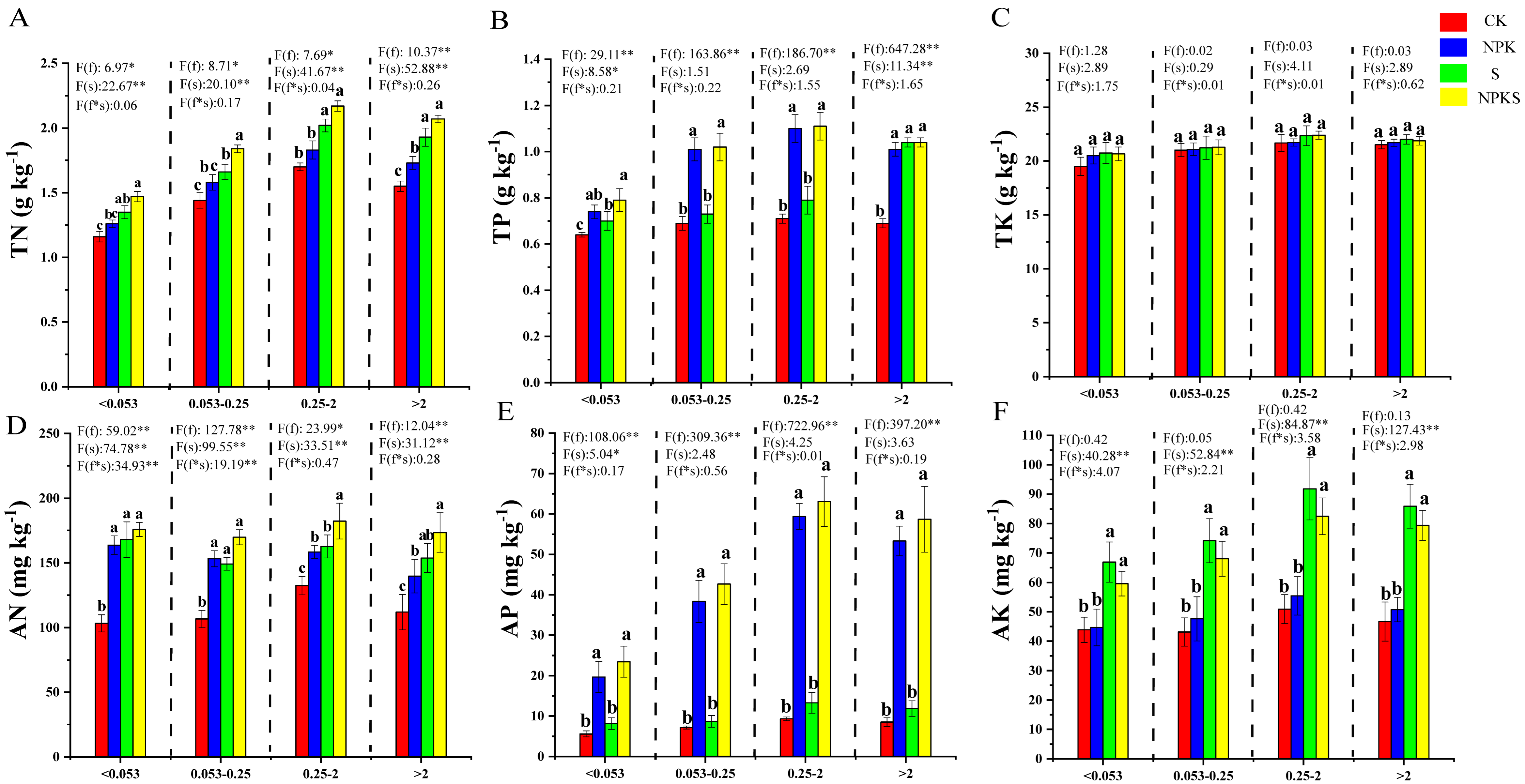
3.4. Effects of Straw Return and Fertilizer Application on the Nutrients of Soil Aggregates
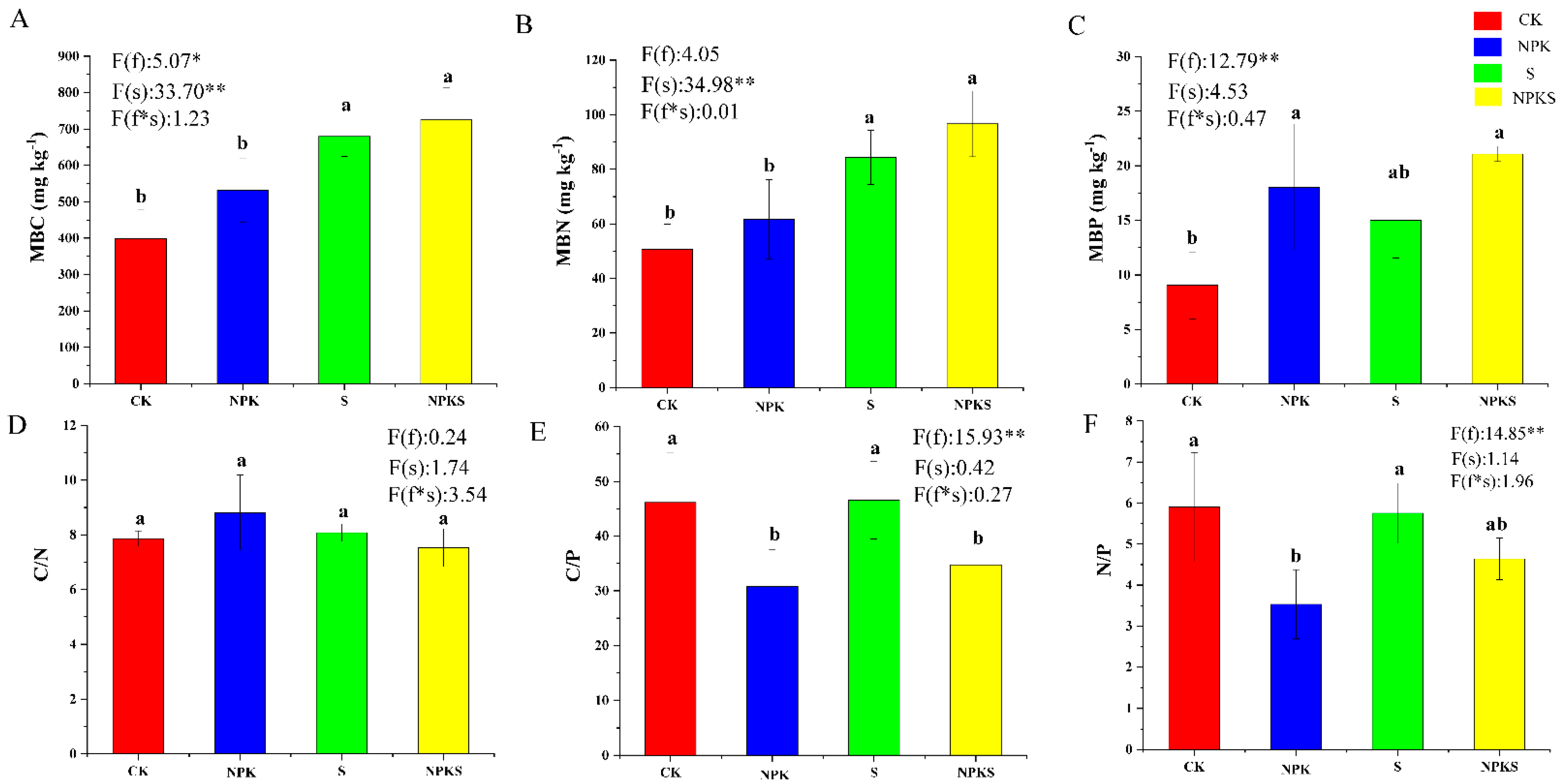
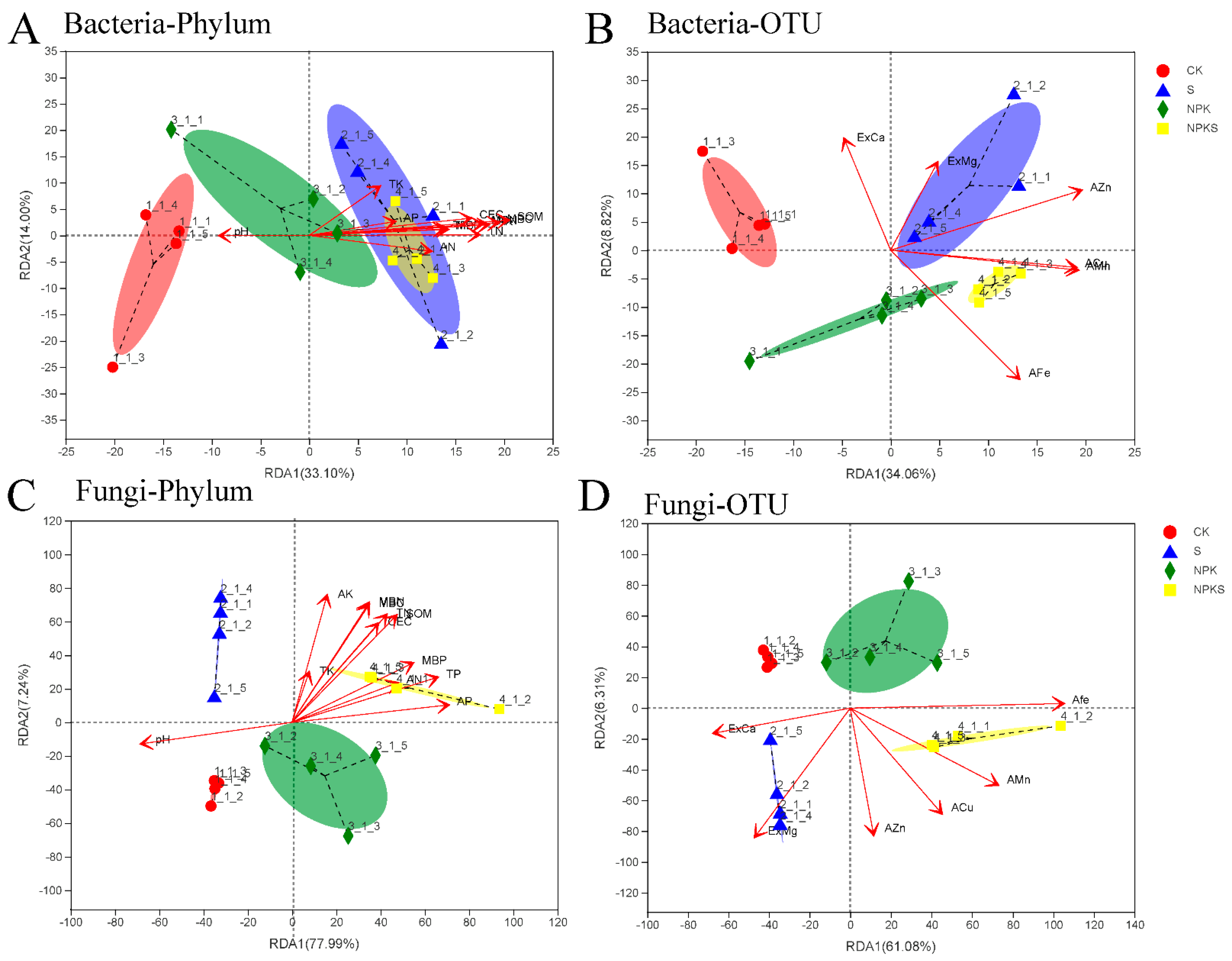
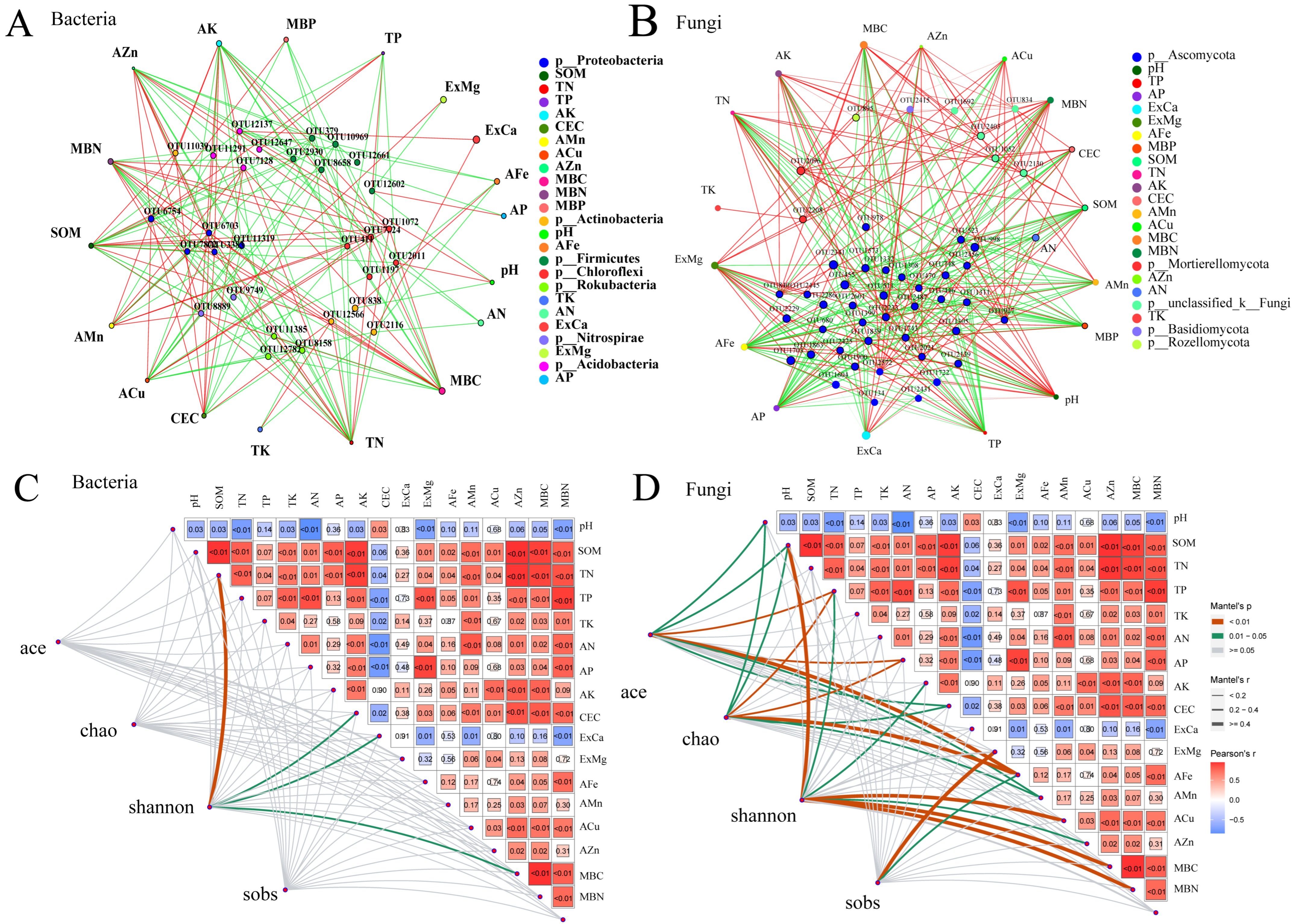
3.5. Effects of Straw Return and Fertilization on Soil Microorganisms
4. Discussion
4.1. Effects of Straw Return and Fertilization on the Growth of Crops
4.2. Effects of Straw Return and Fertilization on the Soil Physical Properties
4.3. Effects of Straw Return and Fertilization on the Soil Microorganisms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Wang, D.; Wen, X.; Yu, G.; He, N.; Wang, R. Differences in SOM decomposition and temperature sensitivity among soil aggregate size classes in a temperate grasslands. PLoS ONE 2015, 10, e0117033. [Google Scholar] [CrossRef] [PubMed]
- Regelink, I.C.; Stoof, C.R.; Rousseva, S. Linkage between aggregate formation, porosity and soil chemical properties. Geoderma 2015, 247–248, 24–37. [Google Scholar] [CrossRef]
- Zhang, M.; Riaz, M.; Zhang, L.; Xia, H.; El-Desouki, Z.; Jiang, C.C. Response of fungal communities in different soils to biochar and chemical fertilizers under simulated rainfall conditions. Sci. Total Environ. 2019, 691, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Mikha, M.M.; Rice, C.W.; Milliken, G.A. Carbon and nitrogen mineralization as affected by drying and wetting cycles. Soil Biol. Biochem. 2005, 37, 339–347. [Google Scholar] [CrossRef]
- Kushwaha, C.P.; Tripathi, S.K.; Sigh, K.P. Soil organic matter and water-stable aggregates under different tillage and residue conditions in a tropical dryland agroecosystem. Appl. Soil Ecol. 2001, 16, 229–241. [Google Scholar] [CrossRef]
- Chen, J.S.; Chiu, C.Y. Characterization of soil organic matter in different particle-size fractions in humid subalpine soils by CP/MAS 13c nmr. Geoderma 2003, 117, 129–141. [Google Scholar] [CrossRef]
- Liu, Z.L.; Yu, W.T. Review of researches on soil aggregate and soil organic carbon. Chin. J. Eco-Agric. 2011, 19, 447–455. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 2014, 32, 2099–2103. [Google Scholar] [CrossRef]
- Ghosh, B.N.; Meena, V.S.; Singh, R.J.; Alam, N.M.; Patra, S.; Bhattacharyya, R.; Sharma, N.K.; Dadhwal, K.S.; Mishra, P.K. Effects of fertilization on soil aggregation, carbon distribution and carbon management index of maize-wheat rotation in the north-western Indian Himalayas. Ecol. Indic. 2019, 105, 415–424. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Garau, G.; Morillas, L.; Roales, J.; Castaldi, P.; Mangia, N.P.; Spano, D.; Mereu, S. Effect of monospecific and mixed Mediterranean tree plantations on soil microbial community and biochemical functioning. Appl. Soil Ecol. 2019, 140, 78–88. [Google Scholar] [CrossRef]
- Zhu, H.; Ao, Y.; Xu, H.; Zhou, Z.; Wang, Y.; Yang, L. Determinants of Farmers’ Intention of Straw Recycling: A Comparison Analysis Based on Different Pro-Environmental Publicity Modes. Int. J. Environ. Res. Public Health 2021, 18, 11304. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Q.B. Study on potential of straw resource nutrient return to field and application technology in China. Soil Fertil. Sci. China 2020, 1, 119–126. (In Chinese) [Google Scholar]
- Wang, C.; Zhang, Y.X. Implementation Pathway and Policy System of Carbon Neutrality Vision. Chin. J. Environ. Manag. 2020, 12, 58–64. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Li, C.L.; Ristovski, Z.; Milic, A.; Gu, Y.T.; Islam, M.S.; Wang, S.X.; Hao, J.M.; Zhang, H.F.; He, C.R.; et al. A review of biomass burning: Emissions and impacts on air quality, health and climate in China. Sci. Total Environ. 2017, 579, 1000–1034. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.M.; Wang, H.Y.; Liu, X.W.; Zhao, X.L.; Lu, D.J.; Zhou, J.M.; Li, C.Z. Changes in soil microbial community and organic carbon fractions under short-term straw return in a rice-wheat cropping system. Soil Tillage Res. 2017, 165, 121–127. [Google Scholar] [CrossRef]
- Liu, B.; Xia, H.; Jiang, C.C.; Riaz, M.; Yang, L.; Chen, Y.F.; Fan, X.P.; Xia, X.G. 14 year applications of chemical fertilizers and crop straw effects on soil labile organic carbon fractions, enzyme activities and microbial community in rice-wheat rotation of middle China. Sci. Total Environ. 2022, 841, 156608. [Google Scholar] [CrossRef]
- Zhang, J.B.; Song, C.C.; Yang, W.Y. Influence of land-use type on soil dissolved organic carbon in the Sanjiang Plain. China Environ. Sci. 2005, 25, 343–347. (In Chinese) [Google Scholar]
- Xia, H.Y.; Wang, K.R.; Zhao, Q.L.; Zhang, Z. Effects of straw addition on decomposition, transformation and composition of soil organic carbon pool. Chin. J. Eco-Agric. 2014, 22, 386–393. (In Chinese) [Google Scholar]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, G.; Feng, H. Effects of straw and biochar amendments on aggregate stability, soil organic carbon, and enzyme activities in the Loess Plateau, China. Environ. Sci. Pollut. Res. 2017, 24, 10108–10120. [Google Scholar]
- Luo, L.; Meng, H.; Gu, J.D. Microbial extracellular enzymes in biogeochemical cycling of ecosystems. J. Environ. Manag. 2017, 197, 539–549. [Google Scholar] [CrossRef]
- Reddy, A.P.; Simmons, C.W. Discovery ofmicroorganisms and enzymes involved in high-solids decomposition of rice straw using metagenomic analyses. PLoS ONE 2013, 8, e77985. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yan, C.R.; He, W.Q.; Chen, B.Q.; Zhang, Y.Q.; Liu, Q.; Liu, E.K. Effects of different tillage practices on soil water-stable aggregation and organic carbo distribution in dryland farming in Northern China. Acta Ecol. Sin. 2015, 35, 65–69. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, Q.; Ai, C. Nitrogen enrichment regulates straw decomposition and its associated microbial community in a double rice cropping system. Sci. Rep. 2018, 8, 1847. [Google Scholar] [CrossRef] [PubMed]
- Elliott, E.T. Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Sci. Soc. Am. J. 1986, 50, 627–633. [Google Scholar] [CrossRef]
- Barreto, R.C.; Madari, B.E.; Machado, P.; Maddock, J.; Costa, A.R. The impact of soil management on aggregation, carbon stabilization and carbon loss as CO2 in the surface layer of a rhodic ferralsol in southern brazil. Agric. Ecosyst. Environ. 2019, 132, 243–251. [Google Scholar] [CrossRef]
- Liu, Z.P.; Xu, J.N.; She, D.L.; Li, X.L.; Wang, J.F. Effects of Biochar Addition on Thermal Properties of Loamy Soil. Acta Pedol. Sin. 2018, 55, 933–944. (In Chinese) [Google Scholar]
- Huang, R.L.; Crowther, T.W.; Sui, Y.Y.; Sun, B. High stability and metabolic capacity of bacterial community promote the rapid reduction of easily decomposing carbon in soil. Commun. Biol. 2021, 4, 1376. [Google Scholar] [CrossRef]
- Choudhury, S.G.; Srivastava, S.; Singh, R.; Chaudhari, S.K.; Sarkar, D. Tillage and residue management effects on soil aggregation, organic carbon dynamics and yield attribute in rice–wheat cropping system under reclaimed sodic soil. Soil Tillage Res. 2014, 136, 76–83. [Google Scholar] [CrossRef]
- Jing, X.A.; Hha, B.; Tn, A.; Zl, A.; Rl, B. Long-term effects of tillage and straw management on soil organic carbon, crop yield, and yield stability in a wheat-maize system. Field Crops Res. 2019, 233, 33–40. [Google Scholar]
- Bu, R.; Ren, T.; Lei, M.; Liu, B.; Lu, J. Tillage and straw-returning practices effect on soil dissolved organic matter, aggregate fraction and bacteria community under rice-rice-rapeseed rotation system. Agric. Ecosyst. Environ. 2020, 287, 106681. [Google Scholar] [CrossRef]
- Wang, X.; Wu, H.; Dai, K.; Zhang, D.; Feng, Z.; Zhao, Q. Tillage and crop residue effects on rainfed wheat and maize production in northern China. Field Crops Res. 2012, 132, 106–116. [Google Scholar] [CrossRef]
- Yin, Y.F.; He, X.H.; Ma, H.L.; Yang, Y.S. Effects of rice straw and its biochar addition on soil labile carbon and soil organic carbon. J. Integr. Agric. 2014, 13, 491–498. [Google Scholar] [CrossRef]
- Xu, S.Q.; Cui, S.Y.; Chen, F.; Xiao, X.P.; Zhang, H.L. Effect of tillage on content of density fractions of paddy soil organic carbon and its spatial distribution. J. Agro-Environ. Sci. 2011, 30, 127–132. (In Chinese) [Google Scholar]
- Liu, D.B.; Xia, X.G.; Fan, X.P.; Yang, L.; Zhang, F.L.; Xia, Y. Effect of long-term application of straw to soil on crop yield and nutrients absorption in rice-wheat rotation system. Hubei Agric. Sci. 2017, 56, 4731–4736. (In Chinese) [Google Scholar]
- Dai, Z.G.; Lu, J.W.; Li, X.K.; Lu, M.X.; Yang, W.B.; Gao, X.Z. Nutrient release characteristics of different crop straws manure. Trans. Chin. Soc. Agric. Eng. 2010, 26, 272–276. (In Chinese) [Google Scholar]
- Takahashi, S.; Uenosono, S.; Ono, S. Short- and long-term effects of rice straw application on nitrogen uptake by crops and nitrogen mineralization under flooded and upland conditions. Plant Soil 2003, 251, 291–301. [Google Scholar] [CrossRef]
- Huang, S.; Zeng, Y.; Wu, J.; Shi, Q.; Pan, X. Effect of crop residue retention on rice yield in China: A meta-analysis. Field Crops Res. 2013, 154, 188–194. [Google Scholar] [CrossRef]
- Karami, A.; Homaee, M.; Afzalinia, S.; Ruhipour, H.; Basirat, S. Organic resource management: Impacts on soil aggregate stability and other soil physico-chemical properties. Agric. Ecosyst. Environ. 2012, 148, 22–28. [Google Scholar] [CrossRef]
- Yemadje, P.L.; Chevallier, T.; Guibert, H.; Bertrand, I.; Bernoux, M. Wetting-drying cycles do not increase organic carbon and nitrogen mineralization in soils with straw mendment. Geoderma 2016, 304, 68–75. [Google Scholar] [CrossRef]
- Shindo, H.; Nishio, T. Immobilization and remineralization of n following addition of wheat straw into soil: Determination of gross n transformation rates by 15n-ammonium isotope dilution technique. Soil Biol. Biochem. 2005, 37, 425–432. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, X.L.; Wei, T.; Yang, Z.; Jia, Z.K.; Yang, B.P.; Han, Q.F.; Ren, X.L. Effects of straw incorporation on the soil nutrient contents, enzyme activities, and crop yield in a semiarid region of china. Soil Tillage Res. 2016, 160, 65–72. [Google Scholar] [CrossRef]
- Song, D.; Xi, X.; Zheng, Q.; Liang, G.; Zhou, W.; Wang, X. Soil nutrient and microbial activity responses to two years after maize straw biochar application in a calcareous soil. Ecotoxicol. Environ. Saf. 2019, 180, 348–356. [Google Scholar] [CrossRef]
- Chavarria, D.N.; Perez-Brandan, C.; Serri, D.L.; Merile, J.M. Response of soil microbial communities to agroecological versus conventional systems of extensive agriculture. Agric. Ecosyst. Environ. 2018, 265, 1–8. [Google Scholar] [CrossRef]
- Bastida, F.; Kandeler, E.; Hernandez, T.; Garcia, C. Long-term effect of municipal solid waste amendment on microbial abundance and humus-associated enzyme activities under semiarid conditions. Microb. Ecol. 2008, 55, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Hu, H.; Liu, Z.; Dong, Q.; Sun, K.; Feng, Y. Shifts in microbial community and carbon sequestration in farmland soil under long-term conservation tillage and straw returning. Appl. Soil Ecol. 2018, 136, 43–54. [Google Scholar] [CrossRef]
- Su, Y.; Lv, J.L.; Yu, M.; Ma, Z.H.; Xi, H.; Kou, C.L. Long-term decomposed straw return positively affects the soil microbial community. J. Appl. Microbiol. 2020, 128, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, A.; Tam, H.M.; Wani, S.P.; Long, T.D. Effect of mulch on soil temperature, moisture, weed infestation and yield of groundnut in northern vietnam. Field Crops Res. 2006, 95, 115–125. [Google Scholar] [CrossRef]
- Kader, M.A.; Nakamura, K.; Senge, M.; Mojid, M.A.; Kawashima, S. Numerical simulation of water- and heat-flow regimes of mulched soil in rain-fed soybean field in central Japan. Soil Tillage Res. 2019, 191, 142–155. [Google Scholar] [CrossRef]
- Banwart, S. Save our soils. Nature 2011, 474, 151–152. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, W.; Zheng, J.; Luo, Y.; Li, R.; Wang, H.; Qi, H. Effect of long-term tillage on soil aggregates and aggregate-associated carbon in black soil of Northeast China. PLoS ONE 2018, 13, e0199523. [Google Scholar] [CrossRef] [PubMed]
- Kan, Z.R.; Ma, S.T.; Liu, Q.Y.; Liu, B.Y.; Virk, A.L.; Qi, J.Y.; Zhao, X.; Lal, R.; Zhang, H.L. Carbon sequestration and mineralization in soil aggregates under long-term conservation tillage in the North China Plain. Catena 2020, 188, 104428. [Google Scholar] [CrossRef]
- Piazza, G.; Pellegrino, E.; Moscatelli, M.C.; Ercoli, L. Long-term conservation tillage and nitrogen fertilization effects on soil aggregate distribution, nutrient stocks and enzymatic activities in bulk soil and occluded microaggregates. Soil Tillage Res. 2020, 196, 104482. [Google Scholar] [CrossRef]
- Yan, Y.; Tian, J.; Fan, M.; Zhang, F.; Li, X.; Christie, P.; Chen, H.; Lee, J.; Kuzyakov, Y.; Six, J. Soil organic carbon and total nitrogen in intensively managed arable soils. Agric. Ecosyst. Environ. 2012, 150, 102–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Gregorich, E.G.; McLaughlin, N.B.; Zhang, X.; Guo, Y.; Liang, A.; Fan, R.; Sun, B. No-tillage with continuous maize cropping enhances soil aggregation and organic carbon storage in Northeast China. Geoderma 2018, 330, 204–211. [Google Scholar] [CrossRef]
- Li, Z.Q.; Li, D.D.; Zhao, B.X.; Zhang, J.B. Effects of straw application and crop planting on soil aggregates and microbial community composition. J. Agro-Environ. Sci. 2017, 36, 1557–1565. (In Chinese) [Google Scholar]
- Tao, F.; Taru, P.; Elena, V.; Raisa, M. Cropland soils in China have a large potential for carbon sequestration based on literature survey. Soil Tillage Res. 2019, 186, 70–78. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.J.; Xu, M.G.; Cai, Z.J.; Peng, C.; Wang, B.R. Effects of Long-Term Fertilization on Change of Labile Organic Carbon in Three Typical Upland Soils of China. Sci. Agric. Sin. 2009, 42, 10. (In Chinese) [Google Scholar]
- Deng, Y.S.; Ding, S.W.; Cai, C.F.; Lv, G.A.; Xia, D.; Zhu, Y. Spatial Distribution of the Collapsing Alluvial Soil Physical Properties in Southeastern Hubei. Sci. Agric. Sin. 2014, 47, 4850–4857. (In Chinese) [Google Scholar]
- Xu, Y.; Wang, T.; Li, H.; Ren, C.; Chen, J.; Yang, G.; Han, X.; Feng, Y.; Ren, G.; Wang, X. Variations of soil nitrogen-fixing microorganism communities and nitrogen fractions in a Robinia pseudoacacia chronosequence on the Loess Plateau of China. Catena 2019, 174, 316–323. [Google Scholar] [CrossRef]
- Akhtar, K.; Wang, W.; Ren, G.; Khan, A.; Wang, H. Integrated use of straw mulch with nitrogen fertilizer improves soil functionality and soybean production. Environ. Int. 2019, 132, 105092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, D.; Wu, M.; Xia, Y.; Zhang, F.; Fan, X. Long-term straw returning improve soil k balance and potassium supplying ability under rice and wheat cultivation. Sci. Rep. 2021, 11, 22260. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Schmidt, S.K. Fungal and bacterial responses to phenolic compounds and amino acids in high altitude barren soils. Soil Biol. Biochem. 2002, 34, 989–995. [Google Scholar] [CrossRef]
- Lucas, R.W.; Casper, B.B.; Jackson, J.K.; Balser, T.C. Soil microbial communities and extracellular enzyme activity in the new jersey pinelands. Soil Biol. Biochem. 2007, 39, 2508–2519. [Google Scholar] [CrossRef]
- Bhme, L.; Langer, U.; Bhme, F. Microbial biomass, enzyme activities and microbial community structure in two european long-term field experiments. Agric. Ecosyst. Environ. 2005, 109, 141–152. [Google Scholar] [CrossRef]
- Zhang, J.; Chi, F.; Wei, D.; Zhou, B.; Cai, S.; Li, Y.; Kuang, E.; Sun, L.; Li, L.J. Impacts of Long-term Fertilization on the Molecular Structure of Humic Acid and Organic Carbon Content in Soil Aggregates in Black Soil. Sci. Rep. 2019, 9, 11908. [Google Scholar] [CrossRef] [PubMed]
- Ceja-Navarro, J.A.; Rivera-Orduna, F.N.; Patino-Zuniga, L.; Vila-Sanjurjo, A.; Crossa, J.; Govaerts, B. Phylogenetic and multivariate analyses to determine the effects of different tillage and residue management practices on soil bacterial communities. Appl. Environ. Microb. 2010, 76, 3685–3691. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Glanville, H.C.; Wade, S.C.; Jones, D.L. Life in the ‘charosphere’-Does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol. Biochem. 2013, 65, 287–293. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Chen, H.; Zhao, Z.; Li Wei Du, S.Z.; Qiao, Y.Q.; Cao, C.F. Effects of Different Straw Mulching Levels on Biological Characteristics of Lime Concretion Black Soil. Ecol. Environ. Sci. 2015, 24, 610–616. (In Chinese) [Google Scholar]
- Zhang, M.Y.; Riaz, M.; Liu, B.; Xia, H.; Jiang, C.C. Two-year study of biochar: Achieving excellent capability of potassium supply via alter clay mineral composition and potassium-dissolving bacteria activity. Sci. Total Environ. 2020, 717, 137286. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Lei, X.; Zhao, L.; Brookes, P.C.; Fei, W.; Chen, C. Fungal communities and functions response to long-term fertilization in paddy soils. Appl. Soil Ecol. 2018, 130, 251–258. [Google Scholar] [CrossRef]
- Debosz, K.; Rasmussen, P.H.; Pedersen, A.R. Corrigendum to “temporal variations in microbial biomass c and cellulolytic enzyme activity in arable soils: Effects of organic matter input”. Appl. Soil Ecol. 2000, 14, 81–82. [Google Scholar] [CrossRef]
- Zhang, M.; Riaz, M.; Xia, H.; Li, Y.X.; Wang, X.L.; Jiang, C.C. Four-year biochar study: Positive response of acidic soil microenvironment and citrus growth to biochar under potassium deficiency conditions. Sci. Total Environ. 2022, 813, 152515. [Google Scholar] [CrossRef]
- Ryan, M.; Terry, J.; Thomas, W. Bacterial metataxonomic profile and putative functional behavior associated with C and N cycle processes remain altered for decades after forest harvest. Soil Biol. Biochem. 2018, 119, 184–193. [Google Scholar]

| Treatment | Wheat | Rice | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | P (%) | K (%) | N (%) | P (%) | K (%) | |||||||
| Stem | Seed | Stem | Seed | Stem | Seed | Stem | Seed | Stem | Seed | Stem | Seed | |
| CK | 0.41 ± 0.04 b | 1.53 ± 0.09 a | 0.04 ± 0.01 b | 0.28 ± 0.02 b | 0.84 ± 0.08 c | 0.37 ± 0.02 b | 0.44 ± 0.04 b | 1.02 ± 0.09 ab | 0.10 ± 0.01 a | 0.29 ± 0.03 a | 2.52 ± 0.09 b | 0.35 ± 0.06 b |
| NPK | 0.59 ± 0.09 a | 1.61 ± 0.04 a | 0.10 ± 0.02 a | 0.37 ± 0.02 a | 0.87 ± 0.04 bc | 0.43 ± 0.03 a | 0.45 ± 0.05 b | 1.03 ± 0.06 ab | 0.10 ± 0.01 a | 0.29 ± 0.03 a | 2.66 ± 0.08 ab | 0.39 ± 0.0 3 ab |
| S | 0.38 ± 0.07 b | 1.49 ± 0.09 a | 0.06 ± 0.01 b | 0.35 ± 0.03 a | 0.95 ± 0.08 ab | 0.42 ± 0.03 ab | 0.43 ± 0.03 b | 1.00 ± 0.07 b | 0.10 ± 0.01 a | 0.31 ± 0.02 a | 2.63 ± 0.09 b | 0.42 ± 0.06 ab |
| NPKS | 0.57 ± 0.16 a | 1.59 ± 0.05 a | 0.09 ± 0.02 a | 0.35 ± 0.03 a | 1.01 ± 0.03 a | 0.41 ± 0.03 ab | 0.51 ± 0.03 a | 1.13 ± 0.06 a | 0.11 ± 0.01 a | 0.30 ± 0.02 a | 2.84 ± 0.19 a | 0.46 ± 0.08 a |
| f | 12.92 ** | 5.98 * | 35.36 ** | 10.42 ** | 2.2 | 2.62 | 4.64 | 4.01 | 0.12 | 0.24 | 9.02 * | 2.12 |
| s | 0.24 | 0.70 | 0.17 | 3.07 | 14.38 ** | 0.77 | 2.21 | 1.44 | 1.27 | 0.97 | 5.58 * | 4.74 * |
| f × s | 0.01 | 0.02 | 5.86 * | 13.78 ** | 0.31 | 4.82 * | 2.83 | 2.86 | 1.34 | 0.01 | 0.44 | 0.01 |
| Treatment | pH | SOM (g kg−1) | TN (g kg−1) | TP (g kg−1) | TK (g kg−1) | AN (mg kg−1) | AP (mg kg−1) | AK (mg kg−1) | CEC (cmol kg−1) | Exch. Ca (mg kg−1) | Exch. Mg (mg kg−1) | Avail. Fe (mg kg−1) | Avail. Mn (mg kg−1) | Avail. Cu (mg kg−1) | Avail. Zn (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CK | 7.15 ± 0.10 a | 17.69 ± 1.39 c | 1.41 ± 0.17 c | 0.77 ± 0.04 c | 20.35 ± 0.27 a | 119.17 ± 15.48 b | 15.63 ± 3.37 b | 56.89 ± 6.55 b | 11.87 ± 0.54 b | 3617.62 ± 599.62 a | 315.31 ± 6.23 a | 49.98 ± 10.60 b | 21.38 ± 0.99 b | 3.67 ± 0.31 b | 0.45 ± 0.08 b |
| NPK | 6.89 ± 0.28 ab | 22.06 ± 0.53 b | 1.63 ± 0.12 b | 0.94 ± 0.05 b | 20.56 ± 0.45 a | 133.35 ± 13.81 ab | 31.99 ± 7.53 a | 67.44 ± 7.42 b | 12.93 ± 0.46 a | 2904.56 ± 328.21 a | 297.09 ± 13.78 b | 108.22 ± 16.31 a | 25.04 ± 4.08 b | 3.84 ± 0.38 ab | 0.47 ± 0.08 b |
| S | 7.11 ± 0.18 a | 24.35 ± 1.55 a | 1.76 ± 0.13 ab | 0.84 ± 0.04 c | 21.07 ± 0.61 a | 130.22 ± 8.25 ab | 19.59 ± 3.17 b | 92.06 ± 9.98 a | 13.21 ± 0.43 a | 3510.14 ± 590.66 a | 328.91 ± 10.36 a | 67.92 ± 8.83 b | 27.67 ± 4.76 ab | 4.18 ± 0.27 ab | 0.65 ± 0.09 a |
| NPKS | 6.63 ± 0.20 b | 26.38 ± 1.64 a | 1.87 ± 0.12 a | 1.04 ± 0.08 a | 20.99 ± 0.59 a | 146.45 ± 14.88 a | 35.85 ± 4.91 a | 84.39 ± 6.80 a | 13.46 ± 0.21 a | 2881.99 ± 332.69 a | 314.38 ± 9.55 a | 116.93 ± 16.57 a | 33.99 ± 6.81 a | 4.34 ± 0.36 a | 0.60 ± 0.06 a |
| f | 13.80 ** | 22.29 ** | 5.60 * | 44.17 ** | 0.15 | 5.13 * | 41.62 ** | 0.13 | 9.10 * | 7.76 * | 10.05 ** | 62.94 ** | 4.58 ** | 0.94 | 0.11 |
| s | 2.27 | 65.91 ** | 18.08 ** | 9.99 ** | 0.98 | 3.24 | 2.39 | 44.55 ** | 19.08 ** | 0.07 | 8.93 * | 3.88 | 10.72 ** | 9.21 * | 19.23 ** |
| f × s | 1.22 | 3.00 | 0.77 | 0.15 | 0.07 | 0.02 | 0.01 | 5.44 * | 3.61 | 0.03 | 0.13 | 0.47 | 0.33 | 0.01 | 0.69 |
| Treatment | Alkyl C | O-Alkyl C | Aromatic C | Carbonyl C | ||||
|---|---|---|---|---|---|---|---|---|
| 0–45 ppm | 45–60 ppm | 60–95 ppm | 95–110 ppm | Total | 110–160 ppm | 160–220 ppm | ||
| CH3/CH2 | OCH3/NCH | Carbohydrate | O-C-O | C=O/Aryl | COO/NC=O | |||
| >2 | CK | 23.46 | 19.59 | 51.75 | 3.38 | 74.71 | 1.77 | 0.05 |
| NPK | 23.61 | 10.54 | 45.23 | 10.90 | 66.68 | 9.71 | 0.01 | |
| S | 36.78 | 16.83 | 35.85 | 6.67 | 59.35 | 3.08 | 0.79 | |
| NPKS | 37.03 | 16.64 | 39.32 | 5.12 | 61.08 | 1.46 | 0.42 | |
| 0.25–2 | CK | 25.57 | 13.64 | 34.00 | 7.36 | 55.00 | 11.79 | 7.64 |
| NPK | 28.80 | 16.76 | 41.73 | 4.96 | 63.44 | 3.33 | 4.42 | |
| S | 23.25 | 11.08 | 33.67 | 6.75 | 51.50 | 17.00 | 8.25 | |
| NPKS | 23.00 | 12.00 | 30.10 | 7.10 | 49.20 | 17.40 | 10.40 | |
| 0.05–0.25 | CK | 26.95 | 11.21 | 31.89 | 6.87 | 49.97 | 14.14 | 8.94 |
| NPK | 24.67 | 11.87 | 35.47 | 6.93 | 54.27 | 14.13 | 6.93 | |
| S | 22.69 | 12.38 | 32.92 | 7.38 | 52.69 | 16.00 | 8.62 | |
| NPKS | 20.58 | 11.39 | 32.27 | 7.69 | 51.35 | 16.78 | 11.29 | |
| <0.05 | CK | 30.65 | 13.85 | 31.65 | 5.42 | 50.92 | 9.32 | 9.11 |
| NPK | 34.67 | 13.76 | 27.14 | 4.86 | 45.76 | 10.00 | 9.57 | |
| S | 21.52 | 9.68 | 31.36 | 6.99 | 48.04 | 16.60 | 13.84 | |
| NPKS | 25.13 | 11.00 | 34.00 | 6.87 | 51.87 | 12.47 | 10.53 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Xia, H.; Jiang, C.; Jiang, C.; Riaz, M.; Yang, L.; Chen, Y.; Fan, X.; Zhang, Z.; Duan, X.; et al. Straw Addition Enhances Crop Yield, Soil Aggregation, and Soil Microorganisms in a 14-Year Wheat–Rice Rotation System in Central China. Plants 2024, 13, 985. https://doi.org/10.3390/plants13070985
Liu B, Xia H, Jiang C, Jiang C, Riaz M, Yang L, Chen Y, Fan X, Zhang Z, Duan X, et al. Straw Addition Enhances Crop Yield, Soil Aggregation, and Soil Microorganisms in a 14-Year Wheat–Rice Rotation System in Central China. Plants. 2024; 13(7):985. https://doi.org/10.3390/plants13070985
Chicago/Turabian StyleLiu, Bo, Hao Xia, Chaoqiang Jiang, Cuncang Jiang, Muhammad Riaz, Li Yang, Yunfeng Chen, Xianpeng Fan, Zhiyi Zhang, Xiaoli Duan, and et al. 2024. "Straw Addition Enhances Crop Yield, Soil Aggregation, and Soil Microorganisms in a 14-Year Wheat–Rice Rotation System in Central China" Plants 13, no. 7: 985. https://doi.org/10.3390/plants13070985
APA StyleLiu, B., Xia, H., Jiang, C., Jiang, C., Riaz, M., Yang, L., Chen, Y., Fan, X., Zhang, Z., Duan, X., Wu, M., & Xia, X. (2024). Straw Addition Enhances Crop Yield, Soil Aggregation, and Soil Microorganisms in a 14-Year Wheat–Rice Rotation System in Central China. Plants, 13(7), 985. https://doi.org/10.3390/plants13070985






