Abstract
Self-incompatibility is a widespread genetic mechanism found in flowering plants. It plays a crucial role in preventing inbreeding and promoting outcrossing. The genes that control self-incompatibility in plants are typically determined by the S-locus female determinant factor and the S-locus male determinant factor. In the Solanaceae family, the male determinant factor is often the SLF gene. In this research, we cloned and analyzed 13 S2-LbSLF genes from the L. barbarum genome, which are located on chromosome 2 and close to the physical location of the S-locus female determinant factor S-RNase, covering a region of approximately 90.4 Mb. The amino acid sequence identity of the 13 S2-LbSLFs is 58.46%, and they all possess relatively conserved motifs and typical F-box domains, without introns. A co-linearity analysis revealed that there are no tandemly repeated genes in the S2-LbSLF genes, and that there are two pairs of co-linear genes between S2-LbSLF and the tomato, which also belongs to the Solanaceae family. A phylogenetic analysis indicates that the S2-LbSLF members can be divided into six groups, and it was found that the 13 S2-LbSLFs are clustered with the SLF genes of tobacco and Petunia inflata to varying degrees, potentially serving as pollen determinant factors regulating self-incompatibility in L. barbarum. The results for the gene expression patterns suggest that S2-LbSLF is only expressed in pollen tissue. The results of the yeast two-hybrid assay showed that the C-terminal region of S2-LbSLFs lacking the F-box domain can interact with S-RNase. This study provides theoretical data for further investigation into the functions of S2-LbSLF members, particularly for the identification of pollen determinant factors regulating self-incompatibility in L. barbarum.
1. Introduction
Lycium barbarum, a species of the genus Lycium within the Solanaceae family, is a perennial shrub primarily distributed in Ningxia, Xinjiang, Inner Mongolia, but is also found in other regions. The L. barbarum fruit is valued for its medicinal and culinary benefits, including its ability to lower blood pressure, nourish the kidneys and lungs, protect the liver, and replenish blood, as well as its whitening properties [1,2]. In recent years, L. barbarum has become increasingly popular on the market, leading to a continuous expansion of planting areas and the development of many high-yielding and high-quality varieties. However, most of these varieties still need to be planted with others to facilitate cross-pollination for normal fruit setting, and self-incompatibility is the underlying cause of issues like inconsistent fruit sizes, severe flower and fruit drop, and even complete crop failure when single varieties are grown over large areas [3]. Studies on the breeding systems and self-incompatibility of various L. barbarum varieties have revealed that, aside from ‘Ningqi 1’ and ‘Ningqi 7’, which have higher self-compatibility, most other varieties are predominantly self-incompatible or have very low compatibility. As a result, self-compatibility has emerged as a crucial factor for L. barbarum breeders to consider when selecting new varieties.
Self-incompatibility (SI) is a genetic mechanism that is widespread in many flowering plants [4,5] that helps to mitigate the negative effects of inbreeding by preventing self-fertilization and promoting cross-pollination [6]. This mechanism is usually governed by a single S locus, which comprises at least two genes that are closely associated and exhibit tissue specificity, encoding the male (pollen) and female (stigma) S-determinants [7,8]. Although research has shown that plant self-incompatibility is regulated by the S-RNase gene (responsible for stigma determinants) and the SLF (S-locus F-box) gene, which encodes F-box proteins, in families such as Solanaceae, Plantaginaceae, and Rosaceae [9,10,11], studies have revealed that the functions of SLF/SFB genes vary among these families. In Rosaceae, SLF gene mutations or losses in the Prunus genus can lead to the self-S-RNase accepting pollen, whereas in Solanaceae and Plantaginaceae, SLF genes act as degradative factors to break down non-self S-RNase [5,12]. For instance, Sijacic et al. identified and characterized S-locus F-box genes in Petunia hybrida and found that pollen carrying different S alleles can lose their self-incompatibility, becoming self-compatible [13]. Qiao et al. also discovered, in snapdragons, that when AhSLF-S2 was overexpressed in homozygous S3S3 plants, only pollen carrying both AhSLF-S2 and S3 could grow normally [14]. In Solanaceae L. barbarum plants, self-incompatibility is mediated by a class of ribonucleases (S-RNases), where the recognition of self/non-self between pollen and stigma is determined by highly polymorphic S loci. When both express the same S specificity, the pollen is rejected by the stigma. The S-RNase is considered cytotoxic, and when it enters the self-pollen tubes, it can degrade self-pollen-tube RNA [15], inhibiting pollen tube growth [16,17,18] and leading to self-incompatibility. The SLF/SFB genes closely associated and interacting with S-RNase can ubiquitinize non-self S-RNase through the formation of the SCF complex, and the ubiquitinated S-RNase is eventually degraded by the 26S proteasome [19,20,21], thereby eliminating the cytotoxic effects of S-RNase and allowing the pollen tube to grow normally in the stigma and complete fertilization.
To date, numerous stigma-specific genes, known as S-RNases, have been isolated from various self-incompatible plants, including Solanaceae, Plantaginaceae, and Rosaceae [22], and their mechanisms of action have been investigated in considerable depth. However, research into the key pollen-control genes has lagged behind. Lai et al. were the first to discover the S-RNase gene by sequencing DNA fragments containing the S-RNase gene region in the genomes of snapdragons and identified a closely linked F-box gene, AhSLF-S2, which is specifically expressed in pollen [23]. Subsequently, similar pollen-specific S-locus F-box genes were detected in Rosaceae plants [24,25,26] and Solanaceae plants [13,14,27]. In 2004, PiSLF-S2 was identified in P. inflata and was shown to play a role in controlling pollen self-incompatibility [14,28,29]. In 2007, Wheeler et al. discovered seven S-locus F-box genes in tobacco that were specifically expressed only in pollen [30]. These genes are collectively referred to as SLF genes in Solanaceae and Plantaginaceae or SFB genes in Rosaceae [31]. The N-terminus of SLF genes features an F-box domain, while the C-terminus possesses two highly variable regions (Hva and Hvb) that lack strong hydrophobicity and are primarily responsible for recognizing self and non-self S-RNase genes [32].
Pollen specificity is not governed by a single SLF gene, but rather by multiple SLF genes that collaborate (the precise number remains unknown) [33,34]. Kubo et al. first introduced a model of multiple SLF genes working together to recognize non-self in P. inflata, and they demonstrated through immunocytochemical assays that each SLF protein can interact with various S-RNase proteins across different S-haplotypes [16]. Subsequently, in 2015, a mathematical model validated that between 16 and 20 SLF genes on the S-haplotype of P. inflata are responsible for recognizing the majority of non-self S-RNase proteins [35]. To date, the same 17 polymorphic SLF genes have been identified in both the S2 and S3 haplotypes of P. inflata [13,36], suggesting that multiple SLF genes serve as pollen S-determinants on the S-haplotypes of P. hybrida. In each species, a comprehensive set of SLF proteins is necessary to counteract the toxicity of non-self S-RNase, enabling compatible pollination. However, there have been no studies on the pollen-specific expression of F-box genes in L. barbarum. To comprehend the regulatory factors of self-incompatible pollen in L. barbarum, it is imperative to identify all the SLF genes that are involved in pollen specificity within L. barbarum.
In our prior studies, we developed a hybrid F1 population by mating plants with contrasting phenotypes through the crossing of plants exhibiting extreme phenotypes. We employed a bulked segregant analysis (BSA) to locate the S locus on chromosome 2 in L. barbarum. Subsequent analyses, involving expression profiling and population genotyping, indicated that the S-RNase is a potential determinant of self-incompatibility in the female flowers of L. barbarum [37]. Recent studies on the F-box gene family in L. barbarum have uncovered that 13 F-box genes located on chromosome 2 are exclusively expressed in the stamen and are physically located close to the S-RNase gene (Lba02g01102), indicating a strong association with S-RNase. These genes are believed to function as stamen S-determinants, potentially contributing to the collective decision-making process of self-incompatibility recognition in L. barbarum. To explore the possibility that these stamen S-determinants are involved in self-incompatibility, in the current study, we cloned these 13 F-box genes and conducted a thorough analysis of their gene structures, conserved protein domains, motifs, and regulatory elements, such as promoters and trans-acting factors, using bioinformatics tools. An evolutionary analysis was also conducted to categorize these genes and identify any co-linear genes. The objective was to elucidate the pollen-specific expression patterns of F-box genes and their interactions with the female determinant S-RNase. This research not only yields theoretical insights into the function of the self-incompatibility stamen determinant S2-LbSLF genes but also provides molecular markers that could be useful in the breeding of self-compatible L. barbarum varieties.
2. Results
2.1. Identification and Cloning of the F-Box Gene Family in L. barbarum
In the L. barbarum genome, a total of 283 genes belonging to the F-box gene family were identified, distributed unevenly across 12 chromosomes, with the highest number found on chromosome 2, which harbors 40 genes. Since the stamen determination factor and the pistil determination factor are located at the S locus, we successfully cloned an S-RNase gene encoding the pistil determination factor from L. barbarum, which is located on chromosome 2. It is hypothesized that the male determination factor responsible for self-incompatibility in L. barbarum is also located on chromosome 2. Additionally, 13 F-box genes on chromosome 2 were found to be specifically expressed in the stamens, suggesting that these genes may collectively regulate the self-incompatibility of L. barbarum in conjunction with the S-RNase. Consequently, these 13 F-box genes on chromosome 2 were cloned to obtain the sequences of the S2-LbSLFs, which were named S2-LbSLF1–S2-LbSLF13. The coding sequences of the 13 SLF genes in L. barbarum typically range in length around 1150 base pairs, and the SLF proteins all possess F-box- and F-box-associated domains (F-box-associated) (Figure 1). The 13 SLF genes are highly similar to one another, exhibiting 58.46% amino acid sequence identity.
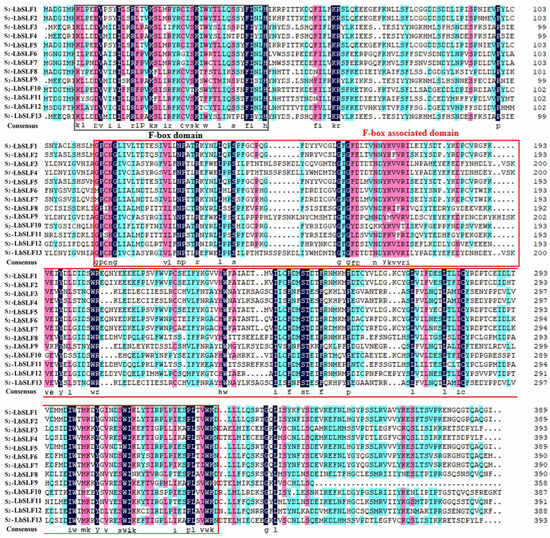
Figure 1.
The amino acid sequence alignment of the S2-LbSLFs. The black-boxed regions represent the F-box structure domain, and the red-boxed regions represent the F-box-associated domain (F-box-associated domain). The conservation strength of amino acids from strong to weak is highlighted with dark blue, purple, and light blue, respectively.
2.2. Physicochemical Properties, Subcellular Localization Prediction, and Functional Prediction of S2-LbSLFs
Utilizing the ExPASy-ProtParam tool to determine protein characteristics such as length, molecular weight, isoelectric point (pI), instability index, lipophilic index, and hydrophilic index (Table 1), the findings indicate that the S2-LbSLFs vary in amino acid length from 358 (S2-LbSLF9) to 393 (S2-LbSLF3, S2-LbSLF4) amino acids. The predicted molecular weights range from 42,018.48 (S2-LbSLF9) to 45,805.57 (S2-LbSLF4) Da. The predicted pI values for the S2-LbSLFs range from 4.81 (S2-LbSLF1) to 7.07 (S2-LbSLF9), with S2-LbSLF4 and S2-LbSLF10 predicted to have pI values above 7, suggesting that they may be basic proteins. The instability indices predict that all the proteins except S2-LbSLF6 and S2-LbSLF7 have indices above 40, indicating potential instability. The lipophilic indices are predicted to range from 86.34 (S2-LbSLF10) to 99.41 (S2-LbSLF8), while the hydrophilic indices for the S2-LbSLFs are expected to range from −0.007 (S2-LbSLF8) to −0.248 (S2-LbSLF9), with all falling below 0, suggesting hydrophilic properties that all S2-LbSLFs have. The subcellular localization predictions using the Cell-PLoc 2.0 online tool suggest that all 13 S2-LbSLF proteins are located in the nucleus.

Table 1.
Predicted physiochemical properties of S2-LbSLF proteins.
The secondary structure analysis of the 13 S2-LbSLF proteins reveals a composition of four structural types: alpha helix, extended strand, beta turn, and random coil. The alpha helix and random coil structures dominate, accounting for over 60%, while the extended strand and beta turn structures account for approximately 30%. The random coil structure is the most abundant, comprising around 50% of the structure, followed by the extended strand (Table 2).

Table 2.
Secondary structure prediction for S2-LbSLF proteins.
2.3. Conservation Motifs, Structural Domains, and Gene Structure of S2-LbSLF Gene Members
The analysis using the MEME online tool revealed that the S2-LbSLF genes in L. barbarum have 10 relatively conserved motifs, with lengths varying from 15 to 50 amino acids (Figure 2B). All 13 members of the S2-LbSLFs proteins possess Motif1, Motif2, Motif3, Motif4, Motif5, and Motif8, indicating that these six motifs are highly conserved. The S2-LbSLF3, S2-LbSLF4, S2-LbSLF9, and S2-LbSLF13 have an additional Motif9 compared to the other S2-LbSLFs, while S2-LbSLF9, S2-LbSLF10, and S2-LbSLF12 lack Motif7, Motif10, and Motif6, respectively (Figure 2A). The conserved regions of the S2-LbSLF family members show that all 13 S2-LbSLF proteins have the typical F-box protein structure, with an N-terminal F-box domain involved in interaction with Skp1 and a C-terminal region involved in interaction with substrate proteins, belonging to the F_box_assoc_1 superfamily (Figure 2C). To understand the structure and genetic diversity of the S2-LbSLFs genes, an exon–intron structure analysis and visualization were performed using the TBtools (v2.042) software, and it was found that none of the S2-LbSLFs contain introns (Figure 2D).
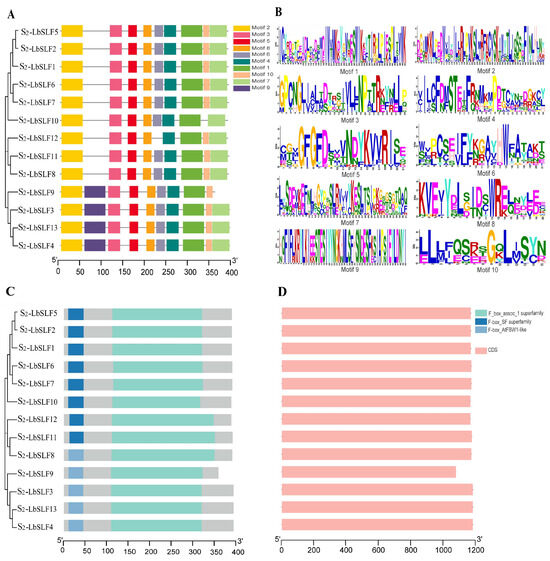
Figure 2.
Analysis of conserved motifs, domains, and gene structure of S2-LbSLF gene members. (A) Analysis of conserved motifs in S2-LbSLFs proteins, where 10 conserved motifs were identified. (B) Sequence logos of the 10 conserved motifs. The motif logo is composed of a stack of letters at each position, with the relative size of the letters indicating their frequency in the sequence. (C) Conserved domains in S2-LbSLFs proteins, with non-domain regions represented in gray. (D) S2-LbSLFs gene structure: exons–introns.
2.4. Analysis of Promoter Cis-Acting Elements
Promoter cis-acting elements are critical binding sites for transcription initiation and play a pivotal role in regulating gene expression. In L. barbarum, the upstream 2000-base-pair promoter regions of the 13 S2-LbSLF family members are extensively annotated, and predictions have uncovered 41 cis-acting elements with potential capabilities for environmental and hormonal responses (Figure 3). These elements encompass photoresponsive elements, various factors related to growth and development, hormone response, and stress resistance (Supplementary Table S1). Many elements associated with hormone-signaling pathways were identified, including drought-response elements and methyl jasmonate (MeJA)-, abscisic-acid-, salicylic-acid-, auxin-, and gibberellin-response elements. Elements related to stress resistance include defense and stress response, cell cycle regulation, anaerobic response, and low-temperature-response elements. The GCN4_motif, O2-site, CAT box, RY-element, MSA-like, and MBSI elements are linked to growth and development. There is considerable diversity in the types and distribution of cis-acting elements within S2-LbSLF promoters. Specifically, 12 S2-LbSLFs contain light-related elements (G-Box), and the predictions also revealed MeJA-response elements (TGACG-motif and CGTCA-motif) in all the promoters except for S2-LbSLF12. Furthermore, MYB-binding sites (MBS) were found in the promoters of S2-LbSLF6, S2-LbSLF7, and S2-LbSLF8, which are associated with drought response. Salicylic-acid-related elements (TCA-element) are exclusively present in the promoters of S2-LbSLF3, S2-LbSLF10, and S2-LbSLF13. Cell-cycle-response elements (MSA-like) were only found in the promoters of S2-LbSLF6 and S2-LbSLF7.
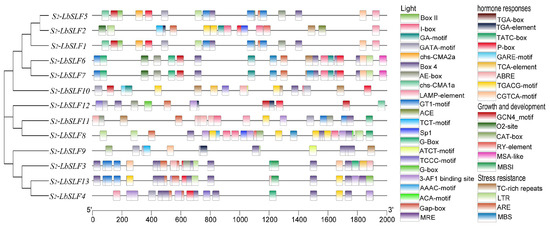
Figure 3.
Analysis of cis-acting elements in the promoter region of S2-LbSLF family members. The colored boxes indicate the types of cis-elements. Different colors represent different numbers of cis-elements.
2.5. Phylogenetic Analysis and Classification of the L. barbarum S2-LbSLF Gene Family
To delve into the similarity and evolutionary connections between the S2-LbSLFs in L. barbarum, a phylogenetic evolutionary tree was constructed using amino acid sequences from 13 individuals from L. barbarum, 15 from P. inflata, and 10 from Nicotiana tabacum. The branch clustering results led to the division of the S2-LbSLFs family into six groups (I–VI) (Figure 4), comprising five, seven, nine, seven, four, and six members, respectively. Group I encompasses four L. barbarum SLFs (S2-LbSLF3, S2-LbSLF4, S2-LbSLF9, and S2-LbSLF13) along with one N. tabacum SLF (NtDD7), suggesting a closer evolutionary relationship between these four SLFs and NtDD7; Group II includes three L. barbarum SLFs (S2-LbSLF8, S2-LbSLF11, and S2-LbSLF12) and four P. inflata SLF family members clustered together; Group V contains one L. barbarum SLF (S2-LbSLF10) and three P. inflata SLFs, with S2-LbSLF10 clustering with EF614187; and Group VI consists of five L. barbarum SLF members (S2-LbSLF1, S2-LbSLF2, S2-LbSLF5, S2-LbSLF6, and S2-LbSLF7), along with one N. tabacum SLF (NtDD6). The clustering analysis revealed that, with the exception of Groups III and IV, which lack L. barbarum SLF members, the remaining four groups all feature members from the families L. barbarum, N. tabacum, or P. inflata. Wheeler et al. established through research on N. tabacum that NtDD6 and NtDD7 are specifically expressed in pollen, with NtDD7 considered a potential SLF homolog [30]. This evolutionary tree suggests that certain candidate F-box genes in L. barbarum (S2-LbSLF1, S2-LbSLF2, S2-LbSLF3, S2-LbSLF4, S2-LbSLF5, S2-LbSLF6, S2-LbSLF7, S2-LbSLF9, and S2-LbSLF13) may act as pollen S-determinants and participate in self-incompatibility responses. Previous studies indicated that seven species of P. inflata SLF family members (AAS79485, KJ670474, EF614187, KF524351, KF524352, KF524353, and EF614188), which have been genetically modified and functionally validated for encoding pollen-specific determinants [13,16,36], clustered with KF524351, KF524352, KF524353, and EF614187 in Groups II and V, further indicating that S2-LbSLF8, S2-LbSLF10, S2-LbSLF11, and S2-LbSLF12 are promising candidates for pollen determinants.
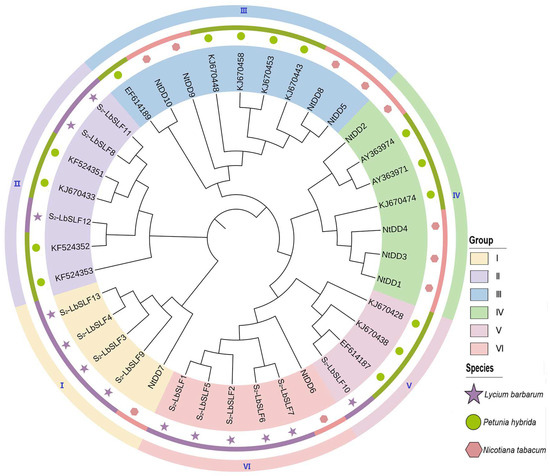
Figure 4.
Phylogenetic tree of F-box protein family of L. barbarum, N. tabacum, and P. inflata. A maximum likelihood (ML) phylogenetic tree was constructed with neighbor joining (NJ) based on bootstrap sampling in MEGA11. Each color region represents a group. The same species are displayed in stripes of the same color. Stars indicate F-box identified in L. barbarum.
2.6. Density and Collinearity Analysis of S2-LbSLF Genes
A collinearity analysis was performed on the L. barbarum genome, resulting in the placement of the 13 S2-LbSLFs and the S-RNase on the second chromosome of L. barbarum. To ascertain whether the neighboring genes were tandem duplicates, a gene duplication analysis was conducted on the L. barbarum genome using Tbtools (Figure 5A). The findings revealed that the identified S2-LbSLFs did not exhibit evidence of tandem duplication. There were two pairs of colinear genes observed between L. barbarum and Solanum lycopersicum, as well as one pair between L. barbarum and Rosa chinensis, with S2-LbSLF9, S2-LbSLF12, and S2-LbSLF13 being the specific genes in L. barbarum (Figure 5B). Upon examination of the collinearity results, it was observed that there was a higher degree of collinearity between L. barbarum, a member of the Solanaceae family, and S. lycopersicum.
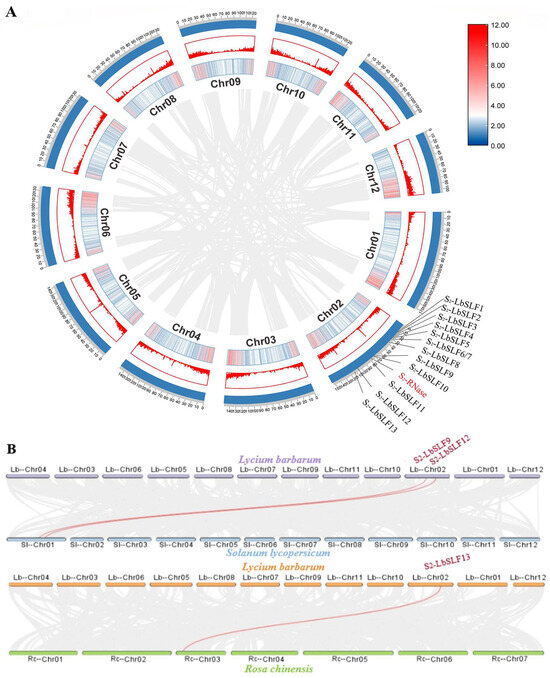
Figure 5.
Localization of S2-LbSLFs in L. barbarum and collinearity with S. lycopersicum and R. chinensis chromosomes. (A) Thirteen S2-LbSLFs are located on chromosome 2. The scale on the outer circle is measured in megabytes. The inner and middle circles show gene density by color or column height. These connections indicate gene tandem. (B) Collinearity of the chromosomes of L. barbarum with those of S. lycopersicum and R. chinensis. These associations indicate a relationship with collinear genes. The labeled S2-LbSLFs are collinear with their SLF connected with a red line. The remaining unlabeled S2-LbSLFs were identified as non-collinear genes of SlSLFs or RcSLFs.
2.7. Analysis of Pollen-Specific Expression Patterns of S2-LbSLFs Genes
Gene expression patterns can offer crucial insights into gene function. To explore the tissue-specific expression patterns of S2-LbSLF genes, we conducted a dedicated analysis of the F-box genes located on the second chromosome and produced a heatmap representing their expression profiles. Our analysis revealed that the transcriptomic data from various tissues pointed to the specific expression of 13 F-box genes in pollen (Figure 6A). To corroborate the integrity of the transcriptomic data, we selected five genes for Qrt-PCR validation. The results aligned with our predictions, demonstrating that the five S2-LbSLFs genes were indeed specifically expressed in pollen (Figure 6B), which confirms the anther-specific expression of S2-LbSLFs genes. These results suggest that F-box genes with elevated expression in particular tissues are most probably engaged in tissue-specific biological processes, and further suggest that these 13 S2-LbSLFs genes may function as pollen determinants involved in the modulation of L. barbarum’s self-incompatibility mechanisms.
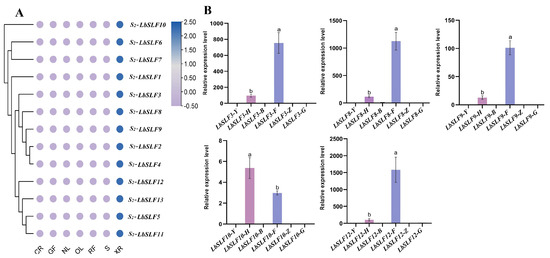
Figure 6.
Analysis of gene expression patterns of S2-LbSLFs. (A) Heatmap showing the relative expression levels of genes in the L. barbarum transcriptome. CR: carpel; XR: anther; S: stem tip; OL: old leaf; NL: new leaf; GF: green fruit; RF: mature fruit. (B) Relative expression levels of S2-LbSLF genes in different tissues of L. barbarum. Y: leaf; H: entire flower; B: petal; F: pollen; Z: stigma; G: flower stalk. Each column represents the mean ± SD from three biological replicates, with error bars denoting the standard deviation. Different lowercase letters indicate significant differences in the relative expression levels (p < 0.05).
2.8. Interaction Analysis of SLF and S-RNase
The S-RNase and SLF/SFB genes have been shown to control the specificity of female organ and pollen self-incompatibility, respectively, with their protein products interacting to determine self-/non-self-specific recognition between female organs and pollen. Furthermore, SLFs can form SCF (SKP1/Cullin1/F-box) complexes, functioning as specific E3 ubiquitin ligases that interact with S-RNase. To elucidate the interaction between SLF and S-RNase, a yeast two-hybrid pair-wise interaction assay was employed to study the interaction between S-RNase and SLF. The S2-LbSLF1–S2-LbSLF13 genes were segmented into full-length, N-terminal (including F-box domain), and C-terminal (F-box associated domain) regions, which were individually tested for interaction with S2-RNase and S5-RNase using yeast two-hybrid (Y2H) experiments. The S2-RNase and S5-RNase were amplified from the entire flower number 6 (S2S5). The interactions were observed through growth on -Leu/-Trp-defective medium and -Ade/-His/-Leu/-Trp-defective media (Supplementary Figures S1–S6). The results revealed that the full-length S2-LbSLF1–S2-LbSLF13 did not interact with S5-RNase, and the C-terminal region of S2-LbSLF7 could interact with S5-RNase. The full-length and C-terminal regions of S2-LbSLF2, as well as the C-terminal region of S2-LbSLF5, S2-LbSLF6, and S2-LbSLF7, could interact with S2-RNase (Figure 7).
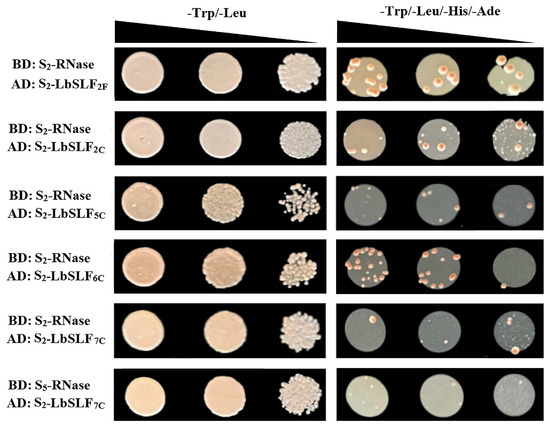
Figure 7.
The interaction between S2-RNase and S5-RNase with S2-LbSLFs was investigated in yeast strains. AD: S2-LbSLFF and S2-LbSLFC denote the full-length and C-terminal regions of S2-LbSLF, respectively, with the numbers indicating the specific genes. BD: S-RNase signifies S2-RNase and S5-RNase. The growth of yeast cells expressing various fusion combinations of BD and AD was assessed on -Trp/-Leu- and -Trp/-Leu/-His/-Ade-minimal media lacking the specified amino acids.
3. Discussion
Solanaceous plants typically exhibit gametophytic self-incompatibility, which is mediated by S-RNase. The female S-determinant is encoded by a class of glycoproteins with ribonuclease activity, known as S-ribonuclease or S-RNase [38], while the pollen S-determinant is encoded by genes containing an F-box structure, referred to as S-locus F-box (SLF) [25]. The F-box proteins generally serve as components of the SCF (SKP1/Cullin1/F-box) ubiquitin ligase complex, which is responsible for mediating the ubiquitination and degradation pathway [39]. The N-terminal region of F-box proteins, typically composed of 40–50 amino acids, acts as the binding site for Skp1- or Skp1-like proteins within the SCF complex. The C-terminal domain of these proteins has the capacity to specifically recognize substrates.
3.1. Cloning of Full-Length Sequences for 13 SLF Genes Located on Chromosome 2 in L. barbarum
In Solanaceous plants, the interaction between pollen and pistil is a complex non-self-recognition process, as evidenced by the functional gain-of-function and loss-of-function experiments conducted by Lee et al. [40]. The female-specific determinant is encoded by a single polymorphic S-RNase gene, whereas multiple polymorphic SLF genes collectively encode the pollen-specific determinant [16,41]. For instance, in P. inflata, approximately 3.1 Mb of the S locus have been cloned to uncover 17 SLF genes and SLFLike1. In citrus, each S haplotype on chromosome 1 harbors one S-RNase and nine SLFs, spanning a region of 198–370 kb [10]. In roses, 30 SLFs are specifically located on chromosome 3, occupying a region of 1.5 to 43.7 Mb [42]. A study on potato chromosome 1 found that the S locus contains 13 SLFs, covering an area of 14.6 Mb [43]. In snapdragons, chromosome 8 contains 32 SLF proteins, spanning 1.2 Mb [44]. However, to date, no published studies have reported SLF genes located on the chromosomes of L. barbarum. In previous work, we cloned the S2-RNase gene, which is an estigmatic determinant regulating self-incompatibility in L. barbarum. Therefore, in this study, we aimed to clone and analyze the SLF pollen determinant located near the S-RNase on chromosome 2 of L. barbarum. The cloned region spans approximately 90.4 Mb of the S locus on this chromosome. Compared to other species, Solanaceous plants have a larger area covered by the S locus, which may be due to their rich, highly repetitive sequences [45]. Through the sequence alignment of the full-length amino acid sequences of 13 L. barbarum F-box genes, a homology of up to 58.46% was observed, with 63.74% identity in the N-terminal and 57.13% in the C-terminal. The sequence alignment revealed that all the genes possess a unique N-terminal (F-box domain) consisting of 42 amino acid residues, and a more distinct F-box-associated domain in the C-terminal.
3.2. Similarity in the Structure of F-Box Genes at the S Locus in Angiosperms
The conserved motifs and structures of the 13 S2-LbSLFs proteins suggest that they all possess an F-box domain at their N-termini and an F-box-related domain (F_box_assoc_1 superfamily domain) at their C-termini, aligning with observations of F-box domains in other species, such as apples [46], pears [47], and tobacco [30]. Furthermore, all 13 members of the S2-LbSLFs family lack introns, corroborating the findings of the study by Wu et al. on P. inflata. F-box genes [48]. The gene structure without introns is conducive to the rapid expression of mRNA [49]. Akash et al. found that 31.8% of the F-box genes in tomatoes lack introns, indicating that the prevalence of gene structures without introns is a unique characteristic of this F-box family [50].
Cis-regulatory elements are essential in plant regulatory networks and offer insights into the functions of related genes [51]. They have been identified as candidates involved in salt tolerance [52], the response to low-temperature stress [49], and drought induction [53]. In this study, we analyzed the cis-regulatory elements within the 2000-base-pair upstream promoter regions of S2-LbSLFs. A variety of light-response elements and various elements responsive to hormone reactions were detected. We also identified cis elements related to abiotic stress, such as those involved in defense and stress responses, as well as elements involved in drought induction and binding sites for MYB. Moreover, elements related to plant growth and development, such as those involved in cell cycle regulation, expression in endosperm, and in the expression of meristematic tissues, deserve special attention. In plants, genes containing F-box domains have been shown to regulate plant growth and development [54,55,56] and play important roles in response to adverse stress [57,58]. Therefore, it is speculated that the analysis of cis-regulatory elements may provide new insights into the study of the functions of S2-LbSLF members, particularly in regulating plant self-incompatibility and related genes, as well as plant development.
3.3. S2-LbSLF1–S2-LbSLF13 Are Good Candidates for the S-Determination Factor of L. barbarum Pollen
The phylogenetic tree analysis reveals that S2-LbSLF1, S2-LbSLF2, S2-LbSLF5, S2-LbSLF6, S2-LbSLF7, and S2-LbSLF3, S2-LbSLF4, S2-LbSLF9, and S2-LbSLF13, respectively, exhibit a strong clustering relationship with NtDD6 and NtDD7. Wheeler et al. [30] demonstrated that out of the ten SLF proteins associated with tobacco that they identified (referred to as DD1-10), seven are exclusively expressed in pollen. Further meticulous mapping found that NtDD2, NtDD7, and NtDD10 are situated in the same chromosomal region as the pollen S-determinant, with NtDD10 also expressed in petals. Since all the SLF genes characterized to date are limited to pollen expression, NtDD10 can be discounted, suggesting that NtDD2 and NtDD7 may function as direct homologs of SLF. In this study, the functions of these nine genes are presumed to be analogous to those of NtDD6 and NtDD7, potentially acting as pollen S-determinants to regulate the self-incompatibility mechanism of L. barbarum. Three L. barbarum SLF members (S2-LbSLF8, S2-LbSLF11, and S2-LbSLF12) in Group II and four P. inflata SLF members cluster together, while Group V encompasses one L. barbarum SLF gene (S2-LbSLF10) and three P. inflata SLF members, with S2-LbSLF10 closely associated with EF614187 and grouped on the same branch. Previously, using RNA-seq, 17 SLF genes (S2-SLF1 to S2-SLF17) and one SLFLike gene (S2-SLFLike1) were identified as being specifically expressed in the pollen transcriptome of P. inflata [36]. In this study, the involved genes include (S2-SLF3 to S2-SLF6 and S2-SLF11 to S2-SLF13), and S2-LbSLF8, S2-LbSLF10, S2-LbSLF11, and S2-LbSLF12 are clustered together with these genes. Overall, these findings indicate that these 13 SLF members can serve as excellent candidates representing the S-determination factors of L. barbarum pollen. These SLF genes are specifically expressed in L. barbarum pollen, aligning with one of the fundamental characteristics of pollen S genes [24,25]. Therefore, S2-LbSLF1–S2-LbSLF13 are further considered as potential pollen determinants and may contribute to self-incompatibility reactions.
3.4. Physical Interactions between S2-LbSLFs and S-RNase
Sun et al. have shown through loss-of-function experiments on phlox flowers that the complete set of F-box proteins is the determinant for pollen specificity, confirming that SLF proteins are the sole cause of pollen self-incompatibility (SI) and revealing their interaction with S-RNase to maintain SI [59]. The yeast two-hybrid assay results from this study indicated that the C-terminal regions of S2-LbSLF2, S2-LbSLF5, S2-LbSLF6, S2-LbSLF7, and the full-length S2-LbSLF2 all exhibited weak interactions with S2-RNase. Furthermore, the C-terminal region of S2-LbSLF7 demonstrated a weak interaction with S5-RNase. Therefore, the 13 identified SLF genes were tested for point-to-point interactions with S-RNase using yeast two-hybrid assays. The results showed that the C-terminal regions of S2-LbSLF2, S2-LbSLF5, S2-LbSLF6, S2-LbSLF7, and the full-length S2-LbSLF2 could weakly interact with S2-RNase; the C-terminal region of S2-LbSLF7 could weakly interact with S5-RNase, indicating that S2-LbSLFs can interact with S-RNase as pollen S-determinant clusters. Qiao et al. have shown that the C-terminal region of AhSLF-S2 specifically interacts with S-RNase in Antirrhinum [14]. A similar phenomenon was also observed in phlox, where Liu et al. demonstrated that the C-terminal region of PhS3L-SLF1 interacts with PhS3-RNase, PhS3L-RNase, and PhSv-RNase in yeast, while the C-terminal region of PhSv-SLF1 only interacts with PhSv-RNase [19]. Xu et al. also discovered, in their study on the interaction between pear PbSLFs and PbS-RNases, that PbS21-RNase interacts with the C-terminal regions of PbSLF3-S34 and PbSLF6-S21, while PbS34-RNases only interact with the C-terminal region of PbSLF3-S34 [60]. This is consistent with the results of this study, in which it was found that S2-RNase specifically interacts with the C-terminal regions of S2-LbSLF5, S2-LbSLF6, and S2-LbSLF7, and that S5-RNase only specifically interacts with the C-terminal region of S2-LbSLF7. This suggests that these interaction relationships depend on the C-terminal regions of S2-LbSLFs without the F-box domain, rather than the full-length S2-LbSLFs and their F-box-domain-containing N-terminal regions. This is in line with the function of the C-terminal domain of F-box proteins in the SCF complex, which is responsible for recognizing specific substrate proteins. However, a difference in this study is the observation that the full-length S2-LbSLF2 can also interact with S2-RNase.
Regarding the self-incompatibility (SI) mechanism of P. inflata, numerous studies have indicated that the S-locus factor (SLF) interacts with non-self S-RNase and specifically neutralizes it [16,35,41,61]. Nonetheless, Liu et al. conducted a yeast double-hybrid assay to study the interaction between SLF and S-RNase in P. hybrida, revealing that PhSLF can specifically engage with both “self” and “non-self” PhS-RNase [19]. Moreover, in P. inflata, the interaction between PiSLF and non-self PiS-RNase is more potent than that with the self S-RNase [62]. The current study demonstrates that only S2-LbSLF7 can interact with non-self S5-RNase, while most of the interaction outcomes involve S2-LbSLF binding to the self S2-RNase, a pattern that contrasts with the majority of the interactions observed in other Solanaceae plants. Additionally, research on Rosaceae and Plantaginaceae has also uncovered interactions between SLF and both “self” and “non-self” S-RNase. For example, Yuan et al. reported in their study on apples that the S2-MdSFBB1 in apples is capable of interacting with both S2- and S3-RNases, S2-MdSFBB2 interacted with S7-RNase, and both S2-MdSFBB3 and S2-MdSFBB4 had the capacity to interact with S3-, S5- and S9-RNases. In addition [63], Qiao et al. utilized yeast two-hybrid tests and co-immunoprecipitation to show that AhSLF-S2 interacts with S2-, S4-, and S5-RNases in Antirrhinum [64].
In conclusion, the data suggest that S2-LbSLFs can selectively interact with both “self” and “non-self” S-RNases in yeast cells. In other Solanaceous plants, S-RNase functions according to a non-self-recognition pattern, meaning that SLFs interact with S-RNases that are not of their own type. In the case of L. barbarum, S2-LbSLFs engage in interactions not only with S2-RNase, but also with S5-RNase. Additionally, S2-LbSLF shows a more robust interaction with its cognate S-RNase than with non-self S-RNase. Consequently, the relationship between S-RNase and SLF may involve intricate interplay.
4. Materials and Methods
4.1. Identification of the L. barbarum F-Box Gene Family
The genomic data for L. barbarum were retrieved from the NCBI online database (https://www.ncbi.nlm.nih.gov/ (accessed on 20 March 2023)). An HMM (PF00646) file corresponding to the F-box gene domain was obtained from the Pfam protein database (http://www.pfam.xfam.org/ (accessed on 20 March 2023)). This F-box HMM file was then used with HMMER 3.0 software to identify the entire F-box gene family within the L. barbarum protein sequences, with the E-value threshold set at 1 × 10−5. The proteins identified were subsequently submitted to the NCBI-CDD (https://www.ncbi.nlm.nih.gov/cdd/ (accessed on 20 March 2023)), SMART (http://smart.embl.de/ (accessed on 20 March 2023)), and Pfam (http://pfam.xfam.org/ (accessed on 20 March 2023)) online databases for conservation domain analysis to verify the presence of the F-box domain. Next, differential expression analysis was performed on all F-box genes located on chromosome 2, leading to the identification of the gene members that were specifically expressed in the stamen on that chromosome.
4.2. Gene-Specific Amplification and Sequence Analysis
The full-length clone of the candidate gene was performed. Genomic DNA was extracted from the leaves of Ningqi 1 (S2S8) using a novel plant genome DNA-extraction kit (Tiangen, Beijing, China), and was stored for future applications. Following the haploid genome data of Ningqi 1 (PRJNA640228), specific forward and reverse primers for the SLF gene were designed based on the sequence flanking the S2-RNase gene located in this study. Primers for the SLF gene were designed based on the predicted sequences derived from genomic sequencing (refer to Supplementary Table S2 for primer details). The PCR amplification was conducted using a 2× Hieff Canace® Gold PCR Master Mix high-fidelity enzyme pre-mix (Yi Sheng, Shanghai, China) with a PCR system containing 100 ng of DNA template, 0.5 μM forward and reverse primers, and 1× Hieff Canace®® Gold PCR Master Mix. The PCR cycling conditions included 5 min of pre-denaturation at 94 °C, 30 s denaturation at 94 °C, 30 s annealing at 60 °C, 45 s extension at 72 °C, and 35 cycles, followed by a final 10-min extension at 72 °C. The PCR products were then separated using 1% agarose gel electrophoresis, and the purified fragments were ligated into the Hieff Clone ZeroTOPO-Blunt Cloning Kit (Yi Sheng, Shanghai, China) vector before being transformed into DH5α competent cells. These transformed cells were sent to the Beijing Uniquehexa Genetic Technology Limited Research Center for sequencing. Sequence-alignment analysis was conducted using DNAMAN software (v10) (the F-box protein sequences of L. barbarum are listed in Supplementary Table S3).
4.3. Physicochemical Property Analysis of S2-LbSLFs Genes
The protein sequences of 13 SLF genes were analyzed using the ExPASy online platform (https://web.expasy.org/pr-otparam/ (accessed on 12 April 2023)) to determine the number of amino acids, molecular weight, isoelectric point, and hydrophobicity of the proteins. The secondary structure of SLF proteins was analyzed using the SOPMA online platform (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?pag-e=npsa_sopma.html/ (accessed on 12 April 2023)). The subcellular localization of S2-LbSLFs proteins was predicted using Cell-PLoc 2.0 software.
4.4. Phylogenetic Tree Development Analysis
To explore the evolutionary relationships of F-box genes within and between species, protein sequences from 10 tobacco F-box genes, 15 morning glory F-box genes, and 13 identified L. barbarum F-box proteins were retrieved from NCBI (https://www.ncbi.nlm.nih.gov/ (accessed on 15 April 2023)) and underwent multiple sequence alignments (the F-box protein sequences of each species are listed in Supplementary Table S3). Clustering was executed employing a distance matrix strategy, utilizing the Poisson correction model for calculations (d = −ln(1 − p), where d signifies the genetic distance between protein sequences and p represents the percentage of sequence variation) [65]. Subsequently, the neighbor-joining method (NJ) [66] from MEGA11 was applied to amalgamate sequences into a singular node based on the shortest distances within the distance matrix, leading to the assembly of a comprehensive phylogenetic tree. This approach was supplemented by the bootstrap method, which generated replicated samples across various datasets, with a bootstrap replication count of 1000, to evaluate the integrity of the constructed phylogenetic tree [67]. The ITOL (https://itol.embl.de/ (accessed on 15 April 2023)) online tool was then used to visualize the evolutionary tree.
4.5. Analysis of Conserved Protein Motifs, Protein Domains, and Gene Structure
The exon distributions of the S2-LbSLFs genes were extracted from the GFF file corresponding to the L. barbarum genome. Default settings were employed in the Tbtools-Simple MEME Wrapper, Tbtools-Visualize Gene Structure, and NCBI-CCD databases to analyze the conserved motifs, gene structures (exons and introns), and protein domains for the S2-LbSLFs. Subsequent visualization was carried out using the Tbtools-Simple Biosequence Viewer.
4.6. Analysis of Cis Elements in SLF Gene Family Members
To explore the potential regulation of S2-LbSLFs, a 2000-base-pair sequence upstream of the ATG start codon for S2-LbSLF genes was isolated from the L. barbarum genome and considered as the promoter region. This sequence was then uploaded to the PlantCARE webtool (http://bioinformatics.psb.ugent.be/webtools/PlantCARE/html/ (accessed on 6 May 2023)) for the prediction of cis-regulatory elements within the promoter. Statistical analyses of the predictions were conducted using Microsoft Excel 2019, and heatmaps were created with TBtools (v2.042) software. These heatmaps were later refined using Adobe Illustrator CC 2019.
4.7. Analysis of S2-LbSLFs Gene Density and Collinearity
Utilizing the L. barbarum genome files and GFF annotations, the One Step MC ScanX feature in Tbtools was utilized to perform gene duplication and collinearity analysis on L. barbarum. Advanced Circos was employed to create circular diagrams that depicted the collinear relationships. The collinearity analysis of the S2-LbSLFs was carried out using Tbtools’ One Step MC ScanX-Supper Fast tool.
4.8. RNA Extraction and Expression Patterns of S2-LbSLFs
To investigate the expression patterns of the F-box gene family, we isolated F-box genes situated on chromosome 2 and created heatmaps of their expression profiles using TBtools (v2.042) software, which is based on transcriptomic data. To validate the accuracy of the expression levels inferred from the transcriptomic data, we then conducted qRT-PCR analysis. Specimens of L. barbarum ‘Ningqi 1’ were harvested from the Goji Germplasm Resource Nursery of Yinchuan Botanical Garden (38°24′ N, 106°10′ E), encompassing leaves, petals, entire flowers, stigma, anthers, and flower stems. These samples were rapidly frozen in liquid nitrogen and subsequently stored in a freezer at −80 °C for RNA extraction and subsequent fluorescence quantitation analysis. The RNA extraction from the plant samples was performed using the method described by Chen et al. [68], and the concentration and purity of the RNA were assessed using a Nano-500B microspectrophotometer (Aosheng, Shanghai, China). Subsequently, the RNA was reverse-transcribed into cDNA using the PrimeScript RT reagent kit with gDNA Eraser (Takara, Dalian, China). Thirteen qRT-PCR primers designed for S2-LbSLFs were created using Primer 5.0, with Actin serving as the internal control gene (the primers for qRT-PCR are detailed in Supplementary Table S4). Real-time fluorescence quantitation was carried out using TB Green®® Premix Ex Taq™ II (Takara, Dalian, China) with a reaction mixture containing 100 ng of cDNA template, 1× TB Green Premix Ex Taq II, and 0.4 μM of forward and reverse primers, supplemented with ddH2O for a final volume of 20 μL. The reaction protocol involved a 30-s pre-denaturation at 95 °C a 5-s denaturation at 95 °C, a 30-s annealing at 60 °C, and 40 cycles of denaturation and annealing. Each experiment was replicated with three biological samples, and the relative expression levels of S2-LbSLFs genes were analyzed using the 2−ΔΔCt method.
4.9. Yeast Two-Hybrid Analysis of Interaction between SLF and S-RNase
To further investigate the interaction between S2-LbSLFs and S-RNases, a yeast two-hybrid assay was employed. We used S2-LbSLFs as prey and S5-RNase and S2-RNase as bait. The S2-LbSLFs were divided into full-length SLFs, N-terminal SLFs, and C-terminal SLFs, which were then cloned and ligated into pGADT7(AD). The S2-RNase and S5-RNase in their full-length forms were ligated into pGBKT7(BD). Vectors containing different SLF fragments were co-transformed into the yeast Y2Hgold strain with S-RNase for interaction experiments. The transformed yeast cells were grown on -Leu/-Trp agar plates at 30 °C for 4 to 5 days. The colonies were further grown on -Leu/-Trp/-Ade/-His agar plates at 30 °C for an additional 3 to 4 days to test for interactions.
4.10. Statistical Analysis
All experiments in this study were conducted with three biological replicates. Data management was facilitated through Excel 2019. To normalize and visualize the transcriptomic data, the logarithm of FPKM values was computed, followed by the generation of clustered heatmaps with the TBtools (v2.042) software. A one-way ANOVA using IBM SPSS Statistics 27.0 was conducted to analyze significant differences, with p-values of less than 0.05 deemed statistically significant. The results presented are represented as the mean ± standard error based on the average of the three biological replicates. Graphical representations were produced using GraphPad Prism 9.5.
5. Conclusions
In this investigation, we identified and cloned 13 S2-LbSLF genes from the L. barbarum genome. The physicochemical properties of these 13 S2-LbSLFs were analyzed, revealing that all the proteins are predicted to be nucleus-localized. The collinearity analysis failed to detect any tandem duplications within the S2-LbSLF gene family. Two pairs of collinear genes were found in L. barbarum and S. lycopersicum, respectively, with a single pair in R. chinensis. The S2-LbSLFs were divided into six groups in the phylogenetic analysis, with four genes clustering with F-box genes encoding pollen determinants in P. inflata, and the remaining nine genes associating with genes specifically expressed in the pollen of N. tabacum, suggesting they may serve as promising candidate genes for pollen determinants. These SLF genes displayed pollen-specific expression. The yeast two-hybrid assays demonstrated an interaction between S-RNase and the C-terminal region of the SLF. These results pave the way for further studies to elucidate the functional roles of SLF genes as pollen determinants regulating self-incompatibility in L. barbarum.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13070959/s1: Supplementary Table S1. Binding site prediction results of S2-LbSLFs promoters; Supplementary Table S2. Cloning primers; Supplementary Table S3. Protein sequence of SLFs from L. barbarum, N. tabacum, and P. inflata; Supplementary Table S4. The qRT-PCR primers; Supplementary Figure S1. The interaction between S2-RNase and S2-LbSLFf was investigated in yeast strains; Supplementary Figure S2. The interaction between S2-RNase and S2-LbSLFn was investigated in yeast strains; Supplementary Figure S3. The interaction between S2-RNase and S2-LbSLFc was investigated in yeast strains; Supplementary Figure S4. The interaction between S5-RNase and S2-LbSLFf was investigated in yeast strains; Supplementary Figure S5. The interaction between S5-RNase and S2-LbSLFn was investigated in yeast strains; Supplementary Figure S6. The interaction between S5-RNase and S2-LbSLFc was investigated in yeast strains.
Author Contributions
Conceptualization, J.W. and C.W.; methodology, J.W., W.X. and C.W.; software, J.W. and C.W.; investigation, J.W., X.N., Z.Y. and X.Z.; resources, X.N.; data curation, H.M. and C.W.; writing—original draft preparation, J.W.; writing—review and editing, J.W. and C.W.; supervision, C.W.; project administration, C.W.; funding acquisition, C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundamental Research Funds for the Central Universities of North Minzu University, grant number 2023QNPY11; West Light Talent Program of the Chinese Academy of Sciences, grant number XAB2022YW08; National Natural Science Foundation of China, grant number 31701878; Innovation Team for Genetic Improvement of Economic Forests, grant number 2022QCXTD04.
Data Availability Statement
The datasets supporting the results presented in this manuscript are included within the article (and its Supplementary Materials).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Skenderidis, P.; Kerasioti, E.; Karkanta, E.; Stagos, D.; Kouretas, D.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Assessment of the antioxidant and antimutagenic activity of extracts from goji berry of Greek cultivation. Toxicol. Rep. 2018, 5, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yan, Y.M.; Zhang, L.T.; Mi, J.; Yu, L.M.; Zhang, F.F.; Lu, L.; Luo, Q.; Li, X.Y.; Zhou, X.; et al. A comprehensive review of goji berry processing and utilization. Food Sci. Nutr. 2023, 11, 7445–7457. [Google Scholar] [CrossRef]
- Jiao, E.; Li, X.Y.; He, J.; Dai, G. Pollination Compatibility of Six Lycium barbarum L. Varieties. Agric. Sci. Technol. 2013, 14, 1542–1544. [Google Scholar]
- Hu, J.B.; Liu, C.C.; Du, Z.Z.; Guo, F.R.; Song, D.; Wang, N.; Wei, Z.M.; Jiang, J.D.; Cao, Z.H.; Shi, C.M.; et al. Transposable elements cause the loss of self-incompatibility in citrus. Plant Biotech. J. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, Y.; Zhang, H.; Song, Y.Z.; Zhao, F.; Zhang, Y.; Zhu, S.H.; Zhang, H.K.; Zhou, Z.D.; Guo, H.; et al. Origin, loss, and regain of self-incompatibility in angiosperms. Plant Cell 2022, 34, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Li, Y.Y.; Zhao, X.W.; Zhang, C.L.; Liu, D.K.; Lan, S.R.; Yin, W.L.; Liu, Z.J. Molecular insights into self-incompatibility systems: From evolution to breeding. Plant Commun. 2023, 100719, 2590–3462. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Carpenter, R.; Dickinson, H.G.; Coen, E.S. Origin of allelic diversity in antirrhinum S locus RNases. Plant Cell 1996, 8, 805–814. [Google Scholar] [PubMed]
- Tian, H.Y.; Zhang, H.K.; Huang, H.Q.; Zhang, Y.; Xue, Y.B. Phase separation of S-RNase promotes self-incompatibility in Petunia hybrida. J. Integr. Plant Biol. 2023. [Google Scholar] [CrossRef]
- Ren, Y.; Hua, Q.Z.; Pan, J.Y.; Zhang, Z.K.; Zhao, J.T.; He, X.H.; Qin, Y.H.; Hu, G.B. SKP1-like protein, CrSKP1-e, interacts with pollen-specific F-box proteins and assembles into SCF-type E3 complex in ‘Wuzishatangju’ (Citrus reticulata Blanco) pollen. PeerJ 2020, 8, e10578. [Google Scholar] [CrossRef]
- Liang, M.; Cao, Z.H.; Zhu, A.D.; Liu, Y.L.; Tao, M.Q.; Yang, H.Y.; Xu, Q.; Wang, S.H.; Liu, J.J.; Li, Y.P.; et al. Evolution of self-compatibility by a mutant Sm-RNase in citrus. Nat. Plants 2020, 6, 131–142. [Google Scholar] [CrossRef]
- Sun, L.H.; Cao, S.Y.; Zheng, N.; Kao, T.H. Analyses of Cullin1 homologs reveal functional redundancy in S-RNase-based self-incompatibility and evolutionary relationships in eudicots. Plant Cell 2023, 35, 673–699. [Google Scholar] [CrossRef]
- Kubo, K.I.; Tsukahara, M.; Fujii, S.; Murase, K.; Wada, Y.; Entani, T.; Iwano, M.; Takayama, S. Cullin1-P is an essential component of Non-self recognition system in self-incompatibility in Petunia. Plant Cell Physiol. 2016, 57, 2403–2416. [Google Scholar] [CrossRef] [PubMed]
- Sijacic, P.; Wang, X.; Skirpan, A.L.; Wang, Y.; Dowd, P.E.; McCubbin, A.G.; Huang, S.; Kao, T.H. Identification of the pollen determinant of S-RNase-mediated selfincompatibility. Nature 2004, 429, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Wang, F.; Zhao, L.; Zhou, J.L.; Lai, Z.; Zhang, Y.S.P.; Robbins, T.P.; Xue, Y.B. The F-box protein AhSLF-S2 controls the pollen function of S-RNase-based self-incompatibility. Plant Cell 2004, 16, 2307–2322. [Google Scholar] [CrossRef]
- Honsho, C. Self-incompatibility related to seedless fruit production in Citrus plants. Hort. J. 2023, 92, 1–12. [Google Scholar] [CrossRef]
- Kubo, K.I.; Entani, T.; Takara, A.; Wang, N.; Fields, A.M.; Hua, Z.H.; Toyoda, M.; Kawashima, S.I.; Ando, T.; Isogai, A.; et al. Collaborative non-self recognition system in S-RNase-based self-incompatibility. Science 2010, 330, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, D.; Tao, R. Recognition of S-RNases by an S locus F-box like protein and an S haplotype-specific F-box like protein in the Prunus-specific self-incompatibility system. Plant Mol. Biol. 2019, 100, 367–378. [Google Scholar] [CrossRef]
- Zhao, H.; Song, Y.Z.; Li, J.H.; Zhang, Y.; Huang, H.Q.; Li, Q.; Zhang, Y.; Xue, Y.B. Primary restriction of S-RNase cytotoxicity by a stepwise ubiquitination and degradation pathway in Petunia hybrida. New Phytol. 2021, 231, 1249–1264. [Google Scholar] [CrossRef]
- Liu, W.; Fan, J.B.; Li, J.H.; Song, Y.Z.; Li, Q.; Zhang, Y.; Xue, Y.B. SCF (SLF)-mediated cytosolic degradation of S-RNase is required for cross-pollen compatibility in S-RNase-based self-incompatibility in Petunia hybrida. Front. Genet. 2014, 5, 228. [Google Scholar] [CrossRef]
- Vieira, J.; Rocha, S.; Vázquez, N.; López-Fernández, H.; Fdez-Riverola, F.; Reboiro-Jato, M.; Vieira, C.P. Predicting specificities under the Non-self gametophytic Self-incompatibility recognition model. Front. Plant Sci. 2019, 10, 879. [Google Scholar] [CrossRef]
- Li, Y.; Duan, X.W.; Wu, C.B.; Yu, J.; Liu, C.S.; Wang, J.; Zhang, X.M.; Yan, G.H.; Jiang, F.; Li, T.Z.; et al. Ubiquitination of S4-RNase by S-locus F-box like 2 contributes to self-compatibility of Sweet Cherry ‘Lapins’. Plant Physiol. 2020, 184, 4. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.C.; Huang, J.; Zhang, Y.Q.; Li, P.X.; Zhang, G.Q.; Xu, Q.; Chen, L.J.; Wang, J.Y.; Luo, Y.B.; Liu, Z.J. Lack of S-RNase-based gametophytic Self-incompatibility in Orchids suggests that this system evolved after the monocot-eudicot split. Front. Plant Sci. 2017, 8, 1106. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Ma, W.S.; Han, B.; Liang, L.Z.; Zhang, Y.S.; Hong, G.F.; Xue, Y.B. An F-box gene linked to the self-incompatibility (S) locus of Antirrhinum is expressed specifically in pollen and tapetum. Plant Mol. Biol. 2002, 50, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Entani, T.; Iwano, M.; Shiba, H.; Che, F.S.; Isogai, A.; Takayama, S. Comparative analysis of the self-incompatibility (S-) locus region of Prunus mume: Identification of a pollen-expressed F-box gene with allelic diversity. Genes Cells 2003, 8, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, K.; Sassa, H.; Dandekar, A.M.; Gradziel, T.M.; Tao, R.; Hirano, H. Structural and transcriptional analysis of the self-incompatibility locus of almond: Identification of a pollen-expressed F-box gene with haplotypespecific polymorphism. Plant Cell 2003, 15, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Yamane, H.; Ikeda, K.; Ushijima, K.; Sassa, H.; Tao, R. A pollen-expressed gene for a novel protein with an F-box motif that is very tightly linked to a gene for S-RNase in two species of cherry, Prunus cerasus and P. avium. Plant Cell Physiol. 2003, 44, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; McCubbin, A.G.; Kao, T.H. Genetic mapping and molecular characterization of the self-incompatibility (S) locus in Petunia inflata. Plant Mol. Biol. 2003, 53, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tsukamoto, T.; Yi, K.W.; Wang, X.; Huang, S.; McCubbin, A.G.; Kao, T.H. Chromosome walking in the Petunia inflata self-incompatibility (S-) locus and gene identification in an 881-kb contig containing S2-RNase. Plant Mol. Biol. 2004, 54, 727–742. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Ando, T.; Watanabe, H.; Marchesi, E.; Kao, T.H. Duplication of the S-locus F-box gene is associated with breakdown of pollen function in an S-haplotype identified in a natural population of self-incompatible Petunia axillaris. Plant Mol. Biol. 2005, 57, 141–153. [Google Scholar] [CrossRef]
- Wheeler, D.; Newbigin, E. Expression of 10 S-class SLF-like genes in Nicotiana alata pollen and its implications for understanding the pollen factor of the S locus. Genetics 2007, 177, 2171–2180. [Google Scholar] [CrossRef]
- Matsumoto, D.; Yamane, H.; Abe, K.; Tao, R. Identification of a Skp1-like protein interacting with SFB, the pollen S determinant of the gametophytic self-incompatibility in prunus. Plant Physiol. 2012, 159, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda-Garcia, E.; Fulton, E.C.; Parlan, E.V.; O’Connor, L.E.; Fleming, A.A.; Replogle, A.J.; Rocha-Sosa, M.; Gendron, J.M.; Thines, B. Unique n-terminal interactions connect F-box stress induced (FBS) proteins to a WD40 repeat-like protein pathway in Arabidopsis. Plants 2021, 10, 2228. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Zhang, Y.; Song, Y.Z.; Zhang, H.; Fan, J.B.; Li, Q.; Zhang, D.F.; Xue, Y.B. Electrostatic potentials of the S-locus F-box proteins contribute to the pollen S specificity in self-incompatibility in Petunia hybrida. Plant J. 2017, 89, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.H.; Williams, J.S.; Wang, N.; Khatri, W.A.; Roman, D.S.; Kao, T.H. Use of domain-swapping to identify candidate amino acids involved in differential interactions between two allelic variants of type-1 S-locus F-box protein and S3-RNase in Petunia inflata. Plant Cell Physiol. 2018, 59, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.I.; Paape, T.; Hatakeyama, M.; Entani, T.; Takara, A.; Kajihara, K.; Tsukahara, M.; Shimizu-Inatsugi, R.; Shimizu, K.K.; Takayama, S. Gene duplication and genetic exchange drive the evolution of S-RNase-based self-incompatibility in Petunia. Nat. Plants 2015, 1, 14005. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.S.; Der, J.P.; DePamphilis, C.W.; Kao, T.H. Transcriptome analysis reveals the same 17 S-Locus F-box genes in two haplotypes of the self-incompatibility locus of Petunia inflata. Plant Cell 2014, 26, 2873–2888. [Google Scholar] [CrossRef]
- Wang, C.P.; Wu, J.L.; Gao, Y.; Dai, G.L.; Shang, X.H.; Ma, H.J.; Zhang, X.; Xu, W.D.; Qin, K. Localization of S-locus-related self-incompatibility in Lycium barbarum Based on BSA Analysis. Horticulturae 2024, 10, 190. [Google Scholar] [CrossRef]
- McClure, B.A.; Haring, V.; Ebert, P.R.; Anderson, M.A.; Simpson, R.J.; Sakiyama, F.; Clarke, A.E. Style self-incompatibility gene products of Nicotlana alata are ribonucleases. Nature 1989, 342, 955–957. [Google Scholar] [CrossRef]
- Zhang, Z.; Qiu, W.M.; Liu, W.; Han, X.J.; Wu, L.H.; Yu, M.; Qiu, X.L.; He, Z.Q.; Li, H.Y.; Zhuo, R.Y. Genome-wide characterization of the hyperaccumulator Sedum alfredii F-box family under cadmium stress. Sci Rep. 2021, 11, 3023. [Google Scholar] [CrossRef]
- Lee, H.S.; Huang, S.; Kao, T.H. S proteins control rejection of incompatible pollen in Petunia inflata. Nature 1994, 367, 560–563. [Google Scholar] [CrossRef]
- Williams, J.S.; Natale, C.A.; Wang, N.; Li, S.; Brubaker, T.R.; Sun, P.; Kao, T.H. Four previously identified Petunia inflata S-Locus F-box genes are involved in pollen specificity in self-incompatibility. Mol. Plant 2014, 7, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.; Pimenta, J.; Gomes, A.; Laia, J.; Rocha, S.; Heitzler, P.; Vieira, C.P. The identification of the Rosa S-locus and implications on the evolution of the Rosaceae gametophytic self-incompatibility systems. Sci Rep. 2021, 11, 3710. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, C.; Ritter, E.; Barone, A.; Debener, T.; Walkemeier, B.; Schachtschabel, U.; Kaufmann, H.; Thompson, R.D.; Bonierbale, M.W.; Ganal, M.W.; et al. RFLP maps of potato and their alignment with the homoeologous tomato genome. Theor. Appl. Genet. 1991, 83, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.H.; Zhang, Y.; Copsy, L.; Han, Q.Q.; Zheng, D.F.; Coen, E.; Xue, Y.B. The snapdragon genomes reveal the evolutionary dynamics of the S-locus supergene. Mol. Biol. Evol. 2023, 40, msad080. [Google Scholar] [CrossRef] [PubMed]
- Bombarely, A.; Moser, M.; Amrad, A.; Bao, M.; Bapaume, L.; Barry, C.S.; Bliek, M.; Boersma, M.R.; Borghi, L.; Bruggmann, R.; et al. Insight into the evolution of the Solanaceae from the parental genomes of Petunia hybrida. Nat. Plants 2016, 2, 16074. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Moriya, S.; Haji, T.; Abe, K. Isolation and characterization of multiple F-box genes linked to the S9—And S10 -RNase in apple (Malus × domestica Borkh.). Plant Reprod. 2013, 26, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.M.; Yin, H.; Qiao, X.; Tan, X.; Gu, C.; Wang, B.H.; Cheng, R.; Wang, Y.Z.; Zhang, S.L. F-box genes: Genome-wide expansion, evolution and their contribution to pollen growth in pear (Pyrus bretschneideri). Plant Sci. 2016, 253, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Williams, J.S.; Sun, L.H.; Kao, T.H. Sequence analysis of the Petunia inflata S-locus region containing 17 S-locus F-box genes and the S-RNase gene involved in self-incompatibility. Plant J. 2020, 104, 1348–1368. [Google Scholar] [CrossRef]
- Feng, C.H.; Niu, M.X.; Liu, X.; Bao, Y.; Liu, S.J.; Liu, M.Y.; He, F.; Han, S.; Liu, C.; Wang, H.L.; et al. Genome-wide analysis of the FBA subfamily of the poplar F-box gene family and its role under drought stress. Int. J. Mol. Sci. 2023, 24, 4823. [Google Scholar] [CrossRef]
- Akash; Parida, A.P.; Srivastava, A.; Mathur, S.; Sharma, A.K.; Kumar, R. Identification, evolutionary profiling, and expression analysis of F-box superfamily genes under phosphate deficiency in tomato. Plant Physiol. Biochem. 2021, 162, 349–362. [Google Scholar] [CrossRef]
- Wu, G.F.; Cao, A.H.; Wen, Y.H.; Bao, W.C.; She, F.W.; Wu, W.Z.; Zheng, S.; Yang, N. Characteristics and functions of MYB (v-Myb avivan myoblastsis virus oncogene homolog)-related genes in Arabidopsis thaliana. Genes 2023, 14, 2026. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.F.; Xia, X.H.; Yao, W.J.; Cheng, Z.H.; Zhang, X.M.; Jiang, J.H.; Zhou, B.R.; Jiang, T.B. Genome-wide identification and expression patterns of the F-box family in poplar under salt stress. Int. J. Mol. Sci. 2022, 23, 10934. [Google Scholar] [CrossRef] [PubMed]
- Chunthong, K.; Pitnjam, K.; Chakhonkaen, S.; Sangarwut, N.; Panyawut, N.; Wasinanon, T.; Ukoskit, K.; Muangprom, A. Differential. Drought responses in F-box gene expression and grain yield between two Rice groups with contrasting drought tolerance. J. Plant Growth. Regul. 2017, 36, 970–982. [Google Scholar] [CrossRef]
- Sun, L.H.; Williams, J.S.; Li, S.; Wu, L.; Khatri, W.A.; Stone, P.G.; Keebaugh, M.D.; Kao, T.H. S-locus F-box proteins are solely responsible for pollen function in S-RNase-based self-incompatibility of Petunia Pollen. Plant Cell 2018, 30, 2959–2972. [Google Scholar] [CrossRef]
- Xu, K.H.; Wu, N.; Yao, W.B.; Li, X.W.; Zhou, Y.G.; Li, H.Y. The biological function and roles in phytohormone signaling of the F-box protein in plants. Agronomy 2021, 11, 2360. [Google Scholar] [CrossRef]
- Saxena, H.; Negi, H.; Sharma, B. Role of F-box E3-ubiquitin ligases in plant development and stress responses. Plant Cell Rep. 2023, 42, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Guo, J.L.; Zhou, F.L.; Singh, S.; Xu, X.; Xie, Q.; Yang, Z.B.; Huang, C.F. F-box protein RAE1 regulates the stability of the aluminum-resistance transcription factor STOP1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2019, 116, 319–327. [Google Scholar] [CrossRef]
- Li, X.C.; Sun, Y.; Liu, N.; Wang, P.; Pei, Y.K.; Liu, D.; Ma, X.W.; Ge, X.Y.; Li, F.G.; Hou, Y.X. Enhanced resistance to Verticillium dahliae mediated by an F-box protein GhACIF1 from Gossypium hirsutum. Plant Sci. 2019, 284, 127–134. [Google Scholar] [CrossRef]
- Sun, P.L.; Li, S.; Lu, D.H.; Williams, J.S.; Kao, T.H. Pollen S-locus F-box proteins of Petunia involved in S-RNase-based self-incompatibility are themselves subject to ubiquitin-mediated degradation. Plant J. 2015, 83, 213–223. [Google Scholar] [CrossRef]
- Xu, C.; Li, M.F.; Wu, J.K.; Guo, H.; Li, Q.; Zhang, Y.; Chai, J.J.; Li, T.Z.; Xue, Y.B. Identification of a canonical SCF(SLF) complex involved in S-RNase-based self-incompatibility of Pyrus (Rosaceae). Plant Mol. Biol. 2013, 81, 245–257. [Google Scholar] [CrossRef]
- Sun, P.L.; Kao, T.H. Self-Incompatibility in Petunia inflata: The relationship between a Self-incompatibility locus F-box protein and its non-self S-RNases. Plant Cell 2013, 25, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.H.; Meng, X.Y.; Kao, T.H. Comparison of Petunia inflata S-locus F-box protein (Pi SLF) with Pi SLF like proteins reveals its unique function in S-RNase based self-incompatibility. Plant Cell 2007, 19, 3593–3609. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Meng, D.; Gu, Z.Y.; Li, W.; Wang, A.D.; Yang, Q.; Zhu, Y.D.; Li, T.Z. A novel gene, MdSSK1, as a component of the SCF complex rather than MdSBP1 can mediate the ubiquitination of S-RNase in apple. J. Exp. Bot. 2014, 65, 3121–3131. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Wang, H.Y.; Zhao, L.; Zhou, J.L.; Huang, J.; Zhang, Y.S.; Xue, Y.B. The F-box protein AhSLF-S2 physically interacts with S-RNases that may be inhibited by the ubiquitin/26S proteasome pathway of protein degradation during compatible pollination in Antirrhinum. Plant Cell 2004, 16, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.H.; Jiang, N.; Xing, W.; Li, T.L.; Yuan, D.; Li, W.T.; Li, J.T.; Luo, L. Cloning and tissue-specific expression analysis of hepcidin gene in koi (Cyprinus carpio). Microbiol. china 2017, 44, 325–335. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Hall, B.G. Building phylogenetic trees from molecular data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef]
- Chen, J.G.; Zhang, Y.Q.; Wang, C.P.; Lü, W.T.; Jin, J.B.; Hua, X.J. Proline induces calcium-mediated oxidative burst and salicylic acid signaling. Amino Acids 2011, 40, 1473–1484. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).