Exploring Intraspecific Trait Variation in a Xerophytic Moss Species Indusiella thianschanica (Ptychomitriaceae) across Environmental Gradients on the Tibetan Plateau
Abstract
1. Introduction
2. Results
2.1. Quantitative Analysis of Intraspecific Morphological Variation
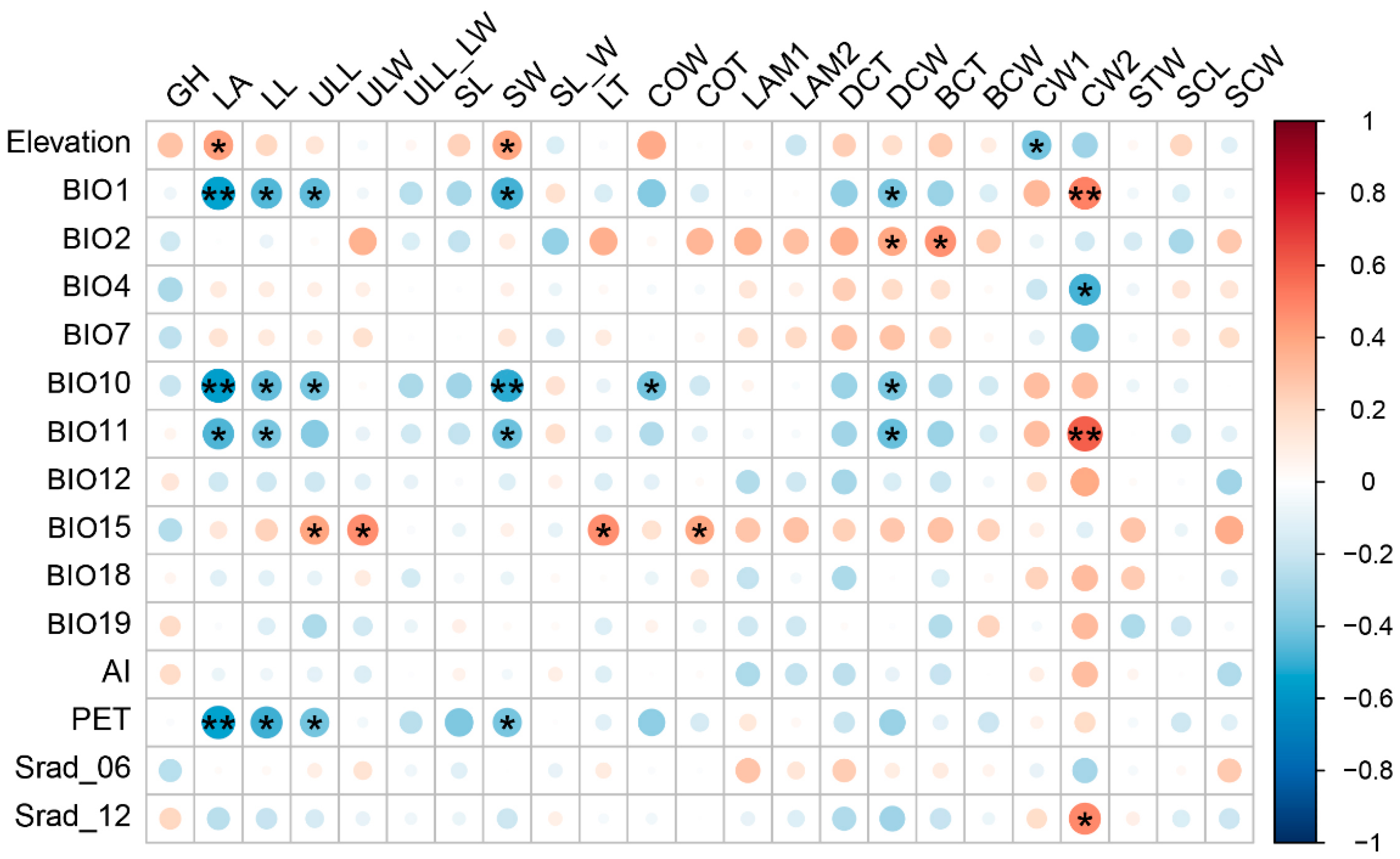
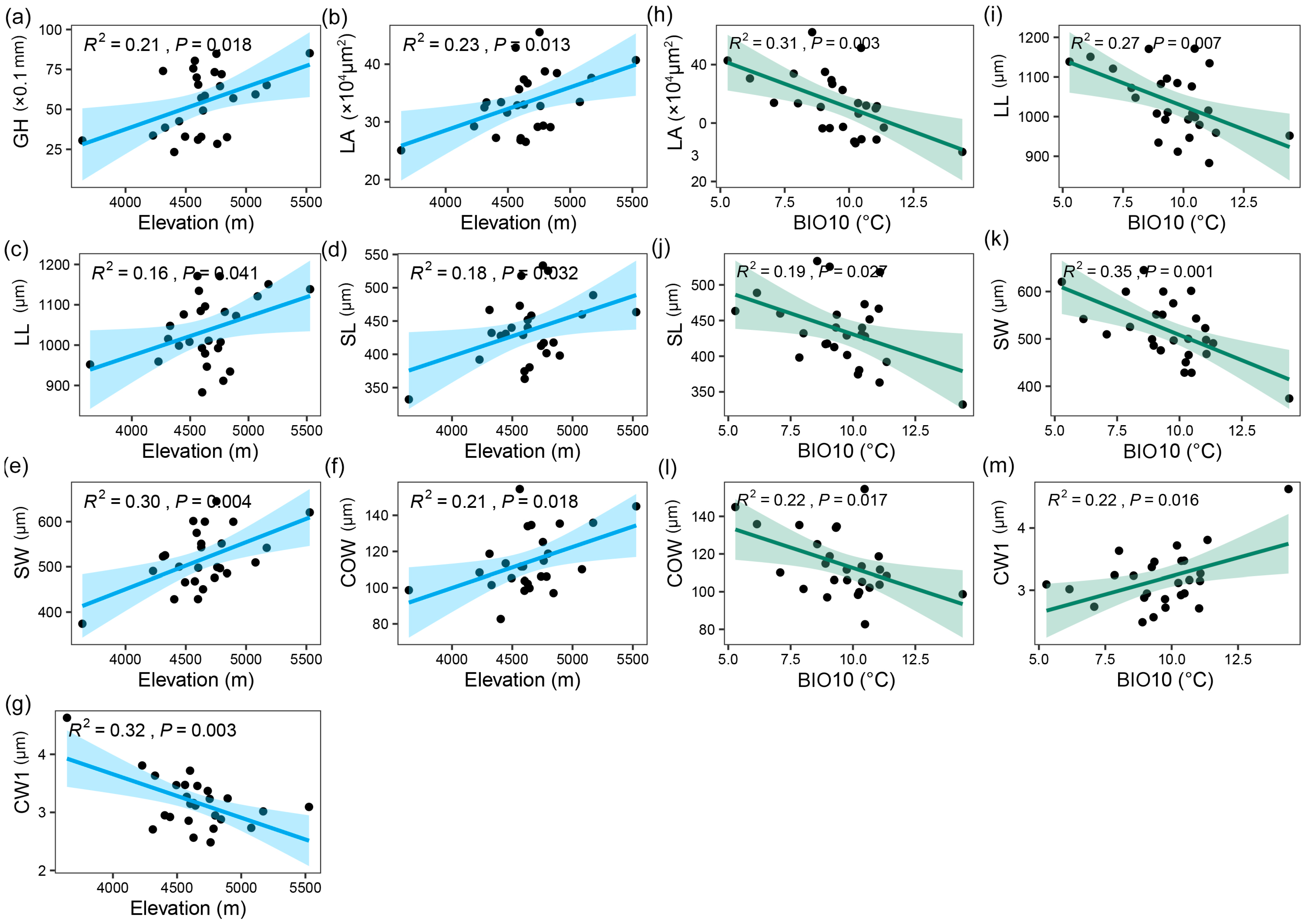
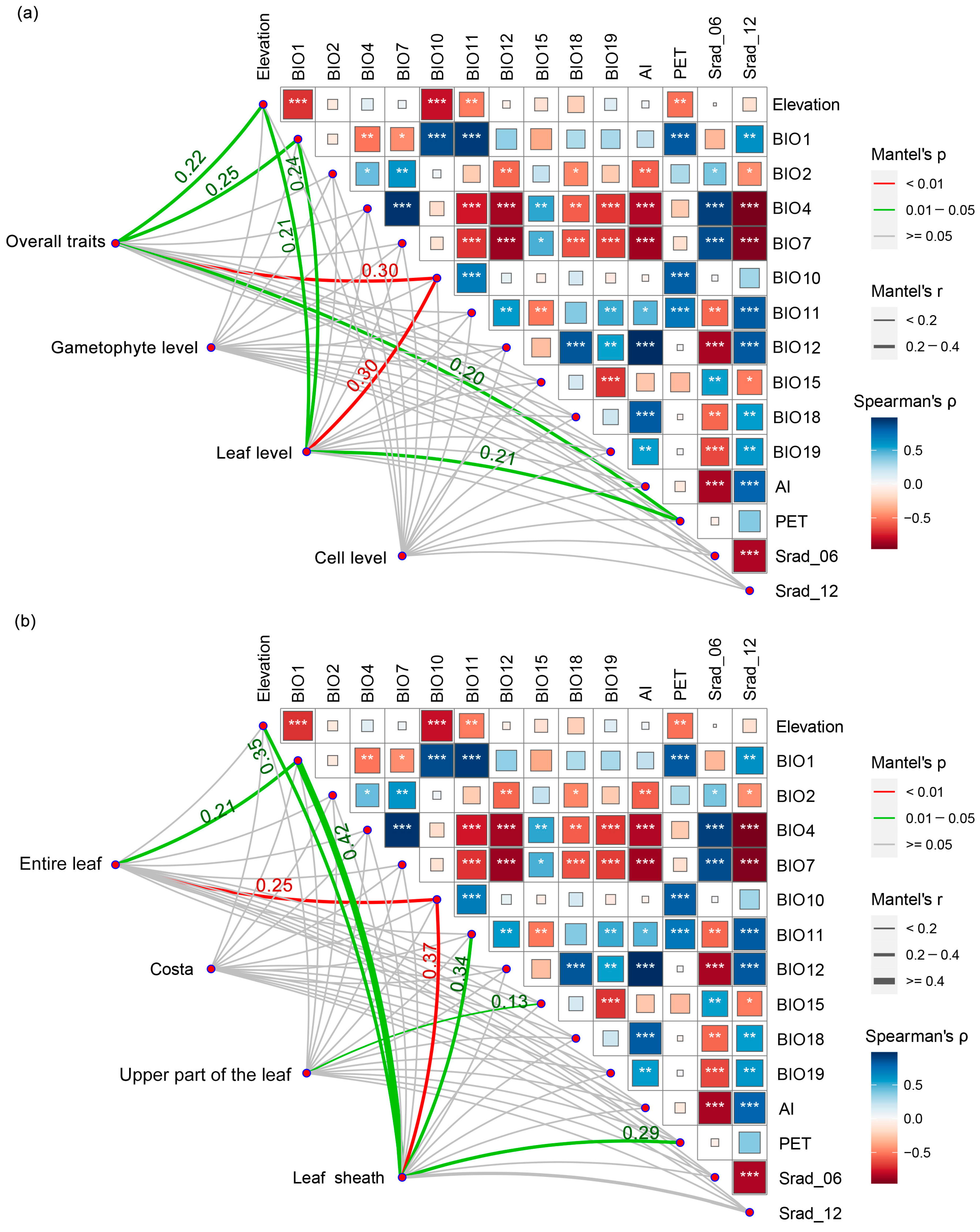
2.2. Relationship between Trait Variation and Environmental Factors
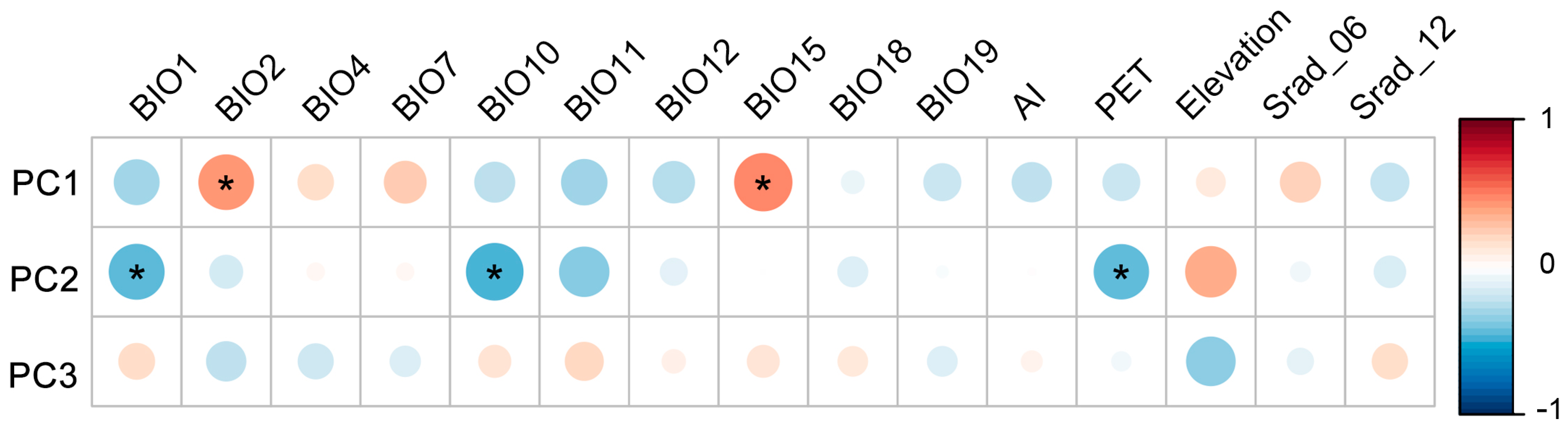
2.3. Interrelationships among Morphological Traits and Trait Covariation
3. Discussion
3.1. Intraspecific Morphological Variation across Environmental Gradients
3.2. The Significant Role of Leaf Sheath: From Environmental Response to Species Adaptation
3.3. Key Environmental Factors Driving Intraspecific Trait Variability
3.4. Trait Covariation and Adaptation Strategies
4. Materials and Methods
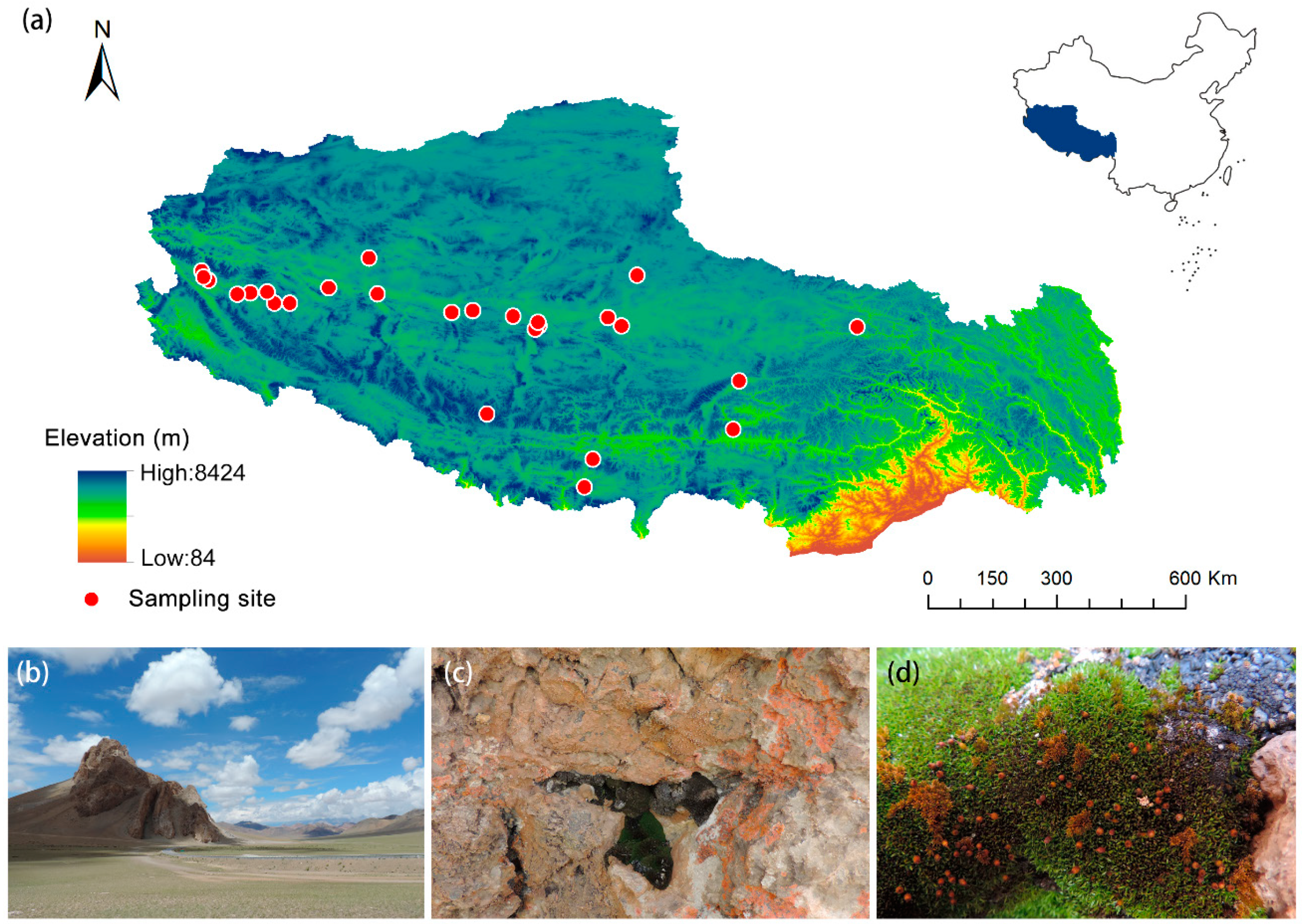
4.1. Research Site and Plant Material Sampling
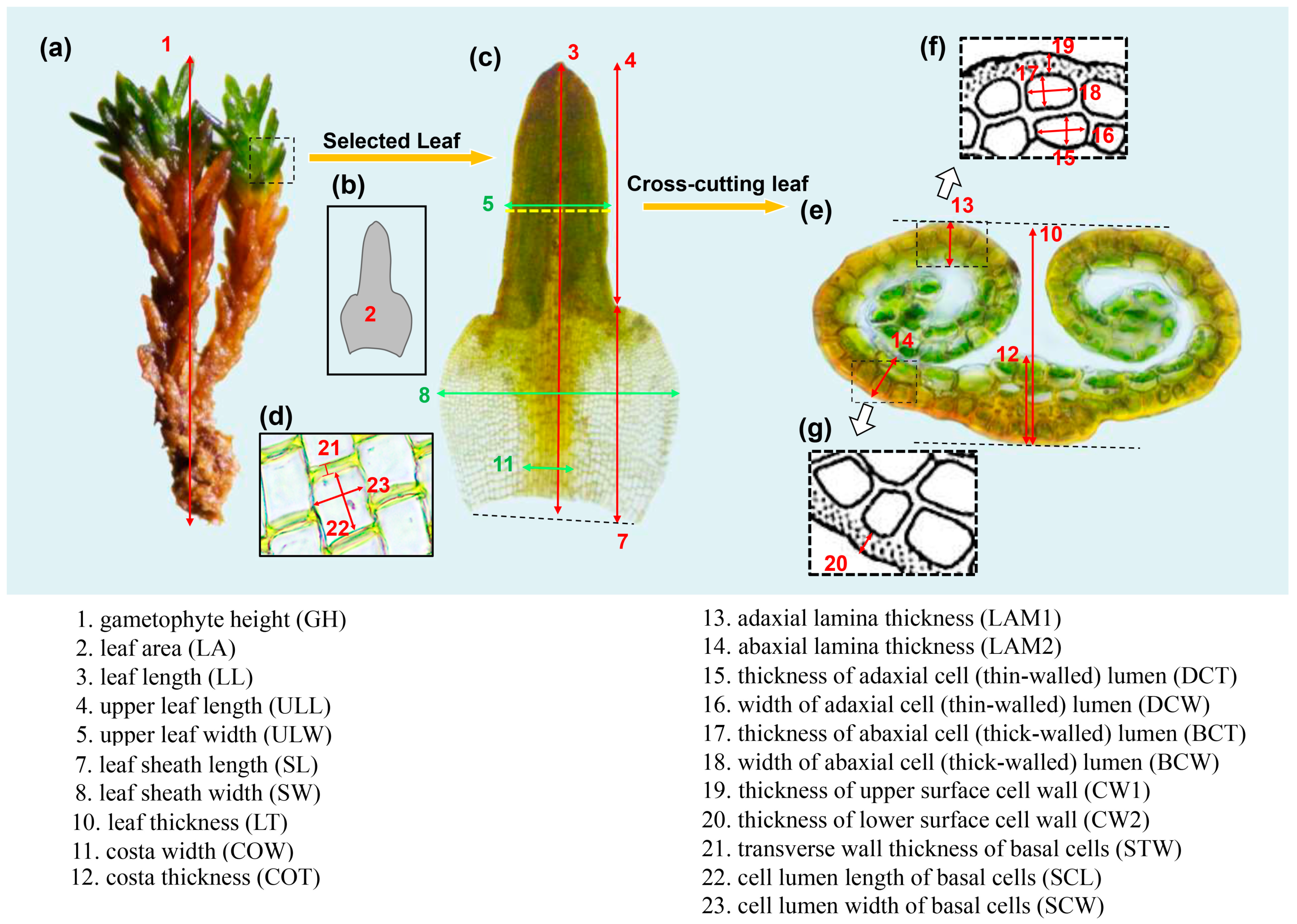
4.2. Trait Measurement
4.3. Environment Variable
4.4. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perez-Harguindeguy, N.; Diaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2016, 64, 715–716. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Jankowski, A.; Wyka, T.P.; Zytkowiak, R.; Nihlgard, B.; Reich, P.B.; Oleksyn, J. Cold adaptation drives variability in needle structure and anatomy in Pinus sylvestris L. along a 1,900km temperate-boreal transect. Funct. Ecol. 2017, 31, 2212–2223. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Bjorkman, A.D.; Myers-Smith, I.H.; Elmendorf, S.C.; Normand, S.; Rueger, N.; Beck, P.S.A.; Blach-Overgaard, A.; Blok, D.; Cornelissen, J.H.C.; Forbes, B.C.; et al. Plant functional trait change across a warming tundra biome. Nature 2018, 562, 57–62. [Google Scholar] [CrossRef]
- Wang, X.; Ji, M.; Zhang, Y.; Zhang, L.; Akram, M.A.; Dong, L.; Hu, W.; Xiong, J.; Sun, Y.; Li, H.; et al. Plant trait networks reveal adaptation strategies in the drylands of China. BMC Plant Biol. 2023, 23, 266. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, C.W.; Andrew, M.E.; Mazanec, R.A.; Ruthrof, K.X.; Challis, A.; Hardy, G.; Byrne, M.; Tissue, D.T.; Rymer, P.D. Plant functional traits differ in adaptability and are predicted to be differentially affected by climate change. Ecol. Evol. 2020, 10, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Moran, E.V.; Hartig, F.; Bell, D.M. Intraspecific trait variation across scales: Implications for understanding global change responses. Global Change Biol. 2016, 22, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Townsend, P.A.; Kruger, E.L. Leaf spectroscopy reveals divergent inter- and intra-species foliar trait covariation and trait-environment relationships across NEON domains. New Phytol. 2022, 235, 923–938. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.E.; Reineking, B.; Muenkemueller, T. Effects of plant functional traits on soil stability: Intraspecific variability matters. Plant Soil 2017, 411, 359–375. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Amarasekare, P.; Araujo, M.S.; Buerger, R.; Levine, J.M.; Novak, M.; Rudolf, V.H.W.; Schreiber, S.J.; Urban, M.C.; Vasseur, D.A. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 2011, 26, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Henn, J.J.; Buzzard, V.; Enquist, B.J.; Halbritter, A.H.; Klanderuds, K.; Maitner, B.S.; Michaletz, S.T.; Potschs, C.; Seltzer, L.; Telford, R.J.; et al. Intraspecific Trait Variation and Phenotypic Plasticity Mediate Alpine Plant Species Response to Climate Change. Front. Plant Sci. 2018, 9, 408249. [Google Scholar] [CrossRef] [PubMed]
- Westerband, A.C.; Funk, J.L.; Barton, K.E. Intraspecific trait variation in plants: A renewed focus on its role in ecological processes. Ann. Bot. 2021, 127, 397–410. [Google Scholar] [CrossRef]
- Anderegg, L.D.L.; Berner, L.T.; Badgley, G.; Sethi, M.L.; Law, B.E.; HilleRisLambers, J. Within-species patterns challenge our understanding of the leaf economics spectrum. Ecol. Lett. 2018, 21, 734–744. [Google Scholar] [CrossRef]
- Lang, B.; Geiger, A.; Oyunbileg, M.; Ahlborn, J.; von Wehrden, H.; Wesche, K.; Oyuntsetseg, B.; Roemermann, C. Intraspecific trait variation patterns along a precipitation gradient in Mongolian rangelands. Flora 2019, 254, 135–146. [Google Scholar] [CrossRef]
- Weemstra, M.; Freschet, G.T.; Stokes, A.; Roumet, C. Patterns in intraspecific variation in root traits are species-specific along an elevation gradient. Funct. Ecol. 2021, 35, 342–356. [Google Scholar] [CrossRef]
- Weemstra, M.; Roumet, C.; Cruz-Maldonado, N.; Anthelme, F.; Stokes, A.; Freschet, G.T. Environmental variation drives the decoupling of leaf and root traits within species along an elevation gradient. Ann. Bot. 2022, 130, 419–430. [Google Scholar] [CrossRef]
- Ahrens, C.W.; Rymer, P.D.; Tissue, D.T. Intra-specific trait variation remains hidden in the environment. New Phytol. 2021, 229, 1183–1185. [Google Scholar] [CrossRef]
- He, J.S.; Wang, Z.H.; Wang, X.P.; Schmid, B.; Zuo, W.Y.; Zhou, M.; Zheng, C.Y.; Wang, M.F.; Fang, J.Y. A test of the generality of leaf trait relationships on the Tibetan Plateau. New Phytol. 2006, 170, 835–848. [Google Scholar] [CrossRef]
- Ke, X.; Kang, H.; Tang, Y. Reduction in leaf size at higher altitudes across 39 broad-leaved herbaceous species on the northeastern Qinghai-Tibetan Plateau. J. Plant Ecol. 2022, 15, 1227–1240. [Google Scholar] [CrossRef]
- McLean, E.H.; Prober, S.M.; Stock, W.D.; Steane, D.A.; Potts, B.M.; Vaillancourt, R.E.; Byrne, M. Plasticity of functional traits varies clinally along a rainfall gradient in Eucalyptus tricarpa. Plant Cell Environ. 2014, 37, 1440–1451. [Google Scholar] [CrossRef]
- Zhong, M.; Wang, J.; Liu, K.; Wu, R.; Liu, Y.; Wei, X.; Pan, D.; Shao, X. Leaf morphology shift of three dominant species along altitudinal gradient in an alpine meadow of the Qinghai-Tibetan plateau. Polish J. Ecol. 2014, 62, 639–648. [Google Scholar] [CrossRef]
- Oliver, M.J.; Velten, J.; Mishler, B.D. Desiccation tolerance in bryophytes: A reflection of the primitive strategy for plant survival in dehydrating habitats? Integr. Comp. Biol. 2005, 45, 788–799. [Google Scholar] [CrossRef]
- Watson, M.A. Patterns of habitat occupation in mosses—Relevance to considerations of the niche. Bull. Torrey Bot. Club 1980, 107, 346–372. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Y.; Song, X.; Tang, J.; Kou, J.; Fan, Y.; Shao, X. Temperature, not precipitation, drives the morphological traits of Didymodon rigidulus in Tibet. Ecol. Indic. 2021, 133, 108401. [Google Scholar] [CrossRef]
- Waite, M.; Sack, L. How does moss photosynthesis relate to leaf and canopy structure? Trait relationships for 10 Hawaiian species of contrasting light habitats. New Phytol. 2010, 185, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, L.; Song, X.; Wang, Q.; Kou, J.; Jiang, Y.; Shao, X. Morphological traits of Bryum argenteum and its response to environmental variation in arid and semi-arid areas of Tibet. Ecol. Eng. 2019, 136, 101–107. [Google Scholar] [CrossRef]
- Cano, M.J.; Jimenez, J.A. Indusiella (Ptychomitriaceae) in South America. Bryologist 2009, 112, 308–314. [Google Scholar] [CrossRef]
- Ivanova, E.I.; Ignatova, E.A.; Isakova, V.G.; Balakirev, I.A.; Kuznetsova, O.I.; Ignatov, M.S. Moss flora of the ust-nera region in the upper course of Indigirka River. East Yakutia. 2018, 27, 18–28. [Google Scholar] [CrossRef]
- Murray, B.M. A revision of the monotypic genera Indusiella, Aligrimmia and Coscinodontella (Musci, Grimmiaceae), with comments on convergent xeromorphological features. Bryologist 1984, 87, 24–36. [Google Scholar] [CrossRef]
- He, N.; Li, Y.; Liu, C.; Xu, L.; Li, M.; Zhang, J.; He, J.; Tang, Z.; Han, X.; Ye, Q.; et al. Plant Trait Networks: Improved Resolution of the Dimensionality of Adaptation. Trends Ecol. Evol. 2020, 35, 908–918. [Google Scholar] [CrossRef]
- Niinemets, U.; Tobias, M. Canopy leaf area index at its higher end: Dissection of structural controls from leaf to canopy scales in bryophytes. New Phytol. 2019, 223, 118–133. [Google Scholar] [CrossRef]
- Cheng, C.; He, N.; Li, M.; Xu, L.; Cai, W.; Li, X.; Zhao, W.; Li, C.; Sun, O.J. Plant species richness on the Tibetan Plateau: Patterns and determinants. Ecography 2023, 2023, e06265. [Google Scholar] [CrossRef]
- Sun, H.; Niu, Y.; Chen, Y.-S.; Song, B.; Liu, C.-Q.; Peng, D.-L.; Chen, J.-G.; Yang, Y. Survival and reproduction of plant species in the Qinghai-Tibet Plateau. J. Syst. Evol. 2014, 52, 378–396. [Google Scholar] [CrossRef]
- Xu, J.; Li, W.; Zhang, C.; Liu, W.; Du, G. The determinants of seed germination in an alpine/subalpine community on the Eastern Qinghai-Tibetan Plateau. Ecol. Eng. 2017, 98, 114–122. [Google Scholar] [CrossRef]
- Pan, Z.; Pitt, W.G.; Zhang, Y.M.; Wu, N.; Tao, Y.; Truscott, T.T. The upside-down water collection system of Syntrichia caninervis. Nat. Plants 2016, 2, 16076. [Google Scholar] [CrossRef]
- Guo, C.Y.; Ma, L.N.; Yuan, S.; Wang, R.Z. Morphological, physiological and anatomical traits of plant functional types in temperate grasslands along a large-scale aridity gradient in northeastern China. Sci. Rep. 2017, 7, 40900. [Google Scholar] [CrossRef]
- Fauset, S.; Freitas, H.C.; Galbraith, D.R.; Sullivan, M.J.P.; Aidar, M.P.M.; Joly, C.A.; Phillips, O.L.; Vieira, S.A.; Gloor, M.U. Differences in leaf thermoregulation and water use strategies between three co-occurring Atlantic forest tree species. Plant Cell Environ. 2018, 41, 1618–1631. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zheng, L.; Qi, D. Variation in leaf traits at different altitudes reflects the adaptive strategy of plants to environmental changes. Ecol. Evol. 2020, 10, 8166–8175. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Clemente-Moreno, M.J.; Bota, J.; Brodribb, T.J.; Gago, J.; Mizokami, Y.; Nadal, M.; Perera-Castro, A.V.; Roig-Oliver, M.; Sugiura, D.; et al. Cell wall thickness and composition are involved in photosynthetic limitation. J. Exp. Bot. 2021, 72, 3971–3986. [Google Scholar] [CrossRef] [PubMed]
- Roig-Oliver, M.; Douthe, C.; Bota, J.; Flexas, J. Cell wall thickness and composition are related to photosynthesis in Antarctic mosses. Physiol. Plant. 2021, 173, 1914–1925. [Google Scholar] [CrossRef]
- Xue, W.; Liu, D.-D.; Tosens, T.; Xiong, D.-L.; Carriqui, M.; Xiong, Y.-C.; Ko, J. Cell wall thickness has phylogenetically consistent effects on the photosynthetic nitrogen-use efficiency of terrestrial plants. Plant Cell Env. 2023, 46, 2323–2336. [Google Scholar] [CrossRef] [PubMed]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Zomer, R.J.; Xu, J.; Trabucco, A. Version 3 of the Global Aridity Index and Potential Evapotranspiration Database. Sci. Data 2022, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Xu, L.; Li, M.; Zhang, J.; He, N. Leaf Trait Networks Based on Global Data: Representing Variation and Adaptation in Plants. Front. Plant Sci. 2021, 12, 710530. [Google Scholar] [CrossRef]


| No. | Column | Mean | Median | Maximum | Minimum | SD 1 | CV 2 | PPI 3 |
|---|---|---|---|---|---|---|---|---|
| 1 | GH (×0.1 mm) | 54.55 | 57.84 | 85.17 | 23.29 | 19.70 | 36.11 | 0.73 |
| 2 | LA (×104 μm2) | 33.32 | 32.94 | 45.55 | 25.06 | 5.31 | 15.95 | 0.45 |
| 3 | LL (μm) | 1036.07 | 1013.11 | 1171.30 | 882.89 | 82.35 | 7.95 | 0.25 |
| 4 | ULL (μm) | 600.39 | 603.18 | 698.36 | 509.89 | 55.63 | 9.27 | 0.27 |
| 5 | ULW (μm) | 214.03 | 217.49 | 245.00 | 175.70 | 16.38 | 7.65 | 0.28 |
| 6 | ULL_LW | 2.82 | 2.84 | 3.51 | 2.32 | 0.32 | 11.36 | 0.34 |
| 7 | SL (μm) | 435.68 | 431.45 | 533.43 | 332.23 | 49.11 | 11.27 | 0.38 |
| 8 | SW (μm) | 517.26 | 504.85 | 645.21 | 374.08 | 64.93 | 12.55 | 0.42 |
| 9 | SL_W | 0.85 | 0.84 | 1.11 | 0.66 | 0.09 | 10.76 | 0.40 |
| 10 | LT (μm) | 115.55 | 114.74 | 132.35 | 97.99 | 8.27 | 7.16 | 0.26 |
| 11 | COW (μm) | 114.36 | 110.88 | 154.54 | 82.69 | 16.90 | 14.78 | 0.46 |
| 12 | COT (μm) | 43.36 | 42.84 | 48.82 | 37.58 | 3.32 | 7.67 | 0.23 |
| 13 | LAM1 (μm) | 22.01 | 22.20 | 25.13 | 17.84 | 1.67 | 7.60 | 0.29 |
| 14 | LAM2 (μm) | 25.72 | 25.95 | 28.70 | 21.50 | 1.62 | 6.30 | 0.25 |
| 15 | DCT (μm) | 11.53 | 11.72 | 13.45 | 8.69 | 1.11 | 9.63 | 0.35 |
| 16 | DCW (μm) | 17.20 | 17.34 | 19.35 | 13.14 | 1.35 | 7.83 | 0.32 |
| 17 | BCT (μm) | 7.42 | 7.37 | 8.67 | 6.22 | 0.74 | 9.93 | 0.28 |
| 18 | BCW (μm) | 9.49 | 9.36 | 11.46 | 7.53 | 0.89 | 9.37 | 0.34 |
| 19 | CW1 (μm) | 3.18 | 3.13 | 4.63 | 2.48 | 0.46 | 14.35 | 0.46 |
| 20 | CW2 (μm) | 4.17 | 3.90 | 7.61 | 2.72 | 1.08 | 25.95 | 0.64 |
| 21 | STW (μm) | 3.58 | 3.58 | 3.96 | 3.14 | 0.21 | 5.91 | 0.21 |
| 22 | SCL (μm) | 15.05 | 15.16 | 20.72 | 11.74 | 2.11 | 14.01 | 0.43 |
| 23 | SCW (μm) | 12.85 | 12.80 | 15.20 | 11.01 | 0.97 | 7.56 | 0.28 |
| No. | Abbreviation | Trait Variable | Unit |
|---|---|---|---|
| 1 | GH | gametophyte height | 0.1 mm |
| 2 | LA | leaf area | 104 μm2 |
| 3 | LL | leaf length | μm |
| 4 | ULL | upper leaf length | μm |
| 5 | ULW | upper leaf width | μm |
| 6 | ULL_LW | ratio of length-to-width of upper leaf (4/5) | / |
| 7 | SL | leaf sheath length | μm |
| 8 | SW | leaf sheath width | μm |
| 9 | SL_W | ratio of length-to-width of leaf sheath (7/8) | / |
| 10 | LT | leaf thickness | μm |
| 11 | COW | costa width | μm |
| 12 | COT | costa thickness | μm |
| 13 | LAM1 | adaxial lamina thickness | μm |
| 14 | LAM2 | abaxial lamina thickness | μm |
| 15 | DCT | thickness of adaxial cell (thin-walled) lumen | μm |
| 16 | DCW | width of adaxial cell (thin-walled) lumen | μm |
| 17 | BCT | thickness of abaxial cell (thick-walled) lumen | μm |
| 18 | BCW | width of abaxial cell (thick-walled) lumen | μm |
| 19 | CW1 | thickness of upper surface cell wall | μm |
| 20 | CW2 | thickness of lower surface cell wall | μm |
| 21 | STW | transverse wall thickness of basal cells | μm |
| 22 | SCL | cell lumen length of basal cells | μm |
| 23 | SCW | cell lumen width of basal cells | μm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Gu, J.; Ye, Y.; Wang, M.; Wang, R.; Ma, H.; Shao, X. Exploring Intraspecific Trait Variation in a Xerophytic Moss Species Indusiella thianschanica (Ptychomitriaceae) across Environmental Gradients on the Tibetan Plateau. Plants 2024, 13, 921. https://doi.org/10.3390/plants13070921
Song X, Gu J, Ye Y, Wang M, Wang R, Ma H, Shao X. Exploring Intraspecific Trait Variation in a Xerophytic Moss Species Indusiella thianschanica (Ptychomitriaceae) across Environmental Gradients on the Tibetan Plateau. Plants. 2024; 13(7):921. https://doi.org/10.3390/plants13070921
Chicago/Turabian StyleSong, Xiaotong, Jiqi Gu, Yanhui Ye, Mengzhen Wang, Ruihong Wang, Heping Ma, and Xiaoming Shao. 2024. "Exploring Intraspecific Trait Variation in a Xerophytic Moss Species Indusiella thianschanica (Ptychomitriaceae) across Environmental Gradients on the Tibetan Plateau" Plants 13, no. 7: 921. https://doi.org/10.3390/plants13070921
APA StyleSong, X., Gu, J., Ye, Y., Wang, M., Wang, R., Ma, H., & Shao, X. (2024). Exploring Intraspecific Trait Variation in a Xerophytic Moss Species Indusiella thianschanica (Ptychomitriaceae) across Environmental Gradients on the Tibetan Plateau. Plants, 13(7), 921. https://doi.org/10.3390/plants13070921






