A Biomimetic Approach to Premyrsinane-Type Diterpenoids: Exploring Microbial Transformation to Enhance Their Chemical Diversity
Abstract
1. Introduction
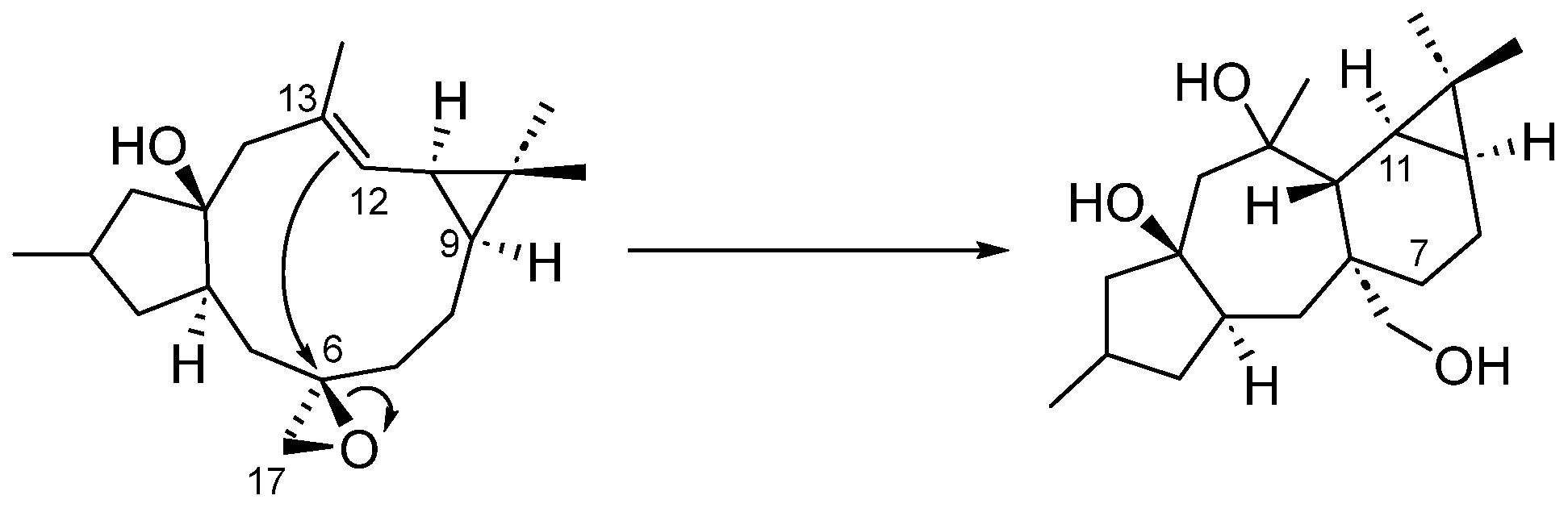
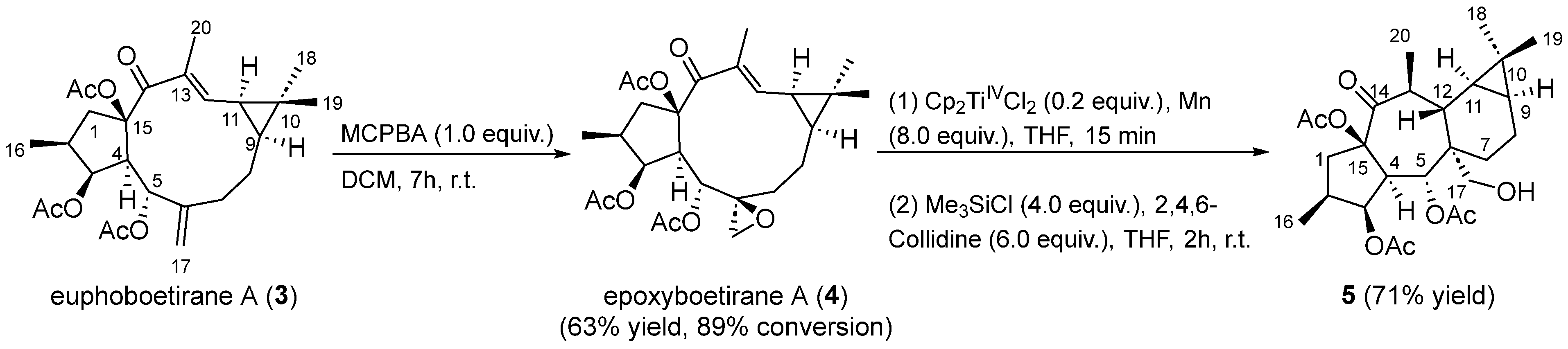
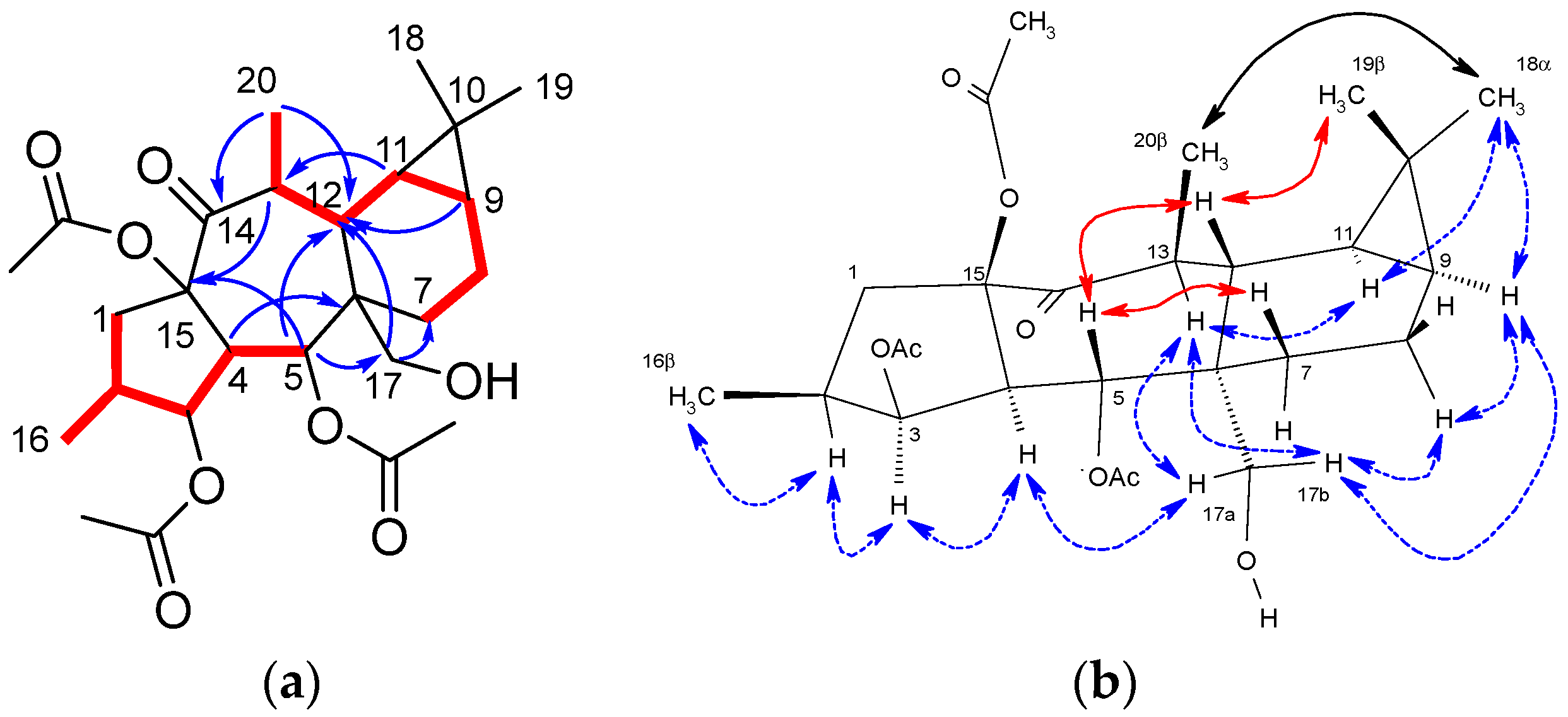
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Microorganism
3.4. Extraction and Isolation of Euphoboetirane A (3) and Epoxyboetirane A (4)
3.5. Preparation of Epoxyboetirane A (4) by Epoxidation of Euphoboetirane A (3)
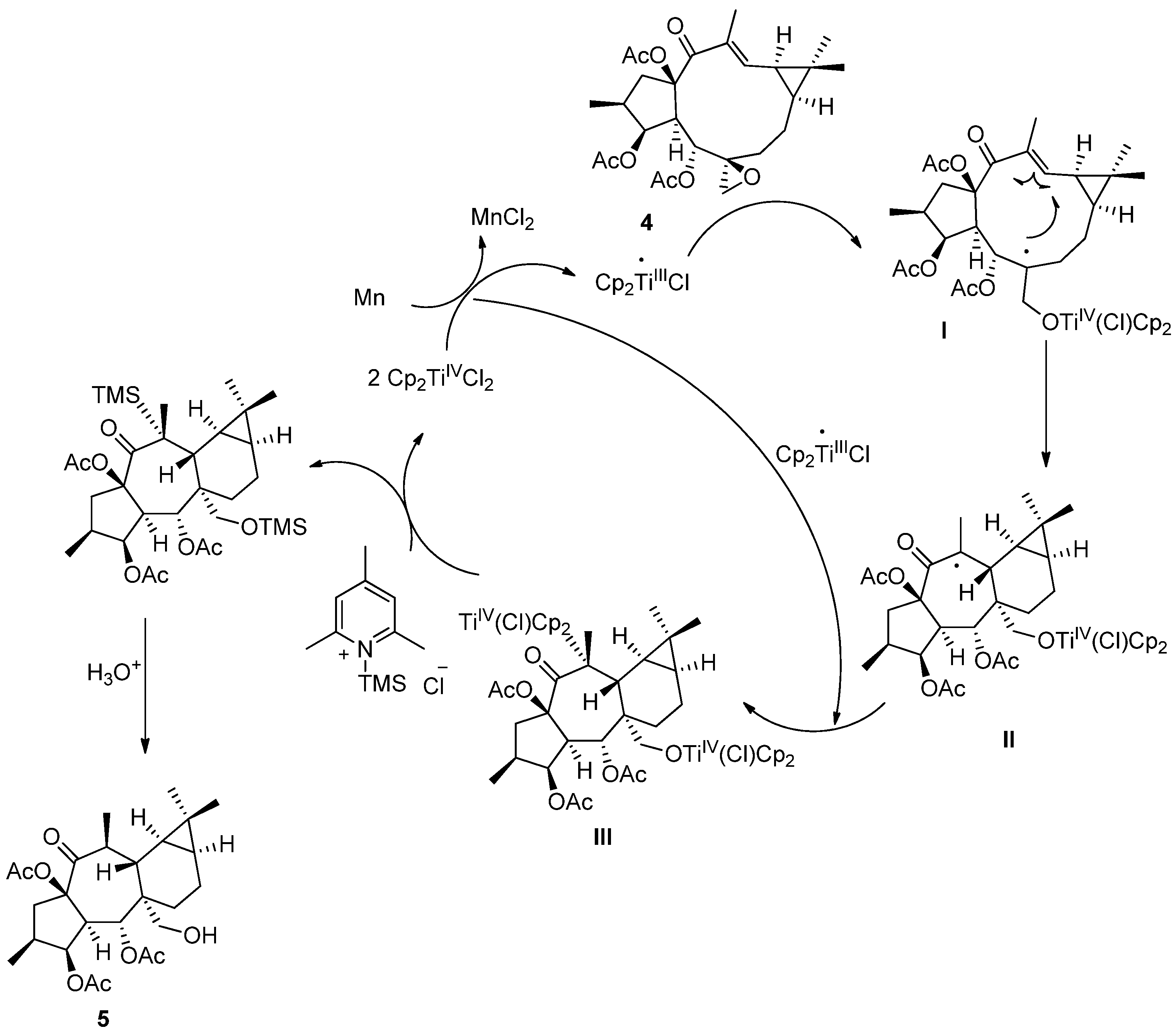
3.6. Preparation of Premyrsinane 5 by Cyclization of Epoxyboetirane A (4) with Cp2TiIIICl
3.7. Biotransformation of Premyrsinane 5 by Mucor circinelloides NRRL3631
3.8. Extraction, Isolation, and Characterization of Biotransformation Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhan, Z.; Li, S.; Chu, W.; Yin, S. Euphorbia Diterpenoids: Isolation, Structure, Bioactivity, Biosynthesis, and Synthesis (2013–2021). Nat. Prod. Rep. 2022, 39, 2132–2174. [Google Scholar] [CrossRef] [PubMed]
- Durán-Peña, M.J.; Botubol Ares, J.M.; Collado, I.G.; Hernández-Galán, R. Biologically Active Diterpenes Containing a gem-Dimethylcyclopropane Subunit: An Intriguing Source of PKC Modulators. Nat. Prod. Rep. 2014, 31, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Vasas, A.; Hohmann, J. Euphorbia Diterpenes: Isolation, Structure, Biological Activity, and Synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef] [PubMed]
- Benjamaa, R.; Moujanni, A.; Kaushik, N.; Choi, E.H.; Essamadi, A.K.; Kaushik, N.K. Euphorbia Species Latex: A Comprehensive Review on Phytochemistry and Biological Activities. Front. Plant Sci. 2022, 13, 1008881. [Google Scholar] [CrossRef]
- Kemboi, D.; Siwe-Noundou, X.; Krause, R.W.M.; Langat, M.K.; Tembu, V.J. Euphorbia Diterpenes: An Update of Isolation, Structure, Pharmacological Activities and Structure–Activity Relationship. Molecules 2021, 26, 5055. [Google Scholar] [CrossRef]
- Fattahian, M.; Ghanadian, M.; Ali, Z.; Khan, I.A. Jatrophane and Rearranged Jatrophane-Type Diterpenes: Biogenesis, Structure, Isolation, Biological Activity and SARs (1984–2019). Phytochem. Rev. 2020, 19, 265–336. [Google Scholar] [CrossRef]
- Wang, H.-B.; Wang, X.-Y.; Liu, L.-P.; Qin, G.-W.; Kang, T.-G. Tigliane Diterpenoids from the Euphorbiaceae and Thymelaeaceae Families. Chem. Rev. 2015, 115, 2975–3011. [Google Scholar] [CrossRef]
- Alves, A.L.V.; da Silva, L.S.; Faleiros, C.A.; Silva, V.A.O.; Reis, R.M. The Role of Ingenane Diterpenes in Cancer Therapy: From Bioactive Secondary Compounds to Small Molecules. Nat. Prod. Commun. 2022, 17, 1–30. [Google Scholar] [CrossRef]
- Vela, F.; Ezzanad, A.; Hunter, A.C.; Macías-Sánchez, A.J.; Hernández-Galán, R. Pharmacological Potential of Lathyrane-Type Diterpenoids from Phytochemical Sources. Pharmaceuticals 2022, 15, 780. [Google Scholar] [CrossRef]
- Mendes, E.; Ramalhete, C.; Duarte, N. Myrsinane-Type Diterpenes: A Comprehensive Review on Structural Diversity, Chemistry and Biological Activities. Int. J. Mol. Sci. 2024, 25, 147. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.-E.; Hamed, A.; Ibrahim, M.; Talat, Z.; Reda, E.; Abdel-Azim, N.; Hammouda, F.; Nakamura, S.; Matsuda, H.; Haggag, E. Euphosantianane A–D: Antiproliferative Premyrsinane Diterpenoids from the Endemic Egyptian Plant Euphorbia sanctae-catharinae. Molecules 2018, 23, 2221. [Google Scholar] [CrossRef] [PubMed]
- Jeske, F.; Jakupovic, J.; Berendsohn, W. Diterpenes from Euphorbia seguieriana. Phytochemistry 1995, 40, 1743–1750. [Google Scholar] [CrossRef]
- Shi, Q.-W.; Su, X.-H.; Kiyota, H. Chemical and Pharmacological Research of the Plants in Genus Euphorbia. Chem. Rev. 2008, 108, 4295–4327. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Cravotto, G.; Jarevång, T.; Sterner, O. Epoxidation Studies on Lathyra-6(17),12-Dienes—Revised Structure of the Euphorbia Factor L1. Eur. J. Org. Chem. 2000, 16, 2933–2938. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, N.; Wan, L.X.; Zhou, X.L.; Li, X.; Gao, F. Iron-Catalyzed Skeletal Conversion of Lathyrane to Premyrsinane Euphorbia Diterpenes and Their Cytotoxic Activities. J. Nat. Prod. 2021, 84, 1838–1842. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, Y.; Ji, W.-S.; Jia, X.-N.; Shan, L.-H.; Li, X.; Liu, Y.-J.; Jiang, T.; Gao, F. Discovery of Myrsinane-Type Euphorbia Diterpene Derivatives through a Skeleton Conversion Strategy from Lathyrane Diterpene for the Treatment of Alzheimer’s Disease. Bioorg. Chem. 2023, 138, 106595. [Google Scholar] [CrossRef]
- Gao, J.; Chen, Q.-B.; Liu, Y.-Q.; Xin, X.-L.; Yili, A.; Aisa, H.A. Diterpenoid Constituents of Euphorbia macrorrhiza. Phytochemistry 2016, 122, 246–253. [Google Scholar] [CrossRef]
- Yazdiniapour, Z.; Sohrabi, M.H.; Motinia, N.; Zolfaghari, B.; Mehdifar, P.; Ghanadian, M.; Lanzotti, V. Diterpenoids from Euphorbia gedrosiaca as Potential Anti-Proliferative Agents against Breast Cancer Cells. Metabolites 2023, 13, 225. [Google Scholar] [CrossRef]
- Zolfaghari, B.; Farahani, A.; Jannesari, A.; Aghaei, M.; Ghanadian, M. New Cytotoxic Premyrsinane-Type Diterpenes from Euphorbia aleppica against Breast Cancer Cells. Iran. J. Pharm. Res. 2022, 21, e127028. [Google Scholar] [CrossRef]
- Vasas, A.; Sulyok, E.; Martins, A.; Rédei, D.; Forgo, P.; Kele, Z.; Zupkó, I.; Molnár, J.; Pinke, G.; Hohmann, J. Cyclomyrsinane and Premyrsinane Diterpenes from Euphorbia falcata Modulate Resistance of Cancer Cells to Doxorubicin. Tetrahedron 2012, 68, 1280–1285. [Google Scholar] [CrossRef]
- Xu, J.; Jin, D.; Guo, Y.; Xie, C.; Ma, Y.; Yamakuni, T.; Ohizumi, Y. New Myrsinol Diterpenes from Euphorbia prolifera and Their Inhibitory Activities on LPS-Induced NO Production. Bioorg. Med. Chem. Lett. 2012, 22, 3612–3618. [Google Scholar] [CrossRef] [PubMed]
- Flores-Giubi, E.; Geribaldi-Doldán, N.; Murillo-Carretero, M.; Castro, C.; Durán-Patrón, R.; Macías-Sánchez, A.J.; Hernández-Galán, R. Lathyrane, Premyrsinane, and Related Diterpenes from Euphorbia boetica: Effect on In Vitro Neural Progenitor Cell Proliferation. J. Nat. Prod. 2019, 82, 2517–2528. [Google Scholar] [CrossRef] [PubMed]
- Botubol-Ares, J.M.; Durán-Peña, M.J.; Hanson, J.R.; Hernández-Galán, R.; Collado, I.G. Cp2Ti(III)Cl and Analogues as Sustainable Templates in Organic Synthesis. Synthesis 2018, 50, 2163–2180. [Google Scholar] [CrossRef]
- Streuff, J. Reductive Umpolung and Defunctionalization Reactions through Higher-Order Titanium(III) Catalysis. Synlett 2023, 34, 314–326. [Google Scholar] [CrossRef]
- Rosales, A.; Rodríguez-García, I.; Muñoz-Bascõn, J.; Roldan-Molina, E.; Padial, N.M.; Morales, L.P.; García-Ocaña, M.; Oltra, J.E. The Nugent Reagent: A Formidable Tool in Contemporary Radical and Organometallic Chemistry. Eur. J. Org. Chem. 2015, 2015, 4567–4591. [Google Scholar] [CrossRef]
- Morcillo, S.P.; Miguel, D.; Campaña, A.G.; Álvarez De Cienfuegos, L.; Justicia, J.; Cuerva, J.M. Recent Applications of Cp2TiCl in Natural Product Synthesis. Org. Chem. Front. 2014, 1, 15–33. [Google Scholar] [CrossRef]
- Martínez, A.R.; Morales, L.P.; Ojeda, E.D.; Rodríguez, M.C.; Rodríguez-García, I. The Proven Versatility of Cp2TiCl. J. Org. Chem. 2021, 86, 1311–1329. [Google Scholar] [CrossRef]
- Green, M.L.H.; Lucas, C.R. Some dl Bis-π-cyclopentadienyl Titanium Complexes with Nitrogen or Phosphorus Ligands. J. Chem. Soc. Dalton Trans. 1972, 8–9, 1000–1003. [Google Scholar] [CrossRef]
- Gansäuer, A.; Bluhm, H.; Pierobon, M. Emergence of a Novel Catalytic Radical Reaction: Titanocene-Catalyzed Reductive Opening of Epoxides. J. Am. Chem. Soc. 1998, 120, 12849–12859. [Google Scholar] [CrossRef]
- Nugent, W.A.; RajanBabu, T.V. Transition-Metal-Centered Radicals in Organic Synthesis. Titanium(III)-Induced Cyclization of Epoxy Olefins. J. Am. Chem. Soc. 1988, 110, 8561–8562. [Google Scholar] [CrossRef]
- Barrero, A.F.; Quílez del Moral, J.F.; Sánchez, E.M.; Arteaga, J.F. Titanocene-Mediated Radical Cyclization: An Emergent Method Towards the Synthesis of Natural Products. Eur. J. Org. Chem. 2006, 2006, 1627–1641. [Google Scholar] [CrossRef]
- Valentová, K. Biotransformation of Natural Products and Phytochemicals: Metabolites, Their Preparation, and Properties. Int. J. Mol. Sci. 2023, 24, 8030. [Google Scholar] [CrossRef] [PubMed]
- Pinedo-Rivilla, C.; Moraga, J.; Pérez-Sasián, G.; Peña-Hernández, A.; Collado, I.G.; Aleu, J. Biocatalytic Preparation of Chloroindanol Derivatives. Antifungal Activity and Detoxification by the Phytopathogenic Fungus Botrytis cinerea. Plants 2020, 9, 1648. [Google Scholar] [CrossRef] [PubMed]
- Rico-Martínez, M.; Medina, F.G.; Marrero, J.G.; Osegueda-Robles, S. Biotransformation of Diterpenes. RSC Adv. 2014, 4, 10627–10647. [Google Scholar] [CrossRef]
- De Sousa, I.P.; Sousa Teixeira, M.V.; Jacometti Cardoso Furtado, N.A. An Overview of Biotransformation and Toxicity of Diterpenes. Molecules 2018, 23, 1387. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yin, C.; Cao, Y.; Liu, X.; Yu, J.; Zhang, J.; Yu, B.; Cheng, Z. Regioselective Hydroxylation of Lathyrane Diterpenoids Biocatalyzed by Microorganisms and Its Application in Integrated Synthesis. J. Mol. Catal. B-Enzym. 2016, 133, S352–S359. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Z. Microbial and Chemical Transformations of Euphorbia Factor L1 and the P-Glycoprotein Inhibitory Activity in Zebrafishes. Nat. Prod. Res. 2023, 37, 871–881. [Google Scholar] [CrossRef]
- Xiao, S.; Xu, X.; Wei, X.; Xin, J.; Li, S.; Lv, Y.; Chen, W.; Yuan, W.; Xie, B.; Zu, X.; et al. Comprehensive Metabolic Profiling of Euphorbiasteroid in Rats by Integrating UPLC-Q/TOF-MS and NMR as Well as Microbial Biotransformation. Metabolites 2022, 12, 830. [Google Scholar] [CrossRef]
- Escobar-Montaño, F.; González-Rodríguez, V.E.; Macías-Sánchez, A.J.; Botubol-Ares, J.M.; Durán-Patrón, R.; Hernández-Galán, R. Enhancing Structural Diversity of Lathyrane Derivatives through Biotransformation by the Marine-Derived Actinomycete Streptomyces puniceus BC-5GB.11. Int. J. Mol. Sci. 2024, 25, 2289. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.; Duarte, N.; Reis, M.A.; Spengler, G.; Madureira, A.M.; Molnár, J.; Ferreira, M.-J.U. Improving the MDR Reversal Activity of 6,17-Epoxylathyrane Diterpenes. Bioorg. Med. Chem. 2014, 22, 6392–6400. [Google Scholar] [CrossRef] [PubMed]
- Engel, D.W. The Determination of Absolute Configuration and Δf” Values for Light-Atom Structures. Acta Crystallog. B 1972, 28, 1496–1509. [Google Scholar] [CrossRef]
- Engel, D.W.; Zechmeister, K.; Hoppe, W. Experience in the Determination of Absolute Configuration of Light Atom Compounds by X-Ray Anomalous Scattering. Tetrahedron Lett. 1972, 13, 1323–1326. [Google Scholar] [CrossRef]
- Lu, J.; Li, G.; Huang, J.; Zhang, C.; Zhang, L.; Zhang, K.; Li, P.; Lin, R.; Wang, J. Lathyrane-Type Diterpenoids from the Seeds of Euphorbia lathyris. Phytochemistry 2014, 104, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Mao, Z.; Dong, W.; Deng, M.; Lu, R.-H. Euphorbia Factor L8: A Diterpenoid from the Seeds of It Euphorbia lathyris. Acta Crystallogr. E 2008, 64, o331. [Google Scholar] [CrossRef] [PubMed]
- Justicia, J.; Rosales, A.; Buñuel, E.; Oller-López, J.L.; Valdivia, M.; Haïdour, A.; Oltra, J.E.; Barrero, A.F.; Cárdenas, D.J.; Cuerva, J.M. Titanocene-Catalyzed Cascade Cyclization of Epoxypolyprenes: Straightforward Synthesis of Terpenoids by Free-Radical Chemistry. Chem.–Eur. J. 2004, 10, 1778–1788. [Google Scholar] [CrossRef] [PubMed]
- Gansaeuer, A.; Pierobon, M.; Bluhm, H. Titanocene Catalysed 5-Exo Cyclisations of Unsaturated Epoxides—Reagent Control in Radical Chemistry. Synthesis 2004, 2001, 2500–2520. [Google Scholar] [CrossRef]
- Estévez, R.E.; Justicia, J.; Bazdi, B.; Fuentes, N.; Paradas, M.; Choquesillo-Lazarte, D.; García-Ruiz, J.M.; Robles, R.; Gansäuer, A.; Cuerva, J.M.; et al. Ti-Catalyzed Barbier-Type Allylations and Related Reactions. Chem.–Eur. J. 2009, 15, 2774–2791. [Google Scholar] [CrossRef]
- Escobar-Montaño, F.; Macías-Sánchez, A.J.; Botubol-Ares, J.M.; Durán-Patrón, R.; Hernández-Galán, R.; Departamento de Química Orgánica, Facultad de Ciencias, Universidad de Cádiz, Puerto Real (Cádiz), Spain. 2024; manuscript in preparation.
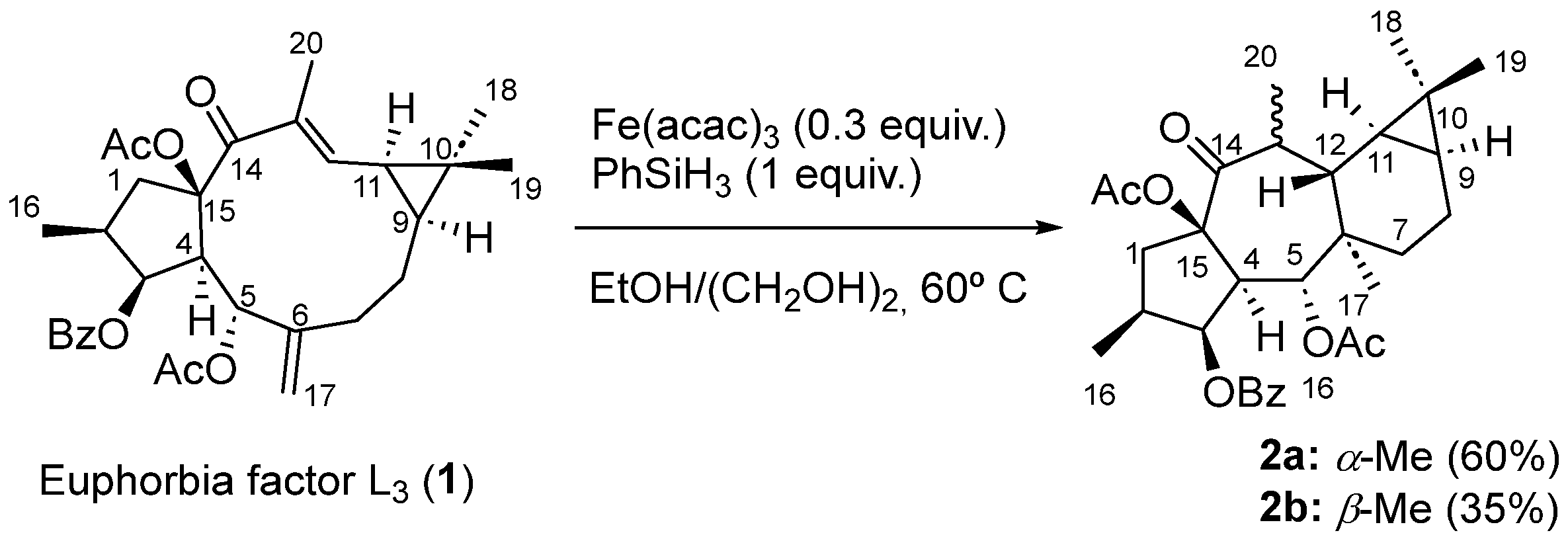

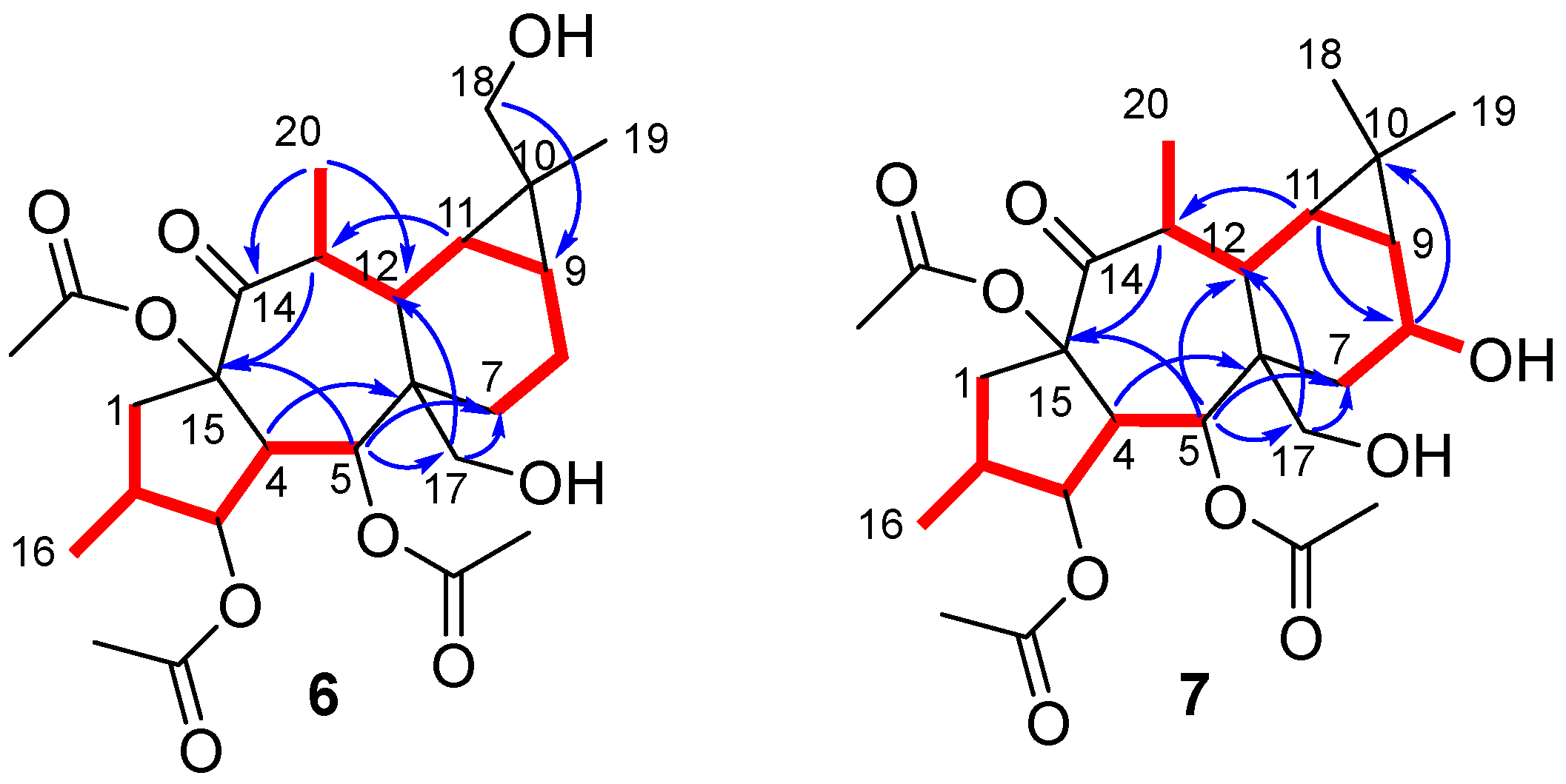
| Position | 5 | 6 | 7 | |||
|---|---|---|---|---|---|---|
| δH, Mult (J in Hz) | δC, Type | δH, Mult (J in Hz) | δC, Type | δH, Mult (J in Hz) | δC, Type | |
| 1α | 3.36, dd (15.1, 9.1) | 42.3, CH2 | 3.35, dd (15.1, 8.9) | 42.3, CH2 | 3.35, dd (15.1, 8.9) | 42.4, CH2 |
| 1β | 1.54, dd (15.1, 11.1) | 1.55, dd (15.1, 11.2) | 1.55, dd (15.1, 11.2) | |||
| 2 | 2.14–2.06, m | 37.0, CH | 2.11–2.06, m | 37.0, CH | 2.13–2.06, m | 37.3, CH |
| 3 | 5.34, t (3.6) | 78.5, CH | 5.34, t (3.5) | 78.4, CH | 5.35, t (3.5) | 78.4, CH |
| 4 | 2.65, dd (11.4, 3.6) | 51.9, CH | 2.66, dd (11.4, 3.5) | 51.9, CH | 2.63, dd (11.4, 3.5) | 51.8, CH |
| 5 | 5.41, d (11.4) | 74.7, CH | 5.42, d (11.4) | 74.7, CH | 5.50, d (11.4) | 75.1, CH |
| 6 | - | 44.1, C | - | 44.1, C | - | 48.0, C |
| 7α | 1.40, dt (14.1, 6.4) | 30.6, CH2 | 1.45, dd (13.8, 5.9) | 30.5, CH2 | 1.92, dd (13.8, 7.6) | 39.2, CH2 |
| 7β | 1.15–1.09, m | 1.12–1.05, m | 1.02, dd (13.8, 11.6) | |||
| 8α | 1.76–1.63, m | 15.8, CH2 | 1.80–1.70, m | 15.4, CH2 | 4.37, dt (11.6, 7.6) | 64.9, CH |
| 8β | 1.33–1.22, m | 1.40–1.33, m | - | |||
| OH-8 | - | - | - | - | 1.31, d (5.2) | - |
| 9 | 0.70, ddd (9.4, 7.9, 4.5) | 21.7, a CH | 0.85, ddd (9.6, 7.5, 3.9) | 18.2, CH | 1.06, dd (9.4, 7.6) | 26.3, CH |
| 10 | - | 18.0, C | - | 24.3, C | - | 20.7, C |
| 11 | 0.11, dd (9.4, 6.7) | 27.1, CH | 0.34, dd (9.6, 6.2) | 24.0, CH | 0.40, dd (9.4, 4.8) | 32.0, CH |
| 12 | 1.76–1.63, m | 38.0, CH | 1.75, dd (11.4, 6.2) | 37.5, CH | 1.88, dd (11.7, 4.8) | 38.9, CH |
| 13 | 2.72–2.61, m | 50.6, CH | 2.73, dq (11.4, 7.5) | 51.0, CH | 2.58, dt (11.7, 7.4) | 50.7, CH |
| 14 | - | 209.0, C | - | 208.7, C | - | 208.3, C |
| 15 | - | 91.8, C | - | 91.7, C | - | 91.4, C |
| 16 | 0.87, d (6.7) | 14.1, CH3 | 0.87, d (6.7) | 14.0, CH3 | 0.88, d (6.7) | 13.9, CH3 |
| 17a | 3.84, d (11.3) | 63.2, CH2 | 3.87, dd (11.3, 2.5) | 62.8, CH2 | 3.86, dd (11.7, 2.5) | 61.8, CH2 |
| 17b | 3.68, d (11.3) | 3.71, dd (11.3, 6.9) | 3.67, dd (11.7, 8.2) | |||
| OH-17 | - | - | 1.67, dd (6.9, 2.5) | - | 1.68, dd (8.2, 2.5) | - |
| 18a | 1.03, s | 28.2, CH3 | 3.41, d (10.4) | 72.8, CH2 | 1.12, s | 29.5, CH3 |
| 18b | 3.30, d (10.4) | |||||
| 19 | 0.99, s | 16.1, CH3 | 1.11, s | 11.7, CH3 | 1.24, s | 17.4, CH3 |
| 20 | 1.10, d (7.5) | 19.7, CH3 | 1.13, d (7.5) | 19.7, CH3 | 1.12, d (7.4) | 20.3, CH3 |
| OCO-3 | - | 171.0, C | - | 170.0, C | - | 171.0, e C |
| OCOMe-3 | 2.04, s | 21.0, b CH3 | 2.03, s | 21.0, c CH3 | 2.04, s | 21.2, d CH3 |
| OCO-5 | - | 170.0, C | - | 171.0, C | - | 170.0, e C |
| OCOMe-5 | 2.03, s | 21.2, b CH3 | 2.04, s | 21.2, c CH3 | 2.04, s | 21.0, d CH3 |
| OCO-15 | - | 169.7, C | - | 169.7, C | - | 170.0, C |
| OCOMe-15 | 2.12, s | 21.6, a CH3 | 2.14, s | 21.6, CH3 | 2.15, s | 21.6, CH3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobar-Montaño, F.; Macías-Sánchez, A.J.; Botubol-Ares, J.M.; Durán-Patrón, R.; Hernández-Galán, R. A Biomimetic Approach to Premyrsinane-Type Diterpenoids: Exploring Microbial Transformation to Enhance Their Chemical Diversity. Plants 2024, 13, 842. https://doi.org/10.3390/plants13060842
Escobar-Montaño F, Macías-Sánchez AJ, Botubol-Ares JM, Durán-Patrón R, Hernández-Galán R. A Biomimetic Approach to Premyrsinane-Type Diterpenoids: Exploring Microbial Transformation to Enhance Their Chemical Diversity. Plants. 2024; 13(6):842. https://doi.org/10.3390/plants13060842
Chicago/Turabian StyleEscobar-Montaño, Felipe, Antonio J. Macías-Sánchez, José M. Botubol-Ares, Rosa Durán-Patrón, and Rosario Hernández-Galán. 2024. "A Biomimetic Approach to Premyrsinane-Type Diterpenoids: Exploring Microbial Transformation to Enhance Their Chemical Diversity" Plants 13, no. 6: 842. https://doi.org/10.3390/plants13060842
APA StyleEscobar-Montaño, F., Macías-Sánchez, A. J., Botubol-Ares, J. M., Durán-Patrón, R., & Hernández-Galán, R. (2024). A Biomimetic Approach to Premyrsinane-Type Diterpenoids: Exploring Microbial Transformation to Enhance Their Chemical Diversity. Plants, 13(6), 842. https://doi.org/10.3390/plants13060842








