Abstract
Algae and bacteria have co-occurred and coevolved in common habitats for hundreds of millions of years, fostering specific associations and interactions such as mutualism or antagonism. These interactions are shaped through exchanges of primary and secondary metabolites provided by one of the partners. Metabolites, such as N-sources or vitamins, can be beneficial to the partner and they may be assimilated through chemotaxis towards the partner producing these metabolites. Other metabolites, especially many natural products synthesized by bacteria, can act as toxins and damage or kill the partner. For instance, the green microalga Chlamydomonas reinhardtii establishes a mutualistic partnership with a Methylobacterium, in stark contrast to its antagonistic relationship with the toxin producing Pseudomonas protegens. In other cases, as with a coccolithophore haptophyte alga and a Phaeobacter bacterium, the same alga and bacterium can even be subject to both processes, depending on the secreted bacterial and algal metabolites. Some bacteria also influence algal morphology by producing specific metabolites and micronutrients, as is observed in some macroalgae. This review focuses on algal-bacterial interactions with micro- and macroalgal models from marine, freshwater, and terrestrial environments and summarizes the advances in the field. It also highlights the effects of temperature on these interactions as it is presently known.
1. Introduction
1.1. Definition, Phylogeny, Distribution, and Relevance of Algae
‘Algae’ refers to a heterogeneous polyphyletic group of photosynthetic eukaryotes excluding land plants (Embryophyta). Characterized by the absence of typical plant organs such as leaves, stems, or roots [1,2], they are derived from primary and secondary endosymbiosis [3], which can be succeeded by higher-order—tertiary, quaternary—endosymbioses [4]. In primary endosymbiosis, a cyanobacterium was engulfed by a eukaryotic cell, while in secondary endosymbiosis, a green or red alga was engulfed by a eukaryotic cell. Algae derived by primary endosymbiosis include the green and red algae, as well as the glaucophytes [2,3,5]. Secondary endosymbionts of the green lineages comprise the Chlorachniophytes and the Euglenoids. The red lineage includes the Cryptophytes, Haptophytes, Dinoflagellates, and Stramenopiles. The Stramenopiles are the most species-rich clade, including diatoms, brown algae, xanthophytes, and other ochrophytes [2,3,5]. Through repeated endosymbiotic events, plastids spread through the eukaryotic tree of life, spanning more than 1.6 billion years of evolution [4].
Algae are also grouped into micro- and macroalgae. Their sizes can range from extremely small, such as the picoalga Ostreococcus (0.8 µm) [6], to giant kelps with fronds up to 60 m in length [7]. The number of algal species has been estimated to exceed one million, with most of them being microalgae [8,9]. Algae are prominent in bodies of water, such as oceans, rivers, and lakes [7,10], and are also common in terrestrial environments [11,12]. Algae can be found on snow and ice [13], and even in the desert [14].
Algae play a significant role in global annual primary production; together with cyanobacteria, they contribute to about 50% of CO2 fixation on Earth [15]. As primary producers, algae are at the base of food webs [16]. Algal activities also affect biogeochemical processes, as observed with the accelerated melting of the Greenland ice sheet [17]. In the ocean, they participate in nutrient [18], carbon [19,20,21], and sulfur cycles [22,23]. Macroalgae and kelps, in particular, are ecosystem engineers and habitat builders that shelter various forms of life [24,25,26].
Algae are strongly affected by climate change; along with the increased temperatures, there is a decrease in natural populations of kelp, such as Saccharina latissima [27,28], and there is coral reef bleaching due to the loss of the algal symbiont (see Section 3.3). Climate-driven shifts in algal-bacterial interactions were also reported over ten years [29].
Algae are used as food [30,31] and feed [32,33], as texture agents [34,35], in biomaterial [36,37], in medicine [38,39], as fertilizer in agriculture [40,41,42], in biofuel and hydrogen production [43,44,45], and in pollution control and bioremediation [46,47].
1.2. The Phycosphere and Algal-Microbial Interactions
The phycosphere is the region immediately surrounding an algal cell within a phytoplankton or in soil. It is enriched in organic molecules exuded by the cell into the surrounding water [48,49]. In this microenvironment, organisms interact via chemical compounds, such as signaling molecules, nutrients, toxins, morphogens, defense compounds, and quorum-sensing signals [50,51,52,53]. These infochemicals can reach significantly higher concentrations than those measured in bulk seawater, generating chemical gradients that can be detected by marine microbes [48,54].
Bacteria can detect and navigate towards favorable chemical gradients in their immediate surroundings through a process known as chemotaxis. This involves the utilization of transmembrane chemoreceptors, which enable bacteria to measure concentrations of chemicals. Subsequently, signal transduction systems process this information, allowing bacteria to precisely modulate their motility in response to perceived chemical gradients [48,55,56]. Algae, especially flagellate ones, also react to chemicals by tactic processes [57,58].
The algal host and its associated microbiota are collectively called holobiont [25,59,60,61], and biotic interactions within such a system are drivers of algal evolution [62]. Host-microbe interactions are grouped into “positive”, “neutral”, and “negative” interactions, called mutualism, commensalism, and antagonism, respectively [63,64,65].
Mutualism refers to a symbiotic relationship between bacteria and microalgae, in which both organisms gain advantages, irrespective of which partner receives greater benefits. This natural symbiosis typically evolves gradually over an extended period, remaining relatively stable across several generations [66].
Commensalism describes a relationship in which only one symbiotic partner benefits, while the other is unaffected. Algal-bacterial associations can transition between mutualism and commensalism, depending on environmental factors [67].
A relationship marked by antagonism occurs when one partner adversely affects or harms the other. Presently, limited research is dedicated to the algal antagonism on bacterial partners. One bulk of research studies focuses on the detrimental effects of extracellular degrading enzymes produced by interacting bacteria, which induces lysis of algal cell walls [68,69], but knowledge about algicidal bacteria that use secondary metabolites as toxins also emerges (e.g., [70,71]).
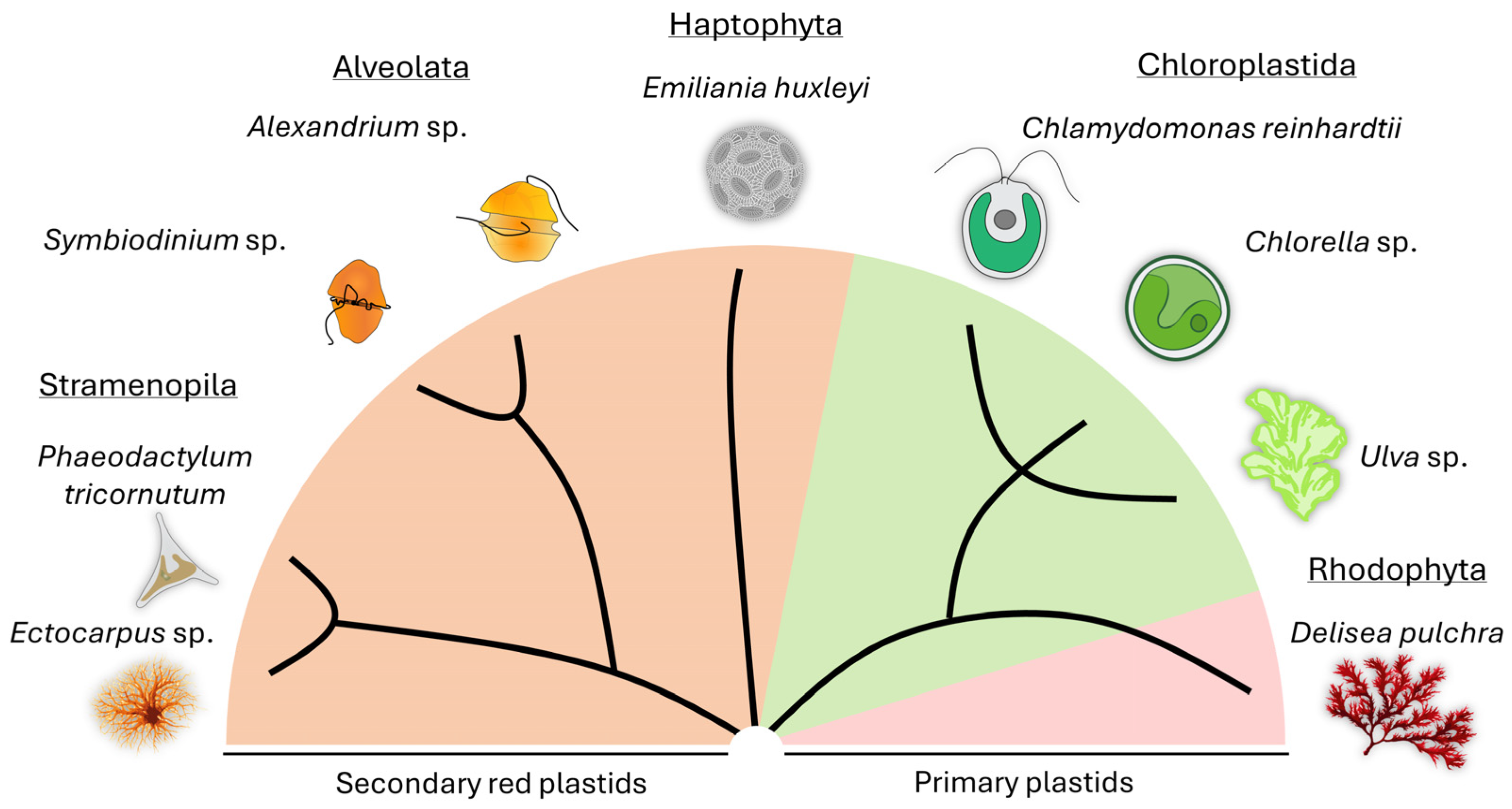
Partners of algae include viruses [72,73,74,75] and bacteria [12,25,76], as well as eukaryotic organisms such as fungi [77,78,79,80] or even sloths [81]. For this review, we will focus on the bacterial partners of algae. While algae and bacteria have coexisted since the early stages of evolution, we still have limited knowledge about their detailed modes of interaction. Here, we have selected exemplary model species of micro- and macroalgae from terrestrial, freshwater, and marine environments and will present the current knowledge about their relationships with bacteria (Figure 1). We are aware that more model species have emerged; however, these are beyond the scope of this review (e.g., [2,82,83]).
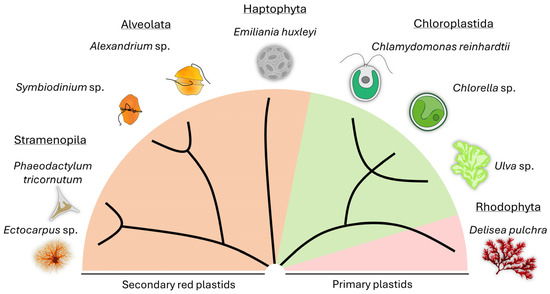
Figure 1.
Representative micro- and macroalgal models used for studying algal-bacterial interactions are highlighted, along with their phylogenetic background. A simplified cladogram scheme derived from a phylogenetic tree [84] was taken as a basis.
2. Terrestrial and Freshwater Microalgae
2.1. The Green Microalga Chlamydomonas reinhardtii Emerges as a Soil Model
For decades, the biflagellate microalga C. reinhardtii has been used as a model for specific biological processes, including, for example, photosynthesis, cilia formation and function, and light-driven processes [85]. The alga can be genetically manipulated, its genome is annotated, and many molecular tools and large-scale mutant libraries are available [86,87,88,89]. Moreover, genetic crosses can be easily performed [90]. Although it is usually grown in liquid culture in the laboratory, in nature, it is mostly found in inhomogeneous wet soil [91]. It was originally isolated from a potato field in Massachusetts, USA [90].
In the past years, this microalga emerged as a model for studying microbial interactions [12,63,92], including algal-bacterial relationships. Its phycosphere was comprehensively studied by cultivating C. reinhardtii in soil [49], and this approach allowed for the identification of the diverse bacterial partners selected by the algal host. The algal phycosphere microbiome was then compared against the rhizosphere microbiome associated with roots of Arabidopsis thaliana. Surprisingly, the analysis revealed a notable overlap in the microbiome between the land plant and the chlorophyte alga, despite their evolutionary distinctiveness. Both host-associated bacterial microbiomes were dominated by Proteobacteria (Pseudomonodata), along with members of the Actinobacteria (Actinomycetota), Bacteroidetes (Bacteroidota), and Firmicutes (Bacillota) phyla [49]. These data provide an important insight into the complexity of the algal rhizosphere and reveal common bacterial communities compared to land plants.
In many investigations, dual interactions of C. reinhardtii and a specific bacterium were studied; mutualistic, as well as antagonistic, interactions were hereby in focus, and examples for both will be presented. In mutualistic interactions, C. reinhardtii has been reported to offer carbon sources in exchange for bacterial metabolites [12]. Cobalamin (vitamin B12) is a critical metabolite required for many cellular processes [93]. Most eukaryotic algae are cobalamin auxotrophs and must receive cobalamin exogenously or through bacterial cooperation. C. reinhardtii requires vitamin B12 for the B12-dependent methionine synthase called METH. As C. reinhardtii also encodes METE, a B12-independent methionine synthase, wild-type algae do not necessarily require exogenous B12; however, metE mutants must obtain vitamin B12 [94]. A recently evolved B12-dependent C. reinhardtii strain, metE7, thus establishes a mutualism with some bacteria, one of them being the rhizobium Mesorhizobium loti [95]. Heat stress represses METE gene expression; consequently, vitamin B12 is essential for heat-treated algal cells [96]. C. reinhardtii becomes thermally tolerant via a mutualistic interaction with vitamin B12-producing bacteria such as Ensifer meliloti. In this context, it is also interesting that C. reinhardtii synthesizes mimics of N-acyl-1-homoserine lactones (AHL) that stimulate quorum sensing in E. meliloti [97]. By quorum sensing, bacteria sense cell population numbers and react to them.
In addition to vitamins, C. reinhardtii profits from nitrogen sources provided by N-fixing bacteria or through N-mineralizing bacteria. For example, the diazotroph Azotobacter chroococcum and other Azotobacter species establish a mutualism with C. reinhardtii when any C- and N-sources are omitted in the growth media (reviewed in [12]). While C. reinhardtii can grow on nitrate, nitrite, ammonium, and certain amino acids, it cannot metabolize proline, hydroxyproline or peptides bearing these amino acids [98]. In coculture with Methylobacterium spp., the algae can grow on these substrates by establishing a mutualistic relationship with the bacterium and offering glycerol. Indole-3-acetic acid (IAA, also known as auxin) is synthesized by the algal cells via an extracellular enzyme; it inhibits algal growth, while Methylobacterium aquaticum degrades auxin in the presence of the alga. Thus, auxin mediates the mutualism between both microorganisms [99]. Recently, it was shown that Microbacterium forte sp. nov. also forms a mutual relationship with C. reinhardtii and even promotes algal hydrogen production [100].
Besides mutualistic interactions, antagonistic interactions with bacteria also emerged for C. reinhardtii. Among three examined species of the genera, Flavobacterium, Xanthomonas, and Pseudomonas, P. protegens (Pf-5) turned out to act algicidal [70]. A cocktail of toxic natural products of P. protegens is involved in the bacterial attack on the algae (Table 1) [70,101,102,103]. They all change the algal cell morphology, or even lyse the cells (in the case of protegencin) and influence algal growth; some secondary metabolites immobilize the algal cells (Table 1). The cyclic lipopeptide orfamide A causes the strongest immobility of algal cells with the lowest IC50 value [102]. Orfamide A triggers an increase in cytosolic Ca2+ that results in the deflagellation of the algal cells and thus renders them immotile [70,103]. Remarkably, this reaction takes place within a minute. In contrast to other cyclic lipopeptides, orfamide A does not seem to cause membrane pores [70]. The action of orfamide A depends on Ca2+ channels of the transient receptor potential (TRP)-type, including TRP5, TRP11, TRP15 (also known as ADF-1), and TRP22 [104]. For its activity, the N-terminal amino acids of the linear part and the terminal fatty acid tail of orfamide A are highly important [104]. One of the most potent toxins of P. protegens is the polyyne protegencin [101]. Protegencin acts within several hours; it blinds the cells by destroying their eyespot and lyses them [101]. This rather unstable toxin could be identified by Raman microspectroscopy, as such triple-bond-containing compounds appear in the silent region of the Raman spectrum [101].

Table 1.
Bacterial toxins from P. protegens and their effects on C. reinhardtii.
Recently, another algicidal bacterium, Paenibacillus polymyxa MEZ6, was detected in soil samples. It acts against C. reinhardtii and other microalgae, including Haematococcus pluvialis and Chlorella ellipsoidea [107]. In this context, it should also be mentioned that orfamide A from P. protegens deflagellates not only C. reinhardtii but also a marine Chlamydomonas sp., as well as Haematococcus pluvialis and Gonium pectorale from the Chlorophyceaea, but not selected members from the Pedinomonaceae and Euglenophyceae [70].
Another bacterium that is antagonistic against C. reinhardtii is Streptomyces iranensis, which secretes the algicidal marginolactone azalomycin F when co-cultivated with the algal cells [108]. In tripartite cultures with the fungus Aspergillus nidulans, the algal cells get shielded from the bacterial toxin. These data emphasize the influence of additional partners in algal-bacterial interactions. An azalomycin F resistant fungus is needed to protect the alga [71]. The alga uses an alternative strategy when no fungus is present. In this case, a specific type of palmelloid, called a gloeocapsoid, is formed [108]. It has been postulated that the margolactone-triggered formation of such cell aggregates may have contributed to the evolution of multicellular colony-forming algae such as Eudorina or Volvox [108].
2.2. The Freshwater Green Alga Chlorella sp. and Its Potential for Biotechnology via Bacterial Interactions
Chlorella is a unicellular, non-motile green alga found in freshwater environments. The genomes of several species, including C. vulgaris [109], C. sorokiniana [110,111], and C. variablis [112] have been completely sequenced and characterized. Unlike Chlamydomonas, Chlorella cannot reproduce sexually; however, various transformation techniques exist to simplify genetic manipulation. The first successful attempt to genetically transform C. vulgaris used the electroporation technique; however, the rigid cell wall of Chlorella can hinder the efficiency of electroporation-based transformations [113]. Thus, cellulase was used to degrade the cell walls of C. ellipsoidea, facilitating the exogenous DNA uptake and leading to transformation [114]. More recently, Agrobacterium [115] and CRISPR-Cas9 [116] systems have been effectively employed to transform C. sorokiniana and C. vulgaris.
Members of the genus Chlorella have found utility across a wide array of biotechnology fields, including human and animal nutrition, bioremediation, and the production of antimicrobial compounds. Thus, there has been a persistent search for bacterial partners capable of improving either the macromolecule properties of Chlorella or nutrient uptake by algal-bacterial consortia.
The foremost study into the Chlorella phycosphere identified 29 distinct bacterial isolates from non-axenic C. sorokiniana cultures. These bacterial strains encompassed various genera, including Pseudomonas, Acinetobacter, Flavobacterium, and Bacillus [117]. Recent studies have investigated the effect of pairwise cocultivation on algal growth and utilized xenic Chlorella cultures to further explore the diversity of bacteria coexisting with Chlorella. Cocultivation with Ralstonia sp. has been shown to promote the development of C. sorokiniana [118], while Brevundimonas sp. has been found to stimulate the growth of C. ellipsoidea [119] and C. vulgaris [120]. Additionally, pairwise cultivation with Bacillus pumilus promotes the growth of C. vulgaris [121]. However, the precise mechanism of growth promotion still needs to be discovered for these pairwise combinations.
One of the most well-studied associations is between Chlorella and Azospirillum brasilense. A. brasilense is a well-established plant growth-promoting diazotrophic bacteria, capable of increasing the growth of 113 plant species [122]. The earliest report of A. brasilense-induced algal growth promotion comes from a study involving the co-immobilization of A. brasilense and C. vulgaris within alginate beads. The co-immobilization increased the total number of algal cells and pigments [123]. Subsequent research confirmed that the exudation of auxin by A. brasilense played a crucial role in promoting the growth of C. vulgaris [124]. Furthermore, transcriptomic investigations identified that IAA exudation also induces the Type 6 secretion system (T6SS1) of A. brasilense [125]. The T6SS is a complex multi-protein machinery crucial for modulating bacterial competition, fostering symbiotic relationships, and discerning between bacteria of the same or different species. Structurally similar to a phage tail, it enables the host bacteria to translocate effector molecules and proteins into both prokaryotic and eukaryotic cells. T6SS1 plays a pivotal role in promoting attachment between A. brasilense and C. sorokiniana, forming a physical bond between the algae and the bacterium consortium [126]. Mutants lacking T6SS1 genes exhibited reduced IAA exudation, indicating a reciprocal relationship between IAA biosynthesis and the T6SS gene cluster. While the mutant A. brasilense could promote algal growth, it could not induce the accumulation of carbohydrates, lipids, and proteins like the wild type, suggesting that the effector molecules and IAA were essential for these processes. However, the specific effector molecules released from T6SS1, which lead to the physiological changes observed in C. sorokiniana, remain elusive.
Interestingly, coculturing is not essential to promote the growth of Chlorella, as A. brasilense can release a whole collection of volatile organic compounds (VOCs). When exposed to these VOCs, C. sorokiniana exhibited enhanced growth and accumulation of total carbohydrates, lipids, and chlorophyll. This enhancement can be attributed to the presence of known plant growth-promoting VOCs such as 2,3-butanediol and acetoin, which are released by A. brasilense [121].
Chlorella finds immense utility as a phytoremediation agent, even though the comparatively low abundance of micro-nutrients such as iron and vitamins can hinder sustained phytoremediation. Also, algae cannot secrete chelating agents to solubilize inorganic iron [127]. In contrast, heterotrophic bacteria can produce low molecular weight chelating agents or siderophores. Siderophore-mediated iron chelation enhances the formation of bioavailable iron complexes, which algae can then absorb through plasma membrane-bound ferrireductase [128]. Consequently, the introduction of siderophore-producing Ralstonia pickettii establishes a mutualistic relationship whereby the bacteria benefit from the photosynthetically fixed algal exopolysaccharides while reciprocally enhancing the growth and degradation of complex azo dyes by C. sorokiniana [129].
It is essential to highlight that not all interactions mutually benefit both partners. In 1972, Gromov and Mamkaeva first described Vampirovibrio chlorellavorus, a predatory cyanobacterium with a distinct evolutionary path compared to other cyanobacteria [130]. Unlike its counterparts, V. chlorellavorus has completely lost its photosynthetic apparatus and instead adapted into an obligate parasite of Chlorella. Upon attachment to the cell wall of Chlorella, it uses a type 4 secretion system to form a conjugative apparatus. It can then release over 100 different hydrolytic enzymes, including proteases and peptidases, to digest its prey. Subsequently, the bacterium ingests algal lysates to aid in bacterial binary fission and plasmid replication, and finally, bacterial offsprings are released from the algal host cells to continue the cycle [131].
3. Marine Microalgae
Phytoplankton communities can be found in the photic zones of the oceans and are characterized by the interactions of microalgae and bacteria that coevolved and shaped the planktonic microbiome [64]. Chemical mediators, such as sulfur metabolites exchanged between the algae and bacteria, play an important role [132,133,134].
3.1. Phaeodactylum tricornutum: A Phytoplankton Diatom Model for Microbial Interactions
Diatoms, prominent microalgae in the oceans, are a key population of phytoplankton. Bacteria have the capacity to either promote or inhibit their growth and proliferation [135,136], and diatoms can also change their metabolic profile in response to bacteria [137]. Among these microalgae, the pennate Phaeodactylum tricornutum, with its diverse morphotypes, alongside the centric Thalassiosira pseudonana, has emerged as a model for diatom research [10,138,139]. The focus here will be on P. tricornutum, as it was used as a model in many algal-bacterial interactions. Its genome is known [140], it can be transformed biolistically and by electroporation, and many molecular tools are available [10,141,142,143]. Ten isolates from nine different geographic locations (seashores, estuaries, rock pools, and tidal creeks) worldwide, spanning from sub-polar to tropical latitudes, have been accessioned and well-described for this diatom [144].
The effect of different bacteria on P. tricornutum was investigated through algal-bacterial cocultures, identifying the presence of both mutualistic and antagonistic bacteria. The algal-bacterial interactions influenced intracellular lipids, as well as extracellular metabolites [145]. Phytoplankton-derived dissolved organic matter (DOM) is dependent on the algal strain [146]. Thus, DOM from some studied photosynthetic bacteria and the diatoms Thalassiosira and Phaeodactylum revealed a high complexity and strain dependence. It was also shown that the micro-environment plays an essential role in the bacterial interaction of Phaeodactylum. Introducing a polymer-based porous microtiter plate increased algal cell abundance and spatially influenced algal-bacterial interactions [147]. Moreover, exometabolites from P. tricornutum can influence bacterial communities, as they can act as selective bacterial growth substrates [148]. Exopolysaccharides were found to represent the largest fraction of microalgal exudates. In a coculture of P. tricornutum and the bacterium Pseudoalteromonas haloplanktis, the exo-environment described by exo-monosaccharide profiles varied depending on the culture condition [149]. This bacterium can utilize diatom-released compounds [150]. Algal-derived dissolved organic carbon and nitrogen and bacterial incorporation of these remineralized compounds were studied with P. tricornutum and several different bacteria. These studies defined three groups: macromolecule remineralizers, macromolecule users, and small-molecule users [151]. The relevance of dissolved organic nitrogen compounds was also shown with Rhodobacteriaceae-type strains that interact with P. tricornutum and can degrade such compounds [152].
Some bacteria act algicidal on P. tricornutum, such as Kordia algicida, which releases an algicidal protease [69]. Moreover, there are also secondary metabolites that act antagonistic. For example, the release of 2-heptyl-4-quinolone from marine bacteria causes inhibition of the cytochrome b6f complex in P. tricornutum and inhibition of its respiration [153].
3.2. The Coccolithophore Emiliania huxleyi: An Algal Chameleon
Coccolithophore haptophyte algae such as E. huxleyi are key contributors to global carbon fluxes and are essential for biogeochemistry [154]. E. huxleyi is ecologically highly relevant; it can form large blooms that are even visible from space. The pangenome of the E. huxleyi reference strain CCMP 1516 and of 13 additional isolates has been sequenced [155]. A transformation method via electroporation based on a virus approach was also established [156].
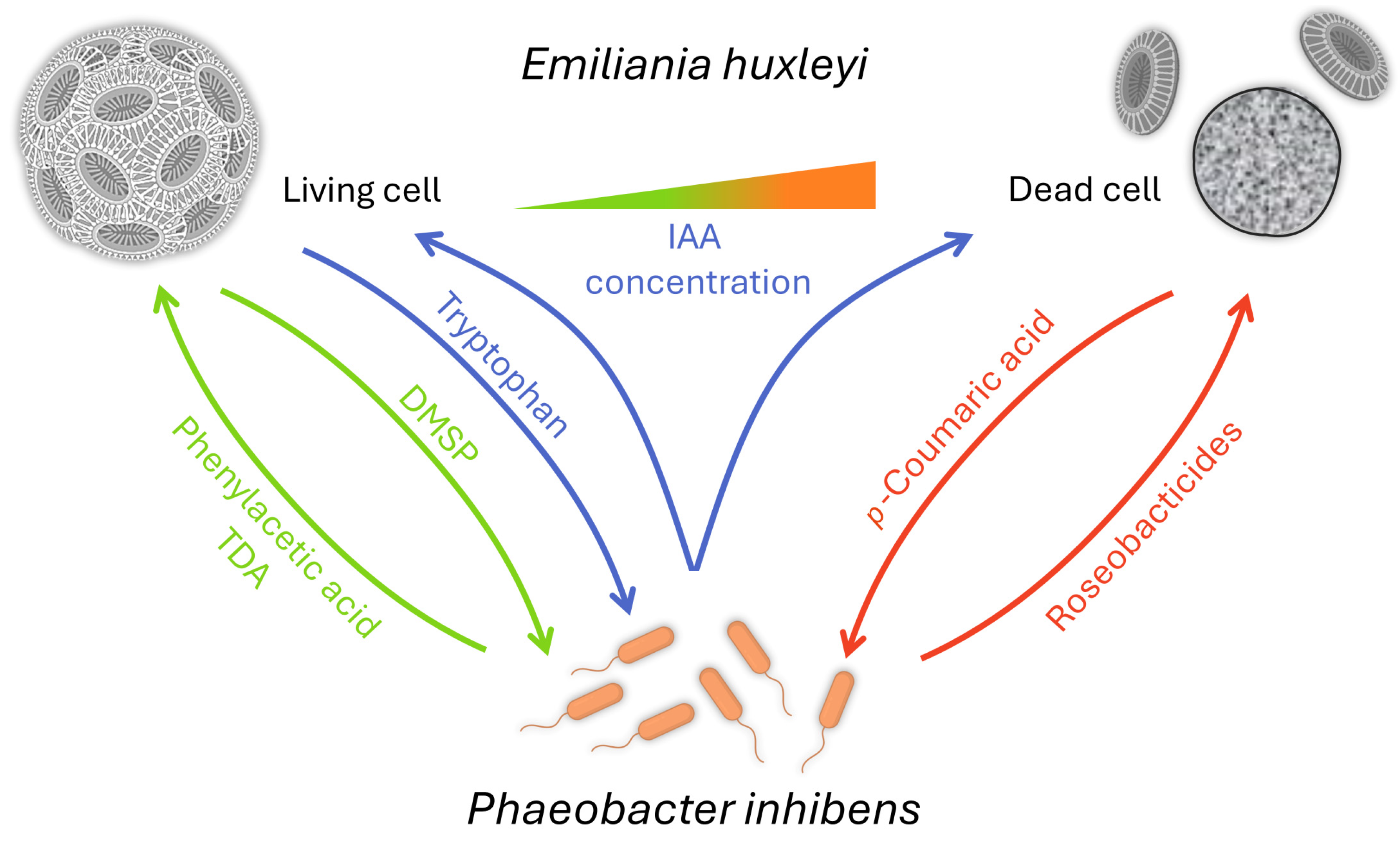
One of the first fascinating reports dealt with the interaction of E. huxleyi with a Roseobacter clade bacterium named Phaeobacter gallaeciensis, also known as P. inhibens [157]. The bacterium supports algal growth via an algal growth promotor, phenylacetic acid, and an antibiotic that protects the host from bacterial pathogens called tropodithietic acid (TDA). In return, the algal cells provide dimethylsulfoniopropionate (DMSP), as C- and S-sources, and a biofilm surface to the bacteria [158,159]. It was also found that auxin plays a role in both the growth promotion of E. huxleyi by P. inhibens and the ultimate death of the algae [160]. E. huxleyi cells exude tryptophan in normal conditions, increasing the bacterial production of IAA and attachment to the algae. When high concentrations of IAA are reached, the algal cell will enter a senescence stage and produce p-coumaric acid, which initiates the synthesis of bacterial roseobacticides. These secondary metabolites are algicidal. Thus, the aging algae convert P. inhibens into a pathogen [158] (Figure 2). Interestingly, the synthesis of the roseobacticides is based on algal and bacterial precursor biomolecules [159]. Moreover, the genes required for TDA synthesis are also necessary for producing roseobacticides. Here, one gene cluster synthesizes two biomolecules that differ in structure and function [161].
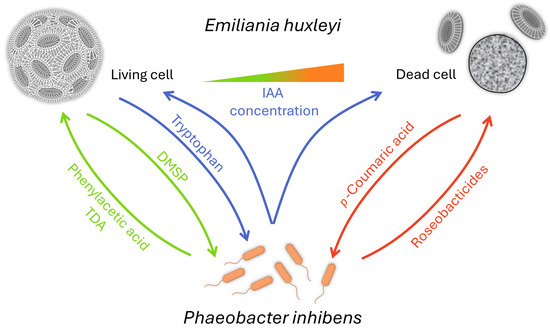
Figure 2.
How bacterial friends turn into foes—from mutualism to antagonism [158,159,162]. E. huxleyi cells provide a biofilm surface and DMSP for its bacterial partner P. inhibens in healthy conditions. In return, the bacteria will provide the growth factor phenylacetic acid and the antibiotic TDA. In normal conditions, E. huxleyi cells also exude tryptophan, increasing the bacterial production of IAA and attachment to algae. When high concentrations of IAA are reached, the algal cell will enter a senescence stage and produce p-coumaric acid, triggering the production of the algicidal roseobactericides by P. inhibens.
Remarkably, associated bacterial communities can protect E. huxleyi from the pathogen, such as the helper bacteria Sulfitobacter pontiacus [163]. P. inhibens can also influence the production of algal alkenone lipids that play a role in estimating sea surface temperature. Unsaturated alkenones are increased in algal-bacterial cocultures, leading to changes in growth temperature up to 2 °C compared to axenic cultures [162]. A bacterial lifestyle switch with E. huxleyi was also observed with Sulfitobacter D7 [164], where algal DMSP is involved in the switch to pathogenicity. Notably, algal-produced benzoate promotes the growth of the bacterium and hinders the DMSP-caused switch [164]. The diversity of bacterial communities present in an E. huxleyi bloom was also studied, and the initial inoculum plays a major role in shaping the microbiome community [165]. These data emphasize that algal-bacterial relationships can transition between mutualistic and antagonistic states within the algal lifespan. Furthermore, they highlight the relevance of the surrounding bacterial microbiome as present in nature.
3.3. Dinoflagellate Symbiotic Symbiodiniaceae and Toxic Alexandrium sp.
Dinoflagellates are found in freshwater and oceans as a significant part of phytoplankton. They are distinguished by their enormous genome sizes, which can be attributed to the presence of hundreds of gene copies. This includes, for example, the gene encoding the luciferin-binding protein found in Gonyaulax polyedra [166], nowadays called Linguludinium polyedra. The genomic and transcriptomic characterization of a dinoflagellate genome from the toxic Alexandrium ostenfeldii revealed tandem repeats covering more than half of the genome [167]. Some of these microalgae are bioluminescent [168,169], and others live in symbiosis with cnidarians in reef-building corals [170]. Coral reef symbionts include mainly members from the dinoflagellate family of Symbiodiniaceae [171], whose genomes have been analyzed [172,173]. Interestingly, the associated bacterial microbiomes are distinct across symbiotic states [174].
Coral reefs are very susceptible to ocean warming, and the loss of the dinoflagellate symbiont due to climate change results in coral bleaching [170]. There is evidence that bacterial communities that are associated with Symbiodiniaceae react to heat selection and may contribute to coral adaptation to altered temperatures [175]. Increased temperature also affects the bacterial microbiome of the red tide-causing Alexandrium minutum [176], highlighting that global temperature increases will perturb dinoflagellate ecology significantly.
As already indicated above, some dinoflagellates, including the Alexandrium species, are highly toxic and dominate harmful algal blooms, also known as red tides [177]. Bacteria are usually associated with these toxic species of Alexandrium, such as Pseudosulfitobacter koreense sp. nov., together with A. pacificum [178] or Haliea alexandrii with A. catenella [179]. A. catenella produces saxitoxin and is related to shellfish poisoning, which is also highly dangerous for human consumption [180]. It was found that some marine lytic bacteria from the Proteobacteria and Cytophaga group are closely associated with this dinoflagellate. Interestingly, a change from a high-nutrient medium to a medium without organic nutrients turns them into symbiotic partners [180]. Harmful algal blooms with A. fundyense influence the relative abundances of bacteria and phytoplankton by repressing and promoting different taxa [181]. Microbiome stability was also investigated with an axenic strain of A. tamarense, to which the intact microbiome was reintroduced. Interestingly, the original microbiome was not restored; instead, a bacterial community shift to the Roseobacter clade was observed [182]. These studies show that understanding dinoflagellate-bacterial interactions is the key to understanding the biology and ecology of these fascinating, but often also highly dangerous microalgae.
4. Marine Macroalgae
Bacteria also colonize marine macroalgae, and these bacterial communities are not fixed and can change temporally and spatially across seasons, lifespans, life stages, and tissue types, and are shaped by biotic and abiotic factors [25,183,184]. The following section describes three macroalgal models: the red, green, and brown algae.
4.1. The Rhodophyceae Delisea pulchra and Its Bacterial Enemies
The red macroalga Delisea pulchra (Rhodophyta, Bonnemaisonales) is a widely distributed, shallow-subtidal red alga occurring throughout Southern Australia, New Zealand, Japan, and Antarctica [185,186,187]. It is one of the best-studied models for the interactions between macroalgae, bacteria, and the environment in the context of disease [188].
Bleaching disease is one of the few well-characterized examples of disease in macroalgae. A bacterial infection causes this disease [189,190], leading to the loss of photosynthetic pigments along the thallus, resulting in tissue necrosis, reduced fecundity, and increased herbivory [191]. The occurrence of bleaching disease is highly correlated with increased seawater temperatures in the summer months, which is thought to reduce the algae’s natural chemical defenses and render it more susceptible to microbial infections [192]. These infections lead to a downregulation of genes coding for predicted protein metabolism, stress response, energy generation, and photosynthesis functions. The rapid repression of genes coding for core cellular processes will likely interfere with the macroalgal antipathogen response, leading to infection, tissue damage, and bleaching symptoms [193].
Furthermore, 16S rRNA gene library analysis showed that D. pulchra bacterial communities contained seven phyla, including 79 species. Alpha-, Delta-, and Gammaproteobacteria are well-represented, and Planctomycetes and Bacteroidetes are relatively common in healthy D. pulchra [194]. Bleached D. pulchra microbiota is enriched in Rhodobacteraceae, Saprospiraceae, and Flavobacteriaceae [195]. Colwelliaceae and Rhodobacteraceae families and Thalassomonas and Parvularcula genera were the most impacted taxa between healthy and bleached communities [196]. Also, comparative metagenomics showed changes in functions associated with transcriptional regulation, cation/multidrug efflux, and nonribosomal peptide synthesis [196]. Several bacterial strains are responsible for bleaching symptoms, e.g., Alteromonas sp. BL110, Aquimarina sp. AD1 and BL5, and Agarivorans sp BL7 [190].
In response to the pathogen, D. pulchra will produce a range of secondary metabolites based on brominated furanones [189,192,197]. These molecules antagonize the same receptor as AHL—a group of widespread intercellular communication signals among bacteria. Halogenated furanones compete with and inhibit bacterial cell-to-cell communication and thus interfere with critical bacterial communication-regulated processes, such as biofilm formation and virulence [188]. They will also help the alga to shape its associated bacterial community by selecting specific groups [198]. Halogenated furanones are localized in the central vesicle of gland cells in D. pulchra [199]. Quantitative variations of D. pulchra furanones are driven by small-scale variations in environmental factors (nutrients and light) and genetic differences among individuals [200,201]. These cells release furanone onto the alga’s surface, and furanones levels on the thallus were highest near the apical tips and decreased towards the base of the alga. Variation in furanone levels within the plant and the number of gland cells followed a similar pattern [199]. These brominated furanones covalently modify and inactivate the LuxS enzyme, which produces autoinducer-2, molecules involved in bacterial quorum-sensing [202]. Furanones can also modulate the cellular concentration of the LuxR protein responsible for the reception of and response to AHLs [203,204]. In parallel to the algal chemical defense, the already established algal microbiota can prevent the pathogenicity of other bacteria, such as Phaeobacter sp. BS52. That bacterial strain, isolated from healthy D. pulchra, was antagonistic towards bleaching pathogens by significantly increasing the proportion of healthy individuals, suggesting a protective activity by preventing dysbiosis rather than direct pathogen inhibition [205].
4.2. The Chlorophyceae Ulva sp. Needs Its Bacteria to Get in Shape
The marine green macroalga Ulva (Chlorophyta, Ulvales), also named sea lettuce or gut weeds, is a model organism for morphogenesis studies, seaweed-bacteria interactions, and chemical ecology. The whole-genome sequence of U. mutabilis is available [206], and its genome contains genes encoding enzymes involved in the production and transformation of DMSP. A gene sequence homologous to the DMSP lyase Alma [207] enables Ulva to convert DMSP to dimethyl sulfide under axenic conditions [208], making this alga an essential component of the marine sulfur cycle because of its rapid growth and high DMSP content. DMSP can play multiple roles as an osmolyte and cryoprotectant in the thallus [209,210], as well as a chemoattractant [208].
In natural populations, Ulva-associated bacterial composition is strongly structured by both salinity and host species [211]. The bacterial communities are mainly composed of Alphaproteobacteria and Bacteroidete members, especially within the Rhodobacteriaceae, Sphingomonadaceae, Flavobacteriaceae, and Sapropiraceae families [212]. Several of these marine bacteria will have an impact on the morphologies and polymorphisms of various Ulva species, such as U. clathrata [213], U. fasciata [214], U. intestinalis [215], U. linza [216], U. mutabilis [215,217], or U. pertusa [218].
The first step in the interaction between Ulva and marine bacteria occurs during the settlement of zoospores, the motile reproductive stage of Ulva. Biofilms that release AHLs attract zoospores [219]. The swimming rate is reduced when zoospores detect AHLs, resulting in the accumulation of cells at the source of the AHLs. AHLs probably act as cues for the settlement of zoospores, rather than being directly involved as a signaling mechanism [50].
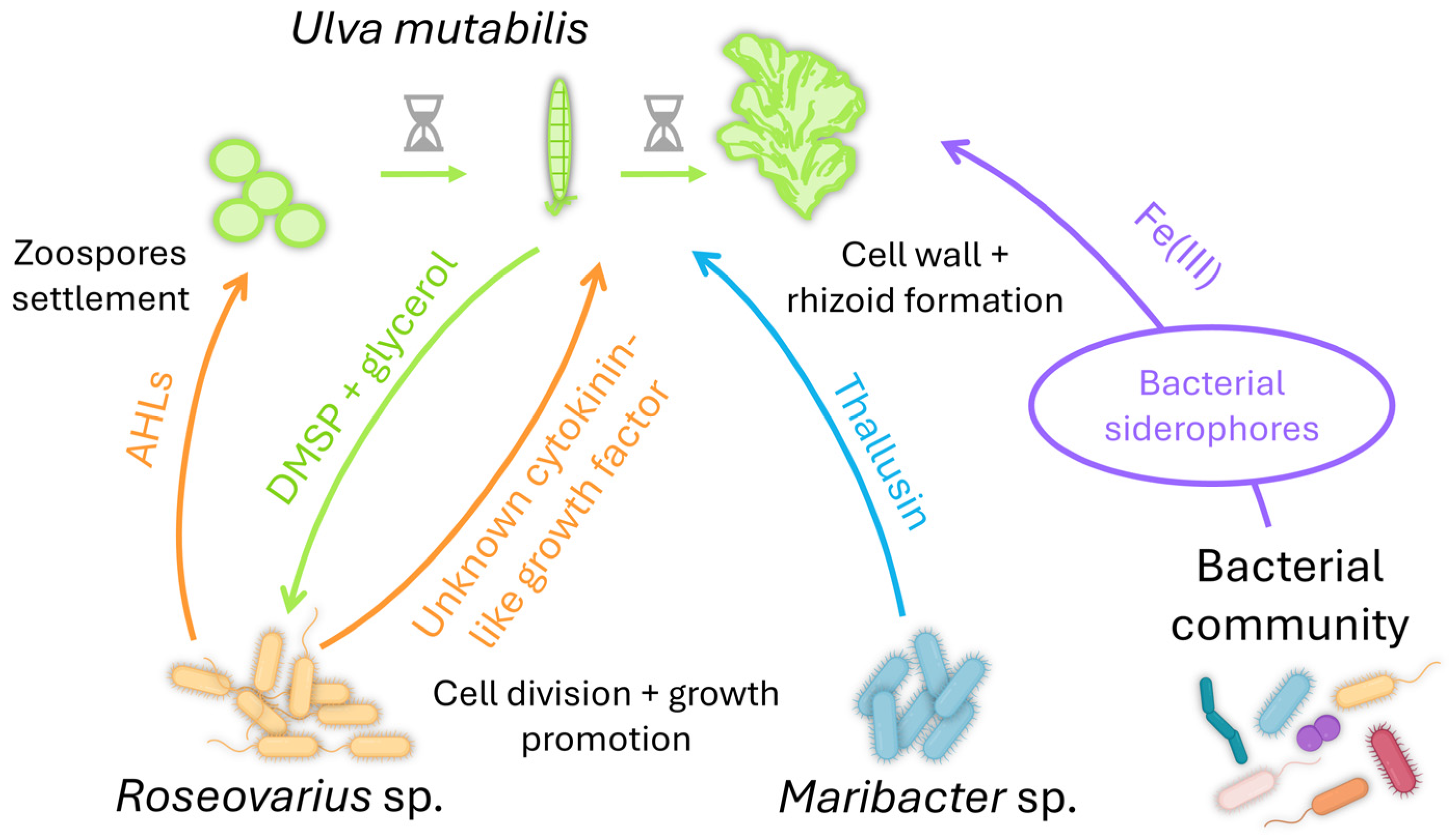
One of the most described interactions of Ulva with bacteria is the tripartite interaction Ulva-Maribacter-Roseovarius (Figure 3; [220,221]). U. mutabilis provides a substrate layer enriched with glycerol, a carbon source for Roseovarius sp., supporting biofilm formation [52]. The macroalga also releases DMSP to attract Roseovarius sp. [208,222], which secretes an unknown cytokinin-like growth factor promoting cell division and growth [217]. The tripartite interaction is completed with Maribacter sp., which is mainly found attached to the rhizoidal zone of the alga [223]. Maribacter sp. produces thallusin, a differentiation inducer that promotes rhizoid and cell wall formation [224]. Thallusin was isolated from the epiphytic marine bacterium YM2-23 (Cytophaga-Flavobacteria-Bacteroides group) of the green alga Monostroma sp. [225,226]. Ulva can also acquire iron from associated bacterial communities [227,228], as its genome contains iron uptake genes coding for putative transporter [206]. Ulva can get iron by importing a 2:1 thallusin-Fe(III)-complex [224]. The role of the Fe(III)-complex remains to be defined, as the cellular uptake of iron facilitated by thallusin derivatives was independent of their morphogenic activity [229]. These results also suggest their active import via siderophore transporters as a shuttle system.
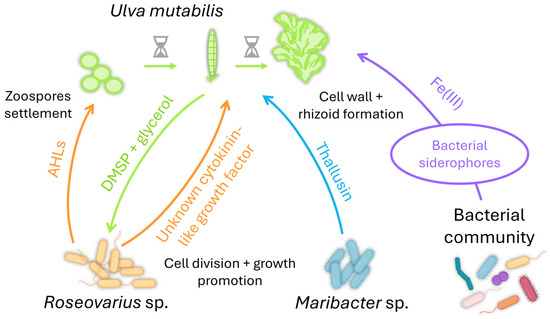
Figure 3.
The beneficial tripartite interaction Ulva-Roseovarius-Maribacter (reviewed in [221]). Roseovarius sp. secrete AHLs that act as cues for the zoospores’ settlement. U. mutabilis also provides a surface enriched in glycerol and DMSP for biofilm formation by Roseovarius sp. This bacterial partner promotes cell division and growth by producing an unknown cytokinin-like factor. The third partner, Maribacter sp., induces cell wall and rhizoid formation via the production of thallusin. Ulva can also acquire iron from its associated bacterial communities via siderophores.
4.3. The Phaeophyceae Ectocarpus sp. Needs Bacteria for Its Shape, Sex, Fitness, and Environmental Adaptation
The brown filamentous macroalga Ectocarpus (Ochrophyta, Ectocarpales) is a cosmopolitan genus that occurs worldwide across temperate and subtropical regions and has been sampled on all continents except Antarctica [230].
Ectocarpus sp. is a genetic and genomic model for brown algae [231]. The genome [232], corresponding metabolic network [233], and transformation protocol by CRISPR-Cas9 [234] are available. Ectocarpus is currently being used as a laboratory model to study several aspects of brown algal biology, including the implementation of in silico approaches to understand brown algal metabolism [233,235,236] and biophysical approaches to study brown algal growth and morphology [237,238]. Ectocarpus is also used to study interactions between brown algae and other organisms, such as symbiotic bacteria [239,240,241] or pathogens [242]. Most studies on the host-bacteria interactions in Ectocarpus use a screening approach by first identifying the macroalgae-associated microorganisms and characterizing potential beneficial or pathogenic effects, then creating synthetic communities and testing in vitro their effects on algal biological parameters.
It has been shown that Ectocarpus depends on its microbiota for its morphology, reproduction, fitness, and adaptation to a changing environment. When cultured under axenic conditions, Ectocarpus sp. loses its branched morphology and grows with a small ball-like appearance [240,243]. Similarly, bacterial isolates significantly promoted the production of new germlings in Ectocarpus [243]. Additionally, adaptation to a salinity gradient is only possible in the presence of an associated bacterial community. Ectocarpus relies on bacteria to facilitate the transition from low to high salinity, and cultures deprived of their associated microbiome fail to survive a transfer to freshwater. However, restoring their microflora also restores their capacity to acclimate to this change [244]. While there are compositional differences in bacterial communities associated with algal hosts, there were no functional differences, suggesting functional redundancy in the associated bacterial community [244].
The microbiota composition of lab-cultured E. subulatus was assessed with isolation techniques and 16S rRNA metabarcoding [239]. The identified isolates belong to 33 genera, with Halomonas (Gammaproteobacteria), Bosea (Alphaproteobacteria), and Limnobacter (Betaproteobacteria) being the most abundant [239]. Metabarcoding sequences data clustered into 48 operational taxonomic units. The genus Alteromonas represents 41.6% of the reads, making Gammaproteobacteria the most abundant class. The microbiota of Ectocarpus subulatus natural populations from Australia was also characterized by 16S rRNA metabarcoding [245]. The bacterial communities were dominated by Alphaproteobacteria (25% of reads), Bacteriodetes (20%), Gammaproteobacteria (8%), Planctomycetes (8%), and Actinobacteria (8%) [245].
Following the characterization of Ectocarpus microbiota, Karimi et al. annotated the draft genomes of seventy-two cultivable Ectocarpus-associated bacteria [246]. Gene clusters related to secondary metabolites production revealed that terpene, bacteriocin, nonribosomal peptide synthetases, polyketide synthases type 1 and type 3, siderophore, and homoserine lactone clusters were abundant in these genomes. Moreover, detoxification and provision of vitamin B pathways have been observed, highlighting potential contributions of bacterial metabolism toward host fitness and survival [246]. Also, coculture experiments with specific bacteria showed a significant increase in algal growth [240,241]. Seven metabolites predicted to be producible by the algae only through metabolic exchanges with specific bacteria [236,247] were selected for targeted metabolite profiling: L-histidine, putrescine, beta-alanine, nicotinic acid, folic acid, auxin, and spermidine. In several cases, these key metabolites were detected only in the inoculated cocultures [240].
5. Conclusions and Futures Directions
This review highlights the importance of bacteria associated with micro- and macroalgae. Bacteria can not only promote or even enable algal growth, but they are also essential for the development of some algae, shaping their morphology, as exemplified with Ulva and Ectocarpus in Section 4.2 and Section 4.3. Moreover, bacteria can be detrimental to algae, ultimately leading to their death. Often, primary and secondary metabolites are involved in these interactions. Beneficial primary metabolites include N- and C-sources or vitamins, and toxins compromise many natural products such as, for example, cyclic lipopeptides, polyynes, or phenolic compounds (see Table 1). Some metabolites are altered depending on the age of the algal culture, thus triggering the change from a mutualistic lifestyle to an antagonistic one (see Figure 2). Abiotic factors such as structured surfaces can influence microbial interactions. Moreover, temperature can play a critical role, as emphasized by coral bleaching due to the loss of the algal symbiont caused by climate change-induced heat waves. All these examples demonstrate how the bacterial microbiome associated with algae profoundly shapes their growth and life, consequently influencing their photosynthetic capacities that are necessary for sustaining life on Earth.
Currently, two major approaches are used for studying algal-microbial interactions, both with pros and cons. On one side, the entire microbiome associated with an alga is characterized, encompassing all microorganisms and their potential effects on algal life. However, such a microbiome is complex, and it is hard to understand the detailed roles of single partners and enemies in the consortium; “-omics” approaches are often used to get a first overview. Genomic and metabolomic tools are being developed to assess these interactions in silico. On the other side, individual bipartite are often examined by coculturing a single alga and a bacterium together. One alga and one bacterium are often put together in coculture. In this case, all exchanged metabolites can be studied; it can usually be predicted from which microorganism they are produced. However, this laboratory scenario does not reflect the natural environment, as we see in the case of those tripartite systems studied so far, where a third partner can already significantly change an alga’s interplay with a bacterium. In the future, it is desirable that we continuously increase the number of microorganisms in cocultures to study the influence of further partners, opportunistic pathogens, and enemies. Ultimately, we should aim to find out if new members may change their positive or negative behavior once confronted with further partners. It is also evident that the interaction of algae with bacteria is just a first step in understanding algal holobionts, as viruses, fungi, and other microorganisms will also be potential partners in the natural ecological environment.
Author Contributions
Conceptualization, B.B.-D. and M.M.; writing—original draft preparation, B.B.-D., P.S. and M.M.; writing—review and editing, B.B.-D., P.S., T.V. and M.M.; figures and table, T.V., B.B.-D. and M.M.; funding acquisition, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
P.S. and T.V. had fellowships from the Carl-Zeiss Foundation under the umbrella of the Jena School for Microbial Communication (JSMC). M.M. received funding from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) within the collaborative research center SFB1127/2 ChemBioSys—Project ID 239748522, subproject A02. M.M. and P.S. received funding from DFG within Germany’s Excellence Strategy—EXC 2051—Project-ID 390713860.
Data Availability Statement
The used information in this review has been referenced.
Acknowledgments
We thank Volker Wagner for valuable comments on the manuscript and Alsatia Lohr for proofreading. The figures were prepared with the help of Biorender.com (accessed 12 February 2024). We thank the funding agencies mentioned above.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AHL | N-acyl-homoserine lactones |
| DMSP | Dimethylsulfoniopropionate |
| DOM | Dissolved organic matter |
| IAA | Indole-3-acetic acid |
| T6SS | Type 6 secretion system |
| TDA | Tropodithietic acid |
| TRP | Transient receptor potential |
| VOC | Volatile organic compounds |
References
- Parker, M.S.; Mock, T.; Armbrust, E.V. Genomic insights into marine microalgae. Annu. Rev. Genet. 2008, 42, 619–645. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.M.; De Clerck, O. Embracing algal models. Semin. Cell Dev. Biol. 2023, 134, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Gentil, J.; Hempel, F.; Moog, D.; Zauner, S.; Maier, U.G. Review: Origin of complex algae by secondary endosymbiosis: A journey through time. Protoplasma 2017, 254, 1835–1843. [Google Scholar] [CrossRef]
- Strassert, J.F.H.; Irisarri, I.; Williams, T.A.; Burki, F. A molecular timescale for eukaryote evolution with implications for the origin of red algal-derived plastids. Nat. Commun. 2021, 12, 1879. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.M.; Cock, J.M. Brown algal model organisms. Annu. Rev. Genet. 2020, 54, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Courties, C.; Vaquer, A.; Troussellier, M.; Lautier, J.; Chrétiennot-Dinet, M.J.; Neveux, J.; Machado, C.; Claustre, H. Smallest eukaryotic organism. Nature 1994, 370, 255. [Google Scholar] [CrossRef]
- Van Den Hoek, C.; Mann, D.G.; Jahns, H.M. Algae: An Introduction to Phycology; Cambridge University Press: Cambridge, MA, USA; New York, NY, USA, 1995; ISBN 978-0-521-30419-1. [Google Scholar]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology, 3rd ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2022; ISBN 978-1-03-203512-3. [Google Scholar]
- Guiry, M.D. How many species of algae are there? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Falciatore, A.; Jaubert, M.; Bouly, J.-P.; Bailleul, B.; Mock, T. Diatom molecular research comes of age: Model species for studying phytoplankton biology and diversity. Plant Cell 2020, 32, 547–572. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Grube, M. Lichens redefined as complex ecosystems. New Phytol. 2020, 227, 1281–1283. [Google Scholar] [CrossRef]
- Calatrava, V.; Tejada-Jimenez, M.; Sanz-Luque, E.; Fernandez, E.; Galvan, A.; Llamas, A. Chlamydomonas reinhardtii, a reference organism to study algal-microbial interactions: Why can’t they be friends? Plants 2023, 12, 788. [Google Scholar] [CrossRef]
- Krug, L.; Erlacher, A.; Markut, K.; Berg, G.; Cernava, T. The microbiome of alpine snow algae shows a specific inter-kingdom connectivity and algae-bacteria interactions with supportive capacities. ISME J. 2020, 14, 2197–2210. [Google Scholar] [CrossRef] [PubMed]
- Treves, H.; Siemiatkowska, B.; Luzarowska, U.; Murik, O.; Fernandez-Pozo, N.; Moraes, T.A.; Erban, A.; Armbruster, U.; Brotman, Y.; Kopka, J.; et al. Multi-omics reveals mechanisms of total resistance to extreme illumination of a desert alga. Nat. Plants 2020, 6, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.H. Marine food webs. In The Structure of Marine Ecosystems; Harvard University Press: Cambridge, MA, USA, 1974; pp. 2–98. ISBN 978-0-674-59251-3. [Google Scholar]
- McCutcheon, J.; Lutz, S.; Williamson, C.; Cook, J.M.; Tedstone, A.J.; Vanderstraeten, A.; Wilson, S.; Stockdale, A.; Bonneville, S.; Anesio, A.M.; et al. Mineral phosphorus drives glacier algal blooms on the Greenland Ice Sheet. Nat. Commun. 2021, 12, 570. [Google Scholar] [CrossRef] [PubMed]
- B-Béres, V.; Stenger-Kovács, C.; Buczkó, K.; Padisák, J.; Selmeczy, G.B.; Lengyel, E.; Tapolczai, K. Ecosystem services provided by freshwater and marine diatoms. Hydrobiologia 2023, 850, 2707–2733. [Google Scholar] [CrossRef]
- Chung, I.K.; Oak, J.H.; Lee, J.A.; Shin, J.A.; Kim, J.G.; Park, K.-S. Installing kelp forests/seaweed beds for mitigation and adaptation against global warming: Korean Project Overview. ICES J. Mar. Sci. 2013, 70, 1038–1044. [Google Scholar] [CrossRef]
- Van Oostende, N.; Moerdijk-Poortvliet, T.C.W.; Boschker, H.T.S.; Vyverman, W.; Sabbe, K. Release of dissolved carbohydrates by Emiliania huxleyi and formation of transparent exopolymer particles depend on algal life cycle and bacterial activity. Environ. Microbiol. 2013, 15, 1514–1531. [Google Scholar] [CrossRef]
- Hartnett, H.E. Dissolved Organic Matter (DOM). In Encyclopedia of Engineering Geology; Bobrowsky, P., Marker, B., Eds.; Encyclopedia of Earth Sciences Series; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–3. ISBN 978-3-319-12127-7. [Google Scholar]
- González, J.M.; Simó, R.; Massana, R.; Covert, J.S.; Casamayor, E.O.; Pedrós-Alió, C.; Moran, M.A. Bacterial community structure associated with a dimethylsulfoniopropionate-producing North Atlantic algal bloom. Appl. Environ. Microbiol. 2000, 66, 4237–4246. [Google Scholar] [CrossRef]
- Durham, B.P.; Sharma, S.; Luo, H.; Smith, C.B.; Amin, S.A.; Bender, S.J.; Dearth, S.P.; Van Mooy, B.A.S.; Campagna, S.R.; Kujawinski, E.B.; et al. Cryptic carbon and sulfur cycling between surface ocean plankton. Proc. Natl. Acad. Sci. USA 2015, 112, 453–457. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 1997, 78, 1946–1957. [Google Scholar] [CrossRef]
- Egan, S.; Harder, T.; Burke, C.; Steinberg, P.; Kjelleberg, S.; Thomas, T. The seaweed holobiont: Understanding seaweed-bacteria interactions. FEMS Microbiol. Rev. 2013, 37, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Steneck, R.S.; Graham, M.H.; Bourque, B.J.; Corbett, D.; Erlandson, J.M.; Estes, J.A.; Tegner, M.J. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ. Conserv. 2002, 29, 436–459. [Google Scholar] [CrossRef]
- Araújo, R.M.; Assis, J.; Aguillar, R.; Airoldi, L.; Bárbara, I.; Bartsch, I.; Bekkby, T.; Christie, H.; Davoult, D.; Derrien-Courtel, S.; et al. Status, trends and drivers of kelp forests in Europe: An expert assessment. Biodivers. Conserv. 2016, 25, 1319–1348. [Google Scholar] [CrossRef]
- Diehl, N.; Li, H.; Scheschonk, L.; Burgunter-Delamare, B.; Niedzwiedz, S.; Forbord, S.; Sæther, M.; Bischof, K.; Monteiro, C. The sugar kelp Saccharina latissima I: Recent advances in a changing climate. Ann. Bot. 2024, 133, 183–212. [Google Scholar] [CrossRef] [PubMed]
- González-Olalla, J.M.; Medina-Sánchez, J.M.; Lozano, I.L.; Villar-Argaiz, M.; Carrillo, P. Climate-driven shifts in algal-bacterial interaction of high-mountain lakes in two years spanning a decade. Sci. Rep. 2018, 8, 10278. [Google Scholar] [CrossRef] [PubMed]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2008, 65, 535–543. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- McHugh, D.J. A Guide to the Seaweed Industry; FAO Fisheries Technical Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; ISBN 978-92-5-104958-7. [Google Scholar]
- Yaakob, Z.; Ali, E.; Zainal, A.; Mohamad, M.; Takriff, M.S. An overview: Biomolecules from microalgae for animal feed and aquaculture. J. Biol. Res. 2014, 21, 6. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. The potential of microalgae and their biopolymers as structuring ingredients in food: A review. Biotechnol. Adv. 2019, 37, 107419. [Google Scholar] [CrossRef]
- Indergaard, M.; Ostgaard, K. Polysaccharides for food and pharmaceutical uses. In Seaweed Resources in Europe: Uses and Potential; Guiry, M.D., Blunden, G., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1991; pp. 169–183. ISBN 0-471-92947-6. [Google Scholar]
- Onen Cinar, S.; Chong, Z.K.; Kucuker, M.A.; Wieczorek, N.; Cengiz, U.; Kuchta, K. Bioplastic production from microalgae: A review. Int. J. Environ. Res. Public. Health 2020, 17, 3842. [Google Scholar] [CrossRef]
- Schmidtchen, L.; Roleda, M.Y.; Majschak, J.-P.; Mayser, M. Processing technologies for solid and flexible packaging materials from macroalgae. Algal Res. 2022, 61, 102300. [Google Scholar] [CrossRef]
- Karuppusamy, S.; Rajauria, G.; Fitzpatrick, S.; Lyons, H.; McMahon, H.; Curtin, J.; Tiwari, B.K.; O’Donnell, C. Biological properties and health-promoting functions of laminarin: A comprehensive review of preclinical and clinical studies. Mar. Drugs 2022, 20, 772. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Microalgae in medicine and human health. In Microalgae in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2018; pp. 195–210. ISBN 978-0-12-811405-6. [Google Scholar]
- Blunden, G. Agricultural uses of seaweeds and seaweed extracts. In Seaweed Resources in Europe; Guiry, M.D., Blunden, G., Eds.; John Wiley & Sons: Chichester, UK, 1991; pp. 65–81. [Google Scholar]
- Castro, J.D.S.; Calijuri, M.L.; Ferreira, J.; Assemany, P.P.; Ribeiro, V.J. Microalgae based biofertilizer: A life cycle approach. Sci. Total Environ. 2020, 724, 138138. [Google Scholar] [CrossRef] [PubMed]
- Sæther, M.; Diehl, N.; Monteiro, C.; Huiru, L.; Niedzwiedz, S.; Burgunter-Delamare, B.; Scheschonk, L.; Bischof, K.; Forbord, S. The sugar kelp Saccharina latissima II: Recent advances in farming and applications. J. Appl. Phycol. 2024. [Google Scholar] [CrossRef]
- Khetkorn, W.; Rastogi, R.P.; Incharoensakdi, A.; Lindblad, P.; Madamwar, D.; Pandey, A.; Larroche, C. Microalgal hydrogen production—A review. Bioresour. Technol. 2017, 243, 1194–1206. [Google Scholar] [CrossRef] [PubMed]
- Branco-Vieira, M.; San Martin, S.; Agurto, C.; Freitas, M.A.V.; Martins, A.A.; Mata, T.M.; Caetano, N.S. Biotechnological potential of Phaeodactylum tricornutum for biorefinery processes. Fuel 2020, 268, 117357. [Google Scholar] [CrossRef]
- Kouhgardi, E.; Zendehboudi, S.; Mohammadzadeh, O.; Lohi, A.; Chatzis, I. Current status and future prospects of biofuel production from brown algae in North America: Progress and challenges. Renew. Sustain. Energy Rev. 2023, 172, 113012. [Google Scholar] [CrossRef]
- Broch, O.; Ellingsen, I.; Forbord, S.; Wang, X.; Volent, Z.; Alver, M.; Handå, A.; Andresen, K.; Slagstad, D.; Reitan, K.; et al. Modelling the cultivation and bioremediation potential of the kelp Saccharina latissima in close proximity to an exposed salmon farm in Norway. Aquac. Environ. Interact. 2013, 4, 187–206. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.-S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Bell, W.; Mitchell, R. Chemotactic and growth responses of marine bacteria to algal extracellular products. Biol. Bull. 1972, 143, 265–277. [Google Scholar] [CrossRef]
- Durán, P.; Flores-Uribe, J.; Wippel, K.; Zhang, P.; Guan, R.; Melkonian, B.; Melkonian, M.; Garrido-Oter, R. Shared features and reciprocal complementation of the Chlamydomonas and Arabidopsis microbiota. Nat. Commun. 2022, 13, 406. [Google Scholar] [CrossRef] [PubMed]
- Joint, I.; Tait, K.; Wheeler, G. Cross-kingdom signalling: Exploitation of bacterial quorum sensing molecules by the green seaweed Ulva. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Goecke, F.; Labes, A.; Wiese, J.; Imhoff, J. Chemical interactions between marine macroalgae and bacteria. Mar. Ecol. Prog. Ser. 2010, 409, 267–299. [Google Scholar] [CrossRef]
- Alsufyani, T.; Weiss, A.; Wichard, T. Time course exo-metabolomic profiling in the green marine macroalga Ulva (Chlorophyta) for identification of growth phase-dependent biomarkers. Mar. Drugs 2017, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Adouane, E.; Mercier, C.; Mamelle, J.; Willocquet, E.; Intertaglia, L.; Burgunter-Delamare, B.; Leblanc, C.; Rousvoal, S.; Lami, R.; Prado, S. Importance of quorum sensing crosstalk in the brown alga Saccharina latissima epimicrobiome. iScience 2024, 27, 109176. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.R.; Amin, S.A.; Raina, J.-B.; Stocker, R. Zooming in on the phycosphere: The ecological interface for phytoplankton-bacteria relationships. Nat. Microbiol. 2017, 2, 17065. [Google Scholar] [CrossRef] [PubMed]
- Wadhams, G.H.; Armitage, J.P. Making sense of it all: Bacterial chemotaxis. Nat. Rev. Mol. Cell Biol. 2004, 5, 1024–1037. [Google Scholar] [CrossRef]
- Stocker, R.; Seymour, J.R. Ecology and physics of bacterial chemotaxis in the ocean. Microbiol. Mol. Biol. Rev. 2012, 76, 792–812. [Google Scholar] [CrossRef]
- Amsler, C.D. Algal Chemical Ecology; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-74180-0. [Google Scholar]
- Govorunova, E.G.; Sineshchekov, O.A. Chemotaxis in the green flagellate alga Chlamydomonas. Biochemistry 2005, 70, 717–725. [Google Scholar] [CrossRef]
- Bordenstein, S.R.; Theis, K.R. Host biology in light of the microbiome: Ten principles of holobionts and hologenomes. PLoS Biol. 2015, 13, e1002226. [Google Scholar] [CrossRef]
- Rosenberg, E.; Zilber-Rosenberg, I. The hologenome concept of evolution after 10 years. Microbiome 2018, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Dittami, S.M.; Arboleda, E.; Auguet, J.-C.; Bigalke, A.; Briand, E.; Cárdenas, P.; Cardini, U.; Decelle, J.; Engelen, A.H.; Eveillard, D.; et al. A community perspective on the concept of marine holobionts: Current status, challenges, and future directions. PeerJ 2021, 9, e10911. [Google Scholar] [CrossRef] [PubMed]
- Brodie, J.; Ball, S.G.; Bouget, F.; Chan, C.X.; De Clerck, O.; Cock, J.M.; Gachon, C.; Grossman, A.R.; Mock, T.; Raven, J.A.; et al. Biotic interactions as drivers of algal origin and evolution. New Phytol. 2017, 216, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Hom, E.F.Y.; Aiyar, P.; Schaeme, D.; Mittag, M.; Sasso, S. A chemical perspective on microalgal-microbial interactions. Trends Plant Sci. 2015, 20, 689–693. [Google Scholar] [CrossRef]
- Cirri, E.; Pohnert, G. Algae-bacteria interactions that balance the planktonic microbiome. New Phytol. 2019, 223, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Tirichine, L.; Piganeau, G. Editorial: Algal symbiotic relationships in freshwater and marine environments. Front. Plant Sci. 2023, 14, 1155759. [Google Scholar] [CrossRef] [PubMed]
- Chomicki, G.; Kiers, E.T.; Renner, S.S. The evolution of mutualistic dependence. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 409–432. [Google Scholar] [CrossRef]
- Mathis, K.A.; Bronstein, J.L. Our current understanding of commensalism. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 167–189. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Su, J.; Tian, Y.; Ning, X.; Hong, H.; Zheng, T. Lysis of a red-tide causing alga, Alexandrium tamarense, caused by bacteria from its phycosphere. Biol. Control 2010, 52, 123–130. [Google Scholar] [CrossRef]
- Paul, C.; Pohnert, G. Interactions of the algicidal bacterium Kordia algicida with diatoms: Regulated protease excretion for specific algal lysis. PLoS ONE 2011, 6, e21032. [Google Scholar] [CrossRef]
- Aiyar, P.; Schaeme, D.; García-Altares, M.; Carrasco Flores, D.; Dathe, H.; Hertweck, C.; Sasso, S.; Mittag, M. Antagonistic bacteria disrupt calcium homeostasis and immobilize algal cells. Nat. Commun. 2017, 8, 1756. [Google Scholar] [CrossRef] [PubMed]
- Krespach, M.K.C.; García-Altares, M.; Flak, M.; Schoeler, H.; Scherlach, K.; Netzker, T.; Schmalzl, A.; Mattern, D.J.; Schroeckh, V.; Komor, A.; et al. Lichen-like association of Chlamydomonas reinhardtii and Aspergillus nidulans protects algal cells from bacteria. ISME J. 2020, 14, 2794–2805. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, D.C.; McKeown, D.A. Viruses of seaweeds. In Studies in Viral Ecology; Hurst, C.J., Ed.; Wiley: Hoboken, NJ, USA, 2021; pp. 121–138. ISBN 978-1-119-60836-3. [Google Scholar]
- Lauritano, C.; Galasso, C. Microbial interactions between marine microalgae and fungi: From chemical ecology to biotechnological possible applications. Mar. Drugs 2023, 21, 310. [Google Scholar] [CrossRef] [PubMed]
- Schleyer, G.; Kuhlisch, C.; Ziv, C.; Ben-Dor, S.; Malitsky, S.; Schatz, D.; Vardi, A. Lipid biomarkers for algal resistance to viral infection in the ocean. Proc. Natl. Acad. Sci. USA 2023, 120, e2217121120. [Google Scholar] [CrossRef] [PubMed]
- Walde, M.; Camplong, C.; De Vargas, C.; Baudoux, A.-C.; Simon, N. Viral infection impacts the 3D subcellular structure of the abundant marine diatom Guinardia delicatula. Front. Mar. Sci. 2023, 9, 1034235. [Google Scholar] [CrossRef]
- Amin, S.A.; Parker, M.S.; Armbrust, E.V. Interactions between diatoms and bacteria. Microbiol. Mol. Biol. Rev. 2012, 76, 667–684. [Google Scholar] [CrossRef]
- Zuccaro, A.; Mitchell, J. Fungal communities of seaweeds. In The Fungal Community: Its Organization and Role in the Ecosystem; Dighton, J., White, J.F., Eds.; CRC Press: New York, NY, USA, 2005; pp. 533–580. ISBN 978-0-429-11640-7. [Google Scholar]
- Vallet, M.; Strittmatter, M.; Murúa, P.; Lacoste, S.; Dupont, J.; Hubas, C.; Genta-Jouve, G.; Gachon, C.M.M.; Kim, G.H.; Prado, S. Chemically-mediated interactions between macroalgae, their fungal endophytes, and protistan pathogens. Front. Microbiol. 2018, 9, 3161. [Google Scholar] [CrossRef]
- Tourneroche, A.; Lami, R.; Hubas, C.; Blanchet, E.; Vallet, M.; Escoubeyrou, K.; Paris, A.; Prado, S. Bacterial-fungal interactions in the kelp endomicrobiota drive autoinducer-2 quorum sensing. Front. Microbiol. 2019, 10, 1693. [Google Scholar] [CrossRef]
- Tourneroche, A.; Lami, R.; Burgaud, G.; Domart-Coulon, I.; Li, W.; Gachon, C.; Gèze, M.; Boeuf, D.; Prado, S. The bacterial and fungal microbiota of Saccharina latissima (Laminariales, Phaeophyceae). Front. Mar. Sci. 2020, 7, 587566. [Google Scholar] [CrossRef]
- Pauli, J.N.; Mendoza, J.E.; Steffan, S.A.; Carey, C.C.; Weimer, P.J.; Peery, M.Z. A syndrome of mutualism reinforces the lifestyle of a sloth. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133006. [Google Scholar] [CrossRef]
- Carrasco Flores, D.; Fricke, M.; Wesp, V.; Desirò, D.; Kniewasser, A.; Hölzer, M.; Marz, M.; Mittag, M. A marine Chlamydomonas sp. emerging as an algal model. J. Phycol. 2021, 57, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Mock, T. Algal model species for advancing biological sciences. J. Phycol. 2023, 59, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Burki, F.; Roger, A.J.; Brown, M.W.; Simpson, A.G.B. The new tree of Eukaryotes. Trends Ecol. Evol. 2020, 35, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Findinier, J.; Grossman, A.R. Chlamydomonas: Fast tracking from genomics. J. Phycol. 2023, 59, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Merchant, S.S.; Prochnik, S.E.; Vallon, O.; Harris, E.H.; Karpowicz, S.J.; Witman, G.B.; Terry, A.; Salamov, A.; Fritz-Laylin, L.K.; Maréchal-Drouard, L.; et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 2007, 318, 245–250. [Google Scholar] [CrossRef]
- Li, X.; Patena, W.; Fauser, F.; Jinkerson, R.E.; Saroussi, S.; Meyer, M.T.; Ivanova, N.; Robertson, J.M.; Yue, R.; Zhang, R.; et al. A genome-wide algal mutant library and functional screen identifies genes required for eukaryotic photosynthesis. Nat. Genet. 2019, 51, 627–635. [Google Scholar] [CrossRef]
- Fauser, F.; Vilarrasa-Blasi, J.; Onishi, M.; Ramundo, S.; Patena, W.; Millican, M.; Osaki, J.; Philp, C.; Nemeth, M.; Salomé, P.A.; et al. Systematic characterization of gene function in the photosynthetic alga Chlamydomonas reinhardtii. Nat. Genet. 2022, 54, 705–714. [Google Scholar] [CrossRef]
- Craig, R.J.; Gallaher, S.D.; Shu, S.; Salomé, P.A.; Jenkins, J.W.; Blaby-Haas, C.E.; Purvine, S.O.; O’Donnell, S.; Barry, K.; Grimwood, J.; et al. The Chlamydomonas Genome Project, version 6: Reference assemblies for mating-type plus and minus strains reveal extensive structural mutation in the laboratory. Plant Cell 2023, 35, 644–672. [Google Scholar] [CrossRef]
- Harris, E.H. The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use; Academic Press Inc.: San Diego, CA, USA, 1989; ISBN 978-1-4832-8860-4. [Google Scholar]
- Sasso, S.; Stibor, H.; Mittag, M.; Grossman, A.R. From molecular manipulation of domesticated Chlamydomonas reinhardtii to survival in nature. eLife 2018, 7, e39233. [Google Scholar] [CrossRef]
- Cooper, M.B.; Smith, A.G. Exploring mutualistic interactions between microalgae and bacteria in the omics age. Curr. Opin. Plant Biol. 2015, 26, 147–153. [Google Scholar] [CrossRef]
- Croft, M.T.; Lawrence, A.D.; Raux-Deery, E.; Warren, M.J.; Smith, A.G. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 2005, 438, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, K.E.; Collins, S.; Kazamia, E.; Purton, S.; Wheeler, G.L.; Smith, A.G. Fundamental shift in vitamin B12 eco-physiology of a model alga demonstrated by experimental evolution. ISME J. 2015, 9, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Bunbury, F.; Deery, E.; Sayer, A.P.; Bhardwaj, V.; Harrison, E.L.; Warren, M.J.; Smith, A.G. Exploring the onset of B12-based mutualisms using a recently evolved Chlamydomonas auxotroph and B12-producing bacteria. Environ. Microbiol. 2022, 24, 3134–3147. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Bishop, S.; Stessman, D.; Wright, D.; Spalding, M.H.; Halverson, L.J. Chlamydomonas reinhardtii thermal tolerance enhancement mediated by a mutualistic interaction with vitamin B12-producing bacteria. ISME J. 2013, 7, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Teplitski, M.; Chen, H.; Rajamani, S.; Gao, M.; Merighi, M.; Sayre, R.T.; Robinson, J.B.; Rolfe, B.G.; Bauer, W.D. Chlamydomonas reinhardtii secretes compounds that mimic bacterial signals and interfere with quorum sensing regulation in bacteria. Plant Physiol. 2004, 134, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Calatrava, V.; Hom, E.F.Y.; Llamas, Á.; Fernández, E.; Galván, A. OK, thanks! A new mutualism between Chlamydomonas and methylobacteria facilitates growth on amino acids and peptides. FEMS Microbiol. Lett. 2018, 365, fny021. [Google Scholar] [CrossRef] [PubMed]
- Calatrava, V.; Hom, E.F.Y.; Guan, Q.; Llamas, A.; Fernández, E.; Galván, A. Genetic evidence for algal auxin production in Chlamydomonas and its role in algal-bacterial mutualism. iScience 2024, 27, 108762. [Google Scholar] [CrossRef] [PubMed]
- Fakhimi, N.; Torres, M.J.; Fernández, E.; Galván, A.; Dubini, A.; González-Ballester, D. Chlamydomonas reinhardtii and Microbacterium forte sp. nov., a mutualistic association that favors sustainable hydrogen production. Sci. Total Environ. 2024, 913, 169559. [Google Scholar] [CrossRef]
- Hotter, V.; Zopf, D.; Kim, H.J.; Silge, A.; Schmitt, M.; Aiyar, P.; Fleck, J.; Matthäus, C.; Hniopek, J.; Yan, Q.; et al. A polyyne toxin produced by an antagonistic bacterium blinds and lyses a Chlamydomonad alga. Proc. Natl. Acad. Sci. USA 2021, 118, e2107695118. [Google Scholar] [CrossRef]
- Rose, M.M.; Scheer, D.; Hou, Y.; Hotter, V.S.; Komor, A.J.; Aiyar, P.; Scherlach, K.; Vergara, F.; Yan, Q.; Loper, J.E.; et al. The bacterium Pseudomonas protegens antagonizes the microalga Chlamydomonas reinhardtii using a blend of toxins. Environ. Microbiol. 2021, 23, 5525–5540. [Google Scholar] [CrossRef]
- Bando, Y.; Hou, Y.; Seyfarth, L.; Probst, J.; Götze, S.; Bogacz, M.; Hellmich, U.A.; Stallforth, P.; Mittag, M.; Arndt, H. Total synthesis and structure correction of the cyclic lipodepsipeptide Orfamide A. Chem. Eur. J. 2022, 28, e202104417. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Bando, Y.; Carrasco Flores, D.; Hotter, V.; Das, R.; Schiweck, B.; Melzer, T.; Arndt, H.; Mittag, M. A cyclic lipopeptide produced by an antagonistic bacterium relies on its tail and transient receptor potential-type Ca2+ channels to immobilize a green alga. New Phytol. 2023, 237, 1620–1635. [Google Scholar] [CrossRef] [PubMed]
- Gross, H.; Loper, J.E. Genomics of secondary metabolite production by Pseudomonas spp. Nat. Prod. Rep. 2009, 26, 1408. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Kobayashi, H.; Furukawa, J.; Namikoshi, M.; Okuda, S.; Sato, Z.; Matsuda, I.; Noda, T. Studies on macrocyclic lactone antibiotics. VII Structure of a phytotoxin “rhizoxin” produced by Rhizopus chinensis. J. Antibiot. 1984, 37, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yi, L.; Ren, S.; Yin, Q.; Xiang, W.; Zhang, X.; Xie, B. Algicidal interaction between Paenibacillus polymyxa MEZ6 and microalgae. J. Appl. Microbiol. 2022, 133, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Krespach, M.K.C.; Stroe, M.C.; Flak, M.; Komor, A.J.; Nietzsche, S.; Sasso, S.; Hertweck, C.; Brakhage, A.A. Bacterial marginolactones trigger formation of algal gloeocapsoids, protective aggregates on the verge of multicellularity. Proc. Natl. Acad. Sci. USA 2021, 118, e2100892118. [Google Scholar] [CrossRef] [PubMed]
- Cecchin, M.; Marcolungo, L.; Rossato, M.; Girolomoni, L.; Cosentino, E.; Cuine, S.; Li-Beisson, Y.; Delledonne, M.; Ballottari, M. Chlorella vulgaris genome assembly and annotation reveals the molecular basis for metabolic acclimation to high light conditions. Plant J. 2019, 100, 1289–1305. [Google Scholar] [CrossRef]
- Hovde, B.T.; Hanschen, E.R.; Steadman Tyler, C.R.; Lo, C.-C.; Kunde, Y.; Davenport, K.; Daligault, H.; Msanne, J.; Canny, S.; Eyun, S.; et al. Genomic characterization reveals significant divergence within Chlorella sorokiniana (Chlorellales, Trebouxiophyceae). Algal Res. 2018, 35, 449–461. [Google Scholar] [CrossRef]
- Wu, T.; Li, L.; Jiang, X.; Yang, Y.; Song, Y.; Chen, L.; Xu, X.; Shen, Y.; Gu, Y. Sequencing and comparative analysis of three Chlorella genomes provide insights into strain-specific adaptation to wastewater. Sci. Rep. 2019, 9, 9514. [Google Scholar] [CrossRef]
- Blanc, G.; Duncan, G.; Agarkova, I.; Borodovsky, M.; Gurnon, J.; Kuo, A.; Lindquist, E.; Lucas, S.; Pangilinan, J.; Polle, J.; et al. The Chlorella variabilis NC64A genome reveals adaptation to photosymbiosis, coevolution with viruses, and cryptic sex. Plant Cell 2010, 22, 2943–2955. [Google Scholar] [CrossRef]
- Chow, K.-C.; Tung, W.L. Electrotransformation of Chlorella vulgaris. Plant Cell Rep. 1999, 18, 778–780. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Zhang, Y.; Chen, X.; Zhang, P.; Ma, S. Development of a new method for genetic transformation of the green alga Chlorella ellipsoidea. Mol. Biotechnol. 2013, 54, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Goud, V.V.; Yamamoto, Y.; Sahoo, L. Efficient Agrobacterium tumefaciens-mediated stable genetic transformation of green microalgae, Chlorella sorokiniana. 3 Biotech 2021, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-R.; Ng, I.-S. Development of CRISPR/Cas9 system in Chlorella vulgaris FSP-E to enhance lipid accumulation. Enzyme Microb. Technol. 2020, 133, 109458. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, C.D.; Colwell, R.R.; Prescott, J.M. Numerical taxonomy of heterotrophic bacteria growing in association with continuous-culture Chlorella sorokiniana. Appl. Microbiol. 1969, 18, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Takihana, N.; Aoyagi, H.; Hanada, S.; Watanabe, Y.; Ohmura, N.; Saiki, H.; Tanaka, H. Symbiotic association in Chlorella culture. FEMS Microbiol. Ecol. 2005, 51, 187–196. [Google Scholar] [CrossRef]
- Park, Y.; Je, K.-W.; Lee, K.; Jung, S.-E.; Choi, T.-J. Growth promotion of Chlorella ellipsoidea by co-inoculation with Brevundimonas sp. isolated from the microalga. Hydrobiologia 2008, 598, 219–228. [Google Scholar] [CrossRef]
- Thi Vu, H.; Otsuka, S.; Ueda, H.; Senoo, K. Cocultivated bacteria can increase or decrease the culture lifetime of Chlorella vulgaris. J. Gen. Appl. Microbiol. 2010, 56, 413–418. [Google Scholar] [CrossRef][Green Version]
- Amavizca, E.; Bashan, Y.; Ryu, C.-M.; Farag, M.A.; Bebout, B.M.; de-Bashan, L.E. Enhanced performance of the microalga Chlorella sorokiniana remotely induced by the plant growth-promoting bacteria Azospirillum brasilense and Bacillus pumilus. Sci. Rep. 2017, 7, 41310. [Google Scholar] [CrossRef]
- Pereg, L.; de-Bashan, L.E.; Bashan, Y. Assessment of affinity and specificity of Azospirillum for plants. Plant Soil 2016, 399, 389–414. [Google Scholar] [CrossRef]
- Gonzalez, L.E.; Bashan, Y. Increased growth of the microalga Chlorella vulgaris when coimmobilized and cocultured in alginate beads with the plant-growth-promoting bacterium Azospirillum brasilense. Appl. Environ. Microbiol. 2000, 66, 1527–1531. [Google Scholar] [CrossRef] [PubMed]
- De-Bashan, L.E.; Antoun, H.; Bashan, Y. Involvement of indole-3-acetic acid produced by the growth-promoting bacterium Azospirillum spp. in promoting growth of Chlorella vulgaris. J. Phycol. 2008, 44, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Van Puyvelde, S.; Cloots, L.; Engelen, K.; Das, F.; Marchal, K.; Vanderleyden, J.; Spaepen, S. Transcriptome analysis of the rhizosphere bacterium Azospirillum brasilense reveals an extensive auxin response. Microb. Ecol. 2011, 61, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Cassan, F.D.; Coniglio, A.; Amavizca, E.; Maroniche, G.; Cascales, E.; Bashan, Y.; de-Bashan, L.E. The Azospirillum brasilense type VI secretion system promotes cell aggregation, biocontrol protection against phytopathogens and attachment to the microalgae Chlorella sorokiniana. Environ. Microbiol. 2021, 23, 6257–6274. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Wang, Z.; Liu, F.; Wu, Z.; Chen, H.; Tang, D.; Liu, J. Effect of complex iron on the phosphorus absorption by two freshwater algae. Environ. Technol. 2021, 42, 4125–4133. [Google Scholar] [CrossRef]
- Amin, S.A.; Green, D.H.; Hart, M.C.; Küpper, F.C.; Sunda, W.G.; Carrano, C.J. Photolysis of iron-siderophore chelates promotes bacterial-algal mutualism. Proc. Natl. Acad. Sci. USA 2009, 106, 17071–17076. [Google Scholar] [CrossRef] [PubMed]
- Rawat, D.; Sharma, U.; Poria, P.; Finlan, A.; Parker, B.; Sharma, R.S.; Mishra, V. Iron-dependent mutualism between Chlorella sorokiniana and Ralstonia pickettii forms the basis for a sustainable bioremediation system. ISME Commun. 2022, 2, 83. [Google Scholar] [CrossRef]
- Gromov, B.V.; Mamkaeva, K.A. Electron microscopic study of parasitism by Bdellovibrio chlorellavorus bacteria on cells of the green alga Chlorella vulgaris. Tsitologiia 1972, 14, 256–260. [Google Scholar]
- Soo, R.M.; Woodcroft, B.J.; Parks, D.H.; Tyson, G.W.; Hugenholtz, P. Back from the dead; the curious tale of the predatory cyanobacterium Vampirovibrio chlorellavorus. PeerJ 2015, 3, e968. [Google Scholar] [CrossRef]
- Landa, M.; Burns, A.S.; Durham, B.P.; Esson, K.; Nowinski, B.; Sharma, S.; Vorobev, A.; Nielsen, T.; Kiene, R.P.; Moran, M.A. Sulfur metabolites that facilitate oceanic phytoplankton–bacteria carbon flux. ISME J. 2019, 13, 2536–2550. [Google Scholar] [CrossRef]
- Moran, M.A.; Durham, B.P. Sulfur metabolites in the pelagic ocean. Nat. Rev. Microbiol. 2019, 17, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Vallet, M.; Pohnert, G. Temporal and spatial signaling mediating the balance of the plankton microbiome. Annu. Rev. Mar. Sci. 2022, 14, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.A.; Hmelo, L.R.; van Tol, H.M.; Durham, B.P.; Carlson, L.T.; Heal, K.R.; Morales, R.L.; Berthiaume, C.T.; Parker, M.S.; Djunaedi, B.; et al. Interaction and signalling between a cosmopolitan phytoplankton and associated bacteria. Nature 2015, 522, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Branscombe, L.; Harrison, E.L.; Choong, Z.Y.D.; Swink, C.; Keys, M.; Widdicombe, C.; Wilson, W.H.; Cunliffe, M.; Helliwell, K. Cryptic bacterial pathogens of diatoms peak during senescence of a winter diatom bloom. New Phytol. 2024, 241, 1292–1307. [Google Scholar] [CrossRef] [PubMed]
- Shibl, A.A.; Isaac, A.; Ochsenkühn, M.A.; Cárdenas, A.; Fei, C.; Behringer, G.; Arnoux, M.; Drou, N.; Santos, M.P.; Gunsalus, K.C.; et al. Diatom modulation of select bacteria through use of two unique secondary metabolites. Proc. Natl. Acad. Sci. USA 2020, 117, 27445–27455. [Google Scholar] [CrossRef] [PubMed]
- Bowler, C.; Falciatore, A. Phaeodactylum tricornutum . Trends Genet. 2019, 35, 706–707. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.T.; Rogato, A.; Jaubert, M.; Karas, B.J.; Falciatore, A. Phaeodactylum tricornutum: An established model species for diatom molecular research and an emerging chassis for algal synthetic biology. J. Phycol. 2023, 59, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Bowler, C.; Allen, A.E.; Badger, J.H.; Grimwood, J.; Jabbari, K.; Kuo, A.; Maheswari, U.; Martens, C.; Maumus, F.; Otillar, R.P.; et al. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 2008, 456, 239–244. [Google Scholar] [CrossRef]
- Apt, K.E.; Grossman, A.R.; Kroth-Pancic, P.G. Stable nuclear transformation of the diatom Phaeodactylum tricornutum. Mol. Gen. Genet. 1996, 252, 572–579. [Google Scholar] [CrossRef]
- De Riso, V.; Raniello, R.; Maumus, F.; Rogato, A.; Bowler, C.; Falciatore, A. Gene silencing in the marine diatom Phaeodactylum tricornutum. Nucleic Acids Res. 2009, 37, e96. [Google Scholar] [CrossRef]
- Xie, W.-H.; Zhu, C.-C.; Zhang, N.-S.; Li, D.-W.; Yang, W.-D.; Liu, J.-S.; Sathishkumar, R.; Li, H.-Y. Construction of novel chloroplast expression vector and development of an efficient transformation system for the diatom Phaeodactylum tricornutum. Mar. Biotechnol. 2014, 16, 538–546. [Google Scholar] [CrossRef]
- De Martino, A.; Meichenin, A.; Shi, J.; Pan, K.; Bowler, C. Genetic and phenotypic characterization of Phaeodactylum tricornutum (Bacillariophyceae) accessions. J. Phycol. 2007, 43, 992–1009. [Google Scholar] [CrossRef]
- Chorazyczewski, A.M.; Huang, I.; Abdulla, H.; Mayali, X.; Zimba, P.V. The influence of bacteria on the growth, lipid production, and extracellular metabolite accumulation by Phaeodactylum tricornutum (Bacillariophyceae). J. Phycol. 2021, 57, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.W.; Berube, P.M.; Follett, C.L.; Waterbury, J.B.; Chisholm, S.W.; DeLong, E.F.; Repeta, D.J. Closely related phytoplankton species produce similar suites of dissolved organic matter. Front. Microbiol. 2014, 5, 111. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kimbrel, J.A.; Vaiana, C.A.; Wollard, J.R.; Mayali, X.; Buie, C.R. Bacterial response to spatial gradients of algal-derived nutrients in a porous microplate. ISME J. 2022, 16, 1036–1045. [Google Scholar] [CrossRef]
- Brisson, V.; Swink, C.; Kimbrel, J.; Mayali, X.; Samo, T.; Kosina, S.M.; Thelen, M.; Northen, T.R.; Stuart, R.K. Dynamic Phaeodactylum tricornutum exometabolites shape surrounding bacterial communities. New Phytol. 2023, 239, 1420–1433. [Google Scholar] [CrossRef] [PubMed]
- Daly, G.; Decorosi, F.; Viti, C.; Adessi, A. Shaping the phycosphere: Analysis of the EPS in diatom-bacterial co-cultures. J. Phycol. 2023, 59, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Daly, G.; Perrin, E.; Viti, C.; Fondi, M.; Adessi, A. Scaling down the microbial loop: Data-driven modelling of growth interactions in a diatom-bacterium co-culture. Environ. Microbiol. Rep. 2021, 13, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Mayali, X.; Samo, T.J.; Kimbrel, J.A.; Morris, M.M.; Rolison, K.; Swink, C.; Ramon, C.; Kim, Y.-M.; Munoz-Munoz, N.; Nicora, C.; et al. Single-cell isotope tracing reveals functional guilds of bacteria associated with the diatom Phaeodactylum tricornutum. Nat. Commun. 2023, 14, 5642. [Google Scholar] [CrossRef] [PubMed]
- Zecher, K.; Hayes, K.R.; Philipp, B. Evidence of interdomain ammonium cross-feeding from methylamine- and glycine betaine-degrading Rhodobacteraceae to diatoms as a widespread interaction in the marine phycosphere. Front. Microbiol. 2020, 11, 533894. [Google Scholar] [CrossRef]
- Dow, L.; Stock, F.; Peltekis, A.; Szamosvári, D.; Prothiwa, M.; Lapointe, A.; Böttcher, T.; Bailleul, B.; Vyverman, W.; Kroth, P.G.; et al. The multifaceted inhibitory effects of an alkylquinolone on the diatom Phaeodactylum tricornutum. ChemBioChem 2020, 21, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, C.; Langer, G.; Wheeler, G.L. Coccolithophore calcification: Changing paradigms in changing oceans. Acta Biomater. 2021, 120, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Read, B.A.; Kegel, J.; Klute, M.J.; Kuo, A.; Lefebvre, S.C.; Maumus, F.; Mayer, C.; Miller, J.; Monier, A.; Salamov, A.; et al. Pan genome of the phytoplankton Emiliania underpins its global distribution. Nature 2013, 499, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Wang, X.; Su, J.; Li, J.; Zeng, J.; Li, G.; Liu, J. Transformation of coccolithophorid Emiliania huxleyi harboring a marine virus (Coccolithoviruses) serine palmitoyltransferase (SPT) gene by electroporation. J. Oceanol. Limnol. 2021, 39, 693–704. [Google Scholar] [CrossRef]
- Frank, O.; Pradella, S.; Rohde, M.; Scheuner, C.; Klenk, H.-P.; Göker, M.; Petersen, J. Complete genome sequence of the Phaeobacter gallaeciensis type strain CIP 105210T (= DSM 26640T = BS107T). Stand. Genomic Sci. 2014, 9, 914–932. [Google Scholar] [CrossRef] [PubMed]
- Seyedsayamdost, M.R.; Case, R.J.; Kolter, R.; Clardy, J. The Jekyll-and-Hyde chemistry of Phaeobacter gallaeciensis. Nat. Chem. 2011, 3, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Seyedsayamdost, M.R.; Wang, R.; Kolter, R.; Clardy, J. Hybrid biosynthesis of roseobacticides from algal and bacterial precursor molecules. J. Am. Chem. Soc. 2014, 136, 15150–15153. [Google Scholar] [CrossRef]
- Segev, E.; Castañeda, I.S.; Sikes, E.L.; Vlamakis, H.; Kolter, R. Bacterial influence on alkenones in live microalgae. J. Phycol. 2016, 52, 125–130. [Google Scholar] [CrossRef]
- Wang, R.; Gallant, É.; Seyedsayamdost, M.R. Investigation of the genetics and biochemistry of roseobacticide production in the Roseobacter clade bacterium Phaeobacter inhibens. mBio 2016, 7, e02118-15. [Google Scholar] [CrossRef]
- Segev, E.; Wyche, T.P.; Kim, K.H.; Petersen, J.; Ellebrandt, C.; Vlamakis, H.; Barteneva, N.; Paulson, J.N.; Chai, L.; Clardy, J.; et al. Dynamic metabolic exchange governs a marine algal-bacterial interaction. eLife 2016, 5, e17473. [Google Scholar] [CrossRef]
- Beiralas, R.; Ozer, N.; Segev, E. Abundant Sulfitobacter marine bacteria protect Emiliania huxleyi algae from pathogenic bacteria. ISME Commun. 2023, 3, 100. [Google Scholar] [CrossRef]
- Barak-Gavish, N.; Dassa, B.; Kuhlisch, C.; Nussbaum, I.; Brandis, A.; Rosenberg, G.; Avraham, R.; Vardi, A. Bacterial lifestyle switch in response to algal metabolites. eLife 2023, 12, e84400. [Google Scholar] [CrossRef] [PubMed]
- Câmara dos Reis, M.; Romac, S.; Le Gall, F.; Marie, D.; Frada, M.J.; Koplovitz, G.; Cariou, T.; Henry, N.; De Vargas, C.; Jeanthon, C. Exploring the phycosphere of Emiliania huxleyi: From bloom dynamics to microbiome assembly experiments. Mol. Ecol. 2023, 32, 6507–6522. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Mittag, M.; Sczekan, S.; Morse, D.; Hastings, J.W. Molecular cloning and genomic organization of a gene for luciferin-binding protein from the dinoflagellate Gonyaulax polyedra. J. Biol. Chem. 1993, 268, 8842–8850. [Google Scholar] [CrossRef] [PubMed]
- Jaeckisch, N.; Yang, I.; Wohlrab, S.; Glöckner, G.; Kroymann, J.; Vogel, H.; Cembella, A.; John, U. Comparative genomic and transcriptomic characterization of the toxigenic marine dinoflagellate Alexandrium ostenfeldii. PLoS ONE 2011, 6, e28012. [Google Scholar] [CrossRef]
- Fogel, M.; Hastings, J.W. Bioluminescence: Mechanism and mode of control of scintillon activity. Proc. Natl. Acad. Sci. USA 1972, 69, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Hastings, J.W. Chemistries and colors of bioluminescent reactions: A review. Gene 1996, 173, 5–11. [Google Scholar] [CrossRef]
- Jacobovitz, M.R.; Hambleton, E.A.; Guse, A. Unlocking the complex cell biology of coral–dinoflagellate symbiosis: A model systems approach. Annu. Rev. Genet. 2023, 57, 411–434. [Google Scholar] [CrossRef]
- Dougan, K.E.; González-Pech, R.A.; Stephens, T.G.; Shah, S.; Chen, Y.; Ragan, M.A.; Bhattacharya, D.; Chan, C.X. Genome-powered classification of microbial eukaryotes: Focus on coral algal symbionts. Trends Microbiol. 2022, 30, 831–840. [Google Scholar] [CrossRef]
- Liu, H.; Stephens, T.G.; González-Pech, R.A.; Beltran, V.H.; Lapeyre, B.; Bongaerts, P.; Cooke, I.; Aranda, M.; Bourne, D.G.; Forêt, S.; et al. Symbiodinium genomes reveal adaptive evolution of functions related to coral-dinoflagellate symbiosis. Commun. Biol. 2018, 1, 95. [Google Scholar] [CrossRef]
- González-Pech, R.A.; Stephens, T.G.; Chen, Y.; Mohamed, A.R.; Cheng, Y.; Shah, S.; Dougan, K.E.; Fortuin, M.D.A.; Lagorce, R.; Burt, D.W.; et al. Comparison of 15 dinoflagellate genomes reveals extensive sequence and structural divergence in family Symbiodiniaceae and genus Symbiodinium. BMC Biol. 2021, 19, 73. [Google Scholar] [CrossRef] [PubMed]
- Wuerz, M.; Lawson, C.A.; Oakley, C.A.; Possell, M.; Wilkinson, S.P.; Grossman, A.R.; Weis, V.M.; Suggett, D.J.; Davy, S.K. Symbiont identity impacts the microbiome and volatilome of a model cnidarian-dinoflagellate symbiosis. Biology 2023, 12, 1014. [Google Scholar] [CrossRef] [PubMed]
- Maire, J.; Deore, P.; Jameson, V.J.; Sakkas, M.; Perez-Gonzalez, A.; Blackall, L.L.; Van Oppen, M.J.H. Assessing the contribution of bacteria to the heat tolerance of experimentally evolved coral photosymbionts. Environ. Microbiol. 2023, 25, 3298–3318. [Google Scholar] [CrossRef] [PubMed]
- Deschaseaux, E.; O’Brien, J.; Siboni, N.; Petrou, K.; Seymour, J.R. Shifts in dimethylated sulfur concentrations and microbiome composition in the red-tide causing dinoflagellate Alexandrium minutum during a simulated marine heatwave. Biogeosciences 2019, 16, 4377–4391. [Google Scholar] [CrossRef]
- Anderson, D.M. Bloom dynamics of toxic Alexandrium species in the northeastern U.S. Limnol. Oceanogr. 1997, 42, 1009–1022. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Z. Identification and genomic analysis of Pseudosulfitobacter koreense sp. nov. isolated from toxin-producing dinoflagellate Alexandrium pacificum. Arch. Microbiol. 2023, 205, 245. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jiang, Z.; Zhou, X.; Zhang, R.; Xie, Z.; Zhang, S.; Wu, Y.; Ge, Y.; Zhang, X. Haliea alexandrii sp. nov., isolated from phycosphere microbiota of the toxin-producing dinoflagellate Alexandrium catenella. Int. J. Syst. Evol. Microbiol. 2020, 70, 1133–1138. [Google Scholar] [CrossRef]
- Amaro, A.M.; Fuentes, M.S.; Ogalde, S.R.; Venegas, J.A.; Suárez-Isla, B.A. Identification and characterization of potentially algal-lytic marine bacteria strongly associated with the toxic dinoflagellate Alexandrium catenella. J. Eukaryot. Microbiol. 2005, 52, 191–200. [Google Scholar] [CrossRef]
- Hattenrath-Lehmann, T.K.; Gobler, C.J. Identification of unique microbiomes associated with harmful algal blooms caused by Alexandrium fundyense and Dinophysis acuminata. Harmful Algae 2017, 68, 17–30. [Google Scholar] [CrossRef]
- Choi, C.J.; Jauzein, C.; Erdner, D.L. High-resolution phylogenetic analysis reveals long-term microbial dynamics and microdiversity in phytoplankton microbiome. J. Eukaryot. Microbiol. 2023, 70, e12966. [Google Scholar] [CrossRef]
- Hollants, J.; Leliaert, F.; De Clerck, O.; Willems, A. What we can learn from sushi: A review on seaweed-bacterial associations. FEMS Microbiol. Ecol. 2013, 83, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Burgunter-Delamare, B.; Rousvoal, S.; Legeay, E.; Tanguy, G.; Fredriksen, S.; Boyen, C.; Dittami, S.M. The Saccharina latissima microbiome: Effects of region, season, and physiology. Front. Microbiol. 2023, 13, 1050939. [Google Scholar] [CrossRef] [PubMed]
- Ricker, R.W. Taxonomy and Biogeography of Macquarie Island Seaweeds; British Museum (Natural History): London, UK, 1987; ISBN 978-0-565-00998-4. [Google Scholar]
- Bonin, D.R.; Hawkes, M.W. Systematics and life histories of New Zealand Bonnemaisoniaceae (Bonnemaisoniales, Rhodophyta): II. The genus Delisea. N. Z. J. Bot. 1988, 26, 619–632. [Google Scholar] [CrossRef]
- Millar, A. Marine red algae of the Coffs Harbour region, northern New South Wales. Aust. Syst. Bot. 1990, 3, 293. [Google Scholar] [CrossRef]
- Harder, T.; Campbell, A.H.; Egan, S.; Steinberg, P.D. Chemical mediation of ternary interactions between marine holobionts and their environment as exemplified by the red alga Delisea pulchra. J. Chem. Ecol. 2012, 38, 442–450. [Google Scholar] [CrossRef]
- Case, R.J.; Longford, S.R.; Campbell, A.H.; Low, A.; Tujula, N.; Steinberg, P.D.; Kjelleberg, S. Temperature induced bacterial virulence and bleaching disease in a chemically defended marine macroalga: Temperature, disease and algal chemical defense. Environ. Microbiol. 2011, 13, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Zozaya-Valdes, E.; Kjelleberg, S.; Thomas, T.; Egan, S. Multiple opportunistic pathogens can cause a bleaching disease in the red seaweed Delisea pulchra. Environ. Microbiol. 2016, 18, 3962–3975. [Google Scholar] [CrossRef]
- Campbell, A.H.; Vergés, A.; Steinberg, P.D. Demographic consequences of disease in a habitat-forming seaweed and impacts on interactions between natural enemies. Ecology 2014, 95, 142–152. [Google Scholar] [CrossRef]
- Campbell, A.H.; Harder, T.; Nielsen, S.; Kjelleberg, S.; Steinberg, P.D. Climate change and disease: Bleaching of a chemically defended seaweed. Glob. Chang. Biol. 2011, 17, 2958–2970. [Google Scholar] [CrossRef]
- Hudson, J.; Deshpande, N.; Leblanc, C.; Egan, S. Pathogen exposure leads to a transcriptional downregulation of core cellular functions that may dampen the immune response in a macroalga. Mol. Ecol. 2022, 31, 3468–3480. [Google Scholar] [CrossRef]
- Longford, S.; Tujula, N.; Crocetti, G.; Holmes, A.; Holmström, C.; Kjelleberg, S.; Steinberg, P.; Taylor, M. Comparisons of diversity of bacterial communities associated with three sessile marine eukaryotes. Aquat. Microb. Ecol. 2007, 48, 217–229. [Google Scholar] [CrossRef]
- Zozaya-Valdés, E.; Roth-Schulze, A.J.; Egan, S.; Thomas, T. Microbial community function in the bleaching disease of the marine macroalgae Delisea pulchra. Environ. Microbiol. 2017, 19, 3012–3024. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.; Steinberg, P.; Rusch, D.; Kjelleberg, S.; Thomas, T. Community structure and functional gene profile of bacteria on healthy and diseased thalli of the red seaweed Delisea pulchra. PLoS ONE 2012, 7, e50854. [Google Scholar] [CrossRef] [PubMed]
- De Nys, R.; Wright, A.D.; König, G.M.; Sticher, O. New halogenated furanones from the marine alga Delisea pulchra (cf. fimbriata). Tetrahedron 1993, 49, 11213–11220. [Google Scholar] [CrossRef]
- Steinberg, P.D.; De Nys, R.; Kjelleberg, S. Chemical cues for surface colonization. J. Chem. Ecol. 2002, 28, 1935–1951. [Google Scholar] [CrossRef]
- Dworjanyn, S.A.; De Nys, R.; Steinberg, P.D. Localisation and surface quantification of secondary metabolites in the red alga Delisea pulchra. Mar. Biol. 1999, 133, 727–736. [Google Scholar] [CrossRef]
- Wright, J.T.; Zuccarello, G.C.; Steinberg, P.D. Genetic structure of the subtidal red alga Delisea pulchra. Mar. Biol. 2000, 136, 439–448. [Google Scholar] [CrossRef]
- Wright, J.; De Nys, R.; Steinberg, P. Geographic variation in halogenated furanones from the red alga Delisea pulchra and associated herbivores and epiphytes. Mar. Ecol. Prog. Ser. 2000, 207, 227–241. [Google Scholar] [CrossRef]
- Zang, T.; Lee, B.W.K.; Cannon, L.M.; Ritter, K.A.; Dai, S.; Ren, D.; Wood, T.K.; Zhou, Z.S. A naturally occurring brominated furanone covalently modifies and inactivates LuxS. Bioorg. Med. Chem. Lett. 2009, 19, 6200–6204. [Google Scholar] [CrossRef]
- Manefield, M.; de Nys, R.; Naresh, K.; Roger, R.; Givskov, M.; Peter, S.; Kjelleberg, S. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology 1999, 145, 283–291. [Google Scholar] [CrossRef]
- Manefield, M.; Rasmussen, T.B.; Henzter, M.; Andersen, J.B.; Steinberg, P.; Kjelleberg, S.; Givskov, M. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 2002, 148, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Weinberger, F.; Saha, M.; Majzoub, M.E.; Egan, S. Cross-host protection of marine bacteria against macroalgal disease. Microb. Ecol. 2022, 84, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- De Clerck, O.; Kao, S.-M.; Bogaert, K.A.; Blomme, J.; Foflonker, F.; Kwantes, M.; Vancaester, E.; Vanderstraeten, L.; Aydogdu, E.; Boesger, J.; et al. Insights into the evolution of multicellularity from the sea lettuce genome. Curr. Biol. 2018, 28, 2921–2933.e5. [Google Scholar] [CrossRef] [PubMed]
- Alcolombri, U.; Ben-Dor, S.; Feldmesser, E.; Levin, Y.; Tawfik, D.S.; Vardi, A. Identification of the algal dimethyl sulfide–releasing enzyme: A missing link in the marine sulfur cycle. Science 2015, 348, 1466–1469. [Google Scholar] [CrossRef]
- Kessler, R.W.; Weiss, A.; Kuegler, S.; Hermes, C.; Wichard, T. Macroalgal-bacterial interactions: Role of dimethylsulfoniopropionate in microbial gardening by Ulva (Chlorophyta). Mol. Ecol. 2018, 27, 1808–1819. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U.; Kirst, G.O.; Wiencke, C. Dimethylsulphoniopropionate (DMSP) accumulation in green macroalgae from polar to temperate regions: Interactive effects of light versus salinity and light versus temperature. Polar Biol. 1992, 12, 603–607. [Google Scholar] [CrossRef]
- Van Alstyne, K.L.; Puglisi, M.P. DMSP in marine macroalgae and macroinvertebrates: Distribution, function, and ecological impacts. Aquat. Sci. 2007, 69, 394–402. [Google Scholar] [CrossRef]
- Van Der Loos, L.M.; D’hondt, S.; Engelen, A.H.; Pavia, H.; Toth, G.B.; Willems, A.; Weinberger, F.; De Clerck, O.; Steinhagen, S. Salinity and host drive Ulva-associated bacterial communities across the Atlantic-Baltic Sea gradient. Mol. Ecol. 2022, 32, 6260–6277. [Google Scholar] [CrossRef]
- Burke, C.; Thomas, T.; Lewis, M.; Steinberg, P.; Kjelleberg, S. Composition, uniqueness and variability of the epiphytic bacterial community of the green alga Ulva australis. ISME J. 2011, 5, 590–600. [Google Scholar] [CrossRef]
- Gemin, M.; Peña-Rodríguez, A.; Quiroz-Guzmán, E.; Magallón-Servín, P.; Barajas-Sandoval, D.; Elizondo-González, R. Growth-promoting bacteria for the green seaweed Ulva clathrata. Aquac. Res. 2019, 50, 3741–3748. [Google Scholar] [CrossRef]
- Singh, R.; Mantri, V.; Reddy, C.; Jha, B. Isolation of seaweed-associated bacteria and their morphogenesis-inducing capability in axenic cultures of the green alga Ulva fasciata. Aquat. Biol. 2011, 12, 13–21. [Google Scholar] [CrossRef]
- Ghaderiardakani, F.; Coates, J.C.; Wichard, T. Bacteria-induced morphogenesis of Ulva intestinalis and Ulva mutabilis (Chlorophyta): A contribution to the lottery theory. FEMS Microbiol. Ecol. 2017, 93, fix094. [Google Scholar] [CrossRef]
- Vesty, E.F.; Kessler, R.W.; Wichard, T.; Coates, J.C. Regulation of gametogenesis and zoosporogenesis in Ulva linza (Chlorophyta): Comparison with Ulva mutabilis and potential for laboratory culture. Front. Plant Sci. 2015, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Spoerner, M.; Wichard, T.; Bachhuber, T.; Stratmann, J.; Oertel, W. Growth and thallus morphogenesis of Ulva mutabilis (Chlorophyta) depends on a combination of two bacterial species excreting regulatory factors. J. Phycol. 2012, 48, 1433–1447. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Nishijima, M.; Nishimura, M.; Kuwano, K.; Saga, N. Bacteria that induce morphogenesis in Ulva pertusa (Chlorophyta) grown under axenic conditions. J. Phycol. 1996, 32, 479–482. [Google Scholar] [CrossRef]
- Tait, K.; Joint, I.; Daykin, M.; Milton, D.L.; Williams, P.; Camara, M. Disruption of quorum sensing in seawater abolishes attraction of zoospores of the green alga Ulva to bacterial biofilms. Environ. Microbiol. 2005, 7, 229–240. [Google Scholar] [CrossRef]
- Wichard, T. Exploring bacteria-induced growth and morphogenesis in the green macroalga order Ulvales (Chlorophyta). Front. Plant Sci. 2015, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Wichard, T. From model organism to application: Bacteria-induced growth and development of the green seaweed Ulva and the potential of microbe leveraging in algal aquaculture. Semin. Cell Dev. Biol. 2023, 134, 69–78. [Google Scholar] [CrossRef]
- Kessler, R.W.; Crecelius, A.C.; Schubert, U.S.; Wichard, T. In situ monitoring of molecular changes during cell differentiation processes in marine macroalgae through mass spectrometric imaging. Anal. Bioanal. Chem. 2017, 409, 4893–4903. [Google Scholar] [CrossRef]
- Vallet, M.; Kaftan, F.; Grabe, V.; Ghaderiardakani, F.; Fenizia, S.; Svatoš, A.; Pohnert, G.; Wichard, T. A new glance at the chemosphere of macroalgal-bacterial interactions: In situ profiling of metabolites in symbiosis by mass spectrometry. Beilstein J. Org. Chem. 2021, 17, 1313–1322. [Google Scholar] [CrossRef]
- Alsufyani, T.; Califano, G.; Deicke, M.; Grueneberg, J.; Weiss, A.; Engelen, A.H.; Kwantes, M.; Mohr, J.F.; Ulrich, J.F.; Wichard, T. Macroalgal-bacterial interactions: Identification and role of thallusin in morphogenesis of the seaweed Ulva (Chlorophyta). J. Exp. Bot. 2020, 71, 3340–3349. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Suzuki, M.; Kasai, H.; Shizuri, Y.; Harayama, S. Isolation and phylogenetic characterization of bacteria capable of inducing differentiation in the green alga Monostroma oxyspermum. Environ. Microbiol. 2003, 5, 25–35. [Google Scholar] [CrossRef]
- Matsuo, Y.; Imagawa, H.; Nishizawa, M.; Shizuri, Y. Isolation of an algal morphogenesis inducer from a marine bacterium. Science 2005, 307, 1598. [Google Scholar] [CrossRef] [PubMed]
- Wichard, T. Identification of metallophores and organic ligands in the chemosphere of the marine macroalga Ulva (Chlorophyta) and at land-sea interfaces. Front. Mar. Sci. 2016, 3, 131. [Google Scholar] [CrossRef]
- Comba González, N.; Ramírez Hoyos, M.; López Kleine, L.; Montoya Castaño, D. Production of enzymes and siderophores by epiphytic bacteria isolated from the marine macroalga Ulva lactuca. Aquat. Biol. 2018, 27, 107–118. [Google Scholar] [CrossRef]
- Wienecke, P.; Ulrich, J.F.; Morales-Reyes, C.F.; Dhiman, S.; Wichard, T.; Arndt, H. Enantioselective total synthesis of the morphogen (−)-thallusin and mediated uptake of Fe(III) into the green seaweed Ulva. Chem. Eur. J. 2024, e202304007. [Google Scholar] [CrossRef]
- Charrier, B.; Coelho, S.M.; Le Bail, A.; Tonon, T.; Michel, G.; Potin, P.; Kloareg, B.; Boyen, C.; Peters, A.F.; Cock, J.M. Development and physiology of the brown alga Ectocarpus siliculosus: Two centuries of research. New Phytol. 2007, 177, 319–332. [Google Scholar] [CrossRef]
- Coelho, S.M.; Peters, A.F.; Müller, D.; Cock, J.M. Ectocarpus: An evo-devo model for the brown algae. EvoDevo 2020, 11, 19. [Google Scholar] [CrossRef]
- Cock, J.M.; Sterck, L.; Rouzé, P.; Scornet, D.; Allen, A.E.; Amoutzias, G.; Anthouard, V.; Artiguenave, F.; Aury, J.-M.; Badger, J.H.; et al. The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature 2010, 465, 617–621. [Google Scholar] [CrossRef]
- Prigent, S.; Collet, G.; Dittami, S.M.; Delage, L.; Ethis de Corny, F.; Dameron, O.; Eveillard, D.; Thiele, S.; Cambefort, J.; Boyen, C.; et al. The genome-scale metabolic network of Ectocarpus siliculosus (EctoGEM): A resource to study brown algal physiology and beyond. Plant J. 2014, 80, 367–381. [Google Scholar] [CrossRef]
- Badis, Y.; Scornet, D.; Harada, M.; Caillard, C.; Godfroy, O.; Raphalen, M.; Gachon, C.M.M.; Coelho, S.M.; Motomura, T.; Nagasato, C.; et al. Targeted CRISPR-Cas9-based gene knockouts in the model brown alga Ectocarpus. New Phytol. 2021, 231, 2077–2091. [Google Scholar] [CrossRef] [PubMed]
- Prigent, S.; Frioux, C.; Dittami, S.M.; Thiele, S.; Larhlimi, A.; Collet, G.; Gutknecht, F.; Got, J.; Eveillard, D.; Bourdon, J.; et al. Meneco, a topology-based gap-filling tool applicable to degraded genome-wide metabolic networks. PLoS Comput. Biol. 2017, 13, e1005276. [Google Scholar] [CrossRef] [PubMed]
- Frioux, C.; Fremy, E.; Trottier, C.; Siegel, A. Scalable and exhaustive screening of metabolic functions carried out by microbial consortia. Bioinformatics 2018, 34, i934–i943. [Google Scholar] [CrossRef] [PubMed]
- Le Bail, A.; Billoud, B.; Kowalczyk, N.; Kowalczyk, M.; Gicquel, M.; Le Panse, S.; Stewart, S.; Scornet, D.; Cock, J.M.; Ljung, K.; et al. Auxin metabolism and function in the multicellular brown alga Ectocarpus siliculosus. Plant Physiol. 2010, 153, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Rabillé, H.; Billoud, B.; Tesson, B.; Le Panse, S.; Rolland, É.; Charrier, B. The brown algal mode of tip growth: Keeping stress under control. PLOS Biol. 2019, 17, e2005258. [Google Scholar] [CrossRef]
- KleinJan, H.; Jeanthon, C.; Boyen, C.; Dittami, S.M. Exploring the cultivable Ectocarpus microbiome. Front. Microbiol. 2017, 8, 2456. [Google Scholar] [CrossRef] [PubMed]
- Burgunter-Delamare, B.; KleinJan, H.; Frioux, C.; Fremy, E.; Wagner, M.; Corre, E.; Le Salver, A.; Leroux, C.; Leblanc, C.; Boyen, C.; et al. Metabolic complementarity between a brown alga and associated cultivable bacteria provide indications of beneficial interactions. Front. Mar. Sci. 2020, 7, 85. [Google Scholar] [CrossRef]
- Karimi, E.; Dittami, S.M. Maintaining beneficial alga-associated bacterial communities under heat stress: Insights from controlled co-culture experiments using antibiotic-resistant bacterial strains. FEMS Microbiol. Ecol. 2023, 99, fiad130. [Google Scholar] [CrossRef]
- Strittmatter, M.; Grenville-Briggs, L.J.; Breithut, L.; Van West, P.; Gachon, C.M.M.; Küpper, F.C. Infection of the brown alga Ectocarpus siliculosus by the oomycete Eurychasma dicksonii induces oxidative stress and halogen metabolism. Plant Cell Environ. 2016, 39, 259–271. [Google Scholar] [CrossRef]
- Tapia, J.E.; González, B.; Goulitquer, S.; Potin, P.; Correa, J.A. Microbiota influences morphology and reproduction of the brown alga Ectocarpus sp. Front. Microbiol. 2016, 7, 197. [Google Scholar] [CrossRef]
- Dittami, S.M.; Duboscq-Bidot, L.; Perennou, M.; Gobet, A.; Corre, E.; Boyen, C.; Tonon, T. Host-microbe interactions as a driver of acclimation to salinity gradients in brown algal cultures. ISME J. 2016, 10, 51–63. [Google Scholar] [CrossRef]
- Dittami, S.M.; Peters, A.F.; West, J.A.; Cariou, T.; KleinJan, H.; Burgunter-Delamare, B.; Prechoux, A.; Egan, S.; Boyen, C. Revisiting Australian Ectocarpus subulatus (Phaeophyceae) from the Hopkins River: Distribution, abiotic environment, and associated microbiota. J. Phycol. 2020, 56, 821579. [Google Scholar] [CrossRef]
- Karimi, E.; Geslain, E.; KleinJan, H.; Tanguy, G.; Legeay, E.; Corre, E.; Dittami, S.M. Genome sequences of 72 bacterial strains isolated from Ectocarpus subulatus: A resource for algal microbiology. Genome Biol. Evol. 2020, 12, 3647–3655. [Google Scholar] [CrossRef]
- Dittami, S.M.; Eveillard, D.; Tonon, T. A metabolic approach to study algal-bacterial interactions in changing environments. Mol. Ecol. 2014, 23, 1656–1660. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).