Abstract
The spotted-wing drosophila, Drosophila suzukii (Matsumura) (Diptera: Drosophilidae), is a pest that reduces the productivity of small fruits. Entomopathogenic nematodes (EPNs) and chemical insecticides can suppress this pest, but the compatibility of the two approaches together requires further examination. This laboratory study evaluated the compatibility of Steinernema brazilense IBCBn 06, S. carpocapsae IBCBn 02, Heterorhabditis amazonensis IBCBn 24, and H. bacteriophora HB with ten chemical insecticides registered for managing D. suzukii pupae. In the first study, most insecticides at the recommended rate did not reduce the viability (% of living infective juveniles (IJs)) of S. braziliense and both Heterorhabditis species. The viability of S. carpocapsae was lowered by exposure to spinetoram, malathion, abamectin, azadirachtin, deltamethrin, lambda-cyhalothrin, malathion, and spinetoram after 48 h. During infectivity bioassays, phosmet was compatible with all the EPNs, causing minimal changes in infectivity (% pupal mortality) and efficiency relative to EPN-only controls, whereas lambda-cyhalothrin generally reduced infectivity of EPNs on D. suzukii pupae the most, with a 53, 75, 57, and 13% reduction in infectivity efficiency among H. bacteriophora, H. amazonensis, S. carpocapsae, and S. brazilense, respectively. The second study compared pupal mortality caused by the two most compatible nematode species and five insecticides in various combinations. Both Heterorhabditis species caused 78–79% mortality among D. suzukii pupae when used alone, and were tested in combination with spinetoram, malathion, azadirachtin, phosmet, or novaluron at a one-quarter rate. Notably, H. bacteriophora caused 79% mortality on D. suzukii pupae when used alone, and 89% mortality when combined with spinetoram, showing an additive effect. Novaluron drastically reduced the number of progeny IJs when combined with H. amazonensis by 270 IJs and H. bacteriophora by 218. Any adult flies that emerged from EPN–insecticide-treated pupae had a shorter lifespan than from untreated pupae. The combined use of Heterorhabditis and compatible chemical insecticides was promising, except for novaluron.
1. Introduction
The spotted-wing drosophila, Drosophila suzukii (Matsumura) (Diptera: Drosophilidae), is a fruit fly native to Asia that is currently found in North and South America, Europe, Africa, and Oceania. It is a polyphagous quarantine pest with high economic importance due to its ability to infest a variety of fruits. Unlike other drosophilids, which generally lay eggs on damaged or decomposing fruits, D. suzukii can lay eggs inside healthy fruits [1]. Primary damage is caused by the larvae consuming the pulp and softening the fruit. Secondary damage is caused by the entry of phytopathogenic microorganisms once the fruit has been punctured [2].
Though chemical insecticides are effective [3], they may kill non-target species, pollute the environment, lead to insecticide-resistant pest populations, and harm human health [4]. Therefore, biological control using entomopathogenic nematodes (EPNs) is a promising alternative [5] given their efficiency, adaptive capacity, and easy application. Furthermore, nematodes search in the soil for the host through chemoreceptor mechanisms and can be selective for the target insect species [6].
EPNs are often applied with other phytosanitary products (chemical, natural, and biological), fertilizers, and soil correctives, and can be mixed in tanks [7]. For example, Steinernema carpocapsae (Weiser), S. feltiae Filipjev, and Heterorhabditis bacteriophora (Poinar) can survive when exposed to various types of chemical pesticides [8]. The action of pesticides on entomopathogenic organisms varies according to the species and lineage of the pathogens, as well as the chemical nature and concentrations of the products used [9]. The effects of pesticides on EPNs can be evaluated by (1) observing the viability and behavior of infective juveniles (IJs) exposed to various concentrations of a given pesticide for different periods and (2) observing the ability of IJs to infect host insects [10]. The compatibility of EPNs with brief exposures to chemical insecticides is an important factor in successful integrated pest management (IPM) [11].
The objective of this study was to evaluate the compatibility of Steinernema brazilense IBCBn 06 (isolate designation), S. carpocapsae IBCBn 02, Heterorhabditis amazonensis IBCBn 24, and H. bacteriophora HB with different chemical insecticides for the control of D. suzukii pupae, and to evaluate the longevity of surviving adult flies. Steinernema carpocapsae and H. bacteriophora were selected since they are commercially available and laboratory trials with these species have been promising in multiple countries [5]. Steinernema braziliense and H. amazonensis were selected since they are important native nematodes in Brazil. These two Brazilian isolates have shown promise in controlling fruit flies.
2. Results
2.1. Study 1—Compatibility of EPNs with Chemical Insecticides
Insecticides can be incompatible by reducing the viability of IJs and/or infectivity rates. For S. carpocapsae IBCBn 02, deltamethrin, spinetoram, malathion, abamectin, azadirachtin, and lambda-cyhalothrin reduced the viability of the IJs relative to the nematode-only control treatment by 55.1%, 35.5%, 54.8%, 77.30%, 53.26%, and 40.16%, respectively (100%—% viability with insecticide, Table 1). Likewise, deltamethrin, spinetoram, malathion, and lambda-cyhalothrin also reduced infectivity relative to the controls (Table 2) and were considered toxic by IOBC standards since the reduction in infectivity efficiency (∆E%) exceeded 30% [12]. For S. brazilense IBCBn 06, only lambda-cyhalothrin significantly reduced IJ viability by 12.6% (Table 1) but was not classified as toxic since ∆E% was 12.5% (Table 2). Overall, S. brazilense had low infectivity rates, causing 6–16% pupal mortality which was lower than the 10–42% rates seen with the other isolates (Table 2). Furthermore, S. braziliense was only negatively affected by thiamethoxam and acetamiprid, which were slightly toxic to the nematodes lowering infectivity efficiency by 38 and 63%, respectively (∆E% in Table 2).

Table 1.
Viability (mean % living IJs ± SE) of S. brazilense IBCBn 06, S. carpocapsae IBCBn 02, H. amazonensis IBCBn 24, and H. bacteriophora HB after 48 h of exposure to insecticides.

Table 2.
Infectivity (mean % pupal mortality ± SE), ∆E% and class of S. brazilense IBCBn 06, S. carpocapsae IBCBn 02, H. amazonensis IBCBn 24, and H. bacteriophora HB after 48 h of exposure to insecticides (IOBC/WPRS protocol—15).
For H. amazonensis IBCBn 24, only abamectin reduced the viability of IJs by 15.5% relative to the nematode-only control (Table 1). Heterhabditis amazonensis was highly affected by nine insecticides regarding infectivity; only phosmet did not affect the nematodes’ infectivity, with a 0% change in efficiency. For H. bacteriophora, only malathion and abamectin significantly lowered IJ viability by 12.64% and 11.71% relative to the control, respectively (Table 1). Five insecticides affected infectivity, as H. bacteriophora infectivity was lowered with spinetoram, abamectin, azadirachtin, novaluron, and lambda-cyhalothrin, and efficiency was lowered by 32, 37, 47, 53, and 53%, respectively (∆E% in Table 2).
Generally, H. amazonensis and H. bacteriophora showed more compatibility with the insecticides. In comparisons across the four species, H. bacteriophora exhibited higher infectivity rates with all ten of the insecticides, whereas S. braziliense exhibited lower infectivity with nine insecticides (see comparison using capital letters in Table 2). Steinernema carpocasae had the lowest viability with all ten insecticides (see comparison using capital letters in Table 1). Thus, the nematodes with lower viability and infectivity responses were not tested in the second study.
2.2. Study 2—Effectiveness of Selected EPNs + Insecticides
The isolates H. amazonensis IBCBn 24 and H. bacteriophora HB and spinetoram, malathion, azadirachtin, phosmet, and novaluron, either separately or combined, caused 7–95% pupal mortality in D. suzukii (Table 3). Mortality caused by the EPN + insecticide combinations was significantly higher than the negative control of water (Table 3). A combination of H. amazonensis + spinetoram resulted in the greatest mortality of D. suzukii pupae at 95%, with a significant 17.5% increase from the EPN alone with 77.5% mortality. Heterorhabditis bacteriophora caused 78.75% mortality of the pupae when used alone, and 88.75% mortality with spinetoram combined, causing a 10% numerical increase (Table 3).

Table 3.
Mortality of pupae and number of emerging IJs (mean ± SE) from D. suzukii pupae in combination with insecticides and H. amazonensis IBCBn 24 or H. bacteriophora HB.
The addition of all the tested insecticides reduced the number of IJs developing in the treated pupae (Table 3). Novaluron caused the most drastic reduction with a 270 IJ/pupa reduction when combined with H. amazonensis, and 218 IJ/pupa reduction with H. bacteriophora. While novaluron did not reduce D. suzukii pupal mortality when combined with either Heterorhabditis species compared to the EPN-only treatments, novaluron negatively affected the developing IJs in D. suzukii pupae (Table 3). The other four insecticides reduced nematode production by 114–210 IJ/pupa with H. amazonensis, and by 48–172 IJ/pupa with H. bacteriophora.
Longevity was shortened among the surviving adult D. suzukii from all the treatments with EPN and/or insecticides compared to the untreated control pupae (F = 41.94; d.f = 17, 126; p ˂ 0.0001) (Figure 1). The surviving adults lived ~3.36 days less when exposed to both EPN and insecticides than insecticide alone. Also, the adults lived ~5 days or less when exposed to H. amazonensis + azadirachtin, H. amazonensis + phosmet, H. bacteriophora + spinetoram, H. bacteriophora + malathion, and H. bacteriophora + phosmet.
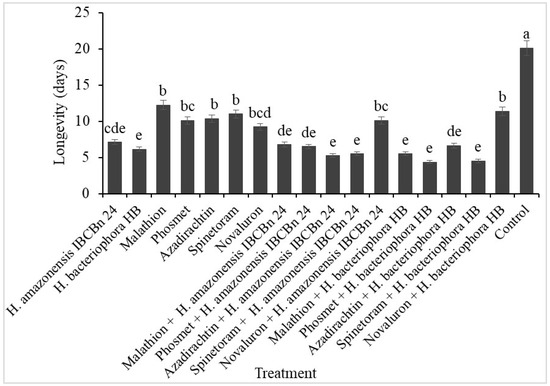
Figure 1.
Longevity of Drosophila suzukii adults exposed to a combination of insecticides and H. amazonensis IBCBn 24 or H. bacteriophora HB. Different letters denote differences identified by the Tukey test, p < 0.05.
3. Discussion
The isolates H. bacteriophora HB, H. amazonensis IBCBn 24, S. brazilense IBCBn 06, and S. carpocapsae IBCBn 02 were often compatible with the ten chemical insecticides tested, as 48 h of exposure resulted in no significant reduction in the viability of IJs in 31 out of the 40 EPN–insecticide combinations tested. Of the four species, S. carpocaspsae experienced the most reduction in viability with six out of ten insecticides. In contrast, the pesticides applied directly to S. carpocapsae of all the strains at the recommended dose did not affect viability after 3 h of exposure [13]. The different outcomes between the studies may be due to exposure durations of 48 versus 3 h. Other studies showed that the IJs of other Steinernema spp. were tolerant to insecticides. Botanical and chemical insecticides at the recommended doses did not affect the viability of S. feltiae 72 h after exposure [14], nor did phosmet, fipronil, and thiamethoxam affect the viability of Steinernema sp. [15]. On the other hand, lambda-cyhalothrin affected the viability of S. carpocapsae and S. amazonensis in this study, which corroborates Negrisoli Jr. et al.’s [16] study with S. carpocapsae and S. glaseri (Steiner).
In addition to minimal changes in IJ viability, a compatible insecticide should not reduce the subsequent infectivity rate of EPNs. Though IJs may remain alive, an insecticide can still reduce infectivity rates by hampering the nematode’s dispersal ability and attraction to the host [17]. Phosmet was the most compatible out of the ten insecticides tested and did not reduce the infectivity of the four nematode species. Abamectin was somewhat incompatible as it reduced the infectivity of S. braziliense and H. amazonensis but not H. bacteriophora or S. carpocapsae in this study. Koppenhöfer et al. [18] and Kary et al. [6] observed that S. feltiae was negatively affected by abamectin, while the effect on H. bacteriophora was very slight. Since the thickness of the epicuticle, cortical, and median cuticle layers of IJs differs between species [19], the different susceptibilities to abamectin between species may be due to differences in cuticles. Abamectin may damage the juvenile cuticle by affecting its permeability and loss of annulations and grooves in the body [6]. The harmful effects of abamectin can lower the viability and infectivity of IJs [20].
Next, thiamethoxam was more toxic among the insecticides tested: it reduced the infectivity of three out of the four nematode species. Thiamethoxam is a widely used systemic insecticide in orchards worldwide, especially in Brazil, for psyllid, sharpshooter, mealybug, aphid, and leafminer control in fruit orchards. Thiamethoxam is applied by soil-drench, where D. suzukii often pupate [21]. Hence, its application to soil combined with EPN applications might compromise their persistence in agroecosystems [21]. Lastly, lambda-cyhalothrin was the most toxic of the tested insecticides; it reduced infectivity for all four EPN species. Likewise, negative results were obtained for the EPNs after being exposed to lambda-cyhalothrin [22].
Our study supports integrating both forms of protection into agronomic practices. A one-quarter dose of spinetoram provided an additive effect when combined with H. amazonensis by increasing D. suzukii pupal mortality by 17.5%. Likewise, Kary et al. [6] reported that H. bacteriophora and S. feltiae were effective control agents against the tuber moth when used with abamectin, providing an increase of 17% in protection. Also, Navarro et al. [23] reported that imidacloprid worked additively with H. sonorensis, and dinotefuran worked additively with S. riobrave and H. sonorensis. For the other EPN–insecticide combinations in Study 2, there were no additive effects nor negative effects. This is similar to the conclusions of Polavarapu et al. [24], who found that Steinernema scarabaei and H. bacteriophora in combination with thiamethoxam and phosmet against scarab did not have an additive effect nor a negative effect. While our study focused on integrating EPNs and insecticides, other chemicals that EPNs encounter in the field require consideration. For example, H. bacteriophora was found to maintain high infectivity in G. mellonella caterpillars when exposed to the fungicides mancozeb and metalaxyl + folpet [24,25,26].
The compatibility obtained by combining EPNs and insecticides can be caused by the chemical ingredient stressing the insect, affecting its physiology and humoral defense, and consequently making it more susceptible to infections by nematodes [27]. Also, the increased efficacy of EPN–insecticide combinations may also be due to the nematodes’ increased movement and nictation activity after exposure to an insecticide [28]. Lastly, Gaugler et al. [29] observed that the compatibility of H. bacteriophora + phosmet on the mortality of scarabeid larvae Cyclocephala sp. (Coleoptera: Scarabaeidae) was caused by changes in the insect’s behavior prompted by the insecticide. Scarab larvae ceased to clean their cuticle or mandibles and did not remove nematodes and other natural enemies in the process.
In this study, the longevity of D. suzukii adults was shortened by the presence of IJs of H. bacteriophora HB, and H. amazonensis IBCBn 24, either applied alone or in combination with insecticides. After penetrating the insect’s integument, IJs usually cause mortality between 24 and 48 h. The emergence of infected adults indicates resistance to infection during the pupal period [30].
4. Materials and Methods
Experiments were performed in the Insect Ecology Laboratory of the Federal University of Pelotas, in the state of Rio Grande Sul, Brazil. Drosophila suzukii were reared by placing adults in flat-bottomed glass containers (85 mm high × 25 mm in diameter ± 0.5 mm) in a climate chamber (ELETROLab®, model EL 212, São Paulo, Brazil) at 25 ± 1 °C, 70 ± 10% RH, and a 12 h:12 h L:D photoperiod. Adults were given a diet that consisted of 500 mL of water, agar (4 g), yeast (20 g), corn flour (40 g), sugar (50 g), 1.5 mL of propionic acid, and Nipagin (10%; 3.5 mL) [31] for food as well as an egg laying substrate. Pupae less than 24 h old were used in the experiments. EPNs were obtained from the ‘Oldemar Cardim Abreu’ Entomopathogenic Nematode Bank of the Biology Institute of São Paulo. The isolates S. brazilense IBCBn 06, S. carpocapsae IBCBn 02, H. amazonensis IBCBn 24, and H. bacteriophora HB were multiplied separately in caterpillars of the fourth and fifth instars Galleria mellonella L. (Lepidoptera: Pyralidae) [32]. The infective juveniles (IJs) used in the experiments were within six days of emergence.
4.1. Chemical Insecticides
We used 10 insecticides from different chemical groups, prepared at the concentration recommended by the vendor for use in strawberry crops; this fruit is attacked the most by D. suzukii in Brazil (Table 4). Based on this concentration, 350 mL solutions of each product were prepared. The insecticides were chosen based on their availability in the market and reported efficacy for the control of D. suzukii in Brazil.

Table 4.
Insecticides used in bioassays to evaluate their compatibility with S. brazilense IBCBn 06, S. carpocapsae IBCBn 02, H. amazonensis IBCBn 24, and H. bacteriophora HB.
4.2. Study 1—Compatibility of EPNs with Chemical Insecticides
The compatibility of S. brazilense, S. carpocapsae, H. bacteriophora, or H amazonesis with the chemical insecticides was evaluated by following Negrisoli Jr. et al. [10]. First, 1000 mL of each insecticide solution was prepared in water (Table 4). Then, 1 mL of the insecticide solution was placed in an 8 mL glass test tube (2.5 cm diam. × 8 cm high), followed by the addition of 2500 IJs in 1 mL of distilled water. Each insecticide–nematode treatment combination was replicated in five tubes. The tubes were agitated and maintained in a climate chamber at 22 ± 1 °C and RH of 70 ± 10%. First, nematode ‘viability’ was evaluated 48 h after exposure. An aliquot of 0.1 mL of the suspension was removed from each tube and approximately 100 IJs were observed with a stereomicroscope at 40×. The IJs were considered dead when they did not respond to the stimulus of a stylus.
The ‘infectivity’ of the nematodes was also tested in the same replicates set up for measuring viability. To wash off the insecticides, the tubes were filled with 3 mL of distilled water, and the solutions were left to settle for 30 min in a refrigerator. About 3 mL of the supernatant was decanted and the remaining substance was washed three times with distilled water. After the last washing, 0.2 mL with ~100 IJs was pipetted into the bottom of a Petri dish (90 diam. × 15 mm), where 10 24-h-old D. suzukii pupae were added. The dishes were then placed in a climate chamber at 22 ± 1 °C and RH of 70 ± 10% for five days, after which pupal mortality was recorded. ‘Infectivity’ is the percentage of dead pupae.
The reduction in efficiency ‘∆E%’ reflects whether nematodes infect D. suzukii pupae less when nematodes were previously exposed to insecticides. ∆E% is calculated by the following: ∆E% = (1 − mt/mc) × 100, where mt is the pupal mortality of the treatment and mc is the mortality of the control [33], based on guidelines from the International Organization for Biological and Integrated Control (IOBC). ∆E% values were classified according to IOBC/WPRS [12] as follows: 1—nontoxic insecticides that reduce infectivity efficiency by less than 30%, 2—slightly toxic (30–79%), 3—moderately toxic (80–99%), and 4—toxic (>99%). Insecticides compatible with EPNs should cause high pupal mortality (infectivity) and cause minimal changes in infectivity efficiency (∆E%).
4.3. Study 2—Effectiveness of Selected EPNs + Insecticides
Based on Study 1, we continued trials with the two most compatible nematode species, H. bacteriophora HB and H. amazonensis IBCBn 24, in combination with either spinetoram, azadirachtin, malathion, phosmet, or novaluron at ¼ of the recommended dose for strawberries (Table 4). The sub-dose of ¼ was used to reduce the environmental impacts of the insecticides and to observe the potential compatibility of the combinations with nematodes; otherwise, a full dose of an insecticide alone may already kill most D. suzukii pupae, masking any positive impacts of EPN combinations. Concentrations of 5400 IJs for H. bacteriophora and 1800 IJs for H. amazonensis were used since these concentrations caused the greatest mortality in D. suzukii pupae in Study 1.
Treatments comprised each nematode and each insecticide either alone or in combination and were as follows: (1) H. amazonensis (H. a.); (2) H. bacteriophora (H. b.); (3) spinetoram; (4) azadirachtin; (5) malathion; (6) phosmet; (7) novaluron; (8) H.a. + spinetoram; (9) H.a. + azadirachtin; (10) H. a. + malathion; (11) H. a. + phosmet; (12) H. a + novaluron; (13) H. b. + spinetoram; (14) H. b. + azadirachtin; (15) H. b. + malathion; (16) H. b. + phosmet; (17) H. b. + novaluron, and (18) water control. Each treatment had eight replications, each consisting of 10 pupae grouped in a 50 mL plastic jar, filled with 50 g of sterilized sand with 10% moisture by weight.
The 1 mL nematode suspension or water (no EPN) was mixed with 3 mL of distilled water in a vial. Then, 1 mL of insecticide or water was added and the vial shaken; then, all 5 mL was pipetted into each plastic jar containing pupae. As a negative control, only sterile water was inoculated, and as a positive control, only the insecticide solution was used without nematodes. The jars were incubated at 22 ± 1 °C and 70 ± 10% RH for six days, after which we recorded the number of dead pupae. In the treatments with nematodes, the dead pupae were dissected to count the IJs.
The surviving adult D. suzukii that emerged from the pupal treatment were placed in individual 300 mL plastic cups and observed for longevity. The cups had a 5 cm diameter hole in the lid covered with voile fabric to allow air circulation. The adults were fed 10 g of artificial diet and 1 mL of distilled water in a cotton wick. The flies were incubated at 22 ± 1 °C and 70 ± 10% RH until death.
4.4. Statistical Analysis
A generalized linear model (GLM) with an appropriate distribution analyzed the treatment effects on the viability %, and infectivity % of the EPNs in Study 1. The treatments were compared in two manners: differences between the insecticides for a given nematode species, and differences between the nematode species with a given insecticide. In Study 2, the pupal mortality %, number of IJs that emerged per D. suzukii pupa, and longevity of the surviving D. suzukii adults were compared in a GLM. The goodness of fit of the data to the model was assessed by using a half-normal probability plot with a simulated envelope [34]. When significant differences between the treatments were detected, multiple comparisons (Tukey HSD test, p < 0.05) were performed using the glht function of the Multicomp package, with adjustment of p-values. These analyses were performed in R software version 4.2.3 [35].
5. Conclusions
In summary, many of the nematode–insecticide combinations tested resulted in viable IJs, with high infectivity rates, particularly among H. amazonensis IBCBn 24 and H. bacteriophora HB. Further testing showed that the combined use of the EPNs and compatible chemical insecticides had neutral or additive effects, except for novaluron, which negatively affected EPN propagation within the treated D. suzukii pupae. The use of some Brazilian isolates of EPNs with insecticides is promising against D. suzukii within an integrated pest management approach.
Author Contributions
A.L.d.B. and F.R.M.G. planned and designed the project. S.d.C.D., M.C.J.-B. and A.L.d.B. performed the experiments. All the authors performed the data analysis. A.L.d.B. and F.R.M.G. provided technical support. All the authors prepared the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CAPES #001 to scholarship for SCF an MCJB, CNPq #402373/2022-7 to scholarship for FRMG, and USDA base funds CRIS 2072-22000-044-00D for JCL.
Data Availability Statement
The data is contained within the manuscript can be made available upon request.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Atallah, J.; Teixeira, L.; Salazar, R.; Zaragoza, G.; Kopp, A. The making of a pest: The evolution of a fruit-penetrating ovipositor in Drosophila suzukii and related species. Proc. Biol. Sci. 2014, 1781, 20132840. [Google Scholar] [CrossRef]
- Hamby, K.A.; Becher, P.G. Current knowledge of interactions between Drosophila suzukii and microbes, and their potential utility for pest management. J. Pest Sci. 2016, 89, 621–630. [Google Scholar] [CrossRef]
- Shawer, R. Chemical control of Drosophila suzukii. In Drosophila suzukii Management; Garcia, F.R.M., Ed.; Springer: Cham, Switzerland, 2020; pp. 133–142. [Google Scholar]
- Sial, A.A.; Roubos, C.R.; Gautam, B.K.; Fanning, P.D.; Van Timmeren, S.; Spies, J.; Petran, A.; Rogers, M.A.; Liburd, O.E.L.; Little, B.A.; et al. Evaluation of organic insecticides to manage spotted-wing drosophila (Drosophila suzukii) in berry crops. J. Appl. Entomol. 2019, 143, 593–608. [Google Scholar] [CrossRef]
- Dias, S.C.; de Brida, A.L.; Jean-Baptiste, M.C.; Leite, L.G.; Ovruski, S.M.; Garcia, F.R.M. Pathogenicity and virulence of different concentrations of brazilian isolates of entomopathogenic nematodes against Drosophila suzukii. Neotrop. Entomol. 2023, 52, 986–992. [Google Scholar] [CrossRef]
- Kary, N.E.; Sanatipour, Z.; Mohammadi, D.; Dillon, A.B. Combination effects of entomopathogenic nematodes, Heterorhabditis bacteriophora, and Steinernema feltiae, with Abamectin on developmental stages of Phthorimaea operculella (Lepidoptera, Gelechiidae). Crop Protec. 2021, 143, 105543. [Google Scholar] [CrossRef]
- Krishnayya, P.V.; Grewal, P.S. Effect of neem and selected fungicides on viability and virulence of the entomopathogenic nematode Steinernema feltiae. Biocontrol. Sci. Technol. 2002, 12, 259–266. [Google Scholar] [CrossRef]
- Koppenhöfer, A.M.; Brown, I.M.; Gaugler, R.; Grewal, P.S.; Kaya, H.K.; Klein, M.G. Synergism of entomopathogenic nematodes and imidacloprid against white grubs: Greenhouse and field evaluation. Biol. Control 2000, 19, 245–252. [Google Scholar] [CrossRef]
- Souza, J.K.A.; Venâncio, R.A.A.; Souza, J.A.; Santos, J.S.; Nunes, M.G.S. Ensaio de compatibilidade de espécie de nematoide entomopatogênicos (Rhabditida: Heterorhabditidae) a produtos fitossanitários. Jornacitec 2019, 8, 9. [Google Scholar]
- Negrisoli, A.S., Jr.; Barbosa, C.R.C.; Moino, A., Jr. Comparação entre metodologias de avaliação da compatibilidade de produtos fitossanitarios com nematoides entomopatogênicos (Nematoda: Rhabditida). Nematol. Bras. 2008, 32, 65–76. [Google Scholar]
- Laznik, Z.; Trdan, S. The influence of insecticides on the viability of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) under laboratory conditions. Pest Manag. Sci. 2013, 70, 784–789. [Google Scholar] [CrossRef]
- Vainio, A. Guideline for laboratory testing of the side-effects of pesticides on entomophagous nematodes Steinernema spp. IOBC/WPRS Bull. 1992, 15, 145–147. [Google Scholar]
- Alumai, A.; Grewal, P.S. Tank-mix compatibility of the entomopathogenic nematodes, Heterorhabditis bacteriophora, and Steinernema carpocapsae, with selected chemical pesticides used in turfgrass. Biocontrol Sci. Technol. 2004, 14, 725–730. [Google Scholar] [CrossRef]
- Mahmoud, M.F.; Osman, M.A.M. Pathogenicity of entomopathogenic nematode, Steinernema feltiae against larvae and pupae of the Peach Fruit Fly Bactrocera zonata (Saunders). Agric. Res. J. 2006, 6, 89–93. [Google Scholar]
- Negrisoli, A.S., Jr.; Garcia, M.S.; Negrisoli, C.R.C.B. Compatibility of entomopathogenic nematodes (Nematoda: Rhabditida) with registered insecticides for Spodoptera frugiperda (Lepidoptera: Noctuidae) under laboratory conditions. Crop Protect. 2010, 29, 545–549. [Google Scholar] [CrossRef]
- Tavares, F.M.; Batista Filho, L.G.; Leite, L.C.; Almeida, A.C. Efeitos sinérgicos de combinações entre entomopathogenic nematodes (Nemata: Rhabditida) e chemical insecticides na mortalidade de Sphenophorus levis (Vaurie) (Coleoptera: Curculionidae). BioAssay 2009, 4, 1–10. [Google Scholar] [CrossRef]
- Laznik, Ž.; Vidrih, M.; Trdan, S. The effects of different fungicides on the viability of entomopathogenic nematodes Steinernema feltiae (Filipjev), S. carpocapsae Weiser, and Heterorhabditis downesi Stock, Griffin and Burnell (Nematoda: Rhabditida) under laboratory conditions. Chil. J. Agric. Res. 2012, 72, 62–67. [Google Scholar]
- Koppenhöfer, A.M.; Cowles, R.S.; Cowles, E.A.; Fuzy, E.M.; Baumgartner, L. Comparison of neonicotinoid insecticides as synergists for entomopathogenic nematodes. Biol. Control 2002, 24, 90–97. [Google Scholar] [CrossRef]
- Patel, M.N.; Wright, D.J. The ultrastructure of the cuticle and sheath of infective juveniles of entomopathogenic steinernematid nematodes. J. Helminthol. 1998, 72, 257–266. [Google Scholar] [CrossRef]
- Laznik, Ž.; Trdan, S. The influence of herbicides on the viability of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae). Int. J. Pest Manag. 2017, 263, 105–111. [Google Scholar] [CrossRef]
- Woltz, J.M.; Lee, J.C. Pupation behavior and larval and pupal biocontrol of Drosophila suzukii in the field. Biol. Control 2017, 110, 62–69. [Google Scholar] [CrossRef]
- Miranda, M.P.; Yamamoto, P.T.; Garcia, R.B.; Lopes, J.P.; Lopes, J.R. Thiamethoxam and imidacloprid drench applications on sweet orange nursery trees disrupt the feeding and settling behavior of Diaphorina citri (Hemiptera: Liviidae). Pest Manag. Sci. 2016, 72, 1785–1793. [Google Scholar] [CrossRef]
- Navarro, P.D.; McMullen, J.; Stock, S.P. Interactions between the entomopathogenic nematode Heterorhabditis sonorensis (Nematoda: Heterorhabditidae) and the saprobic fungus Fusarium oxysporum (Ascomycota: Hypocreales). J. Invertebr. Pathol. 2014, 115, 41–47. [Google Scholar] [CrossRef]
- Polavarapu, S.; Koppenhöfer, A.M.; Barry, J.D.; Holdcraft, R.J.; Fuzy, E.M. Entomopathogenic nematodes and neonicotinoids for remedial control of oriental beetle, Anomala orientalis (Coleoptera: Scarabaeidae), in highbush blueberry. Crop Prot. 2007, 26, 1266–1271. [Google Scholar] [CrossRef]
- Rovesti, L.; Heinzpeter, E.E.W.; Tagliente, F.; Deseö, K.V. Compatibility of pesticides with entomopathogenic nematode Heterorhabditis bacteriophora Poinar (Nematoda: Heterorhabditidae). Nematologica 1988, 34, 462–476. [Google Scholar]
- Rovesti, L.; Deseo, K.V. Compatibility of chemical pesticides with the entomopathogenic nematodes, Steinernema carpocapsae Weiser and S. feltiae Filipjev (Nematoda: Steinernematidae). Nematologica 1990, 36, 237–245. [Google Scholar] [CrossRef]
- Wang, Y.; Campbell, J.F.; Gaugler, R. Infection of entomopathogenic nematodes Steinernema glaseri and Heterorhabditis bacteriophora against Popillia japonica (Coleoptera: Scarabaeidae) larvae. J. Inverter. Pathol. 1994, 66, 178–184. [Google Scholar] [CrossRef]
- Ishibashi, N.; Akki, S. Effects of insecticides on movement, nictation, and infectivity of Steinernema carpocapsae. J. Nematol. 1993, 25, 204–213. [Google Scholar]
- Gaugler, R.; Wang, Y.; Campbell, J.F. Aggressive and evasive behaviors in Popillia japonica (Coleoptera: Scarabaeidae) larvae: Defenses against entomopathogenic nematode attack. J. Invert. Pathol. 1994, 64, 193–199. [Google Scholar] [CrossRef]
- Garriga, A.; Morton, A.; Ribes, A.; Garcia-del-Pino, F. The emergence of Drosophila suzukii adults is a susceptible period for entomopathogenic nematode infection. J. Pest Sci. 2019, 93, 639–646. [Google Scholar] [CrossRef]
- Dalton, P.T.; Walton, V.M.; Shearer, P.W.; Walsh, D.B.; Caprile, J.; Isaacs, R. Laboratory survival of Drosophila suzukii under simulated winter conditions of the Pacific Northwest and seasonal field trapping in five primary regions of small and stone fruit production in the United States. Pest Manag. Sci. 2011, 67, 1368–1374. [Google Scholar] [CrossRef]
- Woodring, J.L.; Kaya, H.K. Steinernematid and Heterorhabditid Nematodes: A Handbook of Biology and Techniques; Agricultural Experiment Station: Fayetteville, NC, USA, 1988; p. 331. [Google Scholar]
- Peters, A.; Poullot, D. Side effects of surfactants and pesticides on entomopathogenic nematodes assessed using advanced IOBC guidelines. IOBC/WPRS Bull. 2004, 27, 67–72. [Google Scholar]
- Moral, R.A.; Hinde, J.; Demétrio, C.G.B. Half-normal plots and overdispersed models in R: The hnp package. J. Stat. Softw. 2017, 8, 12–33. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).