Abstract
Erigeron represents the third largest genus on the Juan Fernández Islands, with six endemic species, five of which occur exclusively on the younger Alejandro Selkirk Island with one species on both islands. While its continental sister species is unknown, Erigeron on the Juan Fernández Islands appears to be monophyletic and most likely evolved from South American progenitor species. We characterized the complete chloroplast genomes of five Erigeron species, including accessions of E. fernandezia and one each from Alejandro Selkirk and Robinson Crusoe Islands, with the purposes of elucidating molecular evolution and phylogenetic relationships. We found highly conserved chloroplast genomes in size, gene order and contents, and further identified several mutation hotspot regions. In addition, we found two positively selected chloroplast genes (ccsA and ndhF) among species in the islands. The complete plastome sequences confirmed the monophyly of Erigeron in the islands and corroborated previous phylogenetic relationships among species. New findings in the current study include (1) two major lineages, E. turricola–E. luteoviridis and E. fernandezia–E. ingae–E. rupicola, (2) the non-monophyly of E. fernandezia occurring on the two islands, and (3) the non-monophyly of the alpine species E. ingae complex.
1. Introduction
The Juan Fernández Archipelago in the Pacific Ocean, located 667 km off the west coast of Chile, is composed of two major islands: Masatierra (or Isla Robinson Crusoe (RC)) and Masafuera (Isla Alejandro Selkirk (AS)) [1]. These two islands, RC and AS, are ca. 4 and 1 million years (my) old, respectively [2], and have estimated areas of 48 and 50 km2, respectively. The islands are separated by 181 km in an east–west orientation. Despite their relatively small size and young geological age, the Juan Fernández Islands harbor a flora with 65% percent endemic species for angiosperms) [3,4,5]. A combination of small flora and the simplicity of the biogeographic setting makes this archipelago ideal for biogeographic and evolutionary studies, especially for assessing the patterns of the two modes of speciation (i.e., cladogenesis and anagenesis) and their genetic consequences [6,7].
After the two endemic genera Dendroseris D. Don and Robinsonia DC., Erigeron L. is the third most species-rich genus on Juan Fernández with six endemic species: E. fernandezia (Colla) Harling, E. ingae Skottsb., E. luteoviridis Skottsb., E. rupicola Phil., E. stuessyi Valdeb. and E. turricola Skottsb. [8]. Unlike Robinsonia and Dendroseris species that primarily occur on the older island (Robinson Crusoe), five of the six Erigeron species are restricted to the younger island (Alejandro Selkirk); E. fernandezia also occurs on Robinson Crusoe [8]. Morphological and limited nucleotide sequence data suggested that the group in the islands has resulted from a single introduction, likely from a single continental ancestor in South America, where the genus is diverse [8,9,10]. All endemic species are polyploid (n = 27) [11,12,13,14]. Species differ in leaf shape (lanceolate to spatulate), capitulum size and number, and growth form (semiglobose subshrubs to erect shrubs over 1 m tall) [8]. In addition, ecological differentiation can be observed from near sea level to over 1000 m, from dry and exposed areas near the coast to wet environments usually covered by fog [8]. A cladistic analysis of morphological characters revealed two major clades: one containing two species, E. rupicola–E. stuessyi, and the other containing the remaining four species, i.e., the E. ingae complex. Additionally, a sister relationship between E. luteoviridis and E. fernandezia was suggested [8]. Recently, two nuclear markers, amplified fragment length polymorphisms (AFLPs) and simple sequence repeats (SSRs), were explored to determine genetic variation within and differentiation among six species of Erigeron [15]. This study found the following three distinct genetic lineages within the genus: (1) E. rupicola and E. stuessyi, (2) E. fernandezia, and (3) the E. ingae complex (including E. ingae, E. luteoviridis and E. turricola). In the case of E. fernandezia on two islands, the results are suggestive of its origin on the younger Alejandro Selkirk Island with the subsequent dispersal and establishment on the older Robinson Crusoe Island [15].
The rapid accumulation of the complete plastome sequences has provided valuable information both for resolving phylogenetic relationships at various taxonomic levels and for building reference genomes. As examples, we have gained pivotal information from plastome sequences for inferring the origin and evolution of plant endemics in island settings: Zanthoxylum [16], Rubus [17], Fagus [18], Dendroseris [19], Viola [20], the woody Sonchus alliance [21], etc. In particular, the plastid phylogenomic analysis of Dendroseris, the largest endemic genus in the Juan Fernández Islands, supported the monophyly of the genus and the full resolution of interspecific and intersubgeneric relationships [19]. In addition, mutation hotspots and highly variable chloroplast microsatellites (simple sequence repeats (SSRs)) were identified as potential chloroplast markers.
While complete plastome sequences for certain plant groups have recently been generated as genomic references and for phylogenomic investigations at various taxonomic levels [17,19,22,23,24,25], very little is known about the plastomic genomes reference of Erigeron and related genera, including Conyza [26,27]. In particular, the plastomes of tribe Astereae from the Southern Hemisphere, including subtribe Conyzinae, are poorly represented, and both specific and higher-level relationships in Erigeron are poorly understood [10]. Although some insights into species relationships and genetic variation among endemic species of Erigeron in the Juan Fernández Islands have been provided in previous studies, no attempt has been made to assemble complete plastomes to generate genomic reference, to understand molecular evolution, nor to further confirm species relationships. Therefore, the purposes of this study are to (1) characterize much needed plastomes of Erigeron species to build genomic references for future phylogenomic analysis of the genus on a global scale, (2) conduct a comprehensive comparative plastome analysis among five species of Erigeron to understand plastome evolution on oceanic islands and (3) assess the first phylogenetic relationships among species based on complete plastome sequences to gain insights into species relationships.
2. Results
2.1. Genome Size and Features
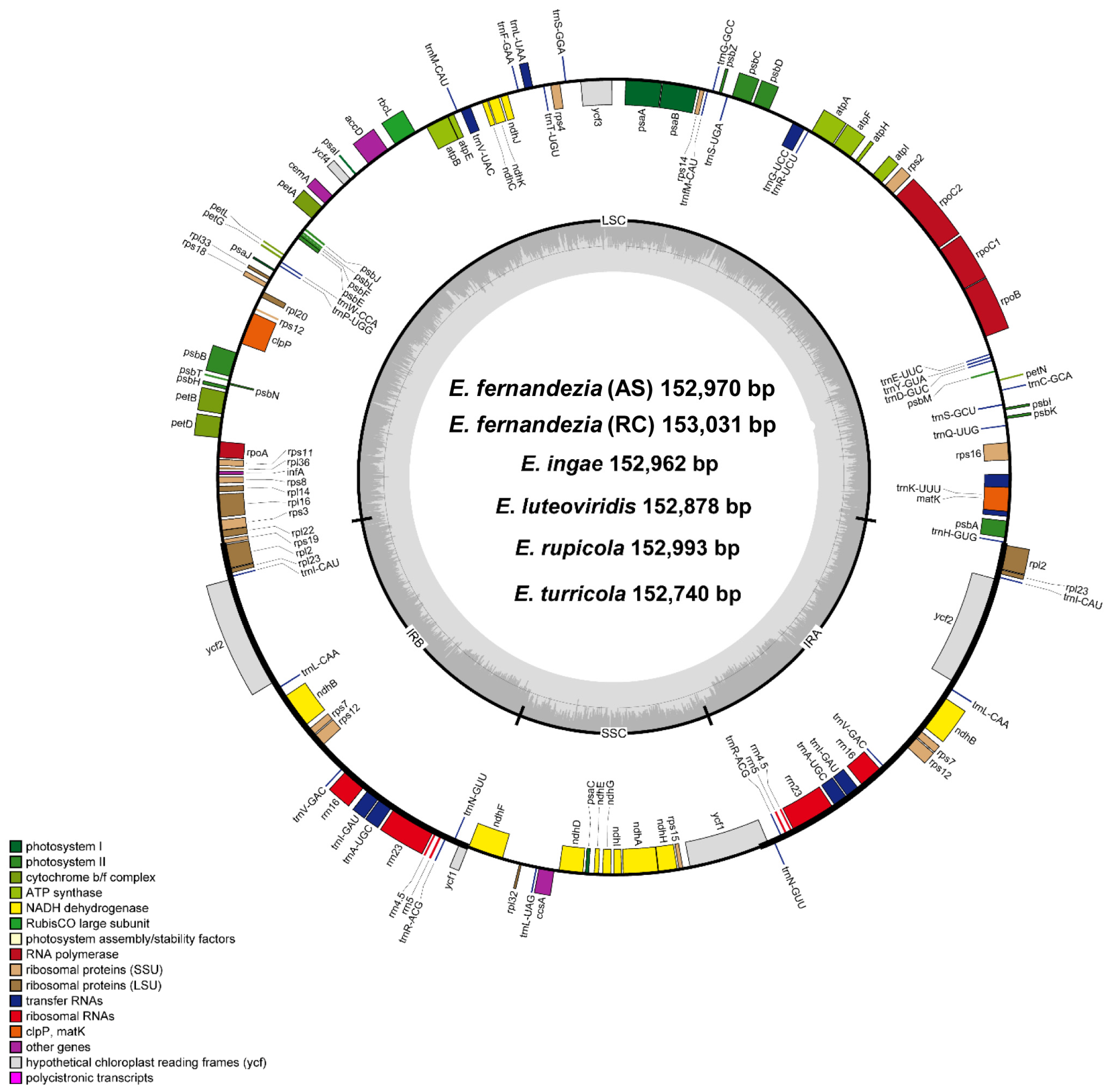
With the exception of E. stuessyi, the six complete plastomes of five Erigeron species from the Juan Fernández Islands were characterized for the first time: two accessions of E. fernandezia in Alejandro Selkirk (AS) and Robinson Crusoe Island (RC), E. ingae, E. luteoviridis, E. rupicola and E. turricola. The length of complete plastome sequences ranged from 152,740 bp (E. turricola) to 153,031 bp (E. fernandezia_RC), and the plastomes were highly conserved with no structural variation or content rearrangements (Figure 1). The large single-copy (LSC) region, small single-copy (SSC) region, and two inverted repeat (IR) regions ranged from 84,864 bp (E. turricola) to 84,504 bp (E. fernandezia_RC), from 18,355 bp (E. fernandezia_RC) to 18,219 bp (E. luteoviridis), and from 25,027 bp (E. luteoviridis) to 24,770 bp (E. turricola), respectively (Table 1). The plastomes of Erigeron species on the islands contained 130 genes, including 85 protein-coding genes, except for E. rupicola which contained 131 genes (one additional rps19 pseudogene present). All six newly sequenced plastomes of Erigeron contained eight rRNA and 36 tRNA genes, and their overall guanine–cytosine (GC) content was identical, i.e., 37.2%. Also, all six plastomes contained a total of 17 duplicated genes in the IR regions, including seven tRNA, four rRNA and six protein-coding genes. Fifteen genes (ndhA, ndhB, petB, petD, rpl2, rpl16, rpoC1, rps12, rps16, trnA-UGC, trnG-UCC, trnI-GAU, trnK-UUU, trnL-UAA and trnV-UAC) contained a single intron, whereas clpP and ycf3 each contained two introns.
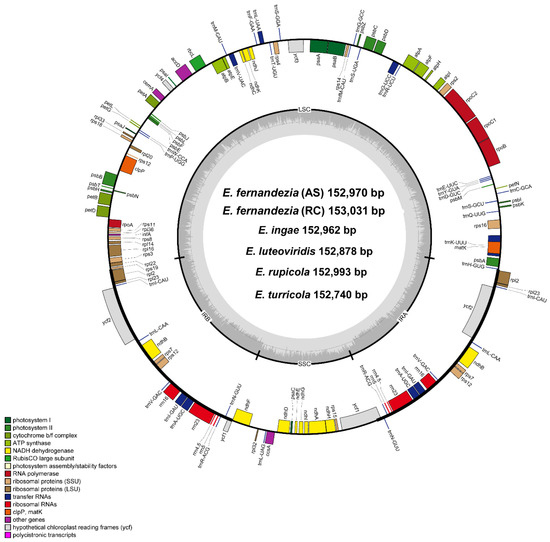
Figure 1.
Complete plastome map of six Erigeron species. The genes located outside of the circle are transcribed counterclockwise, while those located inside are transcribed clockwise.

Table 1.
Summary of the characteristics of the six Erigeron chloroplast genomes on the Juan Fernández Islands.
For the pseudogenized ycf1 gene, which is positioned in the IRb/SSC junction region, E. luteoviridis contained the longest one (732 bp), while the others contained the shorter and same size of 597 bp. As for the complete ycf1 gene in the SSC/IRa junction, E. rupicola and E. turricola contained the short gene (4524 bp and 4470 bp, respectively), whereas the others all had the same length of 5055 bp. Interestingly, five plastomes of Erigeron contained one single complete rps19 gene (279 bp), whereas E. rupicola retained two rps19 genes, including one pseudogenized gene (123 bp) and one single complete gene (279 bp).
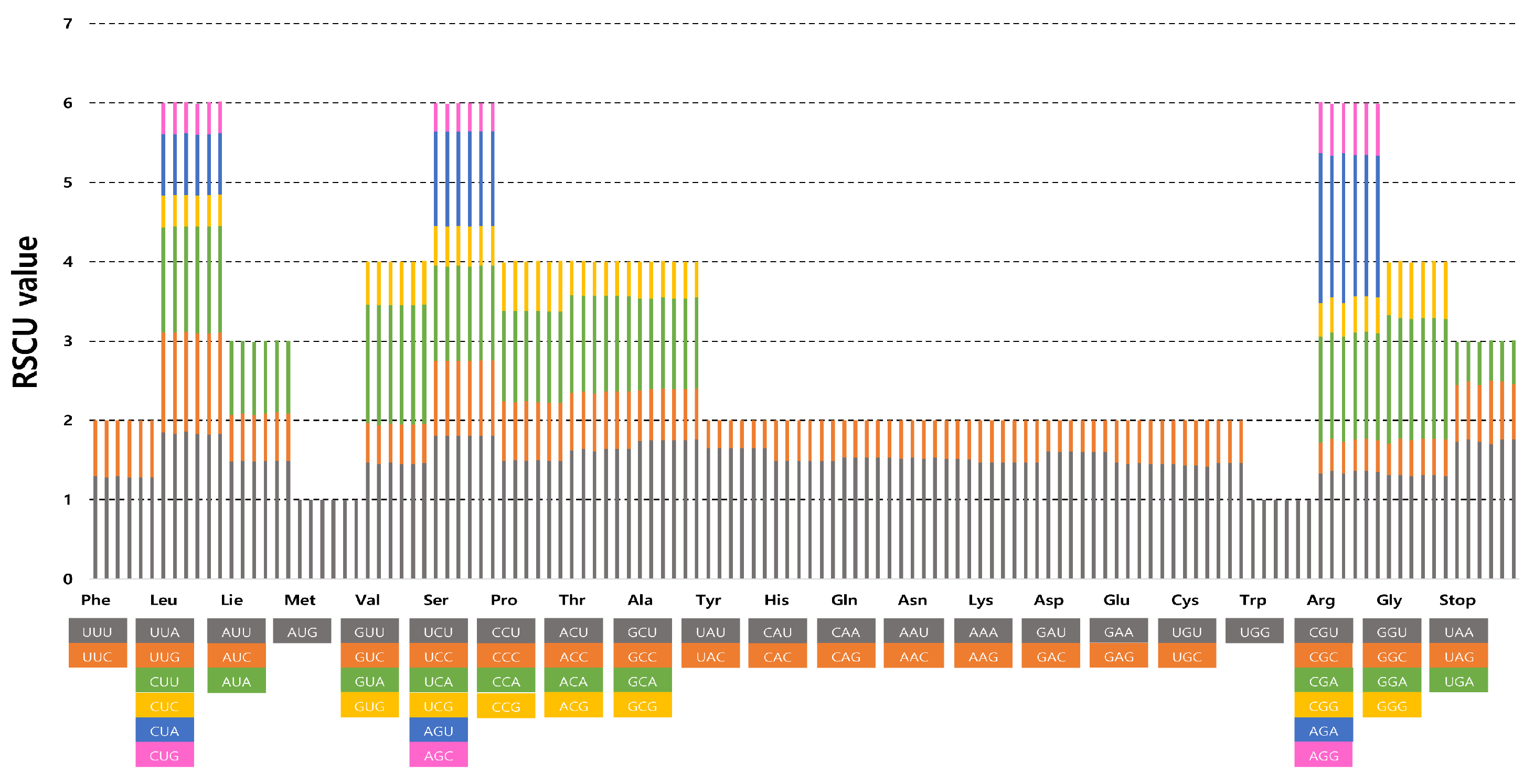
The frequency of codon usage of the six complete plastomes of Erigeron was calculated based on the sequences of protein-coding and tRNA genes. The results showed that the average codon usage among the six accessions ranged from 23,892 (E. turricola) to 25,766 (E. ingae) (Supplementary Table S1). The average codon usage for the remaining species was 25,656 for E. fernandezia (AS), 24,024 for E. fernandezia (RC), 25,766 for E. luteoviridis, 24,002 for E. rupicola and 23,892 for E. turricola. The highest RSCU value was indicated in the usage of the UUA codon for leucine (1.82–1.86) followed by GCU for alanine (1.75–1.76) and AGA for arginine (1.79–1.89). The lowest RSCU value was indicated in the usage of AGC for serine (0.35–0.36) and GAC for aspartic acid (0.39–0.40). We found the distribution of codon types to be consistent (Figure 2), and codons AUG (M) and UGG (W) encoded methionine and tryptophan, respectively, showing no bias (RSCU = 1) (Supplementary Table S1).
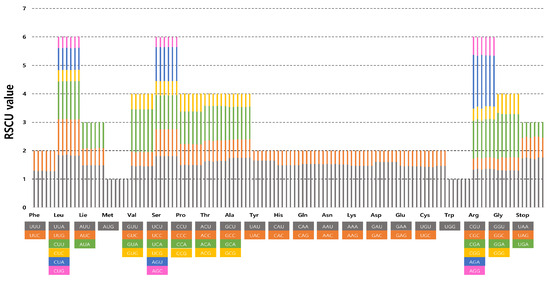
Figure 2.
Relative synonymous codon usage in six newly sequenced Erigeron accessions. The list of species from left to right columns represent E. fernandezia (AS), E. fernandezia (RC), E. ingae, E. luteoviridis and E. turricola.
2.2. Comparative Analysis of Genome Structure
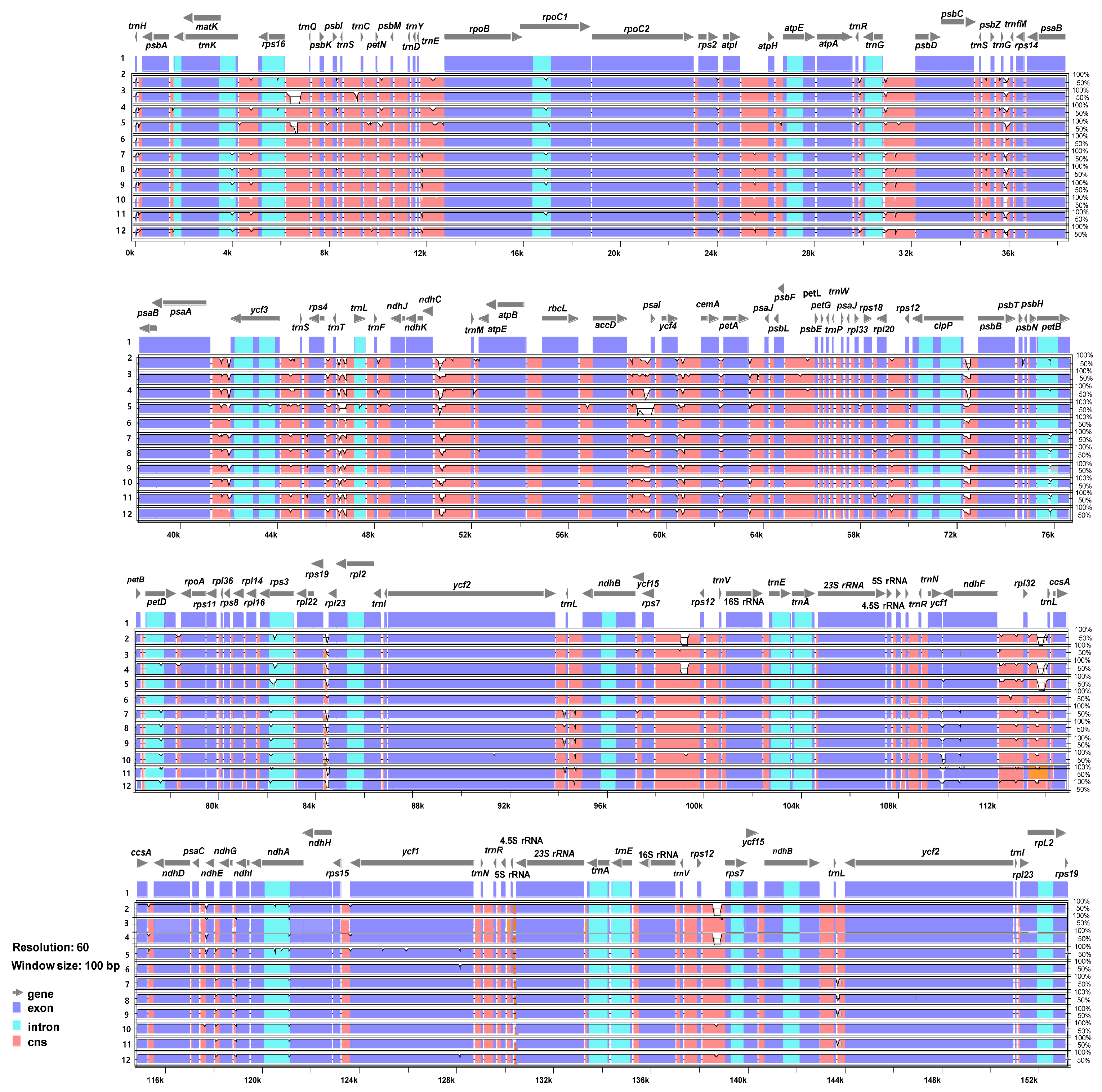
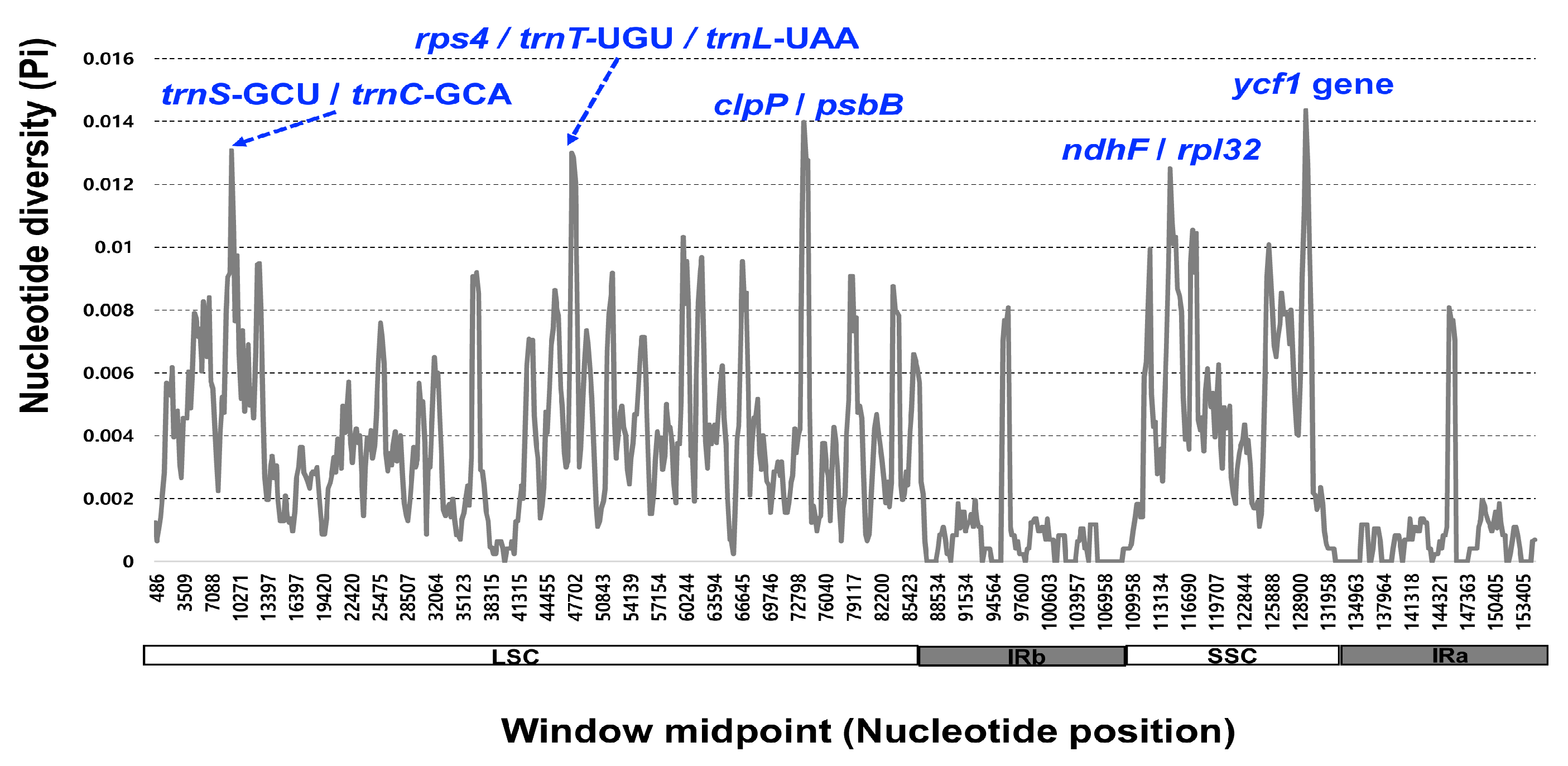
The 11 complete plastome sequences of the Erigeron accessions were plotted using mVISTA analysis, using the annotated E. annuus plastome as a reference (Figure 3). The results indicated that the LSC region was the most divergent, that the two IR regions were highly conserved, and that the non-coding regions were more divergent and variable than the coding regions. A sliding window analysis using the DnaSP program revealed highly variable regions in 11 Erigeron chloroplast genomes (Figure 4). The average value of nucleotide diversity (PI) over the entire cp genome was 0.0033. The most variable region was ycf1 genic region with a PI value of 0.01436. Also, highly variable regions included four other intergenic regions, i.e., clpP/psbB (Pi = 0.01398), rps4/trnT-UGU/trnL-UAA (Pi = 0.013), trnS-GCU/trnC-GCA (Pi = 0.01309) and ndhF/rpl32 (Pi = 0.0125). Therefore, five highly variable regions with PI value of greater than 0.012 were identified in 11 Erigeron plastid genomes, which can be used for population genetic and phylogeographic studies. A positive selection analysis allowed us to identify two positively selected genes, with a statistically significant LRT p value based on the M7 and M8 model, among the six Erigeron plastomes on the Juan Fernández Islands (Table 2); they are the ccsA and ndhF genes, subunits of the cytochrome and NADH-dehydrogenase subunit genes, respectively. However, all but two genes, i.e., 73 of the 75 genes, had an average Ka/Ks ratio below 1, indicating that these genes have been subjected to strong purifying selection in the insular Erigeron chloroplast genomes.
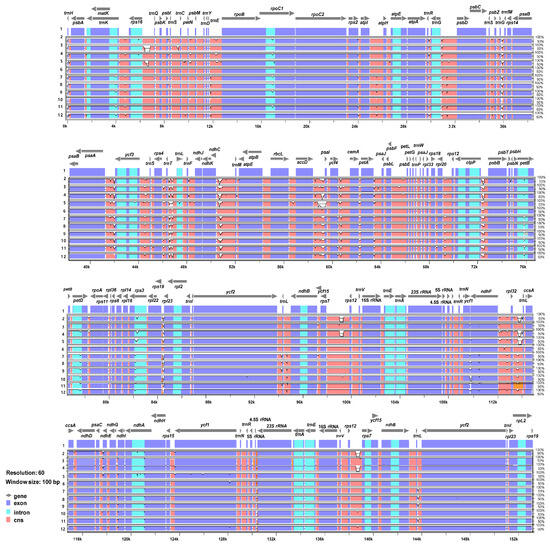
Figure 3.
Visualization of alignment of the 12 plastome sequences of Erigeron accessions. Species: 1. E. annuus MZ361990; 2. E. breviscapus NC043882; 3. E. canadensis NC046789; 4. E. multiradiatus NC056169; 5. E. philadelphicus MT579973; 6. E. strigosus MT579973; 7. E. fernandezia (AS); 8. E. fernandezia (RC); 9. E. ingae; 10. E. luteoviridis; 11. E. rupicola; 12. E. turricola.
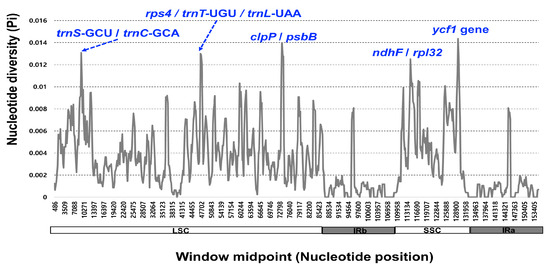
Figure 4.
Sliding window analysis of the 12 whole chloroplast genomes of Erigeron species.

Table 2.
Log-likelihood values of the site-specific models based on six accessions of Erigeron on the Juan Fernández Islands, with detected sites having dN/dS values > 1.
2.3. Phylogenetic Analysis
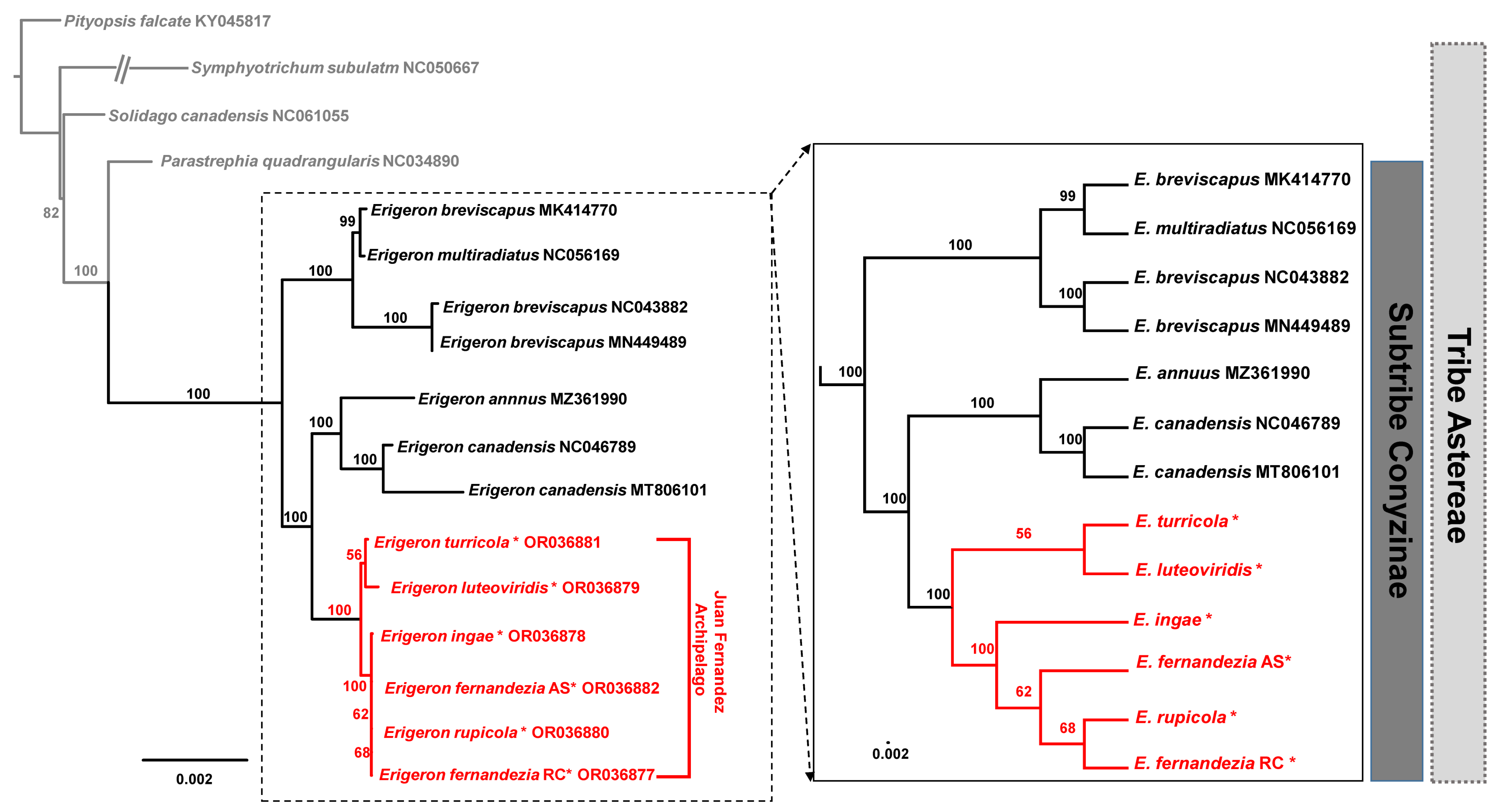
A maximum likelihood (ML) analysis based on the concatenated 80 common protein-coding genes, excluding the ones duplicated in the IR regions, was conducted on the best-fit model of TPM3u+F+I. A total of 62,990 aligned nucleotide bases included 685 parsimony-informative sites. A phylogenetic analysis of 21 representative plastomes within the Astereae tribe confirmed monophyly of the Erigeron genus (100% bootstrap support (BS)) from the outgroup taxa (Parastrephia quadrangularis, Pityopsis aspera var. adenolepis, P. falcata, P. graminifolia, Symphyotrichum subulatum and Solidago canadensis) (Figure 5). Within Erigeron, two Old World species, E. breviscapus and E. multiradiatus, formed an early divergent lineage, but E. breviscapus appeared to be not monophyletic (100% BS). The remaining species of Erigeron formed two major clades, one including continental herbaceous species (E. canadensis, E. philadelphicus, E. annuus and E. strigosus; 100% BS) and the other including Juan Fernández Islands endemics (E. turricola, E. luteoviridis, E. fernandezia, E. ingae and E. rupicola; 100% BS) (Figure 5). Within the monophyletic Erigeron on the Juan Fernández Islands, two major lineages could be further recognized: one includes E. turricola-E. luteoviridis (56%) and the other includes E. fernandezia-E. ingae-E. rupicola (100% BS). The only species found on both Alejandro Selkirk (AS) and Robinson Crusoe Island (RC), E. fernandezia, turned out to be not monophyletic; E. fernandezia on RC is sister to E. rupicola (68% BS).
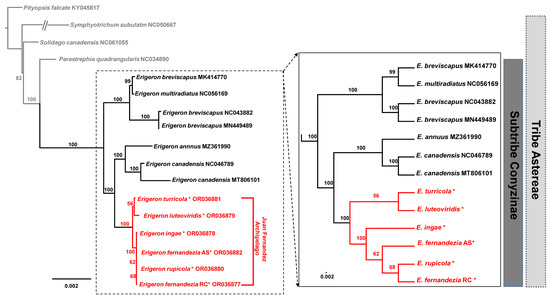
Figure 5.
The maximum-likelihood (ML) tree inferred from 15 accessions of Erigeron and six Astereae tribe plastomes. The bootstrap value based on 1000 replicates is shown on each node. The six newly sequenced accessions in the current study are indicated using red asterisks.
3. Discussion
3.1. Chloroplast Genome Structure and Evolution in Genus Erigeron
In this study, we newly sequenced and characterized five species of Erigeron (six accessions): E. fernandezia (AS), E. fernandezia (RC), E. ingae, E. luteoviridis, E. rupicola and E. turricola. As the cp genomes are generally conserved in sequence and structure in land plants [28], we found highly conserved cp genomes in the genus Erigeron, especially among the species on the Juan Fernández Islands (Table 1). The high conservation of plastomes in Erigeron is consistent with the largest endemic genus Dendroseris on the Juan Fernández Islands, which radiated on the older Robinson Crusoe Island [19].
While the chloroplast genomes were highly conserved amongst the 11 Erigeron species (six continental and five Juan Fernández Islands), we identified five divergence hotspot regions (ycf1, clpP/psbB, rps4/trnT-UGU/trnL-UAA, trnS-GCU/trnC-GCA and ndhF/rpl32) based on mVISTA and sliding window analysis (Figure 3 and Figure 4). With the exception of two regions, these regions are different from the eight hotspot regions based on the 11 continental Erigeron species only: accD/psaI, trnT, ndhC/trnV, clpP/psbB, trnT/trnL, trnG/trnfM, psaA/ycf3 and ccsA/rpl32 [29]. In comparison to the five mutation hotspot regions of insular Erigeron (the Astereae tribe) with those in the genus Dendroseris (the Cichorieae tribe), two regions, ycf1 and trnT/trnL, are found to be highly variable [19]. Highly variable chloroplast regions have been widely utilized for species delimitation, phylogenetic inference, and infraspecific phylogeography [30,31,32,33,34,35,36]. Therefore, we suggest that several variable chloroplast regions found in Erigeron may serve as effective DNA barcoding markers and could be useful for Erigeron phylogenetic and infraspecific phylogeographic studies.
We also found that two chloroplast genes, ccsA and ndhF, were under positive selection, cautiously indicating their adaptive roles during the diversification of Erigeron on the Juan Fernández Islands. The ccsA (ycf5) gene encodes a protein required for cytochrome biogenesis that mediates the attachment of heme to c-type cytochromes [37]. It has been shown that the ccsA gene was under positive selection for epiphytic orchid and fig species as well as insular endemic Cotoneaster wilsonii [38,39,40]. Although ccsA may play an important role in the adaptation of epiphytes to special habitats, it is unclear how this gene is under positive selection for Erigeron species on the Juan Fernández Islands. It is plausible that positive selection on ccsA may contribute to adaptation to specific environmental conditions, such as light intensity, moisture, and/or temperature on oceanic islands [39]. The other selected ndhF gene encodes NADH dehydrogenase subunit proteins and has been known to be under positive selection in various plant groups, including the ones adapted to different altitudinal habitats and in shade-tolerant and sun-loving plants [17,18,41,42,43,44,45,46]. The adaptive evolution of the ndhF gene may affect energy transformation and resistance to photo-oxidative stress in different environments [47]. Furthermore, the ndhF gene is known to be involved in adaptation to hot and dry climates, likely contributing to adaptation to high light intensity during the evolution of Erigeron on the Juan Fernández Islands [46,48,49].
3.2. Adaptive Radiation of Erigeron on the Younger Island of the Juan Fernández Archipelago
Erigeron is biogeographically and evolutionarily interesting because its colonizing ancestor apparently became established on the younger island Alejandro Selkirk Island and later dispersed to the older island Robinson Crusoe. Upon colonization on the younger island, cladogenetic speciation resulted in the complex of six species within 1 million years [15]. Given the ecological differentiation among species, Erigeron represents the best example of radiation, presumably adaptive, within the archipelago: E. rupicola (coastal rocks), E. stuessyi (lower elevations, but only inside the deep, cool, and moist ravine walls), E. fernandezia (open areas, especially on the middle slopes and ravine margins), and E. ingae/E. luteoviridis/E. turricola (higher elevation, ca. 800–1200 m, in the fern–grassland mosaic zone). Morphologically, E. rupicola is a small rosette herb with short flowering stalks and small solitary heads, and the closely related E. stuessyi can be distinguished from it by having thinner leaves with longer flowering stalks [15]. Erigeron fernandezia is tall, with long, dentate leaves (sometimes woody at the base) and with many medium-sized heads. The remaining three species of the E. ingae complex are morphologically similar, having rosette-bearing and long flowering stalks with many larger flowering heads.
While our overall phylogenetic relationships among species and their biogeographic scenarios tend to be consistent with the previous AFLP and microsatellite data [15], one unexpected finding based on complete plastome data is the position of E. ingae (Figure 5). Rather than forming a clade with two other morphologically similar, higher-elevation species (ca. 800–1200 m), i.e., E. turricola and E. luteoviridis, E. ingae is closely related to lower/middle-elevation species, E. rupicola (50–100 m) and E. fernandezia (below 100 m to ca. 1200 m; AS). Given its unusual position based on complete plastome, it is possible that higher-elevation E. ingae experienced gene flow with either strictly coastal E. rupicola and lower/middle-elevation E. fernandezia or their most recent common ancestor. An unrooted neighbor-joining tree based on microsatellites (Figure 3 of López-Sepúlveda et al. [15]) showed that of the two allopatric populations of E. ingae sampled, one population (population 33) is clustered with the geographically closer E. fernandezia (population 16; AS) and E. luteoviridis (population 36), while the other one is clustered with E. turricola (populations 46 and 47) and E. fernandezia (population 22; AS). Considering the geographical distribution of these species in the western part of AS, E. ingae likely captured the chloroplast from E. fernandezia (AS) rather than from the primarily eastern coastal species E. rupicola. Alternatively, incomplete lineage sorting when undergoing rapid adaptive radiation might likely be the cause of this incongruence, which requires further investigation based on more genome-wide, unlinked markers [50,51].
Both the AFLP and microsatellite data clearly demonstrated the population differentiation of E. fernandezia on the two islands and showed lower diversity in E. fernandezia on RC than on AS, suggesting its origin on the younger AS with subsequent dispersal to the older RC [15]. Morphologically the populations of E. fernandezia do not differ between the two islands, and given their restricted distribution to paths and disturbed areas, the older island RC populations were suggested to have been introduced back even during historical time [8,52]. Differences in the flavonoid profiles further support population differentiation between the two islands; the majority of RC populations contain C-glycosyl-flavones, whereas AS populations lack them [8]. Our current study also finds chloroplast genome divergence between the two islands and thus infraspecific population divergence between the two islands, certainly more than among species of the E. ingae complex [15]. In addition, no significant genetic reduction in RC populations and population differentiation patterns were demonstrated by López-Sepúlveda [15], likely refuting the possibility of the recent, especially during historical time, origin of E. fernandezia on RC.
The three species of E. ingae complex appear to be segregated from other taxa morphologically and genetically, despite not being ecologically partitioned. The taxonomic treatment of E. turricola as a heterotypic synonym of E. ingae [53] was not supported by the previous AFLP/microsatellite study. Erigeron turricola was genetically diverged from E. ingae based on microsatellites (FST = 0.422 vs. FST = 0.269 between E. luteoviridis and E. fernandezia, AS; and FST = 0.231 between E. turricola and E. fernandezia, AS), while the AFLP data showed the lowest genetic differentiation between the two species (FST = 0.095). The current study suggested the sharing of their most recent common ancestor between E. turricola and E. luteoviridis. It is uncertain whether the three alpine species of the E. ingae complex are monophyletic, and the monophyly of each species and their phylogenetic relationships on AS await determination. Given the non-monophyly of the three E. ingae complex species based on the current plastome relationships and morphological similarity between E. fernandezia and E. luteoviridis (even once considered conspecific with E. turricola [53]), it is plausible that E. ingae and E. luteoviridis independently adapted to the alpine conditions and drier subalpine regions, respectively. Owing to the potential difficulty in interpreting banding patterns of AFLPs and microsatellites of hexaploid (n = 27) species such as Erigeron as contrasted interpreting bands in diploid plants, the methods used for inferring phylogenetic relationships of diploids are not adequate for polyploids. The use of new analyses for polyploids [54,55,56] may provide insights into the evolutionary history and adaptive radiation of Erigeron in the Juan Fernández Islands.
In conclusions, we characterized the complete chloroplast genomes of five Erigeron species, building important genomic references for the future global-scale phylogenomic analysis of the genus. Chloroplast genomes were found to be highly conserved and provided novel phylogenetic relationships among species in the Juan Fernández Islands. We identified five divergence hotspot regions of insular Erigeron, which might be useful as effective DNA barcoding markers and in infraspecific phylogeographic studies. Two chloroplast genes, ccsA and ndhF, were positively selected, likely contributing to the adaptation and speciation of Erigeron in the Juan Fernández Islands.
4. Materials and Methods
4.1. Plant Sampling, DNA Isolation, and Plastome Sequencing, Assembly and Annotation
Given the rarity and conservation status of some Erigeron species on the islands, we selected and sequenced six complete plastome of Erigeron accessions in this study: two accessions of E. fernandezia, one on Alejandro Selkirk (AS) and the other on Robinson Crusoe Island (RC), and one accession each of E. ingae, E. luteoviridis, E. rupicola, and E. turricola. We were not able to include E. stuessyi due to sequencing difficulty. Silica gel dried leaf tissues collected in the field during the previous expeditions or herbarium specimens deposited at OS (the Ohio State University herbarium) were used for DNA extraction (Supplementary Table S2). Total DNA was extracted using the DNeasy Plant Mini Kit (Qiagen, Carlsbad, CA, USA) and sequenced using an Illumina HiSeq 4000 (Illumina, Inc., San Diego, CA, USA), yielding a 150 bp paired-end read length, at Macrogen Co. (Seoul, Republic of Korea). The resulting paired-end reads were assembled de novo using Velvet v1.2.10 with multiple k-mers [57] with coverage ranging from 71 to 291. tRNAs were confirmed using tRNAscan-SE [58]. The sequences were annotated using Geneious R10 [59] and deposited in GenBank (OR036877-OR036882). Annotated sequence files in the GenBank format were used to draw a circular map with OGDRAW v1.2 [60].
4.2. Comparative Plastome Analysis
To understand plastome evolution within the genus Erigeron, we selected 11 taxa (12 accessions) including six newly assembled plastomes of Erigeron [six continental species, i.e., E. annus (MZ361990), E. breviscapus (NC043882), E. canadensis (NC046789), E. multiradiatus (NC056169), E. philadelphicus (MT579972), E. strigosus (MT579973), and five insular endemics, i.e., E. fernandezia (two accessions, one each from AS and RC), E. ingae, E. luteoviridis, E. rupicola, and E. turricola)] and compared their genomic features using the Shuffle-LAGAN mode [61] of mVISTA [62]. The sequences of the 12 Erigeron plastomes were aligned using the back-translation approach with MAFFT ver.7 [63] and were manually edited using Geneious R10 [59]. Using DnaSP 6.10 [64], a sliding window analysis was performed with a step size of 200 bp and window length of 800 bp to determine the nucleotide diversity (Pi) of the plastomes. The codon usage frequency was calculated using MEGA 7 [65] based on the relative synonymous codon usage (RSCU) value [66], which is a simple measure of the non-uniform usage of synonymous codons in a coding sequence. The DNA code used by bacteria, archaea, prokaryotic viruses and chloroplast proteins was used [67]. To evaluate the natural selection pressure on the protein-coding genes of six Erigeron plastomes, a site-specific model was tested using EasyCodeML [68] with the CODEML algorithm [69]. Seven codon substitution models (M0, M1a, M2a, M3, M7, M8 and M8a) were compared to detect the positively selected sites based on the likelihood ratio test (LRT).
4.3. Phylogenetic Analysis
For the phylogenetic analysis, complete plastome sequences of 15 accessions of Erigeron and six representative species of outgroup within the Asteraceae tribe were aligned using MAFFT ver.7 [63] in Geneious R10 [59]. The 15 accessions of the ingroup Erigeron included all nine Erigeron plastome sequences currently available in GenBank with two additional accessions of E. breviscapus (MK414770 and MN449489) and E. canadensis (MT806101) and the six newly assembled Erigeron plastome sequences in this study. For the concatenated sequences of 80 common protein-coding genes (excluding the ones duplicated in the IR regions), a maximum likelihood (ML) analysis based on the best-fit model of “TPM3u+F+I” was conducted using IQ-TREE 1.4.2 [70]. Parastrephia quadrangularis (NC034890), Solidago canadensis (NC061055), Symphyotrichum subulatum (NC050667) and three plastomes of Pityopsis (P. aspera var. adenolepis (MW271041), P. falcata (KY045817) and P. graminifolia (MW279328)) were used as the outgroup, and a non-parametric bootstrap analysis was performed with 1000 replicates.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13050612/s1, Table S1: The codon usage and codon–anticodon recognition pattern for tRNA in the six Erigeron plastomes from the Juan Fernández Islands. Table S2: List of six Erigeron accessions obtained from the Juan Fernández Islands. NA = not available. AS = Alejandro Selkirk Island. RC = Robinson Crusoe Island.
Author Contributions
Conceptualization, S.-H.K., J.Y. and S.-C.K.; methodology, S.-H.K., J.Y. and M.-S.C.; software, S.-H.K., J.Y. and M.-S.C.; validation, S.-H.K. and J.Y.; formal analysis, S.-H.K., J.Y. and M.-S.C.; investigation, S.-H.K., J.Y. and M.-S.C.; resources, T.F.S. and D.J.C.; data curation, S.-H.K. and J.Y.; writing—original draft preparation, S.-H.K. and J.Y.; writing—review and editing, T.F.S., D.J.C. and S.-C.K.; visualization, S.-H.K., J.Y. and M.-S.C.; supervision, S.-C.K.; project administration, S.-C.K.; funding acquisition, M.-S.C. and S.-C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study is supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education to M.-S.C. (2022R1I1A2063355) and the Ministry of Education, and the NRF Grant (NRF-2019S1A6A3A02058027).
Data Availability Statement
The data presented in this study are openly available in the NCBI GenBank (https://www.ncbi.nlm.nih.gov/) (accession numbers: OR036877-OR036882O).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stuessy, T.F.; Foland, K.A.; Sutter, J.F.; Sanders, R.W.; Silva, M. Botanical and geological significance of potassium-argon dates from the Juan Fernandez Islands. Science 1984, 225, 49–51. [Google Scholar] [CrossRef]
- Stuessy, T.F.; Crawford, D.J.; Marticorena, C. Patterns of phylogeny in the endemic vascular flora of the Juan Fernandez Islands, Chile. Syst. Bot. 1990, 15, 338–346. [Google Scholar] [CrossRef]
- Skottsberg, C. Derivation of the flora and fauna of Juan Fernandez and Easter Island. Nat. Hist. Juan Fernandez Easter Isl. 1956, 1, 193–438. [Google Scholar]
- Stuessy, T.F. Centres of plant diversity: A guide and strategy of their conservation. In Juan Fernández Islands; Davis, S.D., Heywood, V.H., Hamilton, A.C., Eds.; IUCN Publications Unit: Cambridge, UK, 1995; pp. 565–568. [Google Scholar]
- Marticorena, C.; Stuessy, T.F.; Baeza, M. Catálogo de la flora vascular del Archipiélago de Juan Fernández, Chile. Gayana Bot. 1998, 55, 187–211. [Google Scholar]
- Stuessy, T.F.; Ruiz, E.; Crawford, D.J.; Tremetsberger, K. Testing degrees of genetic divergence and populational variation in oceanic island archipelagos: Juan Fernandez as a model system. Nova Acta Leopold. 2005, 92, 147–165. [Google Scholar]
- Takayama, K.; López-Sepúlveda, P.; Greimler, J.; Crawford, D.J.; Peñailillo, P.; Baeza, M.; Ruiz, E.; Kohl, G.; Tremetsberger, K.; Gatica, A.; et al. Genetic consequences of cladogenetic vs. anagenetic speciation in endemic plants of oceanic islands. AoB Plants 2015, 7, plv102. [Google Scholar] [CrossRef] [PubMed]
- Valdebenito, H.; Stuessy, T.F.; Crawford, D.J.; Silva, M. Evolution of Erigeron (Compositae) in the Juan Fernandez Islands, Chile. Syst. Bot. 1992, 17, 470–480. [Google Scholar] [CrossRef]
- Solbrig, O.T. Note on Gymnosperma glutinosum (Compositae-Astereae). Leafl. West. Bot. 1961, 9, 147–150. [Google Scholar]
- Noyes, R.D. Biogeographical and evolutionary insights on Erigeron and allies (Asteraceae) from ITS sequence data. Plant Syst. Evol. 2000, 220, 93–114. [Google Scholar] [CrossRef]
- Solbrig, O.T.; Anderson, L.C.; Kyhos, D.W.; Raven, P.D.; Rüdenberg, L. Chromosome numbers in Compositae. V. Astereae. II. Am. J. Bot. 1964, 51, 513–519. [Google Scholar] [CrossRef]
- Sanders, R.W.; Stuessy, T.F.; Rodriguez, R. Chromosome numbers from the flora of the Juan Fernandez Islands. Am. J. Bot. 1983, 70, 799–810. [Google Scholar] [CrossRef]
- Spooner, D.M.; Stuessy, T.F.; Crawford, D.J.; Silva, M. Chromosome numbers from the flora of the Juan Fernandez Islands. II. Rhodora 1987, 89, 351–356. [Google Scholar]
- Sun, B.-Y.; Stuessy, T.F.; Crawford, D.J. Chromosome counts from the flora of the Juan Fernandez Islands, Chile. III. Pac. Sci. 1990, 44, 258–264. [Google Scholar]
- López-Sepúlveda, P.; Takayama, K.; Greimler, J.; Crawford, D.J.; Peñailillo, P.; Baeza, M.; Ruiz, E.; Kohl, G.; Tremetsberger, K.; Gatica, A.; et al. Speciation and Biogeography of Erigeron (Asteraceae) in the Juan Fernández Archipelago, Chile, based on AFLPs and SSRs. Syst. Bot. 2015, 40, 888–899. [Google Scholar] [CrossRef]
- Reichelt, N.; Wen, J.; Patzold, C.; Appelhans, M.S. Characterization of the four Zanthoxylum L. species (Sapindales: Rutaceae) from the Caribbean, Madagascar, the Mascarene Islands, and the South Pacific. Microbial Resour. Announc. 2021, 10, e00399-21. [Google Scholar]
- Yang, J.Y.; Chiang, Y.-C.; Hsu, T.-W.; Kim, S.-H.; Pak, J.-H.; Kim, S.-C. Characterization and comparative analysis among plastome sequences of eight endemic Rubus (Rosaceae) species in Taiwan. Sci. Rep. 2021, 11, 1152. [Google Scholar] [CrossRef]
- Yang, J.Y.; Takayama, K.; Youn, J.-S.; Pak, J.-H.; Kim, S.-C. Plastome characterization and phylogenomics of east Asian beeches with a special emphasis on Fagus multinervis on Ulleung Island, Korea. Genes 2020, 11, 1338. [Google Scholar] [CrossRef]
- Cho, M.-S.; Kim, S.-H.; Yang, J.Y.; Crawford, D.J.; Stuessy, T.F.; López-Sepúlveda, P.; Kim, S.-C. Plastid phylogenomics of Dendroseris (Cichorieae; Asteraceae): Insights into structural organization and molecular evolution of an endemic lineage from the Juan Fernández Island. Front. Plant Sci. 2020, 11, 594272. [Google Scholar] [CrossRef]
- Go, A.-R.; Yoo, K.-O. Comparative genomics of Viola selkirkii and V. ulleungdoensis (Violaceae). Korean J. Plant Taxon. 2023, 53, 38–46. [Google Scholar] [CrossRef]
- Cho, M.-S.; Yang, J.Y.; Yang, T.-J.; Kim, S.-C. Evolutionary comparison of the chloroplast genome in the woody Sonchus alliance (Asteraceae) on the Canary Islands. Genes 2019, 10, 217. [Google Scholar] [CrossRef]
- Serna-Sánchez, M.; Pérez-Escobar, O.A.; Bogarín, D.; Torres-Jimenez, M.F.; Alvarez-Yela, A.C.; Arcila-Galvis, J.E.; Hall, C.F.; de Barros, F.; Pinheiro, F.; Dodsworth, S.; et al. Plastid phylogenomics resolves ambiguous relationships within the orchid family and provides a solid timeframe for biogeography and macroevolution. Sci. Rep. 2021, 11, 6858. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Z.; Gichira, A.W.; Muchuku, J.K.; Li, W.; Wangm, G.-X.; Chen, J.-M. Plastid phylogenomics and biogeography of the genus Monochoria (Potederiaceae). J. Sys. Evol. 2021, 59, 1027–1039. [Google Scholar] [CrossRef]
- Ross, T.G.; Barrett, C.F.; Gomez, M.S.; Lam, V.K.Y.; Henriquez, C.L.; Les, D.H.; Davis, J.I.; Cuenca, A.; Petersen, G.; Seberg, O.; et al. Plastid phylogenomics and molecular evolution of Alismatales. Cladistics 2016, 32, 160–178. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yu, W.-B.; Tan, Y.-H.; Jin, J.-J.; Wang, B.; Yang, J.-B.; Liu, B.; Corlett, R.T. Plastid phylogenomics improve phylogenetic resolution in the Lauraceae. J. Syst. Evol. 2020, 58, 423–439. [Google Scholar] [CrossRef]
- Li, Z.-J.; Liu, Y.-Y.; Yang, C.-W.; Qian, Z.-G.; Li, G.-D. The complete chloroplast genome sequences of Erigeron breviscapus and Erigeron multiradiatus (Asteraceae). Mitochondrial DNA B Resour. 2019, 4, 3826–3827. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Wu, H.; Zhu, X.; Lin, J. Species identification of Conyza bonariensis assisted by chloroplast genome sequencing. Front. Genet. 2018, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Wicke, S.; Schneeweiss, G.M.; dePamphilis, C.W.; Muller, K.F.; Quandt, D. The evolution of the plastid chromosome in land plants: Gene content, gene order, gene function. Plant Mol. Biol. 2011, 76, 273–297. [Google Scholar] [CrossRef]
- Oh, S.-H.; Park, J. The complete chloroplast genome of Erigeron canadensis isolated in Korea (Asteraceae): Insight into the genetic diversity of the invasive species. Korean J. Plant Taxon. 2023, 53, 47–53. [Google Scholar] [CrossRef]
- Lihová, J.; Kudoh, H.; Marhold, K. Genetic structure and phylogegraphy of a temperate-boreal herb, Cardamine scutata (Brassicaceae), in northeast Asia inferred from AFLPs and cpDNA haplotypes. Am. J. Bot. 2010, 97, 1058–1070. [Google Scholar] [CrossRef]
- Bi, Y.; Zhang, M.F.; Xue, J.; Dong, R.; Du, Y.P.; Zhang, X.H. Chloroplast genomic resources for phylogeny and DNA barcoding: A case study on Fritillaria. Sci. Rep. 2018, 8, 1184. [Google Scholar] [CrossRef]
- Dastpak, A.; Osaloo, S.K.; Maassoumi, A.A.; Safar, K.N. Molecular phylogeny of Astragalus sect. Ammodendron (Fabaceae) inferred from chloroplast ycf1 gene. Ann Bot. Fenn. 2018, 55, 75–82. [Google Scholar] [CrossRef]
- Gutierréz-Rodríguez, C.; Ornelas, F.J.; Rodríguez-Gómez, F. Chloroplast DNA phylogeography of a distylous shrub (Palicourea padifolia, Rubiaceae) reveals past fragmentation and demographic expansion in Mexican cloud forests. Mol. Phylogenetics Evol. 2011, 61, 603–615. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Z.; Wu, H.; Sun, M. Chloroplast phylogeography of Iris dichotoma (Iridaceae), a widespread herbaceous species in East Asia. Nord. J. Bot. 2020, 38, e02888. [Google Scholar] [CrossRef]
- Dong, S.; Ying, Z.; Yu, S.; Wang, Q.; Liao, G.; Ge, Y.; Cheng, R. Complete chloroplast genome of Stephania tetrandra (Menispermaceae) from Zhejiang Province: Insights into molecular structures, comparative genomic analysis, mutational hotspots, and phylogenetic relationships. BMC Genom. 2021, 22, 880. [Google Scholar] [CrossRef]
- Wang, C.; Yap, Z.-Y.; Wan, P.; Chen, K.; Folk, R.A.; Damrel, D.Z.; Barger, W.; Diamond, A.; Horn, C.; Landry, G.P.; et al. Molecular phylogeography and historical demography of a widespread herbaceous species from eastern North America, Podophyllum peltatum. Am. J. Bot. 2023, 110, 216254. [Google Scholar] [CrossRef]
- Xie, Z.; Merchant, S. The plastid-encoded ccsA gene is required for heme attachment to chloroplast c-type cytochromes. J. Biol. Chem. 1996, 271, 4632–4639. [Google Scholar] [CrossRef]
- Dong, W.-L.; Wang, R.-N.; Zhang, N.-Y.; Fan, W.-B.; Fang, M.-F.; Li, Z.-H. Molecular evolution of chloroplast genomes of orchid species: Insights into phylogenetic relationship and adaptive evolution. Int. J. Mol. Sci. 2018, 19, 716. [Google Scholar] [CrossRef]
- Yang, J.Y.; Kim, S.-H.; Pak, J.-H.; Kim, S.-C. Infrageneric plastid genomes of Cotoneaster (Rosaceae): Implications for the plastome evolution and origin of C. wilsonii on Ulleung Island. Genes 2022, 13, 728. [Google Scholar] [CrossRef]
- Zhang, Z.-R.; Yang, X.; Li, W.-Y.; Peng, Y.-Q.; Gao, J. Comparative chloroplast genome analysis of Ficus (Moraceae): Insight into adaptive evolution and mutational hotspot regions. Front. Plant Sci. 2022, 13, 965335. [Google Scholar] [CrossRef]
- Hu, S.; Sablok, G.; Wang, B.; Qu, D.; Barbaro, E.; Viola, R.; Li, M.; Varotto, S. Plastome organization and evolution of chloroplast genes in Cardamine species adapted to contrasting habitats. BMC Genom. 2015, 16, 306. [Google Scholar] [CrossRef]
- Gao, L.-Z.; Liu, Y.-L.; Zhang, D.; Li, W.; Gao, J.; Liu, Y. Evolution of Oryza chloroplast genome promoted adaptation to diverse ecological habitats. Commun. Biol. 2019, 2, 278. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Li, M.; Xu, W.; Heslop-Harrison, J.S. Comparative chloroplast genome analyses of Avena: Insights into evolutionary dynamics and phylogeny. BMC Plant Biol. 2020, 20, 406. [Google Scholar] [CrossRef]
- Li, J.L.; Tang, J.M.; Zeng, S.Y.; Han, F.; Yuan, J.; Yu, J. Comparative plastid genomics of four Pilea (Urticaceae) species: Insights into interspecific plastid genome diversity in Pilea. BMC Plant Biol. 2021, 21, 25. [Google Scholar] [CrossRef]
- Wen, F.; Wu, X.; Li, T.; Jia, M.; Liu, X.; Liao, L. The complete chloroplast genome of Stauntonia chinensis and compared analysis revealed adaptive evolution of subfamily Lardizabaloideae species in China. BMC Genom. 2021, 22, 161. [Google Scholar] [CrossRef]
- Yang, J.Y.; Kang, G.-H.; Pak, J.-H.; Kim, S.-C. Characterization and comparison of two complete plastomes of Rosaceae species (Potentilla dickinsii var. glabrata and Spiraea insularis) endemic to Ulleung Island, Korea. Int. J. Mol. Sci. 2020, 21, 4933. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, F.; Hong, X.; Li, Z.; Mi, Y.; Zhao, B. Comparative chloroplast genome analyses of Paraboea (Gesneriaceae): Insights into adaptive evolution and phylogenetic analysis. Front. Plant Sci. 2022, 13, 1019831. [Google Scholar] [CrossRef]
- Carbonell-Caballero, J.; Alonso, R.; Ibañez, V.; Terol, J.; Dopazo, J. A phylogenetic analysis of 34 chloroplast genomes elucidates the relationships between wild and domesticated species within the genus Citrus. Mol. Biol. Evol. 2015, 32, 2015–2035. [Google Scholar] [CrossRef]
- Caspermeyer, J. Most comprehensive study to date reveals evolutionary history of Citrus. Mol. Biol. Evol. 2015, 32, 2217–2218. [Google Scholar] [CrossRef]
- Maddison, W.P.; Knowles, L.L. Inferring phylogeny despite incomplete lineage sorting. Syst. Biol. 2006, 55, 21–30. [Google Scholar] [CrossRef]
- Yu, Y.; Than, C.; Degnan, J.H.; Nakhleh, L. Coalescent histories on phylogenetic networks and detection of hybridization despite incomplete lineage sorting. Syst. Biol. 2011, 60, 138–149. [Google Scholar] [CrossRef]
- Stuessy, T.F.; Crawford, D.J.; Greimler, J. Human impacts on the vegetation of the Juan Fernández (Robinson Crusoe) Archipelago. Plants 2023, 12, 4038. [Google Scholar] [CrossRef]
- Solbrig, O.T. The South American species of Erigeron. Contr. Gray Herb. 1962, 191, 3–79. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wang, X.; Li, X.; Hu, J.; Fan, L.; Landis, J.B.; Cannon, S.B.; Grimwood, J.; Schmutz, J.; Jackson, S.A.; et al. Phylogenomics of the genus Glycine sheds light on polyploid evolution and life-strategy transition. Nat. Plants 2022, 8, 233–244. [Google Scholar] [CrossRef]
- Wagner, N.D.; He, L.; Hörandl, E. Phylogenomic relationships and evolution of polyploid Salix species revealed by RAD sequencing data. Front. Plant Sci. 2020, 11, 1077. [Google Scholar] [CrossRef]
- Rothfels, C.J. Polyploid phylogenetics. New Phytol. 2021, 230, 66–72. [Google Scholar] [CrossRef]
- Zerbino, D.R.; Birney, E. Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008, 18, 821–829. [Google Scholar] [CrossRef]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Greiner, S.; Lehwark, P.; Bock, R. Organellar genome DRAW (OGDRAW) version 1.3.1: Expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 2019, 47, W59–W64. [Google Scholar] [CrossRef]
- Brundo, M.; Malde, S.; Poliakov, A.; Do, C.B.; Couronne, O.; Dubchak, I. Global alignment: Finding rearrangements during alignment. Bioinformatics 2003, 19 (Suppl. S1), i54–i62. [Google Scholar] [CrossRef]
- Frazer, K.A.; Pachter, L.; Poliakov, A.; Rubin, E.M.; Dubchak, I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004, 32, W273–W279. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software v7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E. DnaSP v6: DNA sequence polymorphism analysis of large datasets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Sharp, P.M.; Li, W.H. The codon adaptation index-A measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987, 15, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol. Rev. 1983, 47, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chen, C.; Arab, D.A.; Du, Z.; He, Y.; Ho, S.Y.W. EasyCodeML: A visual tool for analysis of selection using CodeML. Ecol. Evol. 2019, 9, 3891–3898. [Google Scholar] [CrossRef]
- Yang, Z. PAML: A program package for phylogenetic analysis by maximum likelihood. Bioinformatics 1997, 13, 555–556. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).