Fatty Acid Profiling as a Tool for Fostering the Traceability of the Halophyte Plant Salicornia ramosissima and Contributing to Its Nutritional Valorization
Abstract
1. Introduction
2. Results
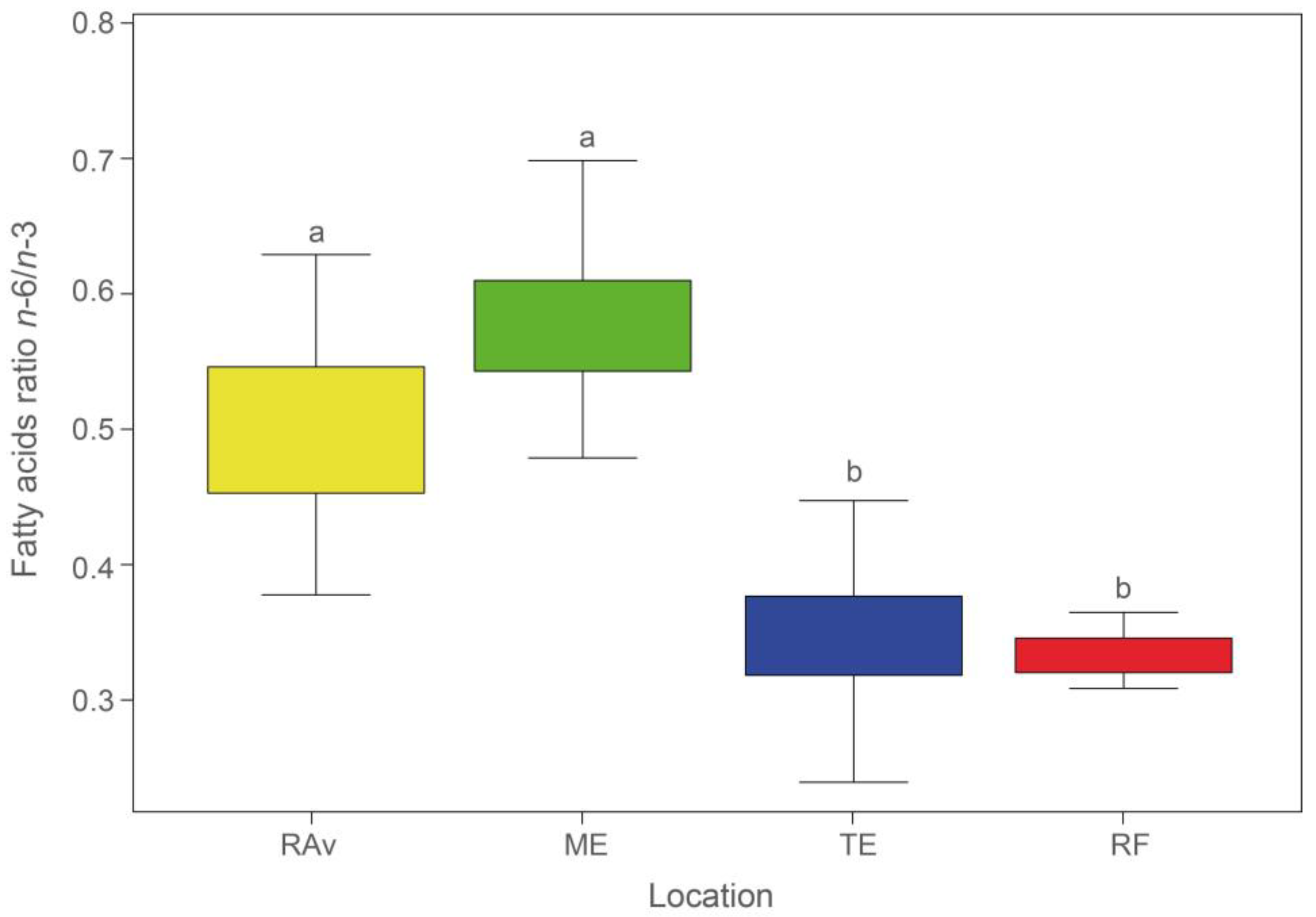
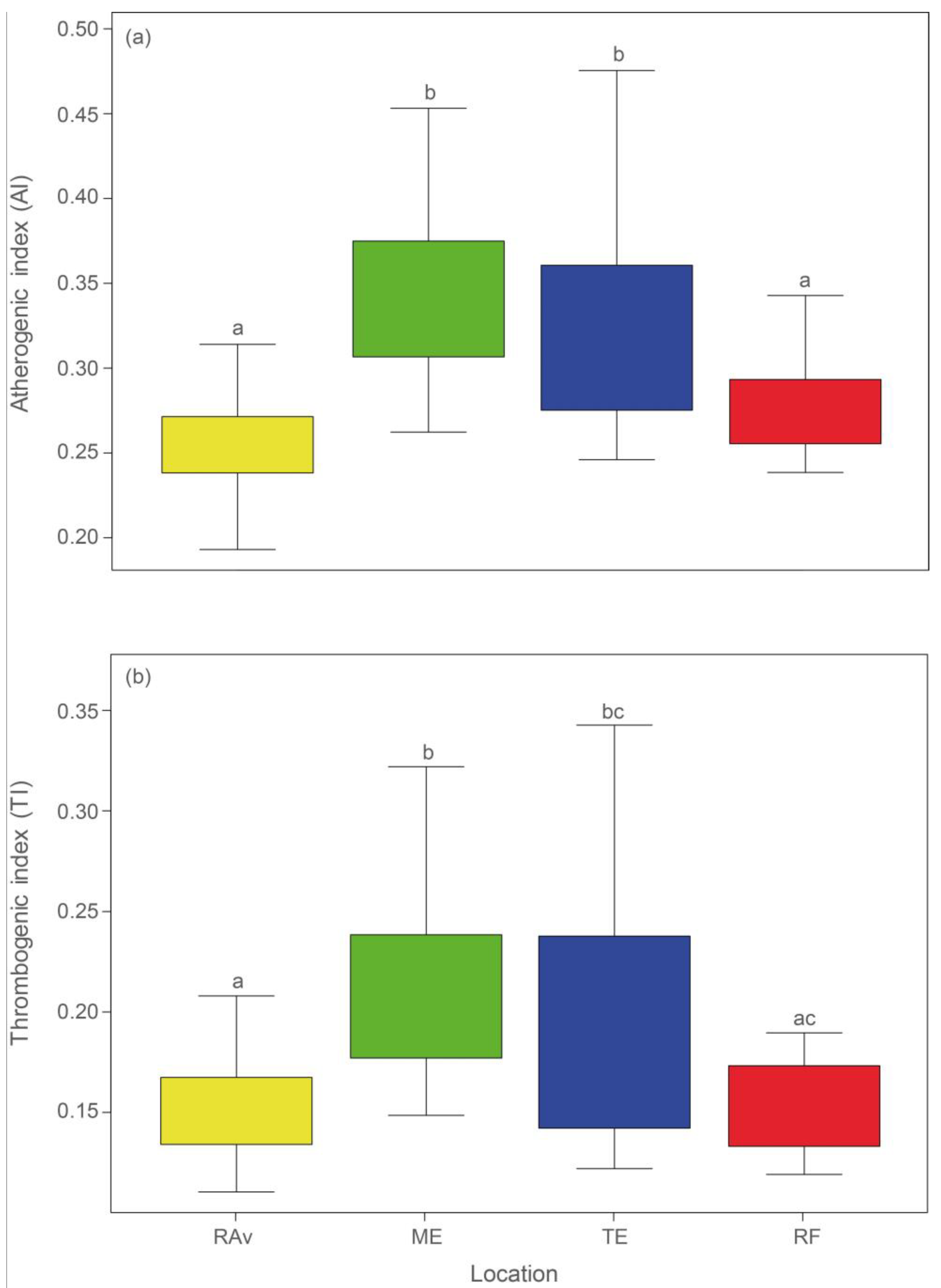
2.1. Fatty Acid Profiles
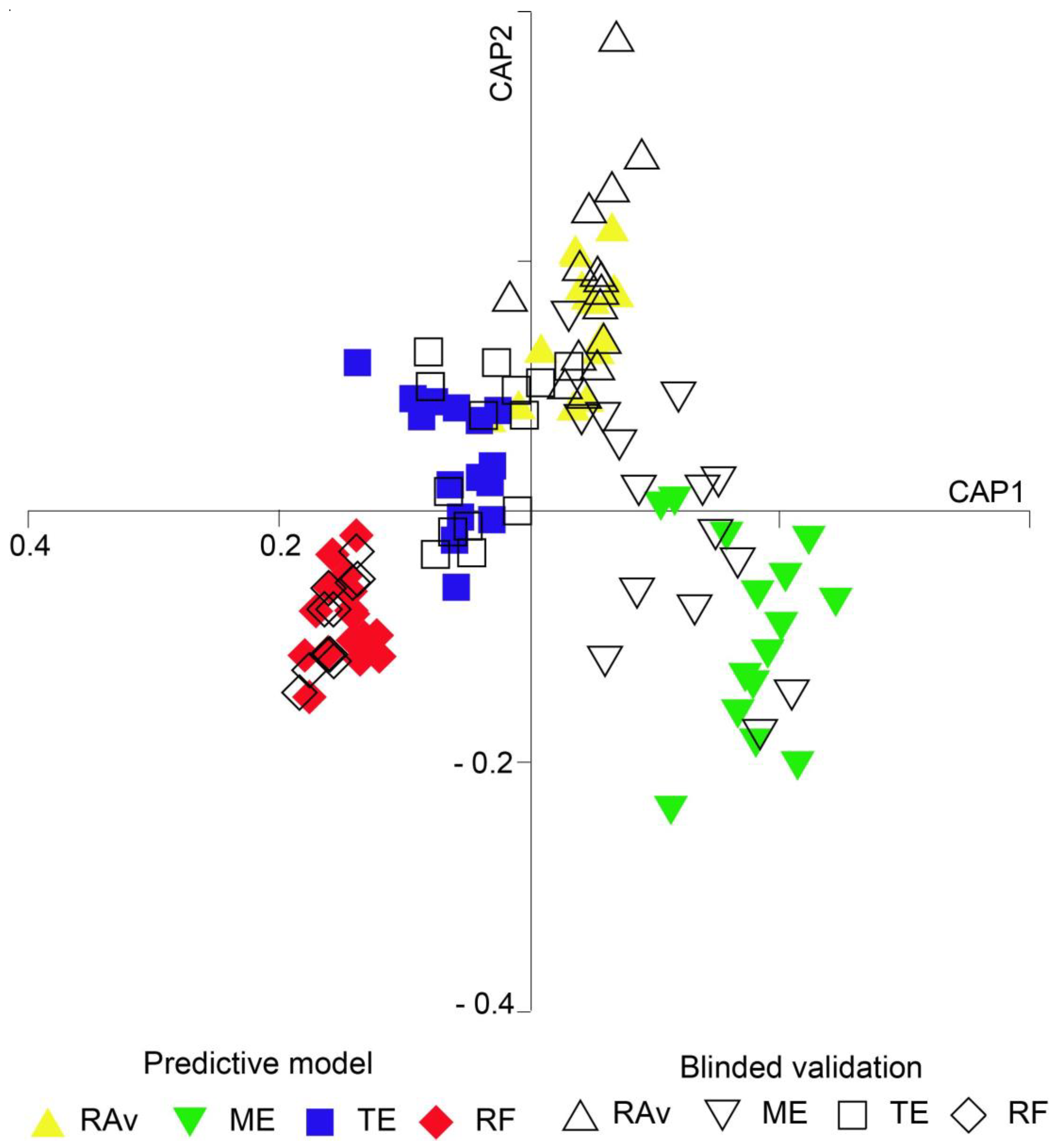
2.2. Geographic Traceability
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Preparation
4.2. Lipid Extraction
4.3. Fatty Acid Analysis
4.4. Fatty Acid Identification and Integration
4.5. Lipid Quality Indices
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumari, A.; Das, P.; Parida, A.K.; Agarwal, P.K. Proteomics, Metabolomics, and Ionomics Perspectives of Salinity Tolerance in Halophytes. Front. Plant Sci. 2015, 6, 537. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity Tolerance in Halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.; Caldeira, G.; Freitas, H. Salicornia ramosissima Population Dynamics and Tolerance of Salinity. Ecol. Res. 2007, 22, 125–134. [Google Scholar] [CrossRef]
- Lopes, M.; Sanches-Silva, A.; Castilho, M.; Cavaleiro, C.; Ramos, F. Halophytes as Source of Bioactive Phenolic Compounds and Their Potential Applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 1078–1101. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lin, Y.; Yang, Y.; Shen, Q.; Huang, J.; Wang, S.; Zhu, X.; Li, Z. Tolerance and Bioaccumulation of Cd and Cu in Sesuvium Portulacastrum. Ecotoxicol. Environ. Saf. 2018, 147, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Munir, N.; Hanif, M.; Dias, D.A.; Abideen, Z. The Role of Halophytic Nanoparticles towards the Remediation of Degraded and Saline Agricultural Lands. Environ. Sci. Pollut. Res. Int. 2021, 28, 60383–60405. [Google Scholar] [CrossRef]
- Beyer, C.P.; Gómez, S.; Lara, G.; Monsalve, J.P.; Orellana, J.; Hurtado, C.F. Sarcocornia Neei: A Novel Halophyte Species for Bioremediation of Marine Aquaculture Wastewater and Production Diversification in Integrated Systems. Aquaculture 2021, 543, 736971. [Google Scholar] [CrossRef]
- Jerónimo, D.; Lillebø, A.I.; Cremades, J.; Cartaxana, P.; Calado, R. Recovering Wasted Nutrients from Shrimp Farming through the Combined Culture of Polychaetes and Halophytes. Sci. Rep. 2021, 11, 6587. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.K.; Pandey, S.; Brahmbhatt, H.R.; Mishra, A.; Jha, B. Lipid Content and Fatty Acid Profile of Selected Halophytic Plants Reveal a Promising Source of Renewable Energy. Biomass Bioenergy 2019, 124, 25–32. [Google Scholar] [CrossRef]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; da Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet Food with Nutritional Health Benefits? J. Food Compost. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Wadood, S.A.; Boli, G.; Xiaowen, Z.; Hussain, I.; Yimin, W. Recent Development in the Application of Analytical Techniques for the Traceability and Authenticity of Food of Plant Origin. Microchem. J. 2020, 152, 104295. [Google Scholar] [CrossRef]
- Leal, M.C.; Pimentel, T.; Ricardo, F.; Rosa, R.; Calado, R. Seafood Traceability: Current Needs, Available Tools, and Biotechnological Challenges for Origin Certification. Trends Biotechnol. 2015, 33, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Jung, D.; Park, S.; Hu, Y.; Huang, K.; Rasco, B.A.; Wang, S.; Ronholm, J.; Lu, X.; Chen, J. Smart Traceability for Food Safety. Crit. Rev. Food Sci. Nutr. 2022, 62, 905–916. [Google Scholar] [CrossRef] [PubMed]
- EC Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying down Procedures in Matters of Food Safety; EC: Brussels, Belgium, 2002.
- Fanelli, V.; Mascio, I.; Miazzi, M.M.; Savoia, M.A.; De Giovanni, C.; Montemurro, C. Molecular Approaches to Agri-Food Traceability and Authentication: An Updated Review. Foods 2021, 10, 1644. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Li, Y.; Zhang, C.; Wang, C.; Wang, J.; Guo, W.; Wang, S. Geographic Origin Discrimination of Pork from Different Chinese Regions Using Mineral Elements Analysis Assisted by Machine Learning Techniques. Food Chem. 2021, 337, 127779. [Google Scholar] [CrossRef] [PubMed]
- Karalis, P.; Poutouki, A.E.; Nikou, T.; Halabalaki, M.; Proestos, C.; Tsakalidou, E.; Gougoura, S.; Diamantopoulos, G.; Tassi, M.; Dotsika, E. Isotopic Traceability (13C and 18O) of Greek Olive Oil. Molecules 2020, 25, 5816. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, F.; Pimentel, T.; Moreira, A.S.P.; Rey, F.; Coimbra, M.A.; Rosário Domingues, M.; Domingues, P.; Costa Leal, M.; Calado, R. Potential Use of Fatty Acid Profiles of the Adductor Muscle of Cockles (Cerastoderma edule) for Traceability of Collection Site. Sci. Rep. 2015, 5, 11125. [Google Scholar] [CrossRef]
- Fonseca, V.F.; Duarte, I.A.; Matos, A.R.; Reis-Santos, P.; Duarte, B. Fatty Acid Profiles as Natural Tracers of Provenance and Lipid Quality Indicators in Illegally Sourced Fish and Bivalves. Food Control 2022, 134, 108735. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, R.; García-González, D.L.; Lobo-Prieto, A.; Aparicio, R. Andalusian Protected Designations of Origin of Virgin Olive Oil: The Role of Chemical Composition in Their Authentication. Eur. J. Lipid Sci. Technol. 2019, 121, 1800133. [Google Scholar] [CrossRef]
- Mehari, B.; Redi-Abshiro, M.; Chandravanshi, B.S.; Combrinck, S.; McCrindle, R.; Atlabachew, M. GC-MS Profiling of Fatty Acids in Green Coffee (Coffea Arabica L.) Beans and Chemometric Modeling for Tracing Geographical Origins from Ethiopia. J. Sci. Food Agric. 2019, 99, 3811–3823. [Google Scholar] [CrossRef]
- Amorello, D.; Orecchio, S.; Pace, A.; Barreca, S. Discrimination of Almonds (Prunus dulcis) Geographical Origin by Minerals and Fatty Acids Profiling. Nat. Prod. Res. 2016, 30, 2107–2110. [Google Scholar] [CrossRef] [PubMed]
- Guyon, F.; Vaillant, F.; Montet, D. Traceability of Fruits and Vegetables. Phytochemistry 2020, 173, 112291. [Google Scholar]
- Upchurch, R.G. Fatty Acid Unsaturation, Mobilization, and Regulation in the Response of Plants to Stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Abd El-Maboud, M.M.; Abd Elbar, O.H. Adaptive Responses of Limoniastrum monopetalum (L.) Boiss. Growing Naturally at Different Habitats. Plant Physiol. Rep. 2020, 25, 325–334. [Google Scholar] [CrossRef]
- He, M.; Ding, N.-Z. Plant Unsaturated Fatty Acids: Multiple Roles in Stress Response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Tian, L.; Chen, M.; Chen, Y.; Wei, A. Low Temperature Affects Fatty Acids Profiling and Key Synthesis Genes Expression Patterns in Zanthoxylum Bungeanum Maxim. Int. J. Mol. Sci. 2022, 23, 2319. [Google Scholar] [CrossRef]
- Vizetto-Duarte, C.; Figueiredo, F.; Rodrigues, M.J.; Polo, C.; Rešek, E.; Custódio, L. Sustainable Valorization of Halophytes from the Mediterranean Area: A Comprehensive Evaluation of Their Fatty Acid Profile and Implications for Human and Animal Nutrition. Sustain. Sci. Pract. Policy 2019, 11, 2197. [Google Scholar] [CrossRef]
- Falcone, D.L.; Ogas, J.P.; Somerville, C.R. Regulation of Membrane Fatty Acid Composition by Temperature in Mutants of Arabidopsis with Alterations in Membrane Lipid Composition. BMC Plant Biol. 2004, 4, 17. [Google Scholar] [CrossRef]
- Duarte, B.; Matos, A.R.; Marques, J.C.; Caçador, I. Lipids in Halophytes: Stress Physiology Relevance and Potential Future Applications. In Halophytes and Climate Change: Adaptive Mechanisms and Potential Uses; CABI: Wallingford Oxfordshire, UK, 2019; pp. 359–371. ISBN 9781786394330. [Google Scholar]
- Isca, V.M.S.; Seca, A.M.L.; Pinto, D.C.G.A.; Silva, H.; Silva, A.M.S. Lipophilic Profile of the Edible Halophyte Salicornia ramosissima. Food Chem. 2014, 165, 330–336. [Google Scholar] [CrossRef]
- Pires-Cabral, P.; Pires-Cabral, P.; Quintas, C. Salicornia ramosissima as a Salt Substitute in the Fermentation of White Cabbage. J. Food Sci. Technol. 2022, 59, 597–605. [Google Scholar] [CrossRef]
- Maciel, E.; Lillebø, A.; Domingues, P.; da Costa, E.; Calado, R.; Domingues, M.R.M. Polar Lipidome Profiling of Salicornia ramosissima and Halimione portulacoides and the Relevance of Lipidomics for the Valorization of Halophytes. Phytochemistry 2018, 153, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.R.; Castañeda-Loaiza, V.; Salazar, M.; Nunes, C.; Quintas, C.; Gama, F.; Pestana, M.; Correia, P.J.; Santos, T.; Varela, J.; et al. Influence of Cultivation Salinity in the Nutritional Composition, Antioxidant Capacity and Microbial Quality of Salicornia ramosissima Commercially Produced in Soilless Systems. Food Chem. 2020, 333, 127525. [Google Scholar] [CrossRef]
- Khalilzadeh, R.; Pirzad, A.; Sepehr, E.; Khan, S.; Anwar, S. Long-Term Effect of Heavy Metal–Polluted Wastewater Irrigation on Physiological and Ecological Parameters of Salicornia europaea L. J. Soil Sci. Plant Nutr. 2020, 20, 1574–1587. [Google Scholar] [CrossRef]
- Tsydendambaev, V.D.; Ivanova, T.V.; Khalilova, L.A.; Kurkova, E.B.; Myasoedov, N.A.; Balnokin, Y.V. Fatty Acid Composition of Lipids in Vegetative Organs of the Halophyte Suaeda altissima under Different Levels of Salinity. Russ. J. Plant Physiol. 2013, 60, 661–671. [Google Scholar] [CrossRef]
- Sui, N.; Han, G. Increases of Unsaturated Fatty Acids in Membrane Lipids Protects Photosystem II from Photoinhibition under Salinity in Different Halophytes. J. Agric. Sci. 2014, 6, 251. [Google Scholar] [CrossRef]
- Ventura, Y.; Wuddineh, W.A.; Myrzabayeva, M.; Alikulov, Z.; Khozin-Goldberg, I.; Shpigel, M.; Samocha, T.M.; Sagi, M. Effect of Seawater Concentration on the Productivity and Nutritional Value of Annual Salicornia and Perennial Sarcocornia Halophytes as Leafy Vegetable Crops. Sci. Hortic. 2011, 128, 189–196. [Google Scholar] [CrossRef]
- Sharkey, A.G.; Shultz, J.L.; Friedel, R.A. Mass Spectra of Esters. Formation of Rearrangement Ions. Anal. Chem. 1959, 31, 87–94. [Google Scholar] [CrossRef]
- Christie, W.W.; Han, X. Lipid Analysis: Isolation, Separation, Identification and Lipidomic Analysis; Elsevier: Amsterdam, The Netherlands, 2010; ISBN 9780857097866. [Google Scholar]
- Pinot, F.; Beisson, F. Cytochrome P450 Metabolizing Fatty Acids in Plants: Characterization and Physiological Roles. FEBS J. 2011, 278, 195–205. [Google Scholar] [CrossRef]
- Kolattukudy, P.E. Structure, Biosynthesis, and Biodegradation of Cutin and Suberin. Annu. Rev. Plant Physiol. 1981, 32, 539–567. [Google Scholar] [CrossRef]
- Pinot, F.; Bosch, H.; Alayrac, C.; Mioskowski, C.; Vendais, A.; Durst, F.; Salaun, J.P. [Omega]-Hydroxylation of Oleic Acid in Vicia Sativa Microsomes (Inhibition by Substrate Analogs and Inactivation by Terminal Acetylenes). Plant Physiol. 1993, 102, 1313–1318. [Google Scholar] [CrossRef][Green Version]
- Rozentsvet, O.A.; Nesterov, V.N.; Bogdanova, E.S. Membrane-Forming Lipids of Wild Halophytes Growing under the Conditions of Prieltonie of South Russia. Phytochemistry 2014, 105, 37–42. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Princípios de Bioquímica de Lehninger, 6th ed.; Artmed: Guelph, ON, Canada, 2014; ISBN 9780716743392. [Google Scholar]
- Stuchlík, M.; Zák, S. Vegetable Lipids as Components of Functional Foods. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2002, 146, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Reiffel, J.A.; McDonald, A. Antiarrhythmic Effects of Omega-3 Fatty Acids. Am. J. Cardiol. 2006, 98, 50i–60i. [Google Scholar] [CrossRef] [PubMed]
- Barceló-Coblijn, G.; Murphy, E.J. Alpha-Linolenic Acid and Its Conversion to Longer Chain n-3 Fatty Acids: Benefits for Human Health and a Role in Maintaining Tissue n-3 Fatty Acid Levels. Prog. Lipid Res. 2009, 48, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Ecker, J. The Opposing Effects of N-3 and n-6 Fatty Acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Garaffo, M.A.; Vassallo-Agius, R.; Nengas, Y.; Lembo, E.; Rando, R.; Maisano, R.; Dugo, G.; Giuffrida, D. Fatty Acids Profile, Atherogenic (IA) and Thrombogenic (IT) Health Lipid Indices, of Raw Roe of Blue Fin Tuna (Thunnus thynnus L.) and Their Salted Product “Bottarga”. Food Nutr. Sci. 2011, 2, 736–743. [Google Scholar]
- IPMA Instituto Português Do Mar e Da Atmosfera. Available online: www.ipma.pt (accessed on 14 November 2023).
- Canvin, D.T. The Effect of Temperature on the Oil Content and Fatty Acid Composition of the Oils from Several Oil Seed Crops. Can. J. Bot. 1965, 43, 63–69. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Nishiyama, Y.; Suzuki, I.; Tasaka, Y.; Murata, N. Genetic Engineering of the Unsaturation of Fatty Acids in Membrane Lipids Alters the Tolerance of Synechocystis to Salt Stress. Proc. Natl. Acad. Sci. USA 1999, 96, 5862–5867. [Google Scholar] [CrossRef]
- Kasamo, K.; Nouchi, I. The Role of Phospholipids in Plasma Membrane ATPase Activity in Vigna radiata L. (Mung Bean) Roots and Hypocotyls. Plant Physiol. 1987, 83, 323–328. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Aued-Pimentel, S.; Lago, J.H.G.; Chaves, M.H.; Kumagai, E.E. Evaluation of a Methylation Procedure to Determine Cyclopropenoids Fatty Acids from Sterculia Striata St. Hil. Et Nauds Seed Oil. J. Chromatogr. A 2004, 1054, 235–239. [Google Scholar] [CrossRef]
- Ulbricht, T.L.; Southgate, D.A. Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021.

| FAs | RAv | ME | TE | RF |
|---|---|---|---|---|
| C14:0 | 0.3 ±0.07 | 0.6 ± 0.17 | 0.4 ± 0.07 | 0.2 ± 0.04 |
| C16:0 | 18.1 ± 1.58 | 20.2 ± 1.7 | 20.3 ± 1.66 | 19.1 ± 1.09 |
| C16:1 | 1.0 ± 0.13 | 0.6 ± 0.16 | 1.0 ± 0.13 | 1.6 ± 0.23 |
| C17:0 | 0.3 ± 0.06 | 0.3 ± 0.10 | 0.4 ± 0.13 | 0.2 ± 0.08 |
| C18:0 | 5.1 ± 2.43 | 6.8 ± 3.22 | 9.0 ± 4.32 | 7.1 ± 4.17 |
| C18:1n-9 | 2.6 ± 0.48 | 3.4 ± 1.10 | 1.6 ± 0.37 | 0.9 ± 0.28 |
| C18:2n-6 | 25.1 ± 2.61 | 22.5 ± 2.63 | 16.9 ± 1.71 | 17.1 ± 1.34 |
| C18:3n-3 | 45.8 ± 3.65 | 38.8 ± 3.47 | 48.6 ± 5.54 | 51.2 ± 3.68 |
| C20:0 | 0.5 ± 0.19 | 0.6 ± 0.15 | 0.5 ± 0.23 | 0.6 ± 0.09 |
| C22:0 | 0.5 ± 0.26 | 1.0 ± 0.35 | 0.7 ± 0.30 | 0.6 ± 0.22 |
| 9,10-epoxy-octadecanoic acid | 4.0 ± 3.57 | |||
| C24:0 | 0.8 ± 0.24 | 1.3 ± 0.53 | 0.7 ± 0.32 | 1.5 ± 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricardo, F.; Veríssimo, A.C.; Maciel, E.; Domingues, M.R.; Calado, R. Fatty Acid Profiling as a Tool for Fostering the Traceability of the Halophyte Plant Salicornia ramosissima and Contributing to Its Nutritional Valorization. Plants 2024, 13, 545. https://doi.org/10.3390/plants13040545
Ricardo F, Veríssimo AC, Maciel E, Domingues MR, Calado R. Fatty Acid Profiling as a Tool for Fostering the Traceability of the Halophyte Plant Salicornia ramosissima and Contributing to Its Nutritional Valorization. Plants. 2024; 13(4):545. https://doi.org/10.3390/plants13040545
Chicago/Turabian StyleRicardo, Fernando, Ana Carolina Veríssimo, Elisabete Maciel, Maria Rosário Domingues, and Ricardo Calado. 2024. "Fatty Acid Profiling as a Tool for Fostering the Traceability of the Halophyte Plant Salicornia ramosissima and Contributing to Its Nutritional Valorization" Plants 13, no. 4: 545. https://doi.org/10.3390/plants13040545
APA StyleRicardo, F., Veríssimo, A. C., Maciel, E., Domingues, M. R., & Calado, R. (2024). Fatty Acid Profiling as a Tool for Fostering the Traceability of the Halophyte Plant Salicornia ramosissima and Contributing to Its Nutritional Valorization. Plants, 13(4), 545. https://doi.org/10.3390/plants13040545







