Gibberellin-Mediated Sensitivity of Rice Roots to Aluminum Stress
Abstract
1. Introduction
2. Results
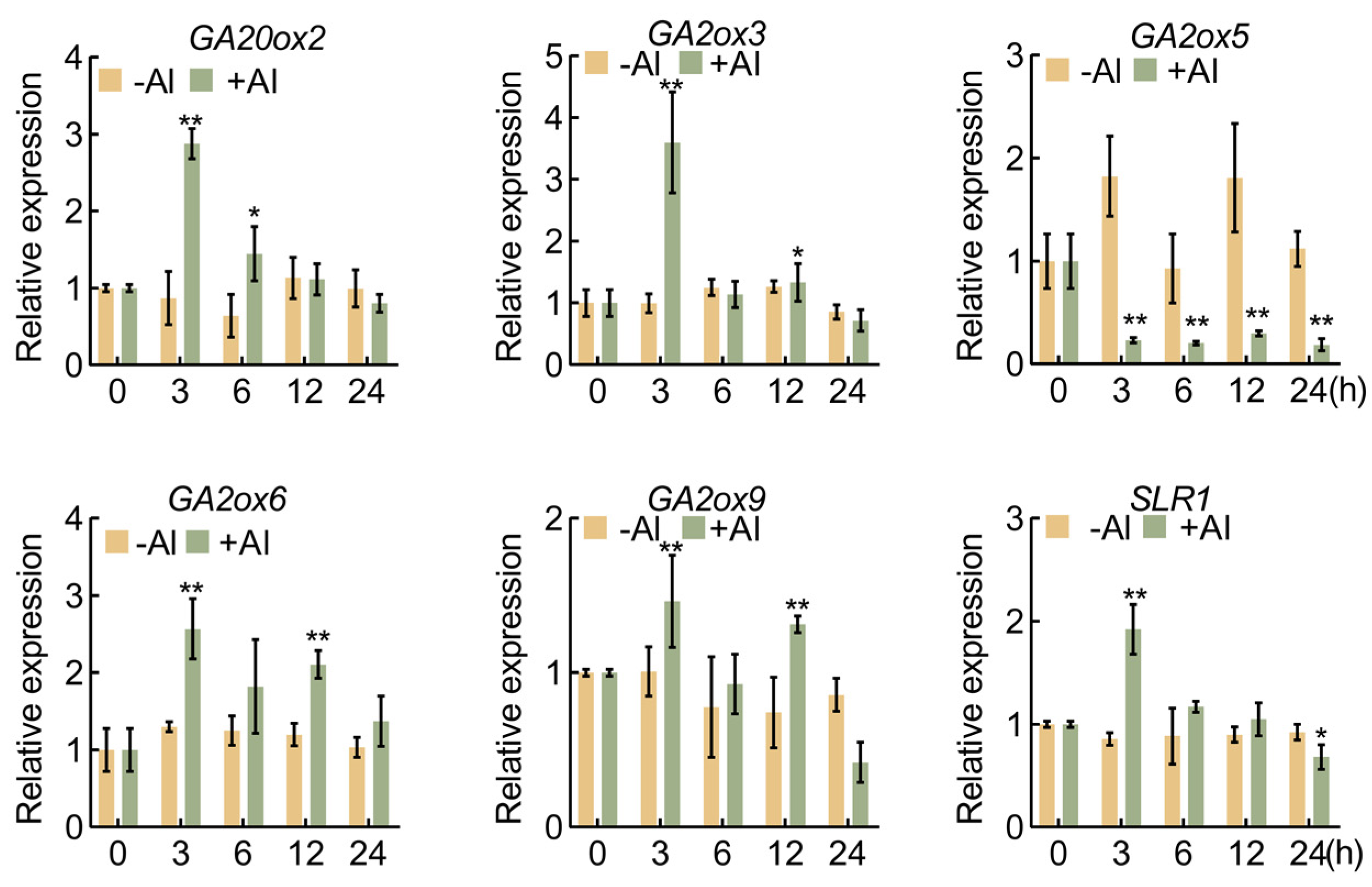
2.1. Expression Patterns of GA Synthesis and Signal Transduction Genes in Response to Aluminum Stress
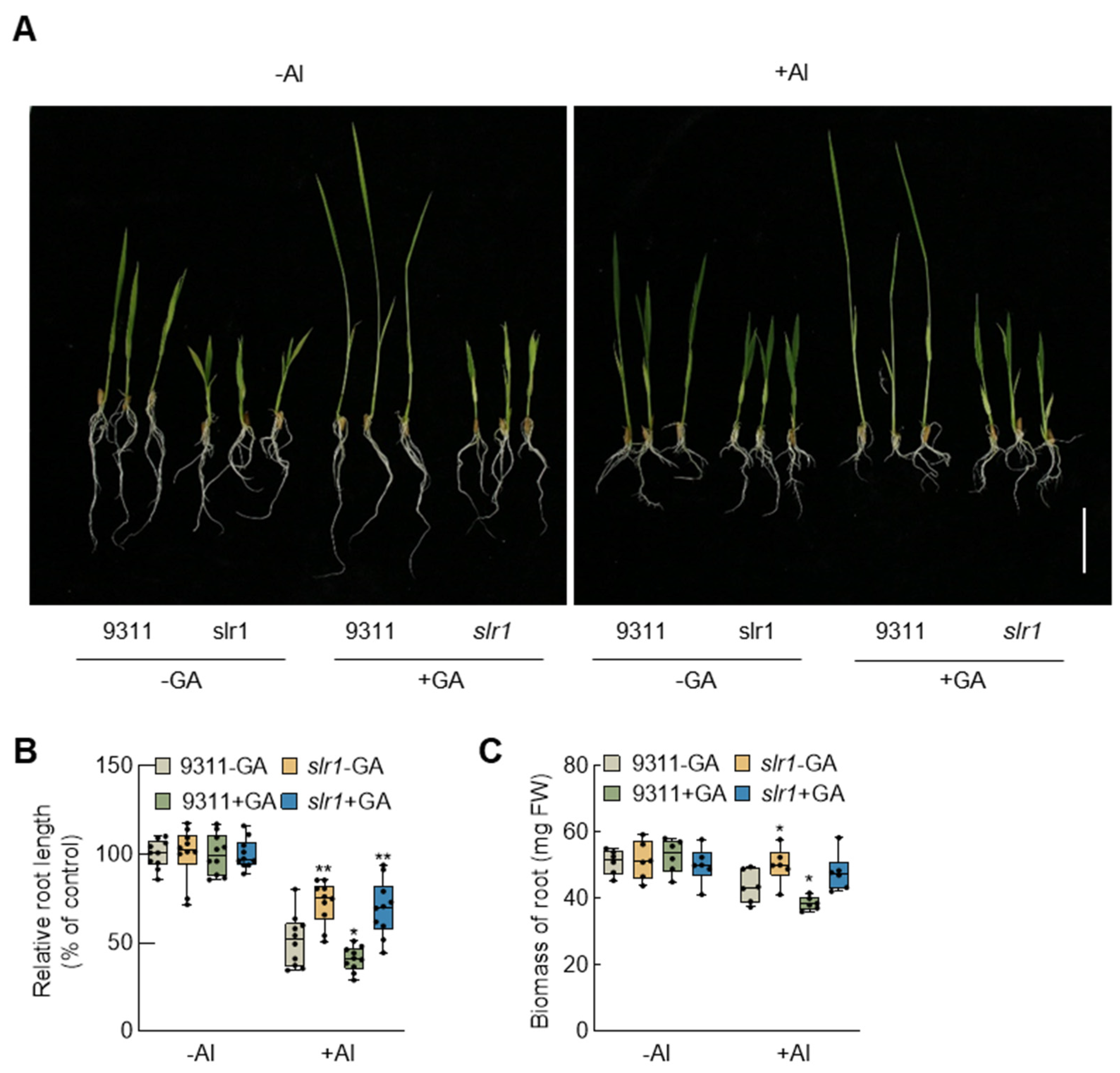
2.2. External Application of GA Exacerbates the Inhibitory Effect of Root Growth by Aluminum
2.3. Overexpression of the GA Biosynthesis Gene SD1 Negatively Modulates Rice Resistance to Aluminum Stress
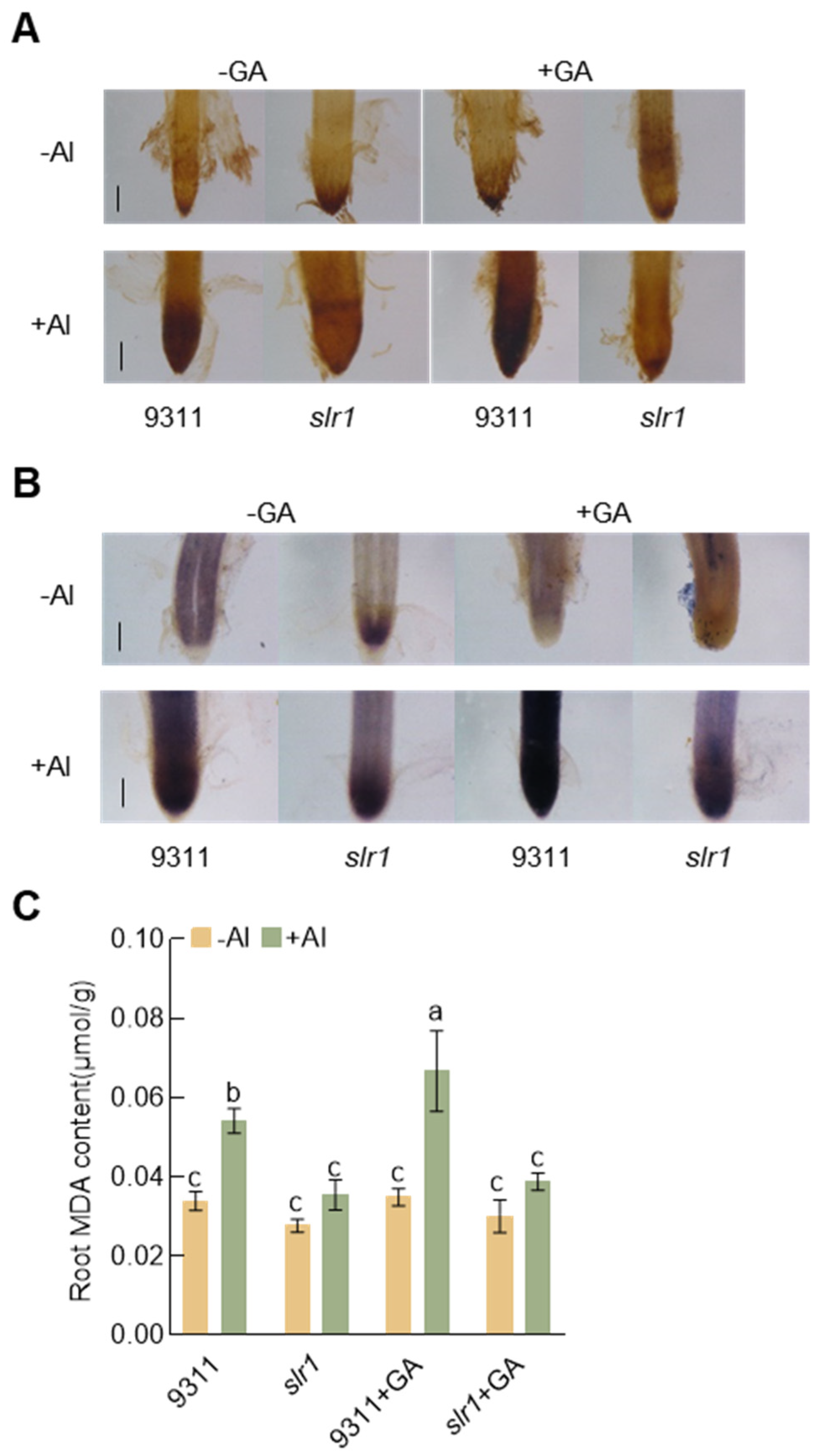
2.4. Disruption of the GA Signaling Enhances Rice Aluminum Resistance
2.5. GA Promotes ROS Accumulation in Rice Roots under Aluminum Stress
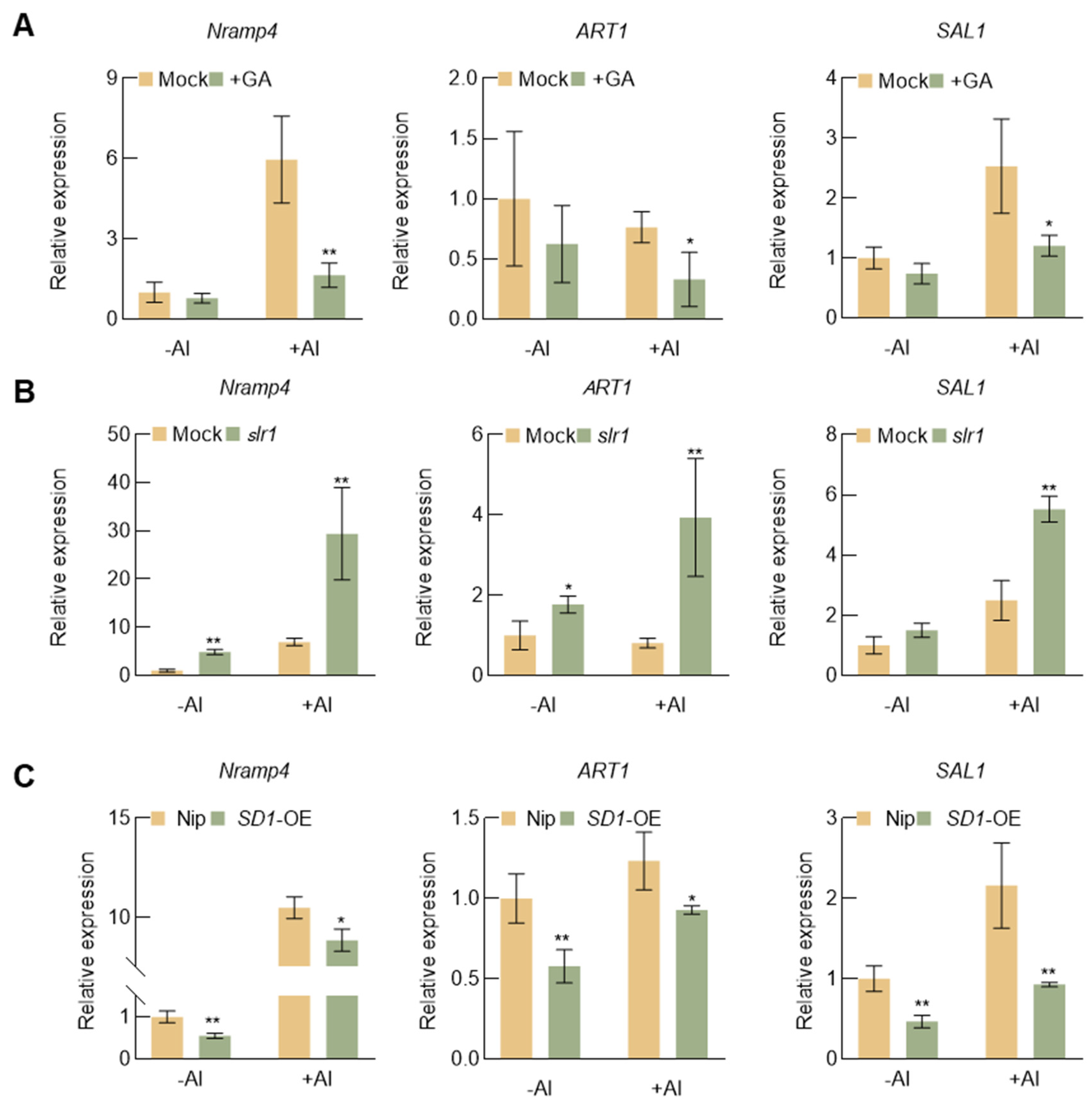
2.6. GA Influences the Expression of Aluminum Tolerance Genes in Rice
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Aluminum Resistance Testing
4.3. Exogenous Gibberellin Application
4.4. RNA Extraction
4.5. Real-Time Fluorescence Quantitative PCR Analysis (RT-qPCR)
4.6. MDA Examination
4.7. ROS Accumulation Measurement
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kochian, L.V. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995, 46, 237–260. [Google Scholar] [CrossRef]
- Matsumoto, H.; Motoda, H. Oxidative stress is associated with aluminum toxicity recovery in apex of pea root. Plant Soil 2013, 363, 399–410. [Google Scholar] [CrossRef]
- Hoekenga, O.; Magalhaes, J. Mechanisms of Aluminum Tolerance; Springer: Berlin/Heidelberg, Germany, 2010; pp. 133–153. [Google Scholar]
- Ma, J.F.; Shen, R.F.; Nagao, S.; Tanimoto, E. Aluminum targets elongating cells by reducing cell wall extensibility in wheat roots. Plant Cell Physiol. 2004, 45, 583–589. [Google Scholar] [CrossRef]
- Ma, J.F. Role of organic acids in detoxification of aluminum in higher plants. Plant Cell Physiol. 2000, 41, 383–390. [Google Scholar] [CrossRef]
- Von Uexküll, H.R.; Mutert, E. Global extent, development and economic impact of acid soils. Plant Soil 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Kochian, L.V.; Pineros, M.A.; Liu, J.; Magalhaes, J.V. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Hiradate, S.; Nomoto, K.; Iwashita, T.; Matsumoto, H. Internal detoxification mechanism of Al in Hydrangea (Identification of Al form in the leaves). Plant Physiol. 1997, 113, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pineros, M.A.; Kochian, L.V. The role of aluminum sensing and signaling in plant aluminum resistance. J. Integr. Plant Biol. 2014, 56, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Ryan, P.R.; Delhaize, E. Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001, 6, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Ligaba, A.; Katsuhara, M.; Ryan, P.R.; Shibasaka, M.; Matsumoto, H. The BnALMT1 and BnALMT2 genes from rape encode aluminum-activated malate transporters that enhance the aluminum resistance of plant cells. Plant Physiol. 2006, 142, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.P.; Magalhaes, J.V.; Shaff, J.; Kochian, L.V. Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J. 2009, 57, 389–399. [Google Scholar] [CrossRef]
- Pellet, D.M.; Grunes, D.L.; Kochian, L.V. Organic acid exudation as an aluminum-tolerance mechanism in maize (Zea mays L.). Planta 1995, 196, 788–795. [Google Scholar] [CrossRef]
- Yang, Z.; Sivaguru, M.; Horst, W.J.; Matsumoto, H. Aluminium tolerance is achieved by exudation of citric acid from roots of soybean (Glycine max). Physiol. Plant. 2001, 110, 72–77. [Google Scholar] [CrossRef]
- Yang, J.L.; Zhu, X.F.; Peng, Y.X.; Zheng, C.; Ming, F.; Zheng, S.J. Aluminum regulates oxalate secretion and plasma membrane H+-ATPase activity independently in tomato roots. Planta 2011, 234, 281–291. [Google Scholar] [CrossRef]
- Kochian, L.V.; Hoekenga, O.A.; Miguel, A.P. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef]
- Ryan, P.; Delhaize, E.; Jones, D. Function and mechanism of organic anion exudation from plant roots. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 527–560. [Google Scholar] [CrossRef]
- Sade, H.; Meriga, B.; Surapu, V.; Gadi, J.; Sunita, M.S.L.; Suravajhala, P.; Kishor, P.B.K. Toxicity and tolerance of aluminum in plants: Tailoring plants to suit to acid soils. Biometals 2016, 29, 187–210. [Google Scholar] [CrossRef]
- Huang, C.F.; Yamaji, N.; Chen, Z.; Ma, J.F. A tonoplast-localized half-size ABC transporter is required for internal detoxification of aluminum in rice. Plant J. 2012, 69, 857–867. [Google Scholar] [CrossRef]
- Xia, J.; Yamaji, N.; Kasai, T.; Ma, J.F. Plasma membrane-localized transporter for aluminum in rice. Proc. Natl. Acad. Sci. USA 2010, 107, 18381–18385. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Sivaguru, M.; Osawa, H.; Chun, G.C.; Matsumoto, H. Aluminum inhibits the H+-ATPase activity by permanently altering the plasma membrane surface potentials in squash roots. Plant Physiol. 2001, 126, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yan, X.; Han, X.; Tang, R.; Chu, M.; Yang, Y.; Yang, Y.H.; Zhao, F.; Fu, A.; Luan, S.; et al. A defective vacuolar proton pump enhances aluminum tolerance by reducing vacuole sequestration of organic acids. Plant Physiol. 2019, 181, 743–761. [Google Scholar] [CrossRef]
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone priming: Regulator for heavy metal stress in plants. J. Plant Growth Regul. 2019, 38, 739–752. [Google Scholar] [CrossRef]
- Kopittke, P.M. Role of phytohormones in aluminium rhizotoxicity. Plant Cell Environ. 2016, 39, 2319–2328. [Google Scholar] [CrossRef]
- Gunsé, B.; Poschenrieder, C.; Barceló, J. The role of ethylene metabolism in the short-term responses to aluminium by roots of two maize cultivars different in Al-resistance. Environ. Exp. Bot. 2000, 43, 73–81. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Q.L.; Geng, M.J.; Guo, Z.H.; Zhao, Z.Q. Effect of indole-3-acetic acid on aluminum-induced efflux of malic acid from wheat (Triticum aestivum L.). Plant Soil 2011, 346, 215–230. [Google Scholar] [CrossRef]
- Saha, I.; Sarkar, B.; Ghosh, A.; De Kumar, A.; Adak, M.K. Abscisic acid induced cellular responses of sub1A QTL to aluminium toxicity in rice (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2019, 183, 109600. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Shen, R.; Zhao, Z.; Wussuwa, M.; Takeuchi, Y.; Ebitani, T.; Yano, M. Response of rice to Al stress and identification of quantitative trait loci for Al tolerance. Plant Cell Physiol. 2002, 43, 652–659. [Google Scholar] [CrossRef]
- Yamaji, N.; Huang, C.F.; Nagao, S.; Yano, M.; Sato, Y.; Nagamura, Y.; Ma, J.F. A zinc finger transcription factor ART1 regulates multiple genes implicated in aluminum tolerance in rice. Plant Cell 2009, 21, 3339–3349. [Google Scholar] [CrossRef]
- Arenhart, R.A.; Bai, Y.; Valter De Oliveira, L.F.; Bucker Neto, L.; Schunemann, M.; Maraschin, F.; Mariath, J.; Silverio, A.; Sachetto-Martins, G.; Margis, R.; et al. New insights into aluminum tolerance in rice: The ASR5 protein binds the STAR1 promoter and other aluminum-responsive genes. Mol. Plant 2014, 7, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Arenhart, R.A.; Schunemann, M.; Bucker Neto, L.; Margis, R.; Wang, Z.Y.; Margis-Pinheiro, M. Rice ASR1 and ASR5 are complementary transcription factors regulating aluminium responsive genes. Plant Cell Environ. 2016, 39, 645–651. [Google Scholar] [CrossRef]
- Yang, Z.B.; He, C.; Ma, Y.; Herde, M.; Ding, Z. Jasmonic acid enhances Al-induced root growth inhibition. Plant Physiol. 2017, 173, 1420–1433. [Google Scholar] [CrossRef]
- Wang, M.; Qiao, J.Y.; Yu, C.L.; Chen, H.; Sun, C.D.; Huang, L.Z.; Li, C.Y.; Geisler, M.; Qian, Q.; Jiang, D.A.; et al. The auxin influx carrier, OsAUX3, regulates rice root development and responses to aluminium stress. Plant Cell Environ. 2019, 42, 1125–1138. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Zhang, X.; Ramesh, S.; Gilliham, M.; Tyerman, S.D.; Zhang, W.-H. Ethylene negatively regulates aluminium-induced malate efflux from wheat roots and tobacco cells transformed with TaALMT1. J. Exp. Bot. 2014, 65, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Camara, M.C.; Vandenberghe LP, S.; Rodrigues, C.; de Oliveira, J.; Faulds, C.; Bertrand, E.; Soccol, C.R. Current advances in gibberellic acid (GA3) production, patented technologies and potential applications. Planta 2018, 248, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Achard, P.; Cheng, H.; De Grauwe, L.; De Grauwe, L.; Decat, J.; Schoutteten, H.; Moritz, T.; Van Der Straeten, D.; Peng, J.; Harberd, N.P. Integration of plant responses to environmentally activated phytohormonal signals. Science 2006, 311, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Mori, Y.; Ishikawa, S.; Furuta, T.; Gamuyao, R.; Niimi, Y.; Hobo, T.; Fukuda, M.; Kojima, M.; Takebayashi, Y.; et al. Antagonistic regulation of the gibberellic acid response during stem growth in rice. Nature 2020, 584, 109–114. [Google Scholar] [CrossRef]
- Kang, S.-M.; Waqas, M.; Hamayun, M.; Asaf, S.; Khan, A.L.; Kim, A.-Y.; Park, Y.-G.; Lee, I.-J. Gibberellins and indole-3-acetic acid producing rhizospheric bacterium Leifsonia xyli SE134 mitigates the adverse effects of copper-mediated stress on tomato. J. Plant Interact. 2017, 12, 373–380. [Google Scholar] [CrossRef]
- Mansour, M.; Kamel, E. Interactive effect of heavy metals and gibberellic acid on mitotic activity and some metabolic changes of Vicia faba L. plants. Cytologia 2005, 70, 275–282. [Google Scholar] [CrossRef]
- Ghorbanli, M.; Kaveh, S.; Farzamisepehr, M. Effects of cadmium and gibberellin on growth and photosynthesis of Glycine max. Photosynthetica 2000, 37, 627–631. [Google Scholar] [CrossRef]
- Kochian, L.; Piñeros, M.; Hoekenga, O. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 2005, 274, 175–195. [Google Scholar] [CrossRef]
- Ma, J.F. Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. In International Review of Cytology; Academic Press: Cambridge, MA, USA, 2007; pp. 225–252. [Google Scholar]
- Itoh, H.; Ueguchi-Tanaka, M.; Sato, Y.; Ashikari, M.; Matsuoka, M. The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell 2002, 14, 57–70. [Google Scholar] [CrossRef]
- Cheng, H.; Qin, L.; Lee, S.; Fu, X.; Richards, D.E.; Cao, D.; Luo, D.; Harberd, N.P.; Peng, J. Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development 2004, 131, 1055–1064. [Google Scholar] [CrossRef]
- Achard, P.; Renou, J.P.; Berthome, R.; Harberd, N.P.; Genschik, P. Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr. Biol. 2008, 18, 656–660. [Google Scholar] [CrossRef]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Lantzouni, O.; Alkofer, A.; Falter-Braun, P.; Schwechheimer, C. GROWTH-REGULATING FACTORS interact with DELLAs and regulate growth in cold stress. Plant Cell 2020, 32, 1018–1034. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, H.; Yang, Z.; Wang, C.; Li, S.; Cao, C.; Yao, T.; Wei, Z.; Li, Y.; Chen, J.; et al. Independently evolved viral effectors convergently suppress DELLA protein SLR1-mediated broad-spectrum antiviral immunity in rice. Nat. Commun. 2022, 13, 6920. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, L.; Su, H.; Guo, L.; Zhang, J.; Li, Y.; Xu, J.; Zhang, X.; Guo, Y.-D.; Zhang, N. Jasmonate and aluminum crosstalk in tomato: Identification and expression analysis of WRKYs and ALMTs during JA/Al-regulated root growth. Plant Physiol. Biochem. 2020, 154, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Yamaji, N.; Mitani, N.; Ma, J.F. A bacterial-type ABC transporter is involved in aluminum tolerance in rice. Plant Cell 2009, 21, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Liu, S.; Gao, H.; Wu, J.; Liu, D.; Kinoshita, T.; Huang, C.-F. PP2C.D phosphatase SAL1 positively regulates aluminum resistance via restriction of aluminum uptake in rice. Plant Physiol. 2023, 192, 1498–1516. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Hong, J.; Chen, X.; Zhang, C.; Chen, M.; Luo, Z.; Chang, S.; Bai, S.; Liang, W.; Liu, Q.; et al. Gibberellins orchestrate panicle architecture mediated by DELLA-KNOX signalling in rice. Plant Biotechnol. J. 2021, 19, 2304–2318. [Google Scholar] [CrossRef]
- Wang, F.; Kong, W.; Wong, G.; Fu, L.; Peng, R.; Li, Z.; Yao, Q. AtMYB12 regulates flavonoids accumulation and abiotic stress tolerance in transgenic Arabidopsis thaliana. Mol. Genet. Genom. 2016, 291, 1545–1559. [Google Scholar] [CrossRef] [PubMed]
- Jabs, T.; Dietrich, R.A.; Dangl, J.L. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 1996, 273, 1853–1856. [Google Scholar] [CrossRef] [PubMed]
- Daudi, A.; O’Brien, J.A. Detection of hydrogen peroxide by DAB staining in Arabidopsis leaves. Bio-Protoc. 2012, 2, e263. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Chen, X.; Tan, Q.; Li, W.; Sun, Y.; Zhang, Z.; Song, Y.; Zeng, R. Gibberellin-Mediated Sensitivity of Rice Roots to Aluminum Stress. Plants 2024, 13, 543. https://doi.org/10.3390/plants13040543
Lu L, Chen X, Tan Q, Li W, Sun Y, Zhang Z, Song Y, Zeng R. Gibberellin-Mediated Sensitivity of Rice Roots to Aluminum Stress. Plants. 2024; 13(4):543. https://doi.org/10.3390/plants13040543
Chicago/Turabian StyleLu, Long, Xinyu Chen, Qinyan Tan, Wenqian Li, Yanyan Sun, Zaoli Zhang, Yuanyuan Song, and Rensen Zeng. 2024. "Gibberellin-Mediated Sensitivity of Rice Roots to Aluminum Stress" Plants 13, no. 4: 543. https://doi.org/10.3390/plants13040543
APA StyleLu, L., Chen, X., Tan, Q., Li, W., Sun, Y., Zhang, Z., Song, Y., & Zeng, R. (2024). Gibberellin-Mediated Sensitivity of Rice Roots to Aluminum Stress. Plants, 13(4), 543. https://doi.org/10.3390/plants13040543






