Almond By-Products Substrates as Sustainable Amendments for Green Bean Cultivation
Abstract
1. Introduction
2. Results and Discussion
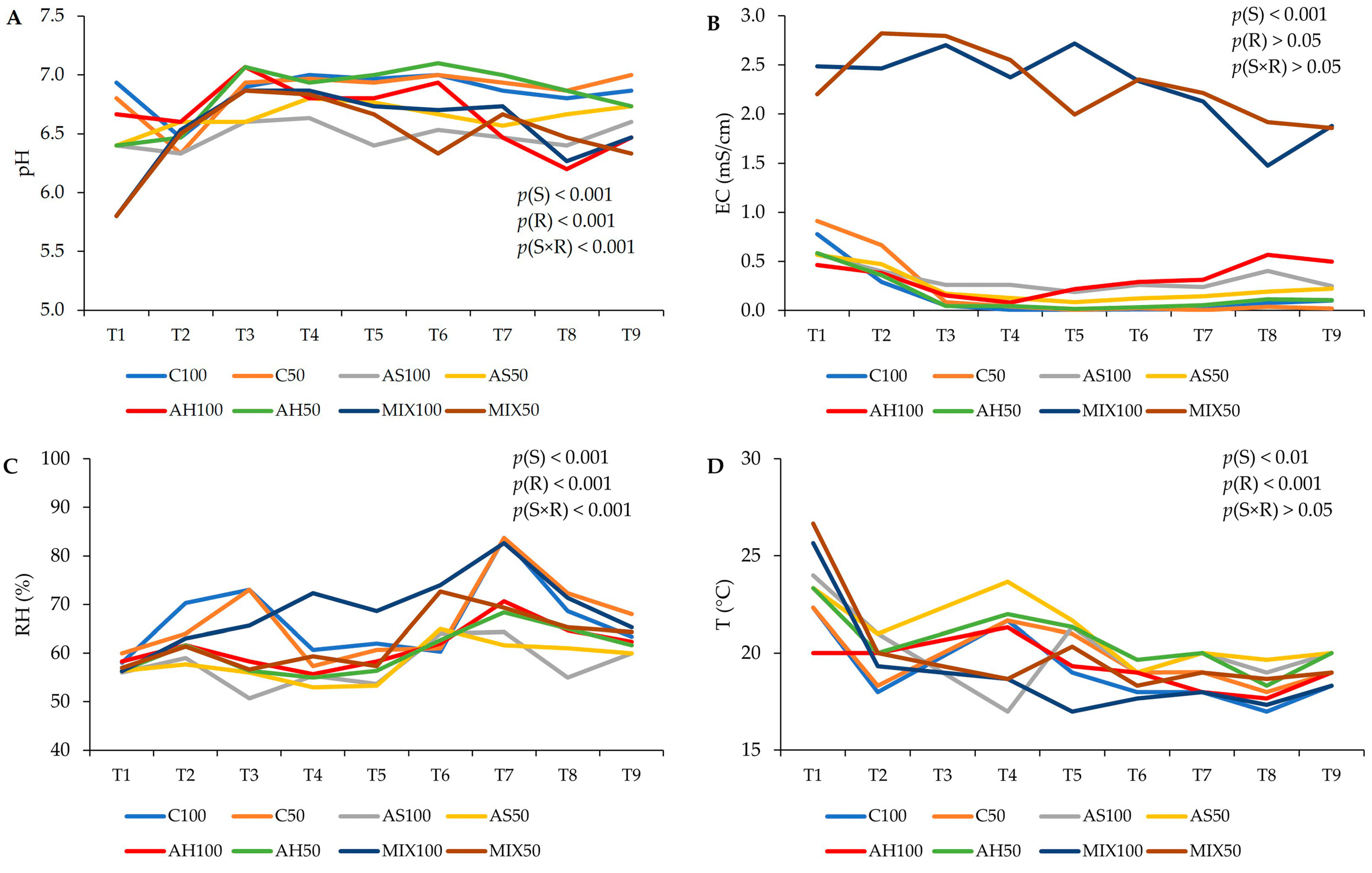
2.1. Substrate Characteristics—pH, Temperature, Relative Humidity, and Electrical Conductivity
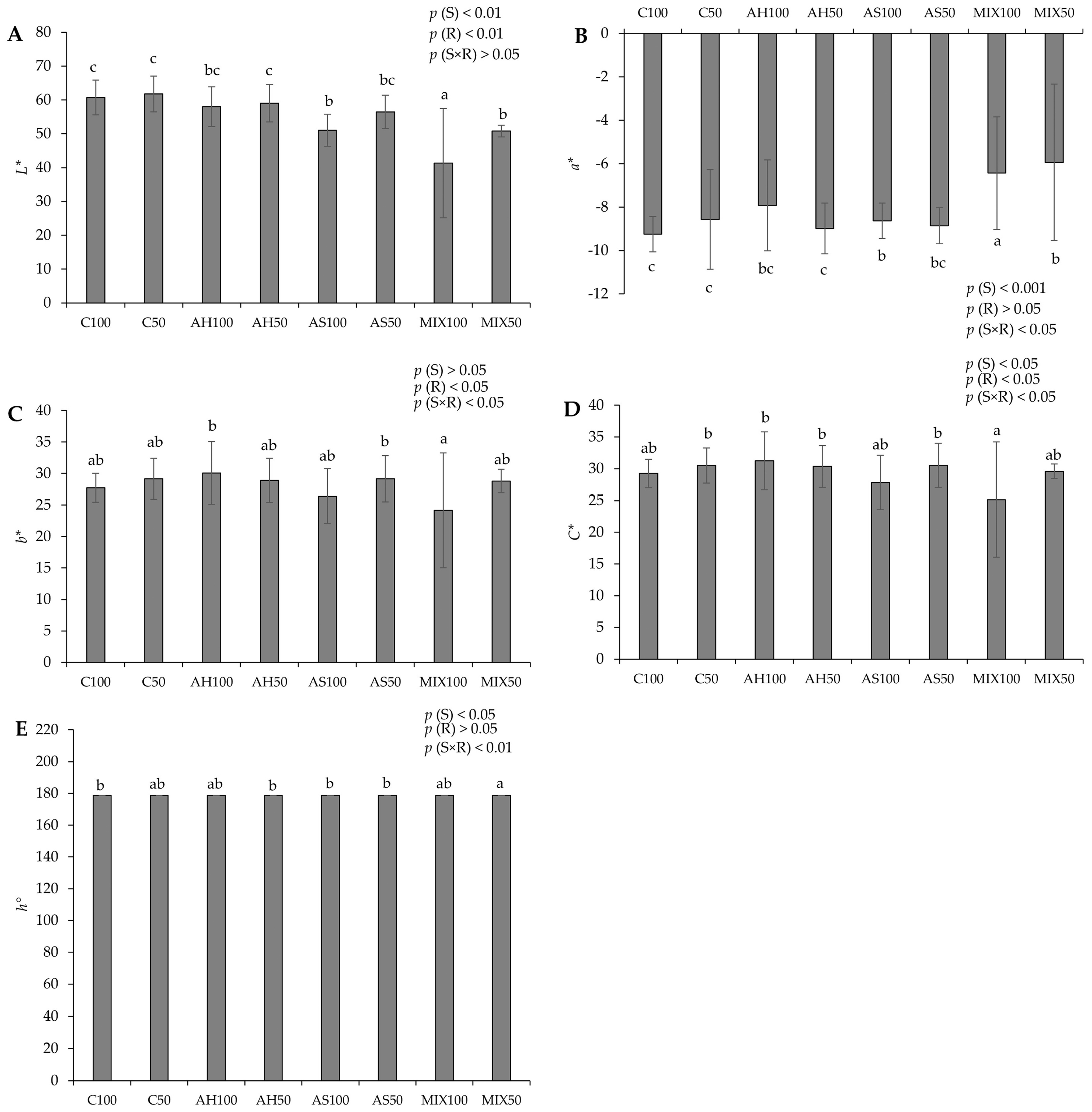
2.2. Chromatic Parameters
2.3. Quantifications in Green Bean Pods
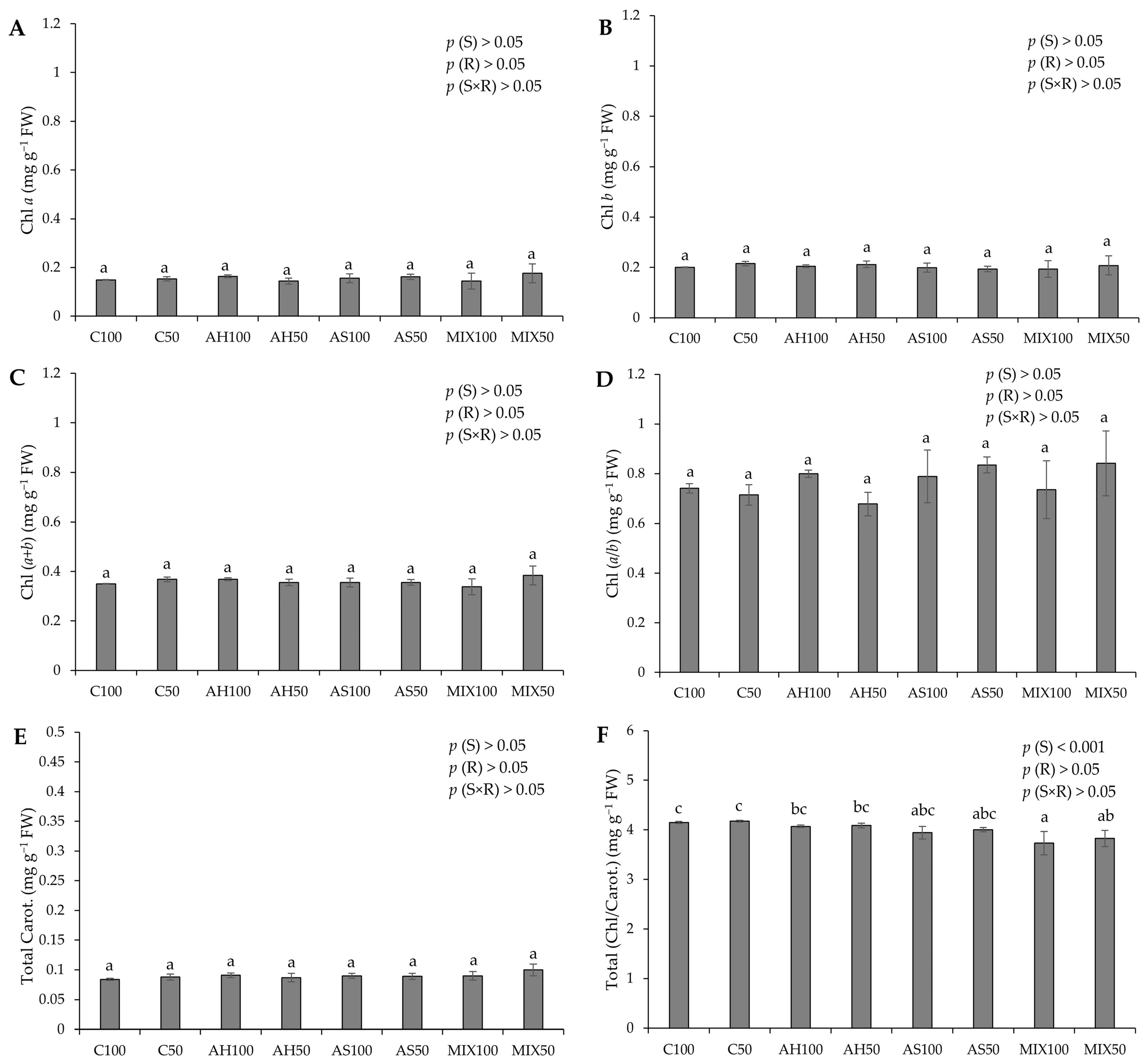
2.3.1. Photosynthetic Pigments
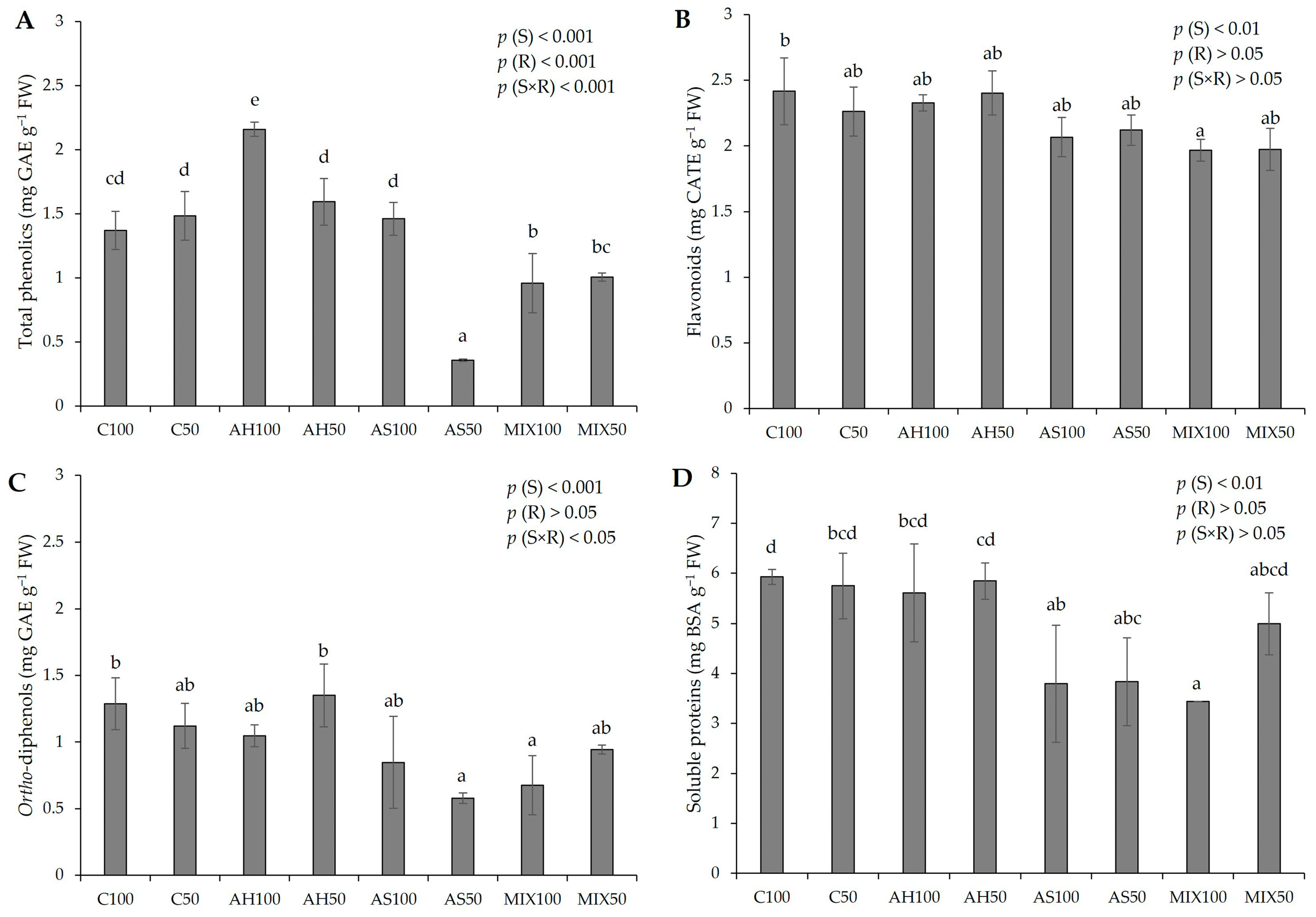
2.3.2. Total Phenolics, Flavonoids, Ortho-Diphenols, and Soluble Proteins
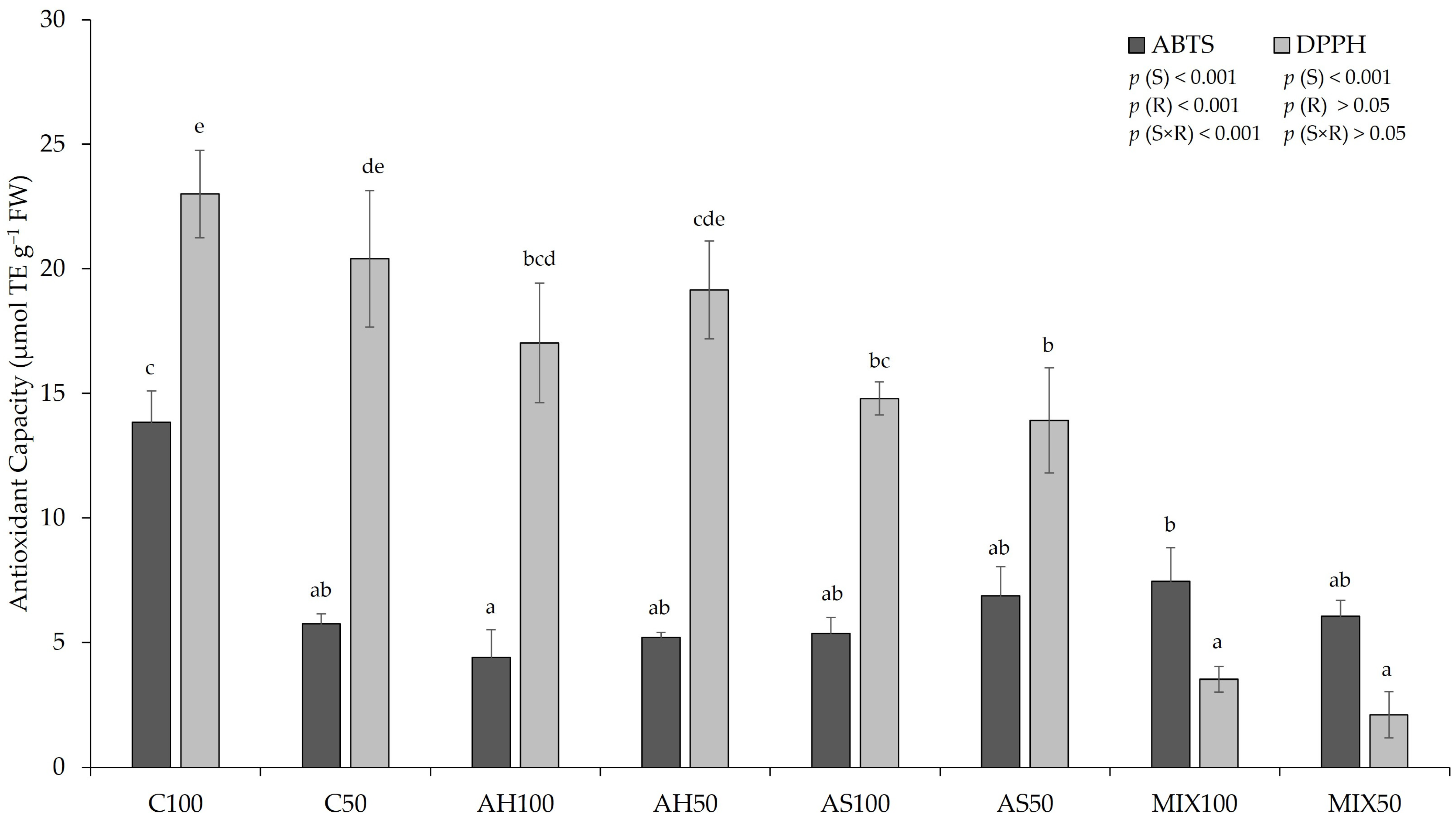
2.4. Antioxidant Capacity (AC)
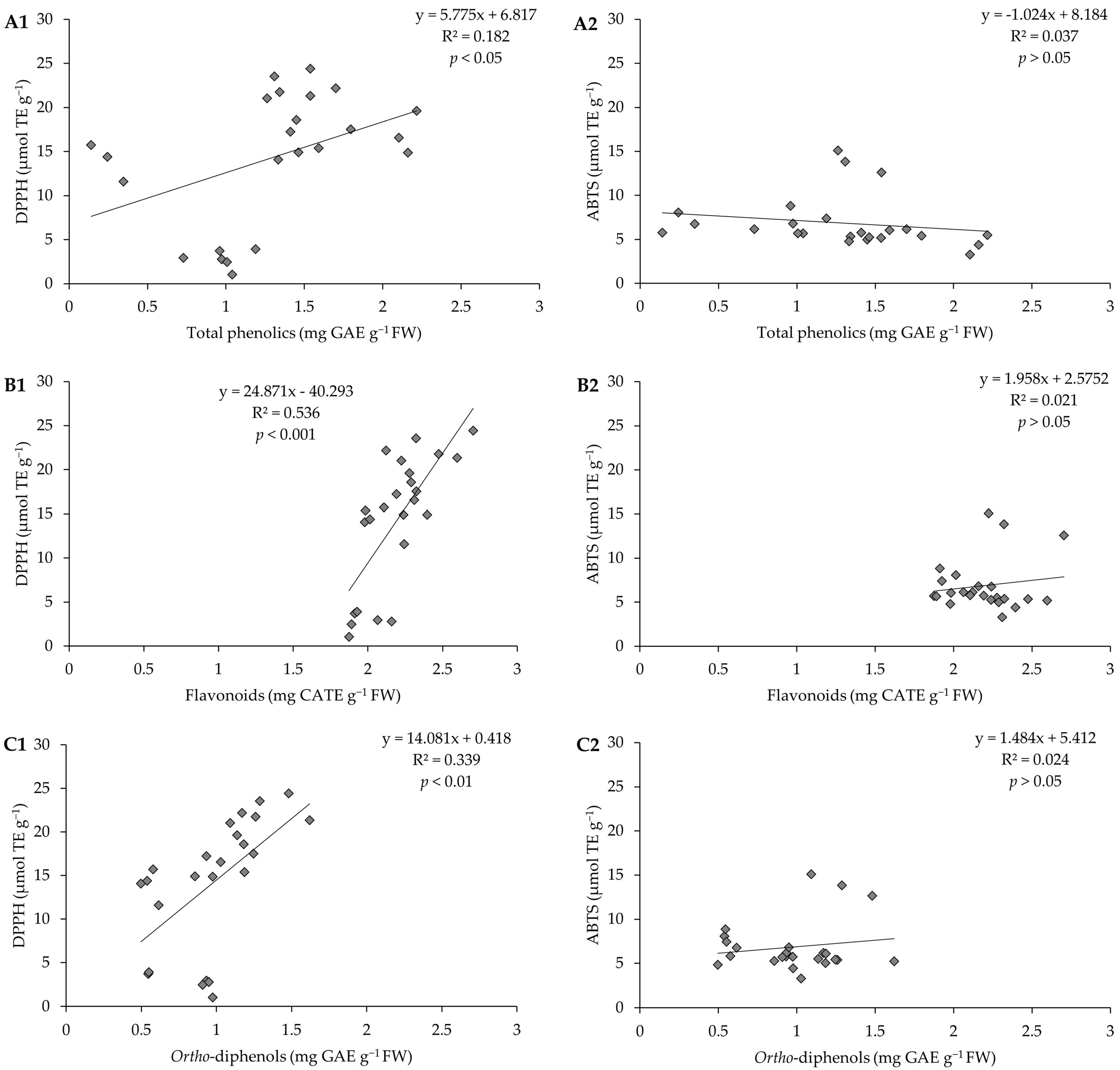
2.5. Pearson Correlation for All Evaluated Parameters
2.6. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Plant Material
3.1.1. Green Bean Seeds
3.1.2. Almond Shell and Almond Hulls
3.2. Almond Shell and Almond Hull Substrates
3.3. Growth Conditions and Experimental Design
3.4. Substrate Characteristics—pH, Temperature, Relative Humidity, and Electrical Conductivity
3.5. Chromatic Parameters
3.6. Quantifications in Green Bean Pods
3.6.1. Photosynthetic Pigments
3.6.2. Total Phenolics, Flavonoids, and Ortho-Diphenols
- Total phenolics
- 2.
- Flavonoids
- 3.
- Ortho-diphenols
3.6.3. Soluble Proteins
3.7. Antioxidant Capacity
- 1.
- DPPH method
- 2.
- ABTS method
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Agriculture Data—Crops and Livestock Products–Almonds in Shell; FAOSTAT: Rome, Italy, 2023; Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 27 December 2023).
- Shahidi, F.; Zhong, Y.; Wijeratne, S.; Ho, C. Almond and almond products: Nutraceutical components and health effects. In Tree Nuts: Composition, Phytochemicals, and Health Effects, 1st ed.; Alasalvar, C., Shahidi, F., Eds.; CRC Press: Boca Raton, FL, USA, 2008; Chapter 8; pp. 1–16. [Google Scholar] [CrossRef]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A.I.R.N.A. Valorization Challenges to Almond Residues: Phytochemical Composition and Functional Application. Molecules 2017, 22, 1774. [Google Scholar] [CrossRef]
- Sakar, E.H.; El Yamani, M.; Boussakouran, A.; Rharrabti, Y. Genotypic and Environmental Variations in Kernel Color Indices in the Main Almond (Prunus dulcis (Mill.) D.A. Webb) Cultivars Grown in North-Eastern Morocco. Scientifica 2021, 2021, 9970223. [Google Scholar] [CrossRef] [PubMed]
- Esfahlan, A.J.; Jamei, R.; Esfahlan, R.J. The importance of almond (Prunus amygdalus L.) and its by-products. Food Chem. 2010, 120, 349–360. [Google Scholar] [CrossRef]
- DePeters, E.J.; Fadel, J.G.; Arana, M.J.; Ohanesian, N.; Etchebarne, M.A.; Hamilton, C.A.; Hinders, R.G.; Maloney, M.D.; Old, C.A.; Riordan, T.J.; et al. Variability in the Chemical Composition of Seventeen Selected By-Product Feedstuffs Used by the California Dairy Industry. Prof. Anim. Sci. 2000, 16, 69–99. [Google Scholar] [CrossRef]
- Valverde, M.; Madrid, R.; García, A.; Del Amor, F.; Rincón, L. Use of almond shell and almond hull as substrates for sweet pepper cultivation. effects on fruit yield and mineral content. Span. J. Agric. Res. 2013, 11, 164–172. [Google Scholar] [CrossRef]
- Ledbetter, C. Shell cracking strength in almond (Prunus dulcis Mill. D.A. Webb.) and its implication in uses as a value-added product. Bioresour. Technol. 2008, 99, 5567–5573. [Google Scholar] [CrossRef]
- Chen, P.; Yanling, C.; Shaobo, D.; Xiangyang, L.; Guangwei, H.; Ruan, R. Utilization of almond residues. Int. J. Agric. Biol. Eng. 2010, 3, 1–18. [Google Scholar]
- Lao, M.T.; Jiménez, S. Evaluation of almond shell as a culture substrate for ornamental plants. II. Ficus benjamina (with 3 tables & 2 figures). Phyton (B. Aires) 2004, 73, 79–84. Available online: http://www.scielo.org.ar/pdf/phyton/v73/v73a08.pdf (accessed on 27 December 2023).
- Prgomet, I.; Goncalves, B.; Domínguez-Perles, R.; Santos, R.; Saavedra, M.J.; Aires, A.; Pascual-Seva, N.; Barros, A. Irrigation deficit turns almond by-products into a valuable source of antimicrobial (poly)phenols. Ind. Crop. Prod. 2019, 132, 186–196. [Google Scholar] [CrossRef]
- Smeriglio, A.; Mandalari, G.; Bisignano, C.; Filocamo, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Polyphenolic content and biological properties of Avola almond (Prunus dulcis Mill. D.A. Webb) skin and its industrial byproducts. Ind. Crop. Prod. 2016, 83, 283–293. [Google Scholar] [CrossRef]
- Monagas, M.; Garrido, I.; Lebrón-Aguilar, R.; Bartolome, B.; Gómez-Cordovés, C. Almond (Prunus dulcis(mill.) D.A. Webb) skins as a potential source of bioactive polyphenols. J. Agric. Food Chem. 2007, 55, 8498–8507. [Google Scholar] [CrossRef]
- Khorairi, A.; Sofian-Seng, A.N.S.; Othaman, N.S.; Abdul Rahman, R.; Mohd Razali, H.; Lim, N.S.; Wan Mustapha, S.J. A Review on Agro-Industrial Waste as Cellulose and Nanocellulose Source and Their Potentials in Food Applications. Food Rev. Int. 2023, 39, 663–688. [Google Scholar] [CrossRef]
- Berenguer, C.V.; Andrade, C.; Pereira, J.A.M.; Perestrelo, R.; Câmara, J.S. Current Challenges in the Sustainable Valorisation of Agri-Food Wastes: A Review. Processes 2023, 11, 20. [Google Scholar] [CrossRef]
- Carpentieri, S.; Larrea-Wachtendorff, D.; Donsì, F.; Ferrari, G. Functionalization of pasta through the incorporation of bioactive compounds from agri-food by-products: Fundamentals, opportunities, and drawbacks. Trends Food Sci. Technol. 2022, 122, 49–65. [Google Scholar] [CrossRef]
- Comunian, T.A.; Silva, M.P.; Souza, C.J.F. The Use of Food By-Products as a Novel for Functional Foods: Their Use as Ingredients and for the Encapsulation Process. Trends Food Sci. Technol. 2021, 108, 269–280. [Google Scholar] [CrossRef]
- Marcillo-Parra, V.; Tupuna-Yerovi, D.S.; González, Z.; Ruales, J. Encapsulation of Bioactive Compounds from Fruit and Vegetable By-Products for Food Application-A Review. Trends Food Sci. Technol. 2021, 116, 11–23. [Google Scholar] [CrossRef]
- Tiwari, A.; Khawas, R. Food waste and Agro by-products: A step towards food sustainability. In Innovation in the Food Sector through the Valorization of Food and Agro-Food By-Products; Barros, A.N., Gouvinhas, I., Eds.; IntechOpen: London, UK, 2021; Chapter 2; pp. 1–14. [Google Scholar] [CrossRef]
- Isah, S.; Ozbay, G. Valorization of food loss and wastes: Feedstocks for biofuels and valuable chemicals. Front. Sustain. Food Syst. 2020, 4, 82. [Google Scholar] [CrossRef]
- Saini, A.; Panesar, P.S.; Bera, M.B. Valorization of fruits and vegetables waste through green extraction of bioactive compounds and their nanoemulsions-based delivery system. Bioresour. Bioprocess. 2019, 6, 26. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- Diaz-Perez, J.C. Bell pepper (Capsicum annum L.) grown on plastic film mulches: Effects on crop microenvironment, physiological attributes, and fruit yield. HortScience 2010, 45, 1196–1204. [Google Scholar] [CrossRef]
- Zhai, Z.; Ehret, D.L.; Forge, T.; Helmer, T.; Lin, W.; Dorais, M.; Papadopoulos, A.P. Organic fertilizers for greenhouse tomatoes: Productivity and substrate microbiology. HortScience 2009, 44, 800–809. [Google Scholar] [CrossRef]
- Fascella, G. Growing Substrates Alternative to Peat for Ornamental Plants. In Soilless Culture—Use of Substrates for the Production of Quality Horticultural Crops; Asaduzzaman, M., Ed.; InTech Publication: Rijeka, Croatia, 2015; Volume 1, pp. 47–67. [Google Scholar] [CrossRef]
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving environmentally sustainable growing media for soilless plant cultivation systems—A review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef]
- Del Amor, F.M.; Gómez-López, M.D. Agronomical response and water use efficiency of sweet pepper plants grown in different greenhouse substrates. HortScience 2009, 44, 810–814. [Google Scholar] [CrossRef]
- Lin, W.; Frey, D.; Nigh, G.D.; Ying, C.C. Combined analysis to characterize yield pattern of greenhouse-grown red sweet peppers. HortScience 2009, 44, 362–365. [Google Scholar] [CrossRef]
- Urrestarazu, M.; Martínez, G.A.; Salas, M.C. Almond shell waste: Possible local rockwool substitute in soilless crop culture. Sci. Hortic. 2005, 103, 453–460. [Google Scholar] [CrossRef]
- Favaro, J.C.; Marano, R.P. Alterations in the physical and physico-chemical properties of a substrate based on composted sawdust and perlite with polycyclic tomato crops. Span. J. Agric. Res. 2003, 1, 105–109. [Google Scholar] [CrossRef]
- Arenas, M.; Vavrina, C.S.; Cornell, J.A.; Hanlon, E.A.; Hochmuth, G.J. Coir as an alternative to peat in media for tomato transplant production. HortScience 2002, 37, 309–312. [Google Scholar] [CrossRef]
- Urrestarazu, M.; Mazuela, P.; Martínez, G. Effect of substrate reutilization on yield and properties of melon and tomato crops. J. Plant. Nutr. 2008, 31, 2031–2043. [Google Scholar] [CrossRef]
- Lao, M.T.; Jiménez, S. Evaluation of almond shell as a culture substrate for ornamental plants. I. Characterization: (with 4 figures & 6 tables). Phyton (B. Aires) 2004, 73, 69–78. Available online: http://www.scielo.org.ar/pdf/phyton/v73/v73a07.pdf (accessed on 27 December 2023).
- Oliveira, I.; Meyer, A.; Aires, A.; Afonso, S.; Gonçalves, B. Enzymatic Activity and Biochemical Composition in Leaves of Green Bean (Phaseolus vulgaris L. cv. Saxa) Grown in Almond Shell Substrates. Waste Biomass Valorization 2019, 10, 1223–1229. [Google Scholar] [CrossRef]
- Oliveira, I.; Meyer, A.; Silva, R.; Afonso, S.; Gonçalves, B. Effect of almond shell addition to substrates in Phaseolus vulgaris L. (cv. Saxa) growth, and physiological and biochemical characteristics. Int. J. Recycl. Org. Waste Agric. 2019, 8, 179–186. [Google Scholar] [CrossRef]
- Smith, M.R.; Dinglasan, E.; Veneklaas, E.; Polania, J.; Rao, I.M.; Beebe, S.E.; Merchant, A. Effect of Drought and Low P on Yield and Nutritional Content in Common Bean. Front. Plant Sci. 2022, 13, 814325. [Google Scholar] [CrossRef] [PubMed]
- Villordo-Pineda, E.; González-Chavira, M.M.; Giraldo-Carbajo, P.; Acosta-Gallegos, J.A.; Caballero-Pérez, J. Identification of novel drought-tolerant-associated SNPs in common bean (Phaseolus vulgaris). Front. Plant Sci. 2015, 6, 546. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Cajiao, C.; Grajales, M. Selection for drought resistance in common bean also improves yield in phosphorus limited and favorable environments. Crop Sci. 2008, 48, 582–592. [Google Scholar] [CrossRef]
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef]
- Gruda, N.; Qaryouti, M.M.; Leonardi, S. Growing media. In Good Agricultural Practices for Greenhouse Vegetable Crops Principles for Mediterranean Climate Areas, 1st ed.; Baudoin, W., Nono-Womdim, R., Lutaladio, N., Hodder, A., Castilla, N., Leonardi, C., De Pascale, S., Qaryouti, M., Duffy, R., Eds.; FAO Technical Papers: Rome, Italy, 2013; Volume 217, pp. 271–302. Available online: https://www.fao.org/3/i3284e/i3284e.pdf (accessed on 30 December 2023).
- Datta, S.; Taghvaeian, S.; Stivers, S. Understanding Soil Water Content and Thresholds for Irrigation Management; Division of Agricultural Sciences and Natural Resources; Oklahoma State University: Oklahoma City, OK, USA, 2017; BAE-1537; Available online: https://www.academia.edu/en/91163065/Understanding_Soil_Water_Content_and_Thresholds_For_Irrigation_Management (accessed on 2 October 2023).
- López, R.; Burgos, P.; Hermoso, J.; Hormaza, J.; González-Fernández, J. Long term changes in soil properties and enzyme activities after almond shell mulching in avocado organic production. Soil Tillage Res. 2014, 143, 155–163. [Google Scholar] [CrossRef]
- De Lucia, B.; Vecchietti, L.; Leone, A. Italian buckthorn response to compost based substrates. Acta Hortic. 2011, 891, 231–236. [Google Scholar] [CrossRef]
- Ministério da Agricultura, do Desenvolvimento Rural e das Pescas—Direção-Geral de Proteção das Culturas (DGPC-DSF). Produção Integrada em Hortícolas, Família das Fabáceas—Ervilha, Fava, Feijão-Verde; Direção-Geral de Proteção das Culturas: Oeiras, Portugal, 2006; pp. 129–190. Available online: https://www.dgadr.gov.pt/mediateca?task=download.send&id=70&catid=8&m=0 (accessed on 2 October 2023).
- Proulx, E.; Yagiz, Y.; Cecilia, M.; Nunes, N.; Emond, J. Quality Attributes Limiting Snap Bean (Phaseolus vulgaris L.) Postharvest Life at Chilling and Non-chilling Temperatures. HortScience Horts 2010, 45, 1238–1249. [Google Scholar] [CrossRef]
- Kasim, R. and Kasim, M-U. Biochemical changes and color properties of fresh-cut green bean (Phaseolus vulgaris L. cv.gina) treated with calcium chloride during storage. Food Sci. Technol. 2015, 35, 266–272. [Google Scholar] [CrossRef]
- Mastrocola, D.; Lerici, C.R. Colorimetric measurements of enzymatic and non enzymatic browning in apple purees. Ital. J. Food Sci. 1991, 3, 219–229. [Google Scholar]
- Oruña-Concha, M.; González-Castro, M.; López-Hernández, J.; Simal-Lozano, J. Effects of freezing on the pigment content in green beans and padrón peppers. Z. Lebensm. Unters. Forsch. 1997, 205, 148–152. [Google Scholar] [CrossRef]
- El-Nafad, R.; El-Bastawesy, A.; Ghoniem, G.; El-Refai, A. Effect of Blanching Process and Frozen Storage on Bioactive Compounds and Some Quality Parameters of Organic and Conventional Green Bean. J. Food Dairy Sci. 2022, 13, 125–131. [Google Scholar] [CrossRef]
- Grecco, E.; Silveira, L.; Lima, V.; Pezzopane, J. Ecophysiological aspects of sun and shade leaves of Ponkan tangerine (Citrus reticulata Blanco). Idesia (Arica) 2014, 32, 113–117. [Google Scholar] [CrossRef]
- Lex Engel, V.; Poggiani, F. Study of foliar chlorophyll concentration and its light absorption spectrum as related to shading at the juvenile phase of four native forest tree species. Rev. Bras. Fisiol. Veg. 1991, 3, 39–45. [Google Scholar]
- Pallardy, S.G. Photosynthesis. In Physiology of Wood Plants, 3rd ed.; Pallardy, S.G., Ed.; Academic Press: London, UK, 2008; Volume 1, pp. 107–167. [Google Scholar] [CrossRef]
- Boardman, N.K. Comparative photosynthesis of sun and shade plants. Ann. Rev. Plant Physiol. 1997, 28, 355–377. [Google Scholar] [CrossRef]
- Muhidin; Syam’Un, E.; Kaimuddin; Musa, Y.; Sadimantara, G.R.; Usman; Leomo, S.; Rakian, T.C. The effect of shade on chlorophyll and anthocyanin content of upland red rice. IOP Conf. Ser. Earth Environ. Sci. 2018, 122, 012030. [Google Scholar] [CrossRef]
- Gross, J. Chlorophylls. In Pigments in Vegetables: Chlorophylls and Carotenoid, 1st ed.; Gross, J., Ed.; Springer: Boston, MA, USA, 1991. [Google Scholar] [CrossRef]
- López, J.C.; Baille, A.; Bonachela, S.; Pérez-Parra, J. Analysis and prediction of greenhouse green bean (Phaseolus vulgaris L.) production in a Mediterranean climate. Biosyst. Eng. 2008, 100, 86–95. [Google Scholar] [CrossRef]
- Tesi, R. Colture Protette-Ortoflorovivaismo in Ambiente Mediterraneo, 6th ed.; Edagricole—Edizioni Agricole de II Sole 24 ORE Business Media s.r.l.: Milan, Italy, 2008; pp. 1–349. [Google Scholar]
- Hadi, H.; Ghassemi-Golezani, K.; Rahimzadeh Khoei, F. Response of common bean (Phaseolus vulgaris L.) to different levels of shade. J. Agron. 2006, 5, 595–599. [Google Scholar] [CrossRef]
- Stirling, C.M.; Williams, J.H.; Black, C.R.; Ong, C.K. The effect of timing of shade on development, dry matter production and light-use efficiency in groundnut (Arachis hypogaea L.) under field conditions. Aust. J. Agric. Res. 1990, 41, 633–644. [Google Scholar] [CrossRef]
- Worku, W.; Skjelvåg, A.O.; Gislerød, H.R. Responses of common bean (Phaseolus vulgaris L.) to photosynthetic irradiance levels during three phenological phases. Agronomie 2004, 24, 267–274. [Google Scholar] [CrossRef]
- Carbas, B.; Machado, N.; Oppolzer, D.; Ferreira, L.; Queiroz, M.; Brites, C.; Rosa, E.A.; Barros, A.I. Nutrients, Antinutrients, Phenolic Composition, and Antioxidant Activity of Common Bean Cultivars and their Potential for Food Applications. Antioxidants 2020, 9, 186. [Google Scholar] [CrossRef] [PubMed]
- Mastura, H.Y.; Hasnah, H.; Dang, T.N. Total phenolic content and antioxidant capacity of beans organic vs inorganic. Int. Food Res. J. 2017, 24, 510–517. Available online: http://www.ifrj.upm.edu.my/volume-24-2017.html (accessed on 30 December 2023).
- Turkmen, N.; Sari, F.; Velioglu, Y. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005, 93, 713–718. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, L. Total phenolic contents and antioxidant properties of commonly consumed vegetables grown in Colorado. Food Sci. Technol. 2006, 39, 1155–1162. [Google Scholar] [CrossRef]
- Sánchez-Mata, M.; Camara, M.; Dıez-Marques, C. Extending shelf life and nutritive value of green beans (Phaseolus vulgaris L.), by controlled atmosphere storage: Macronutrients. Food Chem. 2003, 80, 309–315. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Aspects Med. 2003, 24, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Dissanayaka, C.S. Phenolic-protein interactions: Insight from in-silico analyses—A review. Food Prod. Process Nutr. 2023, 5, 2. [Google Scholar] [CrossRef]
- Prigent, S.V.E.; Gruppen, H.; Visser, A.J.W.G.; van Koningsveld, G.A.; de Jong, G.A.H.; Voragen, A.G.J. Effects of non-covalent interactions with 5-O-caffeoylquinic acid (chlorogenic acid) on the heat denaturation and solubility of globular proteins. J. Agric. Food Chem. 2003, 51, 5088–5095. [Google Scholar] [CrossRef]
- Direção geral de Alimentação e Veterinária (DGAV). Feijão Phaseolus vulgaris L. In Catálogo Nacional de Variedades—Espécies Agrícolas e Hortícolas, 1st ed.; DGAV Publishing: Lisboa, Portugal, 2023; pp. 56–57. Available online: https://www.dgav.pt/destaques/noticias/catalogo-nacional-de-variedades-de-especies-agricolas-e-horticolas-2023/ (accessed on 2 October 2023).
- EU Food Safety. Plants—Plant Reproductive Material—Plant Variety Catalogues, Databases & Information Systems—Agricultural and Vegetable Species—EU Database of Registered Plant Varieties. Common Catalogue of Varieties of Vegetable Species—Consolidated Version 27 January 2023. Europa Union. Available online: https://food.ec.europa.eu/plants/plant-reproductive-material/plant-variety-catalogues-databases-information-systems_en (accessed on 2 October 2023).
- Hoagland, D.R.; Arnon, D.I. The water culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 1–32. Available online: https://ia801405.us.archive.org/24/items/waterculturemeth347hoag/waterculturemeth347hoag.pdf (accessed on 2 October 2023).
- Voss, D.H. Relating colourimeter measurement of plant colour to the royal horticultural society colour chart. HortScience 1992, 27, 1256–1260. [Google Scholar] [CrossRef]
- Hutchings, J.B. Food Colour and Appearance; Blackie Academic & Professional: London, UK, 1994; pp. 1–29. [Google Scholar] [CrossRef]
- Zhang, C.; Whiting, M. Improving ‘Bing’ sweet cherry fruit quality with plant growth regulators. Sci. Hortic. 2011, 127, 341–346. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of objective colour measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Bernalte, M.J.; Hernández, M.T.; Vidal-Aragón, M.C.; Sabio, E. Physical, chemical, flavor and sensory characteristics of two sweet cherry varieties grown in ‘Valle del Jerte’ (Spain). J. Food Qual. 1999, 22, 403–416. [Google Scholar] [CrossRef]
- Gonçalves, B.; Silva, A.P.; Moutinho-Pereira, J.; Bacelar, E.; Rosa, E.; Meyer, A.S. Effect of ripeness and postharvest storage on the evolution of colour and anthocyanins in cherries (Prunus avium L.). Food Chem. 2007, 103, 976–984. [Google Scholar] [CrossRef]
- Giménez, M.J.; Serrano, M.; Valverde, J.M.; Martínez-Romero, D.; Castillo, S.; Valero, D.; Guillén, F. Preharvest salicylic acid and acetylsalicylic acid treatments preserve quality and enhance antioxidant systems during postharvest storage of sweet cherry cultivars. J. Sci. Food Agric. 2016, 97, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts: Polyphenol oxydase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth. Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Santos, R.A.; Queiroz, M.; Leal, C.; Saavedra, M.J.; Domínguez-Perles, R.; Rodrigues, M.; Barros, A.I.R.N.A. Monitoring the antioxidant and antimicrobial power of grape (Vitis vinifera L.) stems phenolics over long-term storage. Ind. Crop. Prod. 2018, 126, 83–91. [Google Scholar] [CrossRef]
- Machado, N.F.L.; Domínguez-Perles, R. Addressing Facts and Gaps in the Phenolics Chemistry of Winery By-Products. Molecules 2017, 22, 286. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvents extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables-evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef]

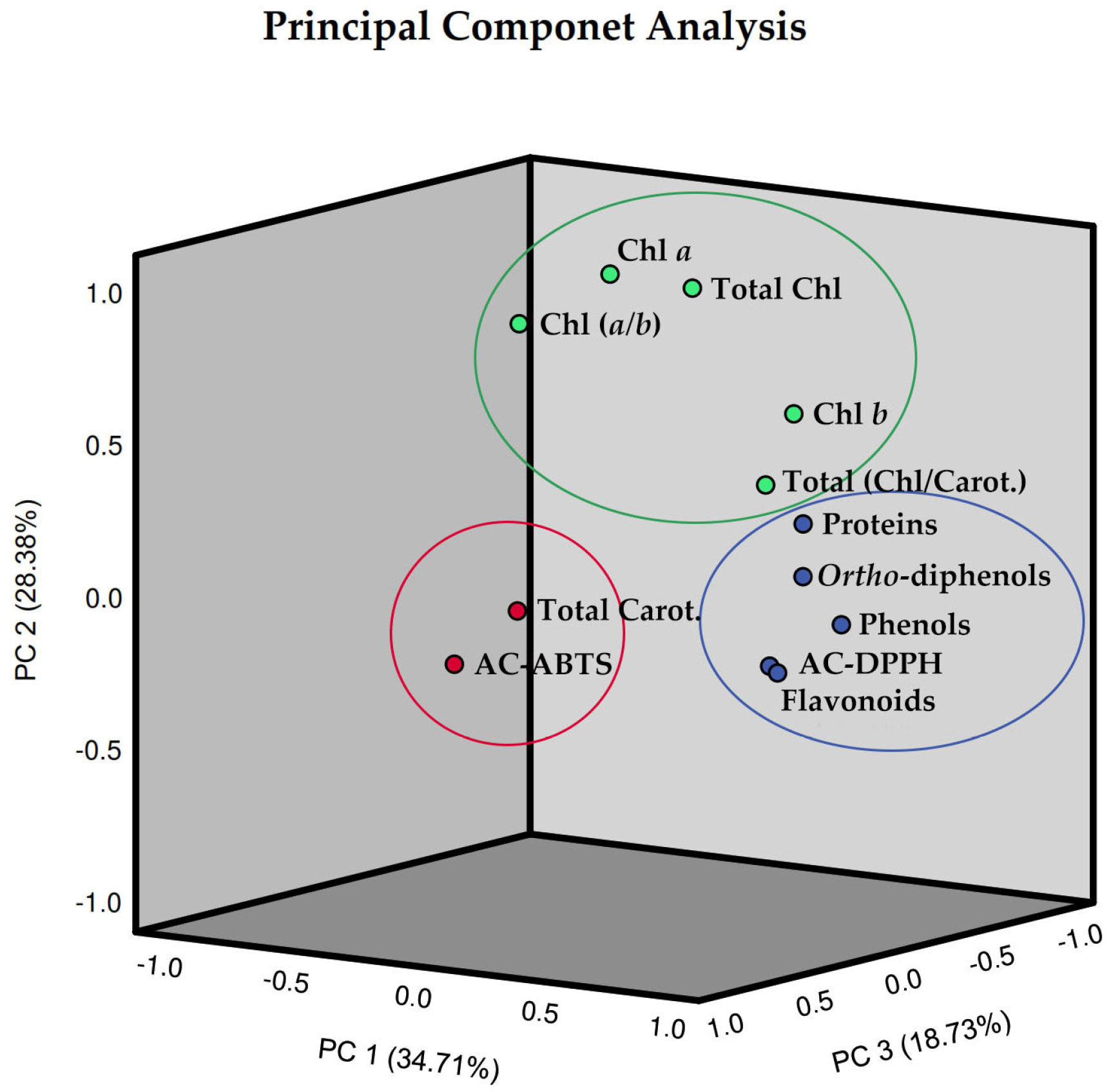

| Variables | PC1 | PC2 | PC3 |
|---|---|---|---|
| Chl a | −0.069 | 0.983 | −0.074 |
| Chl b | 0.533 | 0.560 | −0.249 |
| Total Chl | 0.192 | 0.951 | −0.165 |
| Chl (a/b) | −0.345 | 0.809 | 0.044 |
| Total Carot. | 0.267 | 0.055 | 0.928 |
| Total (Chl/Carot.) | −0.080 | 0.160 | −0.966 |
| Phenolics | 0.726 | −0.113 | −0.242 |
| Ortho-diphenols | 0.884 | 0.125 | 0.201 |
| Flavonoids | 0.797 | −0.197 | 0.220 |
| AC-DPPH | 0.767 | −0.178 | 0.221 |
| AC-ABTS | 0.015 | −0.146 | 0.924 |
| Proteins | 0.852 | 0.289 | 0.154 |
| Eigenvalues | 4.165 | 3.405 | 2.247 |
| Variance (%) | 34.705 | 28.377 | 18.725 |
| Cumulative (%) | 34.705 | 63.082 | 81.807 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, V.; Oliveira, I.; Pereira, J.A.; Gonçalves, B. Almond By-Products Substrates as Sustainable Amendments for Green Bean Cultivation. Plants 2024, 13, 540. https://doi.org/10.3390/plants13040540
Silva V, Oliveira I, Pereira JA, Gonçalves B. Almond By-Products Substrates as Sustainable Amendments for Green Bean Cultivation. Plants. 2024; 13(4):540. https://doi.org/10.3390/plants13040540
Chicago/Turabian StyleSilva, Vânia, Ivo Oliveira, José Alberto Pereira, and Berta Gonçalves. 2024. "Almond By-Products Substrates as Sustainable Amendments for Green Bean Cultivation" Plants 13, no. 4: 540. https://doi.org/10.3390/plants13040540
APA StyleSilva, V., Oliveira, I., Pereira, J. A., & Gonçalves, B. (2024). Almond By-Products Substrates as Sustainable Amendments for Green Bean Cultivation. Plants, 13(4), 540. https://doi.org/10.3390/plants13040540









