Plant Reproductive Success Mediated by Nectar Offered to Pollinators and Defensive Ants in Terrestrial Bromeliaceae
Abstract
1. Introduction
2. Results
2.1. Ant Presence and Behavior
2.2. Floral Pollinators and Nectar Standing Crop
2.3. Plant Reproductive Compatibility System
2.4. Experimental Design Linking Pollination, Defensive Ants, and Plant Reproductive Success
3. Material and Methods
3.1. Plant Material
3.2. Plant Reproductive Compatibility System
3.3. Experimental Design
- Exposed inflorescences (control treatment): ants and pollinators had free access;
- Ant-excluded inflorescences: Spikes prevented ant access and pollinators had free access. Before flowering, the base of the spike was coated with an oil-based insect repellent (Tanglefoot®, Grand Rapids, MI, USA). The repellent was kept at least 20 cm away from the nearest flower, a reasonable distance to avoid its influence on pollinators and flying herbivores. To avoid the formation of natural bridges for ants to the upper sections of the spikes on the ant-excluded plants, the adjacent stems of neighboring plants were removed;
- Bagged inflorescences: ants and pollinators were excluded from the spikes using a voile.
3.4. Pollinators, Ants, and Insects Consuming Plant Tissues
3.5. Data Analysis: Linking Pollination, Defensive Ants, Plant Reproductive Success, and Compatibility System
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Percival, M.S. Types of nectar in angiosperms. New Phytol. 1961, 60, 235–281. [Google Scholar] [CrossRef]
- Baker, H.G.; Baker, I. Floral nectar sugars constituents in relation to pollinator type. In Handbook of Experimental Pollination Biology; Jones, C.E., Little, R.J., Eds.; Van Nostrand Reinhold Co.: New York, NY, USA, 1983; pp. 117–141. [Google Scholar]
- Rosumek, F.B.; Silveira, F.A.; de S. Neves, F.; de U. Barbosa, N.P.; Diniz, L.; Oki, Y.; Pezzini, F.; Fernandes, G.W.; Cornelissen, T. Ants on plants: A meta-analysis of the role of ants as plant biotic defenses. Oecologia 2009, 160, 537–549. [Google Scholar] [CrossRef]
- Del-Claro, K.; Rico-Gray, V.; Torezan-Silingardi, H.M.; Alves-Silva, E.; Fagundes, R.; Lange, D.; Dáttilo, W.; Vilela, A.A.; Aguirre, A.; Rodriguez-Morales, D. Loss and gains in ant–plant interactions mediated by extrafloral nectar: Fidelity, cheats, and lies. Insectes Soc. 2016, 63, 207–221. [Google Scholar] [CrossRef]
- Barônio, G.J.; Del-Claro, K. Increase in ant density promotes dual effects on bee behaviour and plant reproductive performance. Arthropod-Plant Interact. 2018, 12, 201–213. [Google Scholar] [CrossRef]
- Byk, J.; Del-Claro, K. Nectar-and pollen-gathering Cephalotes ants provide no protection against herbivory: A new manipulative experiment to test ant protective capabilities. Acta Ethol. 2010, 13, 33–38. [Google Scholar] [CrossRef]
- Asuncao, M.; Maura, H.; Claro, K. Do ant visitors to extrafloral nectaries of plants repel pollinators and cause indirect cost of mutualism. Flora 2014, 209, 244–249. [Google Scholar] [CrossRef]
- Alves-Silva, E.; Del-Claro, K. On the inability of ants to protect their plant partners and the effect of herbivores on different stages of plant reproduction. Austral. Ecol. 2016, 41, 263–272. [Google Scholar] [CrossRef]
- Dáttilo, W.; Aguirre, A.; De la Torre, P.L.; Kaminski, L.A.; García-Chávez, J.; Rico-Gray, V. Trait-mediated indirect interactions of ant shape on the attack of caterpillars and fruits. Biol. Lett. 2016, 12, 20160401. [Google Scholar] [CrossRef] [PubMed]
- Melati, B.G.; Leal, L.C. Aggressive bodyguards are not always the best: Preferential interaction with more aggressive ant species reduces reproductive success of plant bearing extrafloral nectaries. PLoS ONE 2018, 13, e0199764. [Google Scholar] [CrossRef]
- Bernardello, L.M.; Galetto, L.; Juliani, H.R. Floral nectar, nectary structure and pollinators in some Argentinean Bromeliaceae. Ann. Bot. 1991, 67, 401–411. [Google Scholar] [CrossRef]
- Kroemer, T.; Kessler, M.; Lohaus, G.; Schmidt-Lebuhn, A.N. Nectar sugar composition and concentration in relation to pollination syndromes in Bromeliaceae. Plant Biol. 2008, 10, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Stahl, J.M.; Nepi, M.; Galetto, L.; Guimarães, E.; Machado, S.R. Functional aspects of floral nectar secretion of Ananas ananassoides, an ornithophilous bromeliad from the Brazilian savanna. Ann. Bot. 2012, 109, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Göttlinger, T.; Schwerdtfeger, M.; Tiedge, K.; Lohaus, G. What do nectarivorous bats like? Nectar composition in Bromeliaceae with special emphasis on bat-pollinated species. Front. Plant Sci. 2019, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Galetto, L.; Bernardello, L.M. Extrafloral nectaries that attract ants in Bromeliaceae: Structure and nectar composition. Can. J. Bot. 1992, 70, 1101–1106. [Google Scholar] [CrossRef]
- Ballego-Campos, I.; Forzza, R.C.; Paiva, É.A. Extranuptial nectaries in bromeliads: A new record for Pitcairnia burchellii and perspectives for Bromeliaceae. Sci. Nat. 2022, 109, 28. [Google Scholar] [CrossRef] [PubMed]
- Vesprini, J.L.; Galetto, L.; Bernardello, G. The beneficial effect of ants on the reproductive success of Dyckia floribunda (Bromeliaceae), an extrafloral nectary plant. Can. J. Bot. 2003, 81, 24–27. [Google Scholar] [CrossRef]
- Galetto, L.; Bernardello, L.M. Nectar secretion pattern and removal effects in six Argentinean Pitcairnioideae (Bromeliaceae). Bot. Acta 1992, 105, 292–299. [Google Scholar] [CrossRef]
- Dafni, A. Pollination Ecology: A Practical Approach; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing (Version 4.2.1); R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org (accessed on 18 December 2023).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models using lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Searle, S.R.; Speed, F.M.; Milliken, G.A. Population marginal means in the linear model: An alternative to least squares means. Am. Stat. 1980, 34, 216–221. [Google Scholar] [CrossRef]
- Lüdecke, D. ggeffects: Tidy Data Frames of Marginal Effects from Regression Models. J. Open Source Softw. 2018, 3, 772. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Del-Claro, K.; Oliveira, P.S. Conditional outcomes in a neotropical treehopper-ant association: Temporal and species-specific variation in ant protection and homopteran fecundity. Oecologia 2000, 124, 156–165. [Google Scholar] [CrossRef]
- Blüthgen, N.E.; Stork, N.; Fiedler, K. Bottom-up control and co-occurrence in complex communities: Honeydew and nectar determine a rainforest ant mosaic. Oikos 2004, 106, 344–358. [Google Scholar] [CrossRef]
- Trager, M.D.; Bhotika, S.; Hostetler, J.A.; Andrade, G.V.; Rodriguez-Cabal, M.A.; McKeon, C.S.; Osenberg, C.W.; Bolker, B.M. Benefits for plants in ant-plant protective mutualisms: A meta-analysis. PLoS ONE 2010, 5, e14308. [Google Scholar] [CrossRef] [PubMed]
- Monique, K.; de Souza, G.R.; Calixto, E.S.; Silva, E.A. Temporal variation in the effect of ants on the fitness of myrmecophilic plants: Seasonal effect surpasses periodic benefits. Sci. Nat. 2022, 109, 36. [Google Scholar] [CrossRef] [PubMed]
- Ness, J.H. A mutualism’s indirect costs: The most aggressive plant bodyguards also deter pollinators. Oikos 2006, 113, 506–514. [Google Scholar] [CrossRef]
- Junker, R.; Chung, A.Y.; Blüthgen, N. Interaction between flowers, ants and pollinators: Additional evidence for floral repellence against ants. Ecol. Res. 2007, 22, 665–670. [Google Scholar] [CrossRef]
- Leal, L.C.; Nogueira, A.; Peixoto, P.E. Which traits optimize plant benefits? Meta-analysis on the effect of partner traits on the outcome of an ant–plant protective mutualism. J. Ecol. 2023, 111, 263–275. [Google Scholar] [CrossRef]
- Ferreira, V.C.; Neves, F.S.; Guerra, T.J. Direct and indirect effects of ant–trophobiont interactions on the reproduction of a hummingbird-pollinated mistletoe. Plant Ecol. 2022, 223, 285–296. [Google Scholar] [CrossRef]
- Blüthgen, N.; Menzel, F.; Hovestadt, T.; Fiala, B.; Blüthgen, N. Specialization, constraints, and conflicting interests in mutualistic networks. Curr. Biol. 2007, 17, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Alves-Silva, E.; Bächtold, A.; Barônio, G.J.; Del-Claro, K. Influence of Camponotus blandus (Formicinae) and flower buds on the occurrence of Parrhasius polibetes (Lepidoptera: Lycaenidae) in Banisteriopsis malifolia (Malpighiaceae). Sociobiology 2013, 60, 30–34. [Google Scholar] [CrossRef][Green Version]
- Koptur, S.; Lawton, J.H. Interactions among vetches bearing extrafloral nectaries, their biotic protective agents, and herbivores. Ecology 1988, 69, 278–283. [Google Scholar] [CrossRef]
- Bronstein, J.L.; Alarcón, R.; Geber, M. The evolution of plant–insect mutualisms. New Phytol. 2006, 172, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Marazzi, B.; Bronstein, J.L.; Koptur, S. The diversity, ecology and evolution of extrafloral nectaries: Current perspectives and future challenges. Ann. Bot. 2013, 111, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Filho, L.A.; Calixto, E.S.; Santos, D.F.B.; Del-Claro, K. Negative cascading effects of a predatory fly larva on an ant–plant protective mutualism. Arthropod-Plant Interact. 2022, 16, 373–385. [Google Scholar] [CrossRef]
- Pyke, G.H. What does it cost a plant to produce floral nectar? Nature 1991, 350, 58–59. [Google Scholar] [CrossRef]
- Galetto, L.; Araujo, F.P.; Grilli, G.; Amarilla, L.D.; Torres, C.; Sazima, M. Flower trade-offs derived from nectar investment in female reproduction of two Nicotiana species (Solanaceae). Acta Bot. Bras. 2018, 32, 473–478. [Google Scholar] [CrossRef]
- Ordano, M.; Ornelas, J.F. Generous-like flowers: Nectar production in two epiphytic bromeliads and a meta-analysis of removal effects. Oecologia 2004, 140, 495–505. [Google Scholar] [CrossRef]
- Millán-Cañongo, C.; Orona-Tamayo, D.; Heil, M. Phloem sugar flux and jasmonic acid-responsive cell wall invertase control extrafloral nectar secretion in Ricinus communis. J. Chem. Ecol. 2014, 40, 760–769. [Google Scholar] [CrossRef]
- Grasso, D.A.; Pandolfi, C.; Bazihizina, N.; Nocentini, D.; Nepi, M.; Mancuso, S. Extrafloral-nectar-based partner manipulation in plant–ant relationships. AoB Plants 2015, 7, plv002. [Google Scholar] [CrossRef]

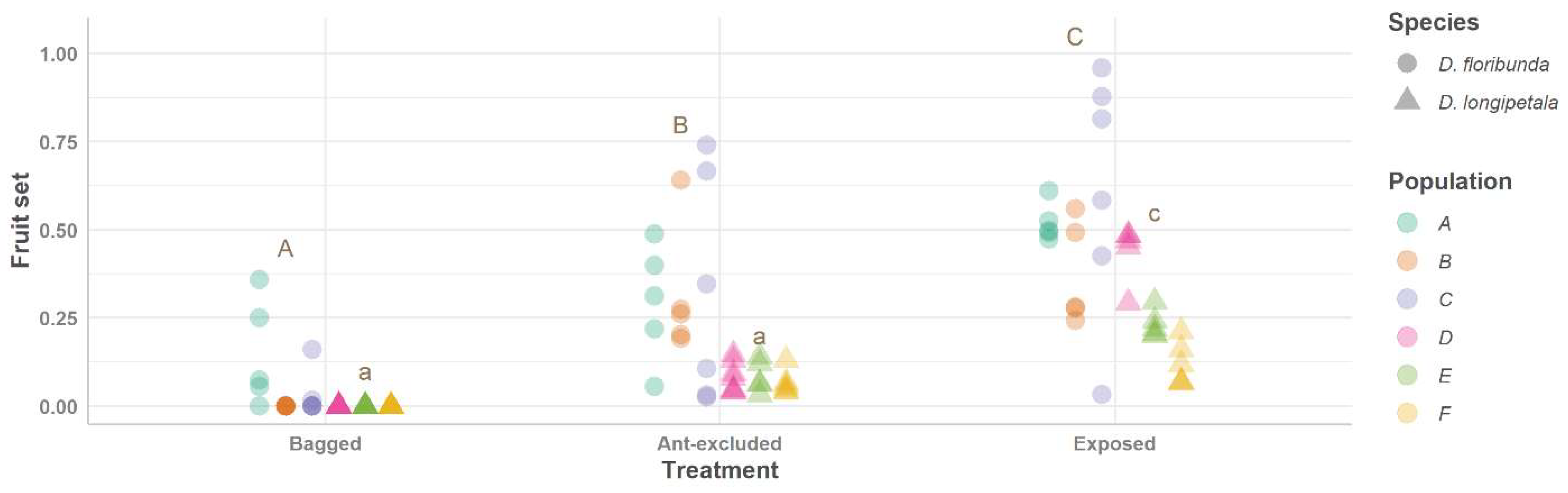
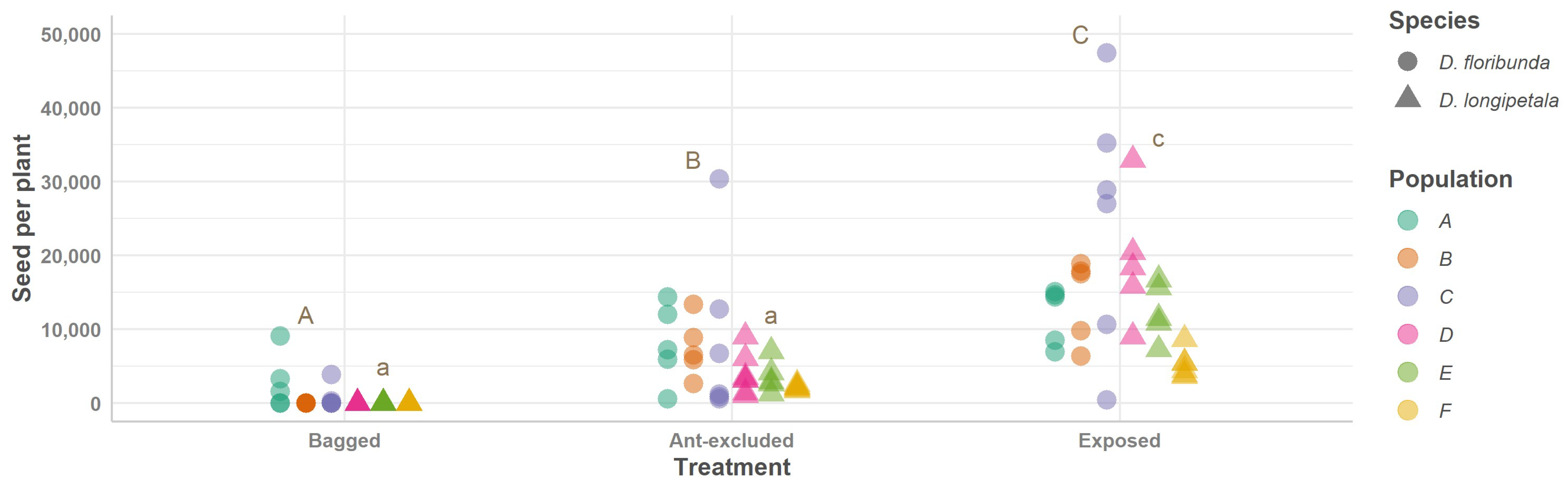
| Species | Variables | Treatments | Means | Confidence Intervals [95% for Predicted Values] |
|---|---|---|---|---|
| Dyckia longipetala | Fruit set | Bagged | 0 | [−0.04, 0.04] |
| Ant-excluded | 0.08 | [0.04, 0.12] | ||
| Exposed | 0.25 | [0.21, 0.30] | ||
| Seeds per plant | Bagged | 0 | [−2315.05, 2337.28] | |
| Ant-excluded | 3209.11 | [1015.86, 5402.36] | ||
| Exposed | 11,851.02 | [9535.97, 14,188.30] | ||
| Dyckia floribunda | Fruit set | Bagged | 0.06 | [−0.06, 0.17] |
| Ant-excluded | 0.31 | [0.20, 0.42] | ||
| Exposed | 0.51 | [0.40, 0.62] | ||
| Seeds per plant | Bagged | 1126.29 | [−3131.42, 5558.08] | |
| Ant-excluded | 8091.56 | [3833.85, 12,523.35] | ||
| Exposed | 17,453.15 | [13,195.44, 21,884.94] |
| Variable | Contrast | Estimate | SE | p-Value |
|---|---|---|---|---|
| Fruit set | Bagged D. floribunda–Ant-excluded D. floribunda) | −0.253 | 0.051 | 8.63 × 10−1 |
| Bagged D. floribunda–Exposed D. floribunda | −0.452 | 0.051 | 4.29 × 10−7 | |
| (Ant-excluded D. floribunda)–Exposed D. floribunda | −0.199 | 0.051 | 0.003 | |
| Bagged D. longipetala–(Ant-excluded D. longipetala) | −0.077 | 0.050 | 0.634 | |
| Bagged D. longipetala–Exposed D. longipetala | −0.252 | 0.051 | 9.06 × 10−1 | |
| (Ant-excluded D. longipetala)–Exposed D. longipetala | −0.175 | 0.050 | 0.010 | |
| Seeds per plant | Bagged D. floribunda–(Ant-excluded D. floribunda) | −6965.26 | 2366.17 | 0.049 |
| Bagged D. floribunda–Exposed D. floribunda | −16,326.86 | 2366.17 | 5.14 × 10−4 | |
| (Ant-excluded D. floribunda)–Exposed D. floribunda | −9361.59 | 2366.17 | 0.003 | |
| Bagged D. longipetala–(Ant-excluded D. longipetala) | −3209.11 | 2301.94 | 0.730 | |
| Bagged D. longipetala–Exposed D. longipetala | −11,851.02 | 2366.17 | 7.11 × 10−1 | |
| (Ant-excluded D. longipetala)–Exposed D. longipetala | −8641.91 | 2301.94 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, C.; Mazzei, M.P.; Vesprini, J.L.; Galetto, L. Plant Reproductive Success Mediated by Nectar Offered to Pollinators and Defensive Ants in Terrestrial Bromeliaceae. Plants 2024, 13, 493. https://doi.org/10.3390/plants13040493
Torres C, Mazzei MP, Vesprini JL, Galetto L. Plant Reproductive Success Mediated by Nectar Offered to Pollinators and Defensive Ants in Terrestrial Bromeliaceae. Plants. 2024; 13(4):493. https://doi.org/10.3390/plants13040493
Chicago/Turabian StyleTorres, Carolina, Mariana P. Mazzei, José L. Vesprini, and Leonardo Galetto. 2024. "Plant Reproductive Success Mediated by Nectar Offered to Pollinators and Defensive Ants in Terrestrial Bromeliaceae" Plants 13, no. 4: 493. https://doi.org/10.3390/plants13040493
APA StyleTorres, C., Mazzei, M. P., Vesprini, J. L., & Galetto, L. (2024). Plant Reproductive Success Mediated by Nectar Offered to Pollinators and Defensive Ants in Terrestrial Bromeliaceae. Plants, 13(4), 493. https://doi.org/10.3390/plants13040493








