Chitosan and Chitosan Nanoparticles Differentially Alleviate Salinity Stress in Phaseolus vulgaris L. Plants
Abstract
1. Introduction
2. Results
2.1. Effects of Cs or CsNPs on Growth and Yield Traits of Common Bean Grown under Salt Stress
2.2. Effects of Cs or CsNPs on Photosynthetic Pigments and Total Carbohydrates Contents of Common Bean Grown under Salt Stress
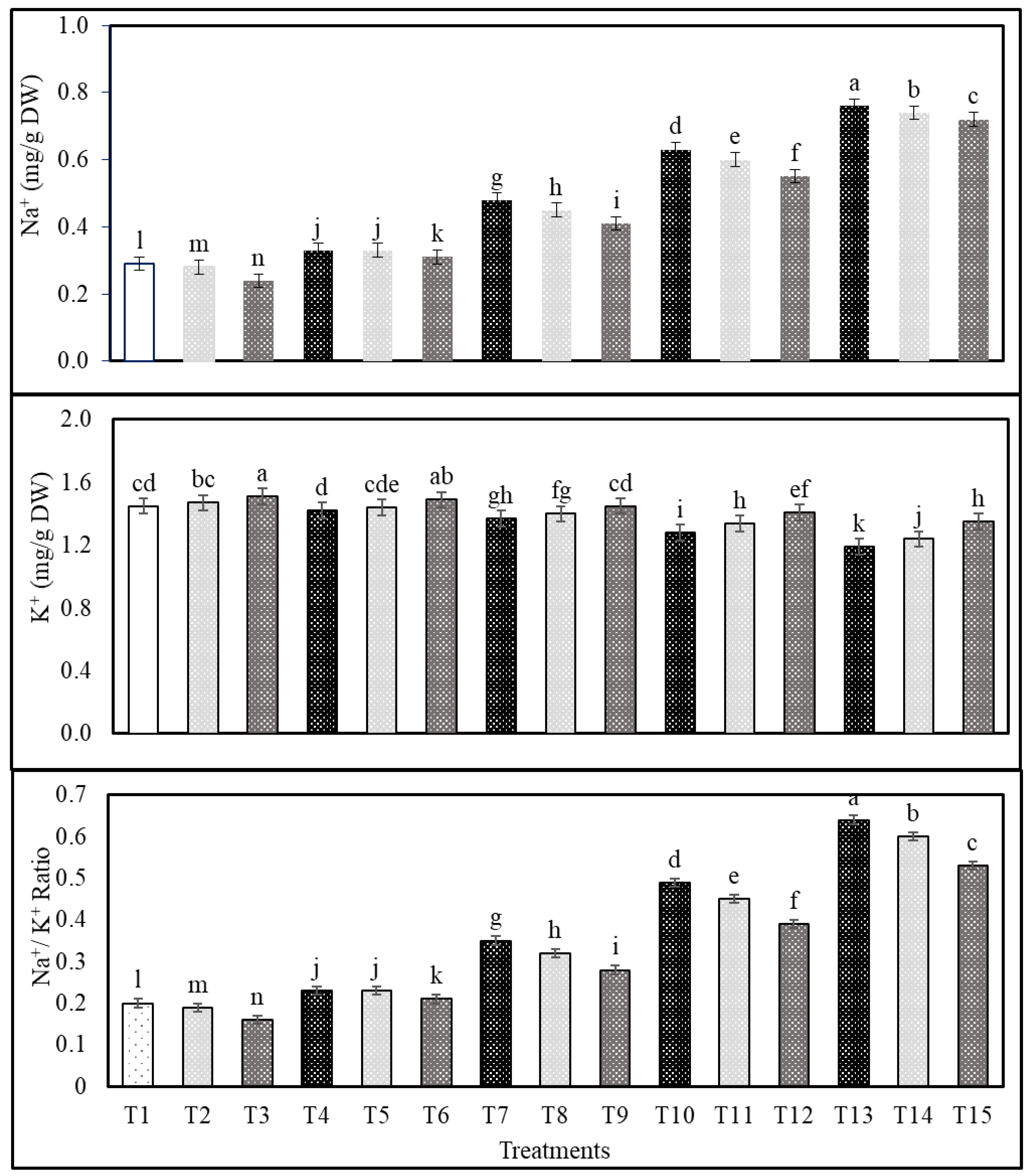
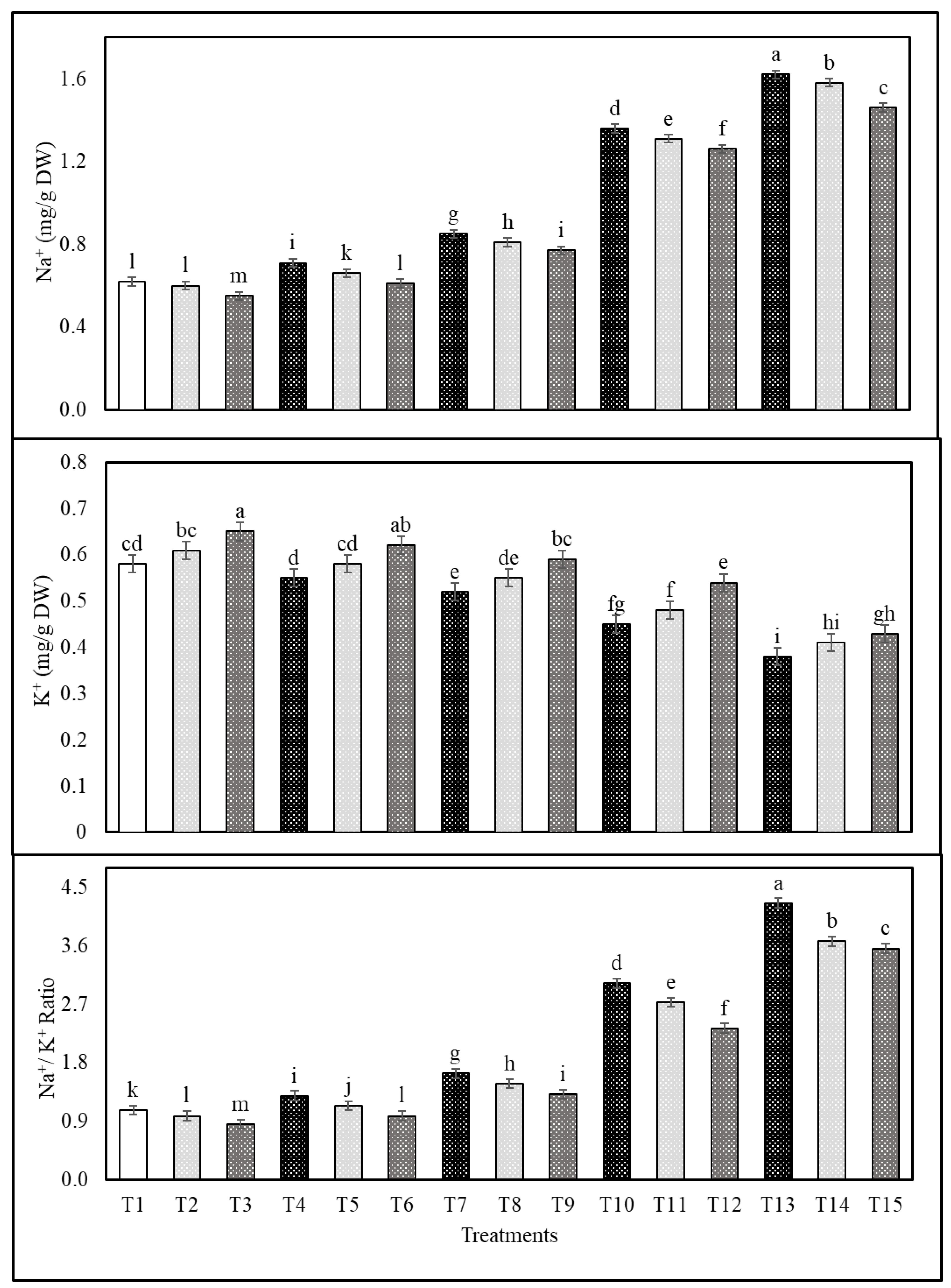
2.3. Effects of Cs or CsNPs on Ions Contents of Common Bean Grown under Salt Stress
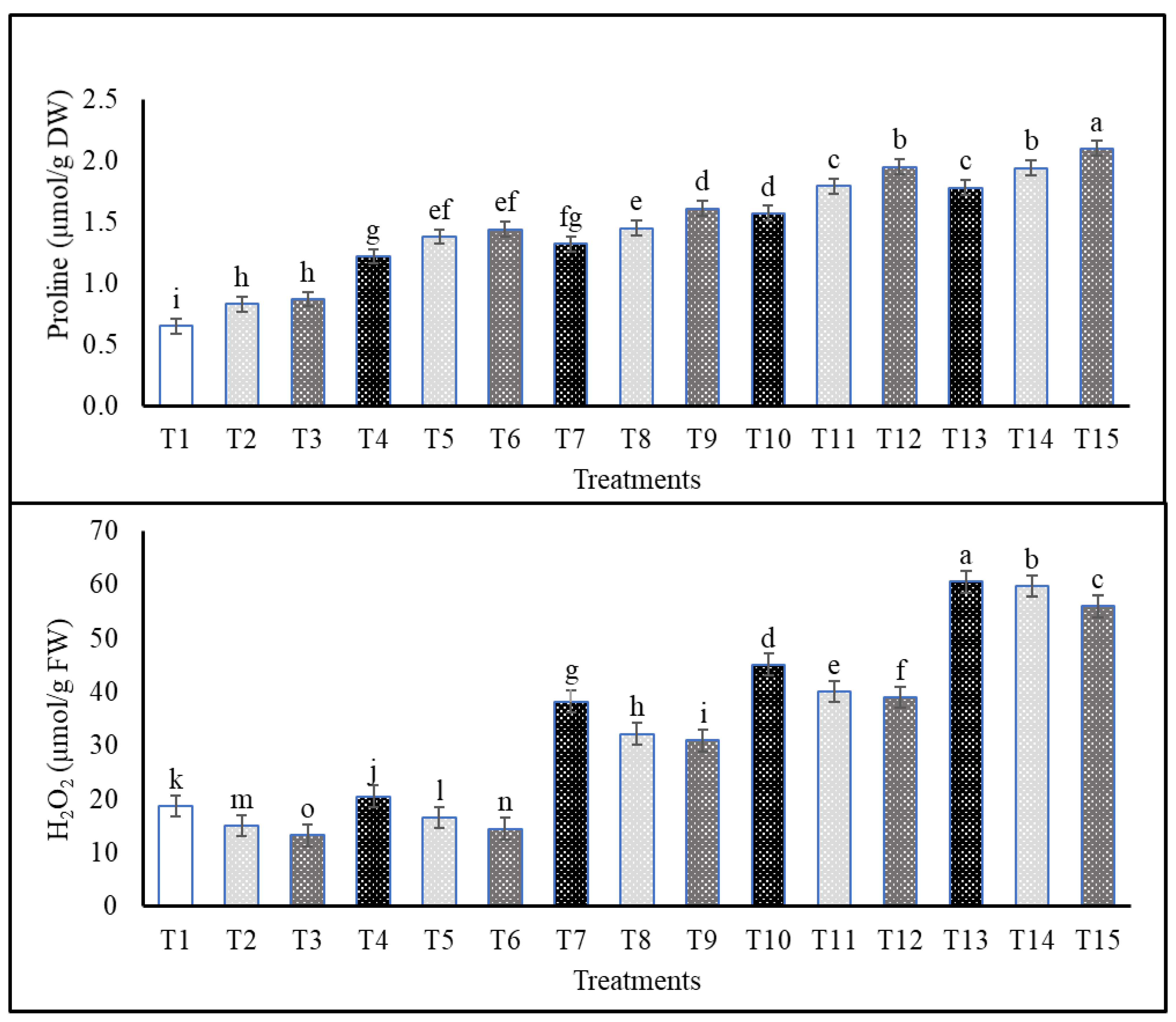
2.4. Effects of Cs or CsNPs on Biochemical Traits of Common Bean Grown under Salt Stress
2.5. Effects of Cs or CsNPs on Antioxidants System in Common Bean Grown under Salt Stress
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Preparation and Characterization of Chitosan Nanoparticles
4.2.1. Morphology and Size of Chitosan Nanoparticles
4.2.2. Zeta Potential
4.2.3. Fourier Transform InfraRed (FTIR) Analysis for CsNPs
4.3. Measurements
4.3.1. Growth and Yield Traits
4.3.2. Photosynthetic Pigment Traits
4.3.3. Carbohydrate Fraction Analysis
4.3.4. Na+ and K+ Contents
4.3.5. Proline Analysis
4.3.6. Hydrogen Peroxide Content
4.3.7. Lipid Peroxidation
4.3.8. Electrolyte Leakage; El of Plant Tissues
4.3.9. Antioxidant Activity
Non-Enzymatic Antioxidant
- Total Phenolic Content
- Ascorbic acid (AsA)
- Reduced glutathione (GSH)
Activity of Antioxidant Enzymes
- Catalase Activity (CAT, EC 1.11.1.6).
- Peroxidase Activity (POX, EC 1.11.1.7).
- Polyphenol Oxidase Activity (PPO, EC 1.10.3.1).
- Ascorbate Peroxidase Activity (APX, EC 1.11.1.11).
- Superoxide Dismutase Activity (SOD, EC 1.15.1.1).
- Glutathione Reductase Activity (GR, EC 1.8.1.7).
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In Ecophysiology and Responses of Plants under Salt Stress; Springer: New York, NY, USA, 2013; pp. 25–87. [Google Scholar]
- Bakhoum, G.S.; Sadak, M.S.; Badr, E.A.E.M. Mitigation of adverse effects of salinity stress on sunflower plant (Helianthus annuus L.) by exogenous application of chitosan. Bull. Natl. Res. Cent. 2020, 44, 79. [Google Scholar] [CrossRef]
- Akladious, S.A.; Mohamed, H.I. Ameliorative effects of calcium nitrate and humic acid on the growth, yield component and biochemical attribute of pepper (Capsicum annuum) plants grown under salt stress. Sci. Hortic. 2018, 236, 244–250. [Google Scholar] [CrossRef]
- Ullah, N.; Basit, A.; Ahmad, I.; Ullah, I.; Shah, S.T.; Mohamed, H.I.; Javed, S. Mitigation the adverse effect of salinity stress on the performance of the tomato crop by exogenous application of chitosan. Bull. Natl. Res. Cent. 2020, 44, 181. [Google Scholar] [CrossRef]
- Alkharabsheh, H.M.; Seleiman, M.F.; Hewedy, O.A.; Battaglia, M.L.; Jalal, R.S.; Alhammad, B.A.; Schillaci, C.; Ali, N.; Al-doss, A. Field crop responses and management strategies to mitigate soil salinity in modern agriculture. Agronomy 2021, 11, 2299. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Taha, R.S.; Seleiman, M.F.; Alhammad, B.A.; Alkahtani, J.; Alwahibi, M.S.; Mahdi, A.H. Activated Yeast extract enhances growth, anatomical structure, and productivity of Lupinus termis L. plants under actual salinity conditions. Agronomy 2021, 11, 74. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Alhammad, B.A.; Ahmad, A.; Seleiman, M.F.; Tola, E. Seed Priming with Nanoparticles and 24-Epibrassinolide Improved Seed Germination and Enzymatic Performance of Zea mays L. in Salt-Stressed Soil. Plants 2023, 12, 690. [Google Scholar] [CrossRef]
- Awais, A.; El-Kamil, T.; Thobayet, S.; Alshahrani, S.; Seleiman, M.F. Enhancement of morphological and physiological performance of Zea mays L. under saline stress using ZnO nanoparticles and 24-epibrassinolide seed priming. Agronomy 2023, 13, 771. [Google Scholar]
- Horie, T.; Kaneko, T.; Sugimoto, G.; Sasano, S.; Panda, S.K.; Shibasaka, M.; Katsuhara, M. Mechanisms of water transport mediated by PIP aquaporins and their regulation via phosphorylation events under salinity stress in barley roots. Plant Cell Physiol. 2011, 52, 663–675. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Ahmad, A.; Alshahrani, T.S. Integrative Effects of Zinc Nanoparticle and PGRs to Mitigate Salt Stress in Maize. Agronomy 2023, 13, 1655. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Ahmad, A.; Battaglia, M.L.; Bilal, H.M.; Alhammad, B.A.; Khan, K. Zinc oxide nanoparticles: A unique saline stress mitigator with the potential to increase future crop production. S. Afr. J. Bot. 2023, 159, 208–218. [Google Scholar] [CrossRef]
- Lin, K.-H.; Lin, F.-W.; Wu, C.-W.; Chang, Y.-S. Biostimulation of maize (Zea mays) and irrigation management improved crop growth and water use under controlled environment. Agronomy 2019, 9, 559. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Chitin- and Chitosan-Based Derivatives in Plant Protection against Biotic and Abiotic Stresses and in Recovery of Contaminated Soil and Water. Polysaccharides 2020, 1, 21–30. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Fundamentals and Applications of Chitosan. In Sustainable Agriculture Reviews 35: Chitin and Chitosan: History, Fundamentals and Innovations; Crini, G., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 49–123. [Google Scholar]
- Maliki, S.; Sharma, G.; Kumar, A.; Moral-Zamorano, M.; Moradi, O.; Baselga, J.; Stadler, F.J.; García-Peñas, A. Chitosan as a Tool for Sustainable Development. Polymers 2022, 14, 1475. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 4. [Google Scholar] [CrossRef]
- Gedda, G.; Lee, C.-Y.; Lin, Y.-C.; Wu, H.-F. Green synthesis of carbon dots from prawn shells for highly selective and sensitive detection of copper ions. Sens. Actuators B Chem. 2016, 224, 396–403. [Google Scholar] [CrossRef]
- Bandara, S.; Du, H.; Carson, L.; Bradford, D.; Kommalapati, R. Agricultural and biomedical applications of chitosan-based nanomaterials. Nanomaterials 2020, 10, 1903. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Chitosan effects on plant systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef]
- Kah, M.; Hofmann, T. Nanopesticide research: Current trends and future priorities. Environ. Int. 2014, 63, 224–235. [Google Scholar] [CrossRef]
- Somdutt, B.; Joshi, H.; Rajawat, K.S.; Choudhary, J. Controlled release action of chitosan nanoparticles to improve nutrient use efficiency. Int. J. Res. Agron. 2020, 3, 36–41. [Google Scholar]
- Malerba, M.; Cerana, R. Recent advances of chitosan applications in plants. Polymers 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Ullah, I.; Zhu, B.; Adnan, M.; Liaqat, R.; Kong, W.-B.; Niu, S. In silico analysis of epitope-based vaccine candidate against tuberculosis using reverse vaccinology. Sci. Rep. 2021, 11, 1249. [Google Scholar] [CrossRef] [PubMed]
- Al-Tawaha, A.R.; Turk, M.A.; Al-Tawaha, A.R.M.; Alu’datt, M.H.; Wedyan, M.; Al-Ramamneh, E.; Hoang, A.T. Using chitosan to improve growth of maize cultivars under salinity conditions. Bulg. J. Agric. Sci. 2018, 24, 437–442. [Google Scholar]
- Ray, S.R.; Bhuiyan, M.J.H.; Hossain, M.A.; Hasan, A.K.; Sharmin, S. Chitosan ameliorates growth and biochemical attributes in mungbean varieties under saline condition. Res. Agric. Livest. Fish. 2016, 3, 45–51. [Google Scholar] [CrossRef]
- Mahdavi, B. Seed germination and growth responses of Isabgol (Plantago ovata Forsk) to chitosan and salinity. Int. J. Agric. Crop Sci. 2013, 5, 1084. [Google Scholar]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Balusamy, S.R.; Rahimi, S.; Sukweenadhi, J.; Sunderraj, S.; Shanmugam, R.; Thangavelu, L.; Mijakovic, I.; Perumalsamy, H. Chitosan, hitosan nanoparticles and modified chitosan biomaterials, a potential tool to combat salinity stress in plants. Carbohydr. Polym. 2022, 284, 119189. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle-based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Dwivedi, P. Chitosan Based Nanoformulation for Sustainable Agriculture with Special Reference to Abiotic Stress. J. Polym. Environ. 2022, 30, 1264–1283. [Google Scholar] [CrossRef]
- Zayed, M.; Elkafafi, S.; Zedan, A.; Dawoud, S. Effect of Nano chitosan on growth, physiological and biochemical parameters of Phaseolus vulgaris under salt stress. J. Plant Prod. Mansoura Univ. 2017, 8, 577–585. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Ali, A.A.; Serry, N.K.H. Effect of nano trace elements and nano chitosan foliar application on productivity and fruits quality of Grapevine CV. ‘Superior Seedless’. J. Hortic. Sci. Ornam. Plants 2019, 11, 7–13. [Google Scholar]
- Didinger, C.; Foster, M.T.; Bunning, M.; Thompson, H.J. Nutrition and human health benefits of dry beans and other pulses. In Dry Beans and Pulses: Production, Processing, and Nutrition; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 481–504. [Google Scholar]
- Uebersax, M.A.; Cichy, K.A.; Gomez, F.E.; Porch, T.G.; Heitholt, J.; Osorno, J.M.; Kamfwa, K.; Snapp, S.S.; Bales, S. Dry beans (Phaseolus vulgaris L.) as a vital component of sustainable agriculture and food security—A review. Legume Sci. 2022, 5, e155. [Google Scholar] [CrossRef]
- Garcia, C.L.; Dattamudi, S.; Chanda, S.; Jayachandran, K. Effect of Salinity Stress and Microbial Inoculations on Glomalin Production and Plant Growth Parameters of Snap Bean (Phaseolus vulgaris). Agronomy 2019, 9, 545. [Google Scholar] [CrossRef]
- Sen, S.K.; Chouhan, D.; Das, D.; Ghosh, R.; Mandal, P. Improvisation of salinity stress response in mung bean through solid matrix priming with normal and nano-sized chitosan. Int. J. Biol. Macromol. 2020, 145, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.M.; Talaat, N.B.; Abdelhamid, M.T.; Shawky, B.T.; Desoky, E.M. Maize (Zea mays L.) grains extract mitigates the deleterious effects of salt stress on common bean (Phaseolus vulgaris L.) growth and physiology. J. Hortic. Sci. Biotechnol. 2019, 94, 777–789. [Google Scholar] [CrossRef]
- Miransari, M.; Smith, D. Sustainable wheat (Triticum aestivum L.) production in saline fields. Crit. Rev. Biotechnol. 2019, 39, 999–1014. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Ali, E.; Gaber, A.; Fetouh, M.I.; Mazrou, R. Chitosan nanoparticles effectively combat salinity stress by enhancing antioxidant activity and alkaloid biosynthesis in Catharanthus roseus (L.) G. Don. Plant Physiol. Biochem. 2021, 162, 291–300. [Google Scholar] [CrossRef]
- Heikal, Y.M.; El-esawi, M.A.; El-ballat, E.M.; Abdel-Aziz, H.M.M. Applications of nanoparticles for mitigating salinity and drought stress in plants: An overview on the physiological, biochemical and molecular genetic aspects. New Zealand J. Crop Hortic. Sci. 2023, 51, 297–327. [Google Scholar] [CrossRef]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; AlMutairi, K.A.; Siddiqui, Z.H. Role of nanomaterials in plants under challenging environments. Plant Physiol. Biochem. 2017, 110, 194–209. [Google Scholar] [CrossRef]
- De Moura, M.R.; Aouada, F.A.; Mattoso, L.H.C. Preparation of chitosan nanoparticles using methacrylic acid. J. Colloid Interface Sci. 2008, 321, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Hasaneen, M.N.A.; Abdel-Aziz, H.M.M.; El-Bialy, D.M.A.; Omer, A.M. Preparation of chitosan nanoparticles for loading with NPK fertilizer. Afr. J. Biotechnol. 2014, 13, 3158–3164. [Google Scholar]
- Gotovac, S.; Song, L.; Kanoh, H.; Kaneko, K. Assembly structure control of single wall carbon nanotubes with liquid phase naphthalene adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2007, 300, 117–121. [Google Scholar] [CrossRef]
- Costa, R.C.L.; Lobato, A.K.S.; Oliveira Neto, C.F.; Maia, P.S.P.; Alves, G.A.R.; Laughinghouse, I.V.H. Biochemical and physiological responses in two Vigna unguiculata (L.) Walp. cultivars under water stress. J. Agron. 2008, 7, 98–101. [Google Scholar] [CrossRef]
- Hassnain, M.; Alam, I.; Ahmad, A.; Basit, I.; Ullah, N.; Alam, I.; Ullah, M.A.; Khalid, B.; Shair, M.M. Efficacy of chitosan on performance of tomato (Lycopersicon esculentum L.) plant under water stress condition. Pak. J. Agric. Res. 2020, 33, 27–41. [Google Scholar] [CrossRef]
- Maggio, A.; Barbieri, G.; Raimondi, G.; De Pascale, S. Contrasting effects of GA3 treatments on tomato plants exposed to increasing salinity. J. Plant Growth Regul. 2010, 29, 63–72. [Google Scholar] [CrossRef]
- Górnik, K.; Grzesik, M.; Romanowska-Duda, B. The effect of chitosan on rooting of grapevine cuttings and on subsequent plant growth under drought and temperature stress. J. Fruit Ornam. Plant Res. 2008, 16, 333–343. [Google Scholar]
- Bittelli, M.; Flury, M.; Campbell, G.S.; Nichols, E.J. Reduction of transpiration through foliar application of chitosan. Agric. For. Meteorol. 2001, 107, 167–175. [Google Scholar] [CrossRef]
- Ramadan, M.E.; El-Saber, M.M.; Adelhamid, A.E.; El-Sayed, A.A. Effect of nano-chitosan encapsulated spermine on growth, productivity and bioactive compounds of chili pepper (Capsicum annuum L.) under salinity stress. Egypt. J. Chem. 2022, 65, 2–4. [Google Scholar] [CrossRef]
- Sheikhalipour, M.; Esmaielpour, B.; Behnamian, M.; Gohari, G.; Giglou, M.T.; Vachova, P.; Rastogi, A.; Brestic, M.; Skalicky, M. Chitosan–selenium nanoparticle (Cs–Se NP) foliar spray alleviates salt stress in bitter melon. Nanomaterials 2021, 11, 684. [Google Scholar] [CrossRef]
- Pandey, H.C.; Baig, M.J.; Bhatt, R.K. Effect of moisture stress on chlorophyll accumulation and nitrate reductase activity at vegetative and flowering stage in Avena species. Agric. Sci. Res. J. 2012, 2, 111–118. [Google Scholar]
- Abd El-Gawad, H.G.; Bondok, A.M. Response of tomato plants to salicylic acid and chitosan under infection with tomato mosaic virus. Am. Eur. J. Agric. Environ. Sci. 2015, 15, 1520–1529. [Google Scholar]
- Khoshbakht, D.; Asghari, M.R.; Haghighi, M. Influence of foliar application of polyamines on growth, gas-exchange characteristics, and chlorophyll fluorescence in Bakraii citrus under saline conditions. Photosynthetica 2018, 56, 731–742. [Google Scholar] [CrossRef]
- Hussain, N.; Sohail, Y.; Shakeel, N.; Javed, M.; Bano, H.; Gul, H.S.; Zafar, Z.U.; Frahat Zaky Hassan, I.; Ghaffar, A.; Athar, H.U.R.; et al. Role of mineral nutrients, antioxidants, osmotic adjustment and PSII stability in salt tolerance of contrasting wheat genotypes. Sci. Rep. 2022, 12, 12677. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.S.; Abdelgawad, Z.A.; El-Bassiouny, H.M.S. Alleviation of the adverse effects of salinity stress using trehalose in two rice varieties. South Afr. J. Bot. 2016, 103, 275–282. [Google Scholar] [CrossRef]
- Hassanein, R.A.; Bassuony, F.M.; Baraka, D.M.; Khalil, R.R. Physiological effects of nicotinamide and ascorbic acid on Zea mays plant grown under salinity stress. 1-Changes in growth, some relevant metabolic activities and oxidative defense systems. Res. J. Agric. Biol. Sci. 2009, 5, 72–81. [Google Scholar]
- Javadipour, Z.; Movahhedi, D.M.; Balouchi, H. Changes in the rate of proline, soluble sugars, glycinebetaine and protein content in leaves of six spring safflower (Carthamus tinctirius L.) under salinity stress. Plant Process Funct. 2012, 1, 13–24. [Google Scholar]
- Sharif, P.; Seyedsalehi, M.; Paladino, O.; Van Damme, P.; Sillanpää, M.; Sharifi, A.A. Effect of drought and salinity stresses on morphological and physiological characteristics of canola. Int. J. Environ. Sci. Technol. 2018, 15, 1859–1866. [Google Scholar] [CrossRef]
- Ma, L.; Li, Y.; Yu, C.; Wang, Y.; Li, X.; Li, N.; Chen, Q.; Bu, N. Alleviation of exogenous oligochitosan on wheat seedlings growth under salt stress. Protoplasma 2012, 249, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Hajihashemi, S.; Kazemi, S. The potential of foliar application of nano-chitosan-encapsulated nano-silicon donor in amelioration the adverse effect of salinity in the wheat plant. BMC Plant Biol. 2022, 22, 148. [Google Scholar] [CrossRef]
- Abdel-Haliem, M.E.; Hegazy, H.S.; Hassan, N.S.; Naguib, D.M. Effect of silica ions and nano silica on rice plants under salinity stress. Ecol. Eng. 2017, 99, 282–289. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhang, X.; Merewitz, E.; Peng, Y.; Ma, X.; Huang, L.; Yan, Y. Metabolic pathways regulated by chitosan contributing to drought resistance in white clover. J. Proteome Res. 2017, 16, 3039–3052. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Hosseini, M.; Naeimi, A.; Boroomand, N.; Aalifar, M.; Farajpour, M. Alleviating the adverse effects of salinity on Roselle plants by green synthesized nanoparticles. Sci. Rep. 2022, 12, 18165. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.T.; Das Bhowmik, S.; Long, H.; Cheng, Y.; Mundree, S.; Hoang, L.T.M. Rapid accumulation of proline enhances salinity tolerance in Australian wild rice Oryza australiensis Domin. Plants 2021, 10, 2044. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Egamberdieva, D. Bioremediation of adverse impact of cadmium toxicity on Cassia italica Mill by arbuscular mycorrhizal fungi. Saudi J. Biol. Sci. 2016, 23, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Saad-Allah, K.M.; Ragab, G.A. Sulfur nanoparticles mediated improvement of salt tolerance in wheat relates to decreasing oxidative stress and regulating metabolic activity. Physiol. Mol. Biol. Plants 2020, 26, 2209–2223. [Google Scholar] [CrossRef] [PubMed]
- Elkarmout, A.F.; Yang, M.; Hassan, F.A. Chitosan Treatment Effectively Alleviates the Adverse Effects of Salinity in Moringa oleifera Lam via Enhancing Antioxidant System and Nutrient Homeostasis. Agronomy 2022, 12, 2513. [Google Scholar] [CrossRef]
- Mehmood, S.; Ahmed, W.; Ikram, M.; Imtiaz, M.; Mahmood, S.; Tu, S.; Chen, D. Chitosan modified biochar increases soybean (Glycine max L.) resistance to salt-stress by augmenting root morphology, antioxidant defense mechanisms and the expression of stress-responsive genes. Plants 2020, 9, 1173. [Google Scholar] [CrossRef]
- Tawfik, M.M.; Badr, E.A.; Ibrahim, O.M.; Abd Elhamid, E.M.; Sadak, M.S. Biomass and some physiological aspects of Spartina patens grown under salt affected environment in South Sinai, inter. J. Agric. Res. 2017, 12, 17–26. [Google Scholar]
- Khalil, H.A.; Eldin, R.M.B. Chitosan improves morphological and physiological attributes of grapevines under deficit irrigation conditions. J. Hortic. Res. 2021, 29, 9–22. [Google Scholar] [CrossRef]
- Sheikhalipour, M.; Esmaielpour, B.; Gohari, G.; Haghighi, M.; Jafari, H.; Farhadi, H.; Kulak, M.; Kalisz, A. Salt Stress Mitigation via the Foliar Application of Chitosan-Functionalized Selenium and Anatase Titanium Dioxide Nanoparticles in Stevia (Stevia rebaudiana Bertoni). Molecules 2021, 26, 4090. [Google Scholar] [CrossRef] [PubMed]
- Safikhan, S.; Khoshbakht, K.; Chaichi, M.R.; Amini, A.; Motesharezadeh, B. Role of chitosan on the growth, physiological parameters and enzymatic activity of milk thistle (Silybum marianum (L.) Gaertn) in a pot experiment. J. Appl. Res. Med. Aromat. Plants 2018, 10, 49–58. [Google Scholar] [CrossRef]
- Haghighi, M.; Afifipour, Z.; Mozafarian, M. The effect of N-Si on tomato seed germination under salinity levels. J. Biol. Environ. Sci. 2012, 6, 87–90. [Google Scholar]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Faisal, M.; Al Sahli, A.A. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 2014, 33, 2429–2437. [Google Scholar] [CrossRef]
- Gohari, G.; Zareei, E.; Rostami, H.; Panahirad, S.; Kulak, M.; Farhadi, H.; Amini, M.; del Carmen Martinez-Ballesta, M.; Fotopoulos, V. Protective effects of cerium oxide nanoparticles in grapevine (Vitis vinifera L.) cv. Flame Seedless under salt stress conditions. Ecotoxicol. Environ. Saf. 2021, 220, 112402. [Google Scholar] [CrossRef] [PubMed]
- Hniličková, H.; Hnilička, F.; Orsák, M.; Hejnák, V. Effect of salt stress on growth, electrolyte leakage, Na+ and K+ content in selected plant species. Plant Soil Environ. 2019, 65, 90–96. [Google Scholar] [CrossRef]
- Xie, W.; Xu, P.; Liu, Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorganic Med. Chem. Lett. 2001, 11, 1699–1701. [Google Scholar] [CrossRef]
- Al Hassan, M.; Fuertes, M.M.; Sánchez, F.J.R.; Vicente, O.; Boscaiu, M. Effects of salt and water stress on plant growth and on accumulation of osmolytes and antioxidant compounds in cherry tomato. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 1–11. [Google Scholar] [CrossRef]
- Ismail, L.M.; Soliman, M.I.; Abd El-Aziz, M.H.; Abdel-Aziz, H.M.M. Impact of silica ions and nano silica on growth and productivity of pea plants under salinity stress. Plants 2022, 11, 494. [Google Scholar] [CrossRef]
- Hertwig, B.; Streb, P.; Feierabend, J. Light dependence of catalase synthesis and degradation in leaves and the influence of interfering stress conditions. Plant Physiol. 1992, 100, 1547–1553. [Google Scholar] [CrossRef]
- Nazar, R.; Iqbal, N.; Syeed, S.; Khan, N.A. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J. Plant Physiol. 2011, 168, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Rausch, T.; Gromes, R.; Liedschulte, V.; Müller, I.; Bogs, J.; Galovic, V.; Wachter, A. Novel insight into the regulation of GSH biosynthesis in higher plants. Plant Biol. 2007, 9, 565–572. [Google Scholar] [CrossRef]
- Chawla, S.; Jain, S.; Jain, V. Salinity induced oxidative stress and antioxidant system in salt-tolerant and salt-sensitive cultivars of rice (Oryza sativa L.). J. Plant Biochem. Biotechnol. 2013, 22, 27–34. [Google Scholar] [CrossRef]
- Wang, H.M.; Xiao, X.R.; Yang, M.Y.; Gao, Z.L.; Zang, J.; Fu, X.M.; Chen, Y.H. Effects of salt stress on antioxidant defense system in the root of Kandelia candel. Bot. Stud. 2014, 55, 57. [Google Scholar] [CrossRef] [PubMed]
- Kaymakanova, M.; Stoeva, N. Physiological reaction of bean plants (Phaseolus vulgaris. L.) to salt stress. Gen. Appl. Plant Physiol. 2008, 34, 177–188. [Google Scholar]
- ALKahtani, M.D.; Attia, K.A.; Hafez, Y.M.; Khan, N.; Eid, A.M.; Ali, M.A.; Abdelaal, K.A. Chlorophyll fluorescence parameters and antioxidant defense system can display salt tolerance of salt acclimated sweet pepper plants treated with chitosan and plant growth promoting rhizobacteria. Agronomy 2020, 10, 1180. [Google Scholar] [CrossRef]
- Raliya, R.; Nair, R.; Chavalmane, S.; Wang, W.N.; Biswas, P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 2015, 7, 1584–1594. [Google Scholar] [CrossRef]
- Morales-Espinoza, M.C.; Cadenas-Pliego, G.; Pérez-Alvarez, M.; Hernández-Fuentes, A.D.; Cabrera de la Fuente, M.; Benavides-Mendoza, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Se nanoparticles induce changes in the growth, antioxidant responses, and fruit quality of tomato developed under NaCl stress. Molecules 2019, 24, 3030. [Google Scholar] [CrossRef]
- Corradini, E.; DeMoura, M.R.; Mattoso, L.H.C. A preliminary study of the incorparation of NPK fertilizer into chitosan nanoparticles. Express Polym. Lett. 2010, 4, 509–515. [Google Scholar] [CrossRef]
- Beadle, C.L. Growth analysis. In Photosynthesis and Production in a Changing Environment; Hall, D.C., Scurlock, J.M.O., Bolhar-Nordenkampf, H.R., Leegod, R.C., Long, S.P., Eds.; A field and laboratory manual; Champman and Hall: London, UK, 1993; pp. 36–46. [Google Scholar]
- Ray, S.; Choudhuri, M.A. Regulation of flage leaf senescence in rice by nutrients and its impacts on yield. RISO 1980, 29, 9–14. [Google Scholar]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Hedge, J.E.; Hofreiter, B.T. Methods of estimating starch and carbohydrates. Carbohydr. Chem. 1962, 17, 163–201. [Google Scholar]
- Yemm, E.W.; Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508. [Google Scholar] [CrossRef] [PubMed]
- Motsara, M.R.; Roy, R.N. Guide to Laboratory Establishment for Plant Nutrient Analysis; Food and Agriculture Organization of the United Nations: Rome, Italy, 2008; pp. 80–90. [Google Scholar]
- Khalil, R.R.; Moustafa, A.N.; Bassuony, F.M.; Haroun, S.A. Kinetin and/or calcium affect growth of Phaseolus vulgaris L. plant grown under heavy metals stress. J. Environ. Sci. 2017, 46, 103–120. [Google Scholar]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Bao, Z.; Zhu, Z.; Ying, Q.; Qian, Q. Effects of different treatments of salicylic acid on heat tolerance, chlorophyll fluorescence, and antioxidant enzyme activity in seedlings of Cucumis sativa L. Plant Growth Regul. 2006, 48, 127–135. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Omaye, S.T.; Turnbull, J.D.; Sauberlich, H.E. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1979; pp. 3–11. [Google Scholar]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Agarwal, S.; Shaheen, R. Stimulation of antioxidant system and lipid peroxidation by abiotic stresses in leaves of Momordica charantia. Braz. J. Plant Physiol. 2007, 19, 149–161. [Google Scholar] [CrossRef]
- Gomes-Junior, R.A.; Gratão, P.L.; Gaziola, S.A.; Mazzafera, P.; Lea, P.J.; Azevedo, R.A. Selenium-induced oxidative stress in coffee cell suspension cultures. Funct. Plant Biol. 2007, 34, 449–456. [Google Scholar] [CrossRef]
- Devi, P. Principles and Methods in Plant Molecular Biology, Biochemistry and Genetics, 1st ed.; Agrobios: Jodhpur, India, 2002. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Nishikimi, M.; Roa, N.A.; Yogi, K. Measurement of superoxide dismutase. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Spooner, R.G. Glutathione reductase. Methods Ezymatic Anal. 1983, 3, 258–265. [Google Scholar]

| Treatments | Plant Height (cm) | Shoot Length (cm) | Pod Length (cm) | Plant Water Content (%) | Number of Pods/Plant | Pod Weight (g/pod) | Number of Seeds/Pod | Seeds Weight (g/pod) | |
|---|---|---|---|---|---|---|---|---|---|
| Control | No Cs | 77.20 d ± 3.20 | 69.80 e ± 2.25 | 11.00 c ± 0.23 | 80.27 f ± 1.22 | 5.1 a ± 0.13 | 3.26 c ± 0.23 | 3.2 a ± 0.11 | 0.29 f ± 0.03 |
| Cs | 85.00 c ± 2.10 | 79.00 c ± 1.23 | 11.60 b ± 0.33 | 78.58 l ± 1.02 | 6.2 a ± 0.12 | 3.52 b ± 0.12 | 4.1 a ± 0.21 | 0.41 b ± 0.04 | |
| CsNPs | 96.00 a ± 3.23 | 88.50 a ± 1.20 | 12.00 a ± 0.43 | 79.88 g ± 1.33 | 6.2 a ± 0.22 | 3.59 a ± 0.16 | 5.0 a ± 0.13 | 0.48 a ± 0.05 | |
| S1 | No Cs | 71.60 e ± 2.25 | 63.50 fg ± 2.20 | 10.25 d ± 0.53 | 79.23 i ± 2.22 | 5.4 a ± 0.13 | 3.18 d ± 0.23 | 3.2 a ± 0.33 | 0.26 g ± 0.06 |
| Cs | 78.00 d ± 3.44 | 66.40 f ± 4.20 | 10.80 c ± 0.27 | 79.25 i ± 0.92 | 5.3 a ± 0.24 | 3.46 b ± 0.16 | 4.1 a ± 0.23 | 0.35 d ± 0.05 | |
| CsNPs | 91.00 b ± 3.23 | 85.25 b ± 3.26 | 11.50 b ± 0.43 | 78.63 k ± 0.98 | 6.1 a ± 0.33 | 3.51 b ± 0.27 | 4.1 a ± 0.25 | 0.46 a ± 0.04 | |
| S2 | No Cs | 64.10 g ± 2.12 | 56.00 h ± 1.60 | 9.75 e ± 0.63 | 79.46 h ± 1.52 | 5.2 a ± 0.13 | 2.85 f ± 0.13 | 3.1 a ± 0.26 | 0.21 h ± 0.03 |
| Cs | 72.50 e ± 1.45 | 63.00 g ± 3.77 | 10.30 d ± 0.33 | 78.40 m ± 1.44 | 5.2 a ± 0.23 | 3.06 e ± 0.23 | 4.1 a ± 0.21 | 0.27 fg ± 0.04 | |
| CsNPs | 89.00 b ± 2.66 | 81.25 c ± 3.28 | 10.90 c ± 0.73 | 79.07 j ± 1.32 | 6.1 a ± 0.25 | 3.13 d ± 0.43 | 4.1 a ± 0.13 | 0.38 c ± 0.05 | |
| S3 | No Cs | 57.50 h ± 5.10 | 49.25 i ± 4.20 | 8.25 h ± 0.23 | 82.79 b ± 0.92 | 3.0 a ± 0.13 | 2.42 h ± 0.23 | 2.0 a ± 0.14 | 0.16 i ± 0.03 |
| Cs | 67.25 f ± 4.20 | 61.25 g ± 2.25 | 9.00 f ± 0.53 | 82.13 c ± 0.82 | 3.0 a ± 0.12 | 2.66 g ± 0.13 | 3.2 a ± 0.13 | 0.19 h ± 0.02 | |
| CsNPs | 79.00 d ± 1.20 | 74.75 d ± 1.70 | 9.50 e ± 0.65 | 81.37 e ± 1.20 | 4.1 a ± 0.24 | 2.89 f ± 0.25 | 4.0 a ± 0.12 | 0.32 e ± 0.01 | |
| S4 | No Cs | 52.40 i ± 3.26 | 46.20 j ± 3.67 | 6.75 i ± 0.67 | 81.60 d ± 1.18 | 3.0 a ± 0.25 | 2.11 k ± 0.33 | 2.2 a ± 0.15 | 0.13 j ± 0.02 |
| Cs | 62.00 g ± 2.66 | 56.50 h ± 2.20 | 8.10 h ± 0.43 | 81.63 d ± 1.09 | 3.1 a ± 0.33 | 2.20 j ± 0.13 | 3.2 a ± 0.16 | 0.14 ij ± 0.03 | |
| CsNPs | 76.80 d ± 1.70 | 70.25 e ± 1.29 | 8.70 g ± 0.53 | 83.37 a ± 1.11 | 4.1 a ± 0.43 | 2.31 i ± 0.11 | 3.2 a ± 0.25 | 0.25 g ± 0.04 | |
| Treatments | Crop Yield (g/Plant) | Seed Yield (g/Plant) | Straw Yield (g/plant) | Harvest Index | Crop Index | Mobilization Index | |
|---|---|---|---|---|---|---|---|
| Control | No Cs | 5.44 e ± 0.23 | 3.53 cd ± 0.13 | 2.52 d ± 0.26 | 1.40 f ± 0.13 | 0.58 e ± 0.03 | 2.16 e ± 0.13 |
| Cs | 6.27 c ± 0.13 | 4.02 b ± 0.21 | 2.81 cd ± 0.33 | 1.43 e ± 0.11 | 0.59 d ± 0.02 | 2.23 b ± 0.12 | |
| CsNPs | 7.54 a ± 0.23 | 5.34 a ± 0.14 | 3.48 a ± 0.13 | 1.53 c ± 0.10 | 0.61 b ± 0.05 | 2.17 d ± 0.15 | |
| S1 | No Cs | 4.75 g ± 0.25 | 3.22 de ± 0.12 | 2.21 e ± 0.21 | 1.46 d ± 0.20 | 0.59 d ± 0.06 | 2.15 f ± 0.17 |
| Cs | 5.76 d ± 0.13 | 3.61 c ± 0.14 | 2.73 cd ± 0.25 | 1.32 g ± 0.13 | 0.57 f ± 0.07 | 2.11 h ± 0.23 | |
| CsNPs | 7.22 b ± 0.27 | 5.10 a ± 0.21 | 3.27 ab ± 0.28 | 1.56 b ± 0.11 | 0.61 b ± 0.02 | 2.21 c ± 0.22 | |
| S2 | No Cs | 4.21 h ± 0.13 | 2.99 ef ± 0.14 | 1.96 ef ± 0.13 | 1.53 c ± 0.12 | 0.60 c ± 0.03 | 2.15 f ± 0.16 |
| Cs | 5.08 f ± 0.25 | 3.25 de ± 0.21 | 2.55 d ± 0.22 | 1.27 h ± 0.14 | 0.56 g ± 0.04 | 1.99 l ± 0.33 | |
| CsNPs | 6.53 c ± 0.13 | 4.21 b ± 0.15 | 3.02 bc ± 0.13 | 1.39 f ± 0.11 | 0.58 e ± 0.03 | 2.16 e ± 0.23 | |
| S3 | No Cs | 3.33 jk ± 0.33 | 2.48 h ± 0.26 | 1.57 g ± 0.21 | 1.58 b ± 0.11 | 0.61 b ± 0.05 | 2.12 g ± 0.33 |
| Cs | 3.65 i ± 0.13 | 2.85 fg ± 0.17 | 1.82 fg ± 0.23 | 1.57 b ± 0.13 | 0.61 b ± 0.02 | 2.01 k ± 0.13 | |
| CsNPs | 4.11 h ± 0.23 | 3.05 ef ± 0.28 | 2.16 e ± 0.16 | 1.41 ef ± 0.11 | 0.59 d ± 0.05 | 1.90 m ± 0.23 | |
| S4 | No Cs | 3.05 k ± 0.24 | 2.07 i ± 0.33 | 1.23 h ± 0.27 | 1.68 a ± 0.10 | 0.63 a ± 0.05 | 2.48 a ± 0.23 |
| Cs | 3.24 k ± 0.13 | 2.39 h ± 0.13 | 1.58 g ± 0.21 | 1.51 c ± 0.14 | 0.60 c ± 0.03 | 2.05 i ± 0.33 | |
| CsNPs | 3.57 ij ± 0.22 | 2.58 gh ± 0.25 | 1.75 fg ± 0.13 | 1.47 d ± 0.15 | 0.60 c ± 0.02 | 2.04 j ± 0.33 | |
| Variable and Source of Variation | df | F | p | Variable and Source of Variation | df | F | p |
|---|---|---|---|---|---|---|---|
| Plant height | Crop yield | ||||||
| Chitosan | 2 | 3842.48 | *** | Chitosan | 2 | 1174.09 | *** |
| Salinity | 4 | 1553.80 | *** | Salinity | 4 | 1942.04 | *** |
| Chitosan × Salinity | 8 | 15.64 | *** | Chitosan × Salinity | 8 | 73.49 | *** |
| Shoot length | Seed yield | ||||||
| Chitosan | 2 | 2356.03 | *** | Chitosan | 2 | 761.92 | *** |
| Salinity | 4 | 733.40 | *** | Salinity | 4 | 794.15 | *** |
| Chitosan × Salinity | 8 | 20.30 | *** | Chitosan × Salinity | 8 | 54.52 | *** |
| Pod length | Straw yield | ||||||
| Chitosan | 2 | 102.96 | *** | Chitosan | 2 | 302.20 | *** |
| Salinity | 4 | 309.90 | *** | Salinity | 4 | 374.26 | *** |
| Chitosan × Salinity | 8 | 2.02 | ns | Chitosan × Salinity | 8 | 7.32 | *** |
| Plant water content | Harvest index | ||||||
| Chitosan | 2 | 4444.85 | *** | Chitosan | 2 | 10.81 | *** |
| Salinity | 4 | 60,066.80 | *** | Salinity | 4 | 8.34 | *** |
| Chitosan × Salinity | 8 | 4000.85 | *** | Chitosan × Salinity | 8 | 7.59 | *** |
| Number of pods/plant | Crop index | ||||||
| Chitosan | 2 | 4.20 | * | Chitosan | 2 | 10.40 | *** |
| Salinity | 4 | 12.20 | *** | Salinity | 4 | 12.30 | *** |
| Chitosan × Salinity | 8 | 0.20 | ns | Chitosan × Salinity | 8 | 8.40 | *** |
| Pod weight | Mobilization index | ||||||
| Chitosan | 2 | 44.92 | *** | Chitosan | 2 | 154.66 | *** |
| Salinity | 4 | 271.85 | *** | Salinity | 4 | 98.94 | *** |
| Chitosan × Salinity | 8 | 1.07 | ns | Chitosan × Salinity | 8 | 82.66 | *** |
| Number of seeds/pod | |||||||
| Chitosan | 2 | 7.80 | ** | ||||
| Salinity | 4 | 2.70 | * | ||||
| Chitosan × Salinity | 8 | 0.30 | ns | ||||
| Seeds weight | |||||||
| Chitosan | 2 | 341.22 | *** | ||||
| Salinity | 4 | 221.19 | *** | ||||
| Chitosan × Salinity | 8 | 5.38 | *** | ||||
| Treatments | Chlorophyll a | Chlorophyll b | Carotenoids | Total Pigments | TSS | Starch | Total Carbohydrates | |
|---|---|---|---|---|---|---|---|---|
| (mg/g FW) | (mg Glucose Equivalent/g DW) | |||||||
| Control | No Cs | 1.92 g ± 0.13 | 0.60 bc ± 0.03 | 1.03 abc ± 0.13 | 3.55 cde ± 0.23 | 56.12 j ± 3.23 | 355.30 c ± 13.23 | 411.42 cd ± 43.33 |
| Cs | 2.28 b ± 0.17 | 0.62 b ± 0.02 | 1.04 ab ± 0.14 | 3.94 b ± 0.25 | 58.82 i ± 4.25 | 401.29 b ± 15.26 | 460.11 b ± 44.14 | |
| CsNPs | 2.49 a ± 0.12 | 0.67 a ± 0.03 | 1.05 a ± 0.17 | 4.21 a ± 0.27 | 62.80 h ± 3.45 | 441.08 a ± 26.33 | 503.88 a ± 35.34 | |
| S1 | No Cs | 1.88 h ± 0.13 | 0.55 ef ± 0.04 | 1.01 c ± 0.16 | 3.44 de ± 0.28 | 69.50 g ± 3.44 | 316.00 e ± 23.27 | 385.50 e ± 43.33 |
| Cs | 2.19 d ± 0.12 | 0.58 cd ± 0.05 | 1.02 bc ± 0.17 | 3.79 bc ± 0.33 | 73.93 f ± 4.26 | 359.16 c ± 27.29 | 433.09 c ± 56.25 | |
| CsNPs | 2.25 bc ± 0.13 | 0.59 c ± 0.06 | 1.04 ab ± 0.13 | 3.88 b ± 0.36 | 76.58 e ± 4.26 | 403.01 b ± 33.43 | 479.59 b ± 33.23 | |
| S2 | No Cs | 1.83 i ± 0.15 | 0.53 fg ± 0.03 | 0.97 d ± 0.14 | 3.36 ef ± 0.24 | 78.89 d ± 3.24 | 273.27 f ± 43.23 | 352.16 fg ± 13.23 |
| Cs | 2.14 e ± 0.16 | 0.56 de ± 0.04 | 1.01 c ± 0.15 | 3.71 bcd ± 0.43 | 80.72 d ± 3.33 | 310.26 e ± 33.25 | 390.98 de ± 33.43 | |
| CsNPs | 2.23 c ± 0.17 | 0.58 cd ± 0.06 | 1.02 bc ± 0.12 | 3.86 bc ± 0.26 | 85.47 c ± 3.43 | 334.61 d ± 43.20 | 420.08 c ± 53.23 | |
| S3 | No Cs | 1.78 j ± 0.13 | 0.47 h ± 0.04 | 0.87 f ± 0.13 | 3.12 fg ± 0.27 | 85.97 c ± 3.53 | 222.27 h ± 45.21 | 308.24 h ± 53.23 |
| Cs | 2.06 f ± 0.15 | 0.51 g ± 0.03 | 0.92 e ± 0.15 | 3.49 de ± 0.43 | 87.34 bc ± 3.33 | 268.05 f ± 34.43 | 355.39 f ± 53.26 | |
| CsNPs | 2.16 de ± 0.12 | 0.54 ef ± 0.02 | 0.95 d ± 0.13 | 3.68 bcd ± 0.33 | 89.39 ab ± 4.23 | 272.10 f ± 45.23 | 361.49 f ± 63.23 | |
| S4 | No Cs | 1.67 l ± 0.11 | 0.35 j ± 0.06 | 0.83 g ± 0.17 | 2.88 g ± 0.43 | 89.07 ab ± 5.27 | 176.95 i ± 55.55 | 266.02 i ± 65.23 |
| Cs | 1.73 k ± 0.12 | 0.38 i ± 0.05 | 0.87 f ± 0.18 | 3.01 g ± 0.23 | 90.53 a ± 6.39 | 220.52 h ± 44.27 | 311.05 h ± 67.23 | |
| CsNPs | 1.82 i ± 0.14 | 0.40 i ± 0.08 | 0.91 e ± 0.12 | 3.16 fg ± 0.13 | 91.12 a ± 5.25 | 239.50 g ± 56.23 | 330.62 gh ± 77.23 | |
| Variable and Source of Variation | df | F | p | Variable and Source of Variation | df | F | p |
|---|---|---|---|---|---|---|---|
| Chlorophyll a | TSS | ||||||
| Chitosan | 2 | 1629.94 | *** | Chitosan | 2 | 436.89 | *** |
| Salinity | 4 | 894.94 | *** | Salinity | 4 | 6140.95 | *** |
| Chitosan × Salinity | 8 | 58.10 | *** | Chitosan × Salinity | 8 | 23.99 | *** |
| Chlorophyll b | Starch | ||||||
| Chitosan | 2 | 117.80 | *** | Chitosan | 2 | 19,333.83 | *** |
| Salinity | 4 | 824.30 | *** | Salinity | 4 | 54,486.97 | *** |
| Chitosan × Salinity | 8 | 2.30 | * | Chitosan × Salinity | 8 | 285.36 | *** |
| Carotenoids | Total Carbohydrates | ||||||
| Chitosan | 2 | 102.20 | *** | Chitosan | 2 | 21,131.50 | *** |
| Salinity | 4 | 491.70 | *** | Salinity | 4 | 36,793.48 | *** |
| Chitosan × Salinity | 8 | 6.45 | *** | Chitosan × Salinity | 8 | 312.38 | *** |
| Total pigments | |||||||
| Chitosan | 2 | 9204.20 | *** | ||||
| Salinity | 4 | 10,190.80 | *** | ||||
| Chitosan × Salinity | 8 | 179.20 | *** | ||||
| Treatments | Total Phenols (mg/g DW) | Ascorbic Acid (mg/g FW) | GSH (mmol/g FW) | CAT (mmol H2O2/g FW/min) | POX | PPO | APX (mmol AsA/g FW/min) | SOD | GR | |
|---|---|---|---|---|---|---|---|---|---|---|
| (U/g FW/min) | (U/g FW/min) | |||||||||
| Control | No Cs | 6.95 j ± 0.13 | 2.41 h ± 0.11 | 1.07 l ± 0.15 | 3.18 f ± 0.18 | 49.60 h ± 0.83 | 20.10 k ± 0.55 | 9.79 i ± 0.33 | 32.31 l ± 0.83 | 4.99 o ± 0.13 |
| Cs | 7.19 ij ± 0.17 | 2.82 fg ± 0.13 | 1.66 k ± 0.13 | 3.30 ef ± 0.13 | 52.00 gh ± 0.99 | 24.85 j ± 0.34 | 10.07 hi ± 0.23 | 37.79 i ± 0.76 | 5.65 n ± 0.14 | |
| CsNPs | 8.88 fg ± 0.16 | 3.23 e ± 0.17 | 1.74 j ± 0.12 | 3.45 e ± 0.12 | 53.30 fg ± 1.13 | 25.10 j ± 0.45 | 10.39 h ± 0.32 | 38.63 h ± 0.45 | 6.63 m ± 0.03 | |
| S1 | No Cs | 7.28 ij ± 0.14 | 2.55 gh ± 0.12 | 1.89 i ± 0.11 | 2.96 g ± 0.18 | 50.60 h ± 0.78 | 25.35 j ± 0.43 | 12.39 g ± 0.54 | 34.94 k ± 0.46 | 7.23 l ± 0.21 |
| Cs | 8.57 g ± 0.23 | 3.04 ef ± 0.13 | 1.93 i ± 0.13 | 4.71 d ± 0.12 | 55.70 ef ± 0.63 | 35.95 g ± 0.23 | 13.25 f ± 0.23 | 38.90 gh ± 0.63 | 9.96 k ± 0.04 | |
| CsNPs | 9.16 f ± 0.24 | 3.57 cd ± 0.15 | 2.05 h ± 0.12 | 4.96 c ± 0.10 | 59.60 cd ± 0.93 | 39.22 f ± 0.45 | 16.39 d ± 0.44 | 39.21 fg ± 0.34 | 11.12 j ± 0.15 | |
| S2 | No Cs | 7.59 i ± 0.17 | 2.73 g ± 0.16 | 2.07 h ± 0.10 | 2.57 h ± 0.23 | 53.70 fg ± 0.63 | 28.75 i ± 0.23 | 13.39 f ± 0.15 | 37.08 j ± 0.66 | 13.36 i ± 0.23 |
| Cs | 9.31 f ± 0.18 | 3.32 de ± 0.23 | 2.26 g ± 0.15 | 4.71 d ± 0.11 | 58.30 d ± 0.55 | 41.70 e ± 0.65 | 14.04 e ± 0.25 | 40.06 e ± 0.76 | 17.21 h ± 0.17 | |
| CsNPs | 10.75 d ± 0.12 | 3.71 c ± 0.21 | 2.65 f ± 0.12 | 5.06 c ± 0.13 | 61.30 c ± 1.13 | 45.10 cd ± 0.53 | 18.93 c ± 0.26 | 43.19 d ± 0.33 | 20.81 f ± 0.12 | |
| S3 | No Cs | 8.14 h ± 0.13 | 3.24 e ± 0.13 | 2.67 f ± 0.13 | 1.97 i ± 0.12 | 57.40 de ± 0.73 | 31.25 h ± 0.52 | 16.07 d ± 0.35 | 39.35 f ± 0.22 | 20.43 g ± 0.19 |
| Cs | 10.03 e ± 0.22 | 3.73 c ± 0.11 | 3.74 d ± 0.12 | 5.79 b ± 0.10 | 61.30 c ± 0.93 | 43.85 d ± 0.46 | 16.32 d ± 0.37 | 43.19 d ± 0.43 | 27.01 d ± 0.20 | |
| CsNPs | 13.04 c ± 0.23 | 4.21 b ± 0.15 | 3.96 c ± 0.10 | 5.86 b ± 0.14 | 71.80 a ± 0.88 | 48.20 b ± 0.54 | 19.13 c ± 0.43 | 46.21 c ± 0.76 | 30.21 c ± 0.23 | |
| S4 | No Cs | 9.16 f ± 0.15 | 3.77 c ± 0.16 | 3.52 e ± 0.22 | 1.54 j ± 0.21 | 58.00 de ± 0.99 | 35.40 g ± 0.35 | 19.00 c ± 0.23 | 40.37 e ± 0.27 | 26.53 e ± 0.25 |
| Cs | 13.91 b ± 0.13 | 4.12 b ± 0.17 | 4.45 b ± 0.14 | 5.89 b ± 0.12 | 67.70 b ± 1.33 | 46.10 c ± 0.36 | 19.55 b ± 0.31 | 47.92 b ± 0.65 | 35.48 b ± 0.17 | |
| CsNPs | 17.73 a ± 0.11 | 4.73 a ± 0.18 | 4.67 a ± 0.25 | 6.13 a ± 0.11 | 73.50 a ± 0.73 | 50.25 a ± 0.67 | 20.17 a ± 0.53 | 50.63 a ± 0.66 | 40.73 a ± 0.34 | |
| Variable and Source of Variation | df | F | p | Variable and Source of Variation | df | F | p |
|---|---|---|---|---|---|---|---|
| Total Phenols | PPO | ||||||
| Chitosan | 2 | 7626.62 | *** | Chitosan | 2 | 1814.62 | *** |
| Salinity | 4 | 5966.54 | *** | Salinity | 4 | 1442.85 | *** |
| Chitosan × Salinity | 8 | 746.09 | *** | Chitosan × Salinity | 8 | 45.55 | *** |
| Ascorbic Acid | APX | ||||||
| Chitosan | 2 | 361.62 | *** | Chitosan | 2 | 4254.31 | *** |
| Salinity | 4 | 296.83 | *** | Salinity | 4 | 13,778.33 | *** |
| Chitosan × Salinity | 8 | 1.18 | ns | Chitosan × Salinity | 8 | 470.63 | *** |
| GSH | SOD | ||||||
| Chitosan | 2 | 386.94 | *** | Chitosan | 2 | 19,347.31 | *** |
| Salinity | 4 | 1832.91 | *** | Salinity | 4 | 15,780.54 | *** |
| Chitosan × Salinity | 8 | 34.41 | *** | Chitosan × Salinity | 8 | 521.59 | *** |
| CAT | GR | ||||||
| Chitosan | 2 | 54,128.93 | *** | Chitosan | 2 | 2744.57 | *** |
| Salinity | 4 | 3745.88 | *** | Salinity | 4 | 16,250.13 | *** |
| Chitosan × Salinity | 8 | 4783.31 | *** | Chitosan × Salinity | 8 | 251.02 | *** |
| POX | |||||||
| Chitosan | 2 | 1219.61 | *** | ||||
| Salinity | 4 | 1048.48 | *** | ||||
| Chitosan × Salinity | 8 | 70.94 | *** | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alenazi, M.M.; El-Ebidy, A.M.; El-shehaby, O.A.; Seleiman, M.F.; Aldhuwaib, K.J.; Abdel-Aziz, H.M.M. Chitosan and Chitosan Nanoparticles Differentially Alleviate Salinity Stress in Phaseolus vulgaris L. Plants. Plants 2024, 13, 398. https://doi.org/10.3390/plants13030398
Alenazi MM, El-Ebidy AM, El-shehaby OA, Seleiman MF, Aldhuwaib KJ, Abdel-Aziz HMM. Chitosan and Chitosan Nanoparticles Differentially Alleviate Salinity Stress in Phaseolus vulgaris L. Plants. Plants. 2024; 13(3):398. https://doi.org/10.3390/plants13030398
Chicago/Turabian StyleAlenazi, Mekhled M., Aya M. El-Ebidy, Omar A. El-shehaby, Mahmoud F. Seleiman, Khalid J. Aldhuwaib, and Heba M. M. Abdel-Aziz. 2024. "Chitosan and Chitosan Nanoparticles Differentially Alleviate Salinity Stress in Phaseolus vulgaris L. Plants" Plants 13, no. 3: 398. https://doi.org/10.3390/plants13030398
APA StyleAlenazi, M. M., El-Ebidy, A. M., El-shehaby, O. A., Seleiman, M. F., Aldhuwaib, K. J., & Abdel-Aziz, H. M. M. (2024). Chitosan and Chitosan Nanoparticles Differentially Alleviate Salinity Stress in Phaseolus vulgaris L. Plants. Plants, 13(3), 398. https://doi.org/10.3390/plants13030398







