Unraveling the Mechanism of Cork Spot-like Physiological Disorders in ‘Kurenainoyume’ Apples Based on Occurrence Location
Abstract
1. Introduction
2. Results
2.1. Quality of Harvested Fruit and Time-Dependent Fruit Size Change
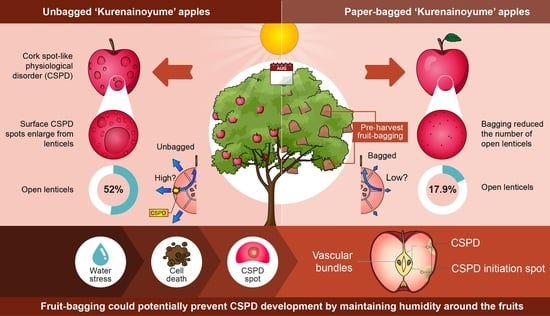
2.2. CSPD Development and Its Relation to Fruit Size
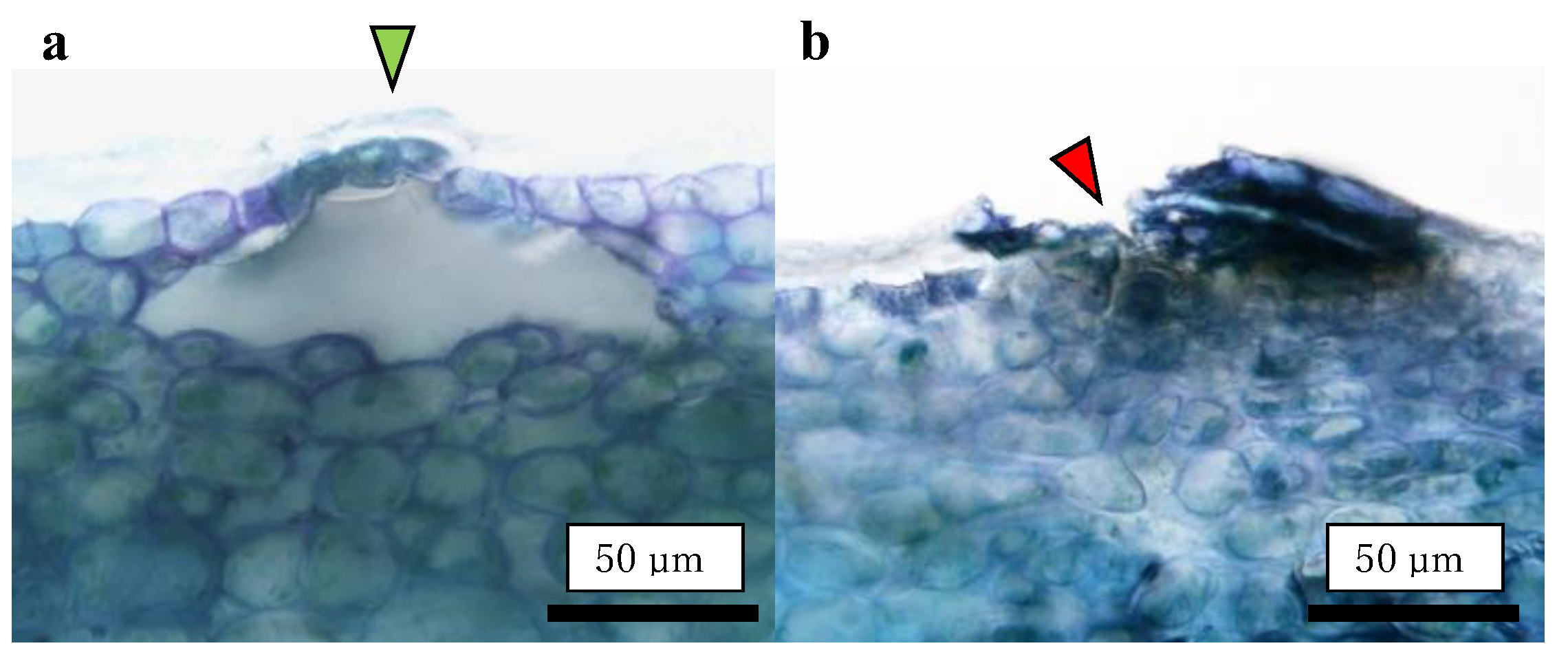
2.3. Effect of Fruit-Bagging Treatment on the Number, Size, and State of Lenticels
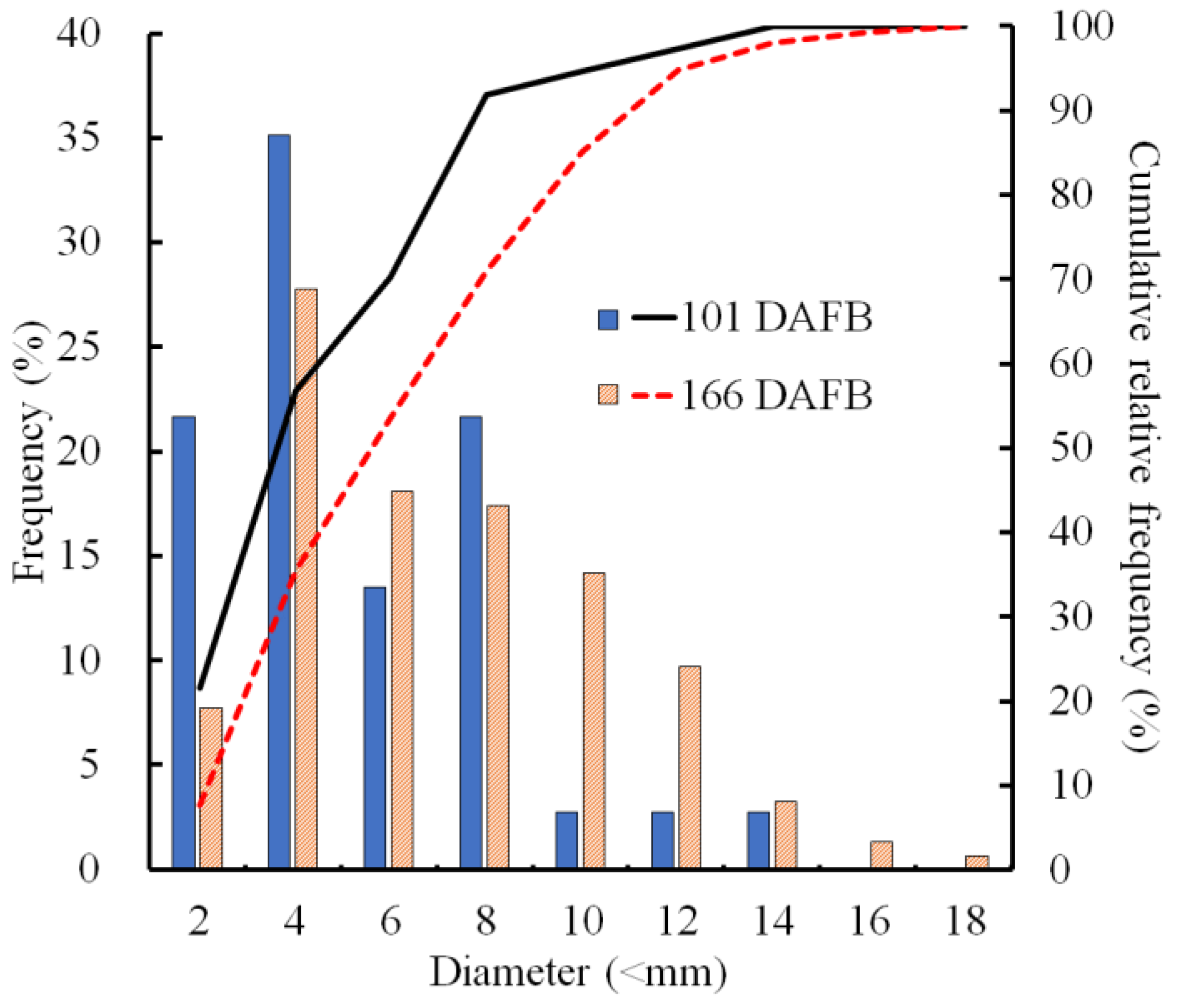
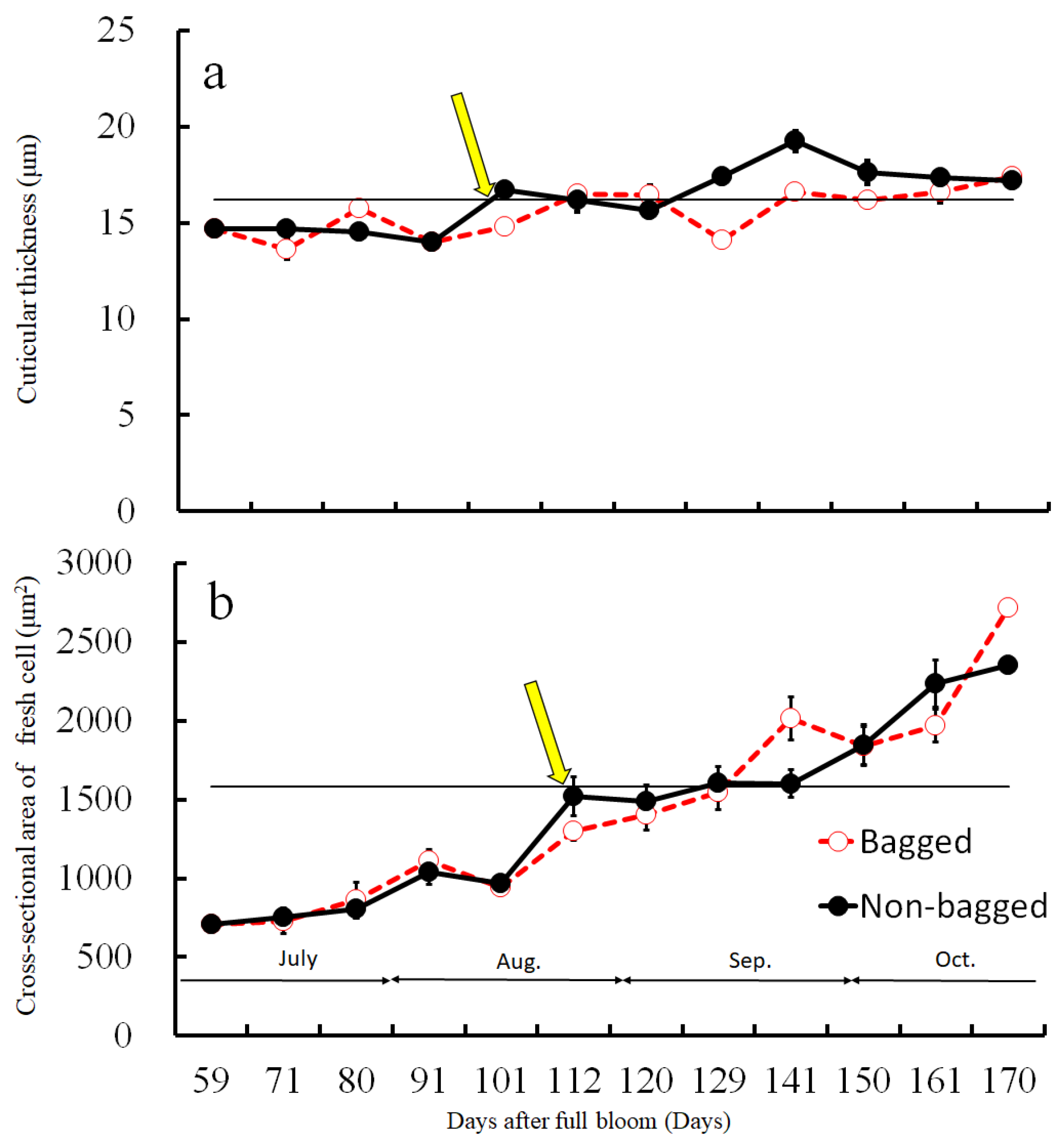
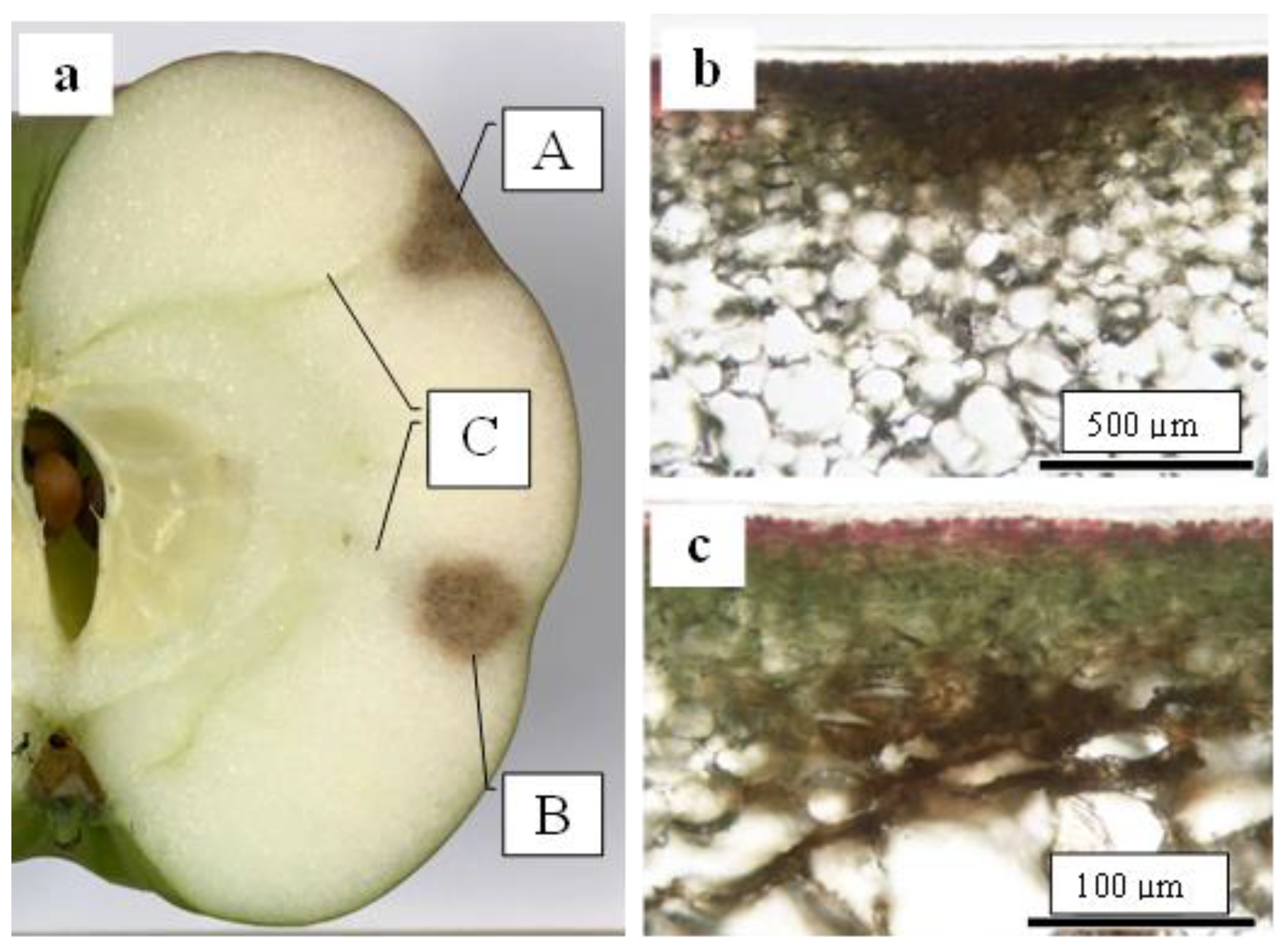
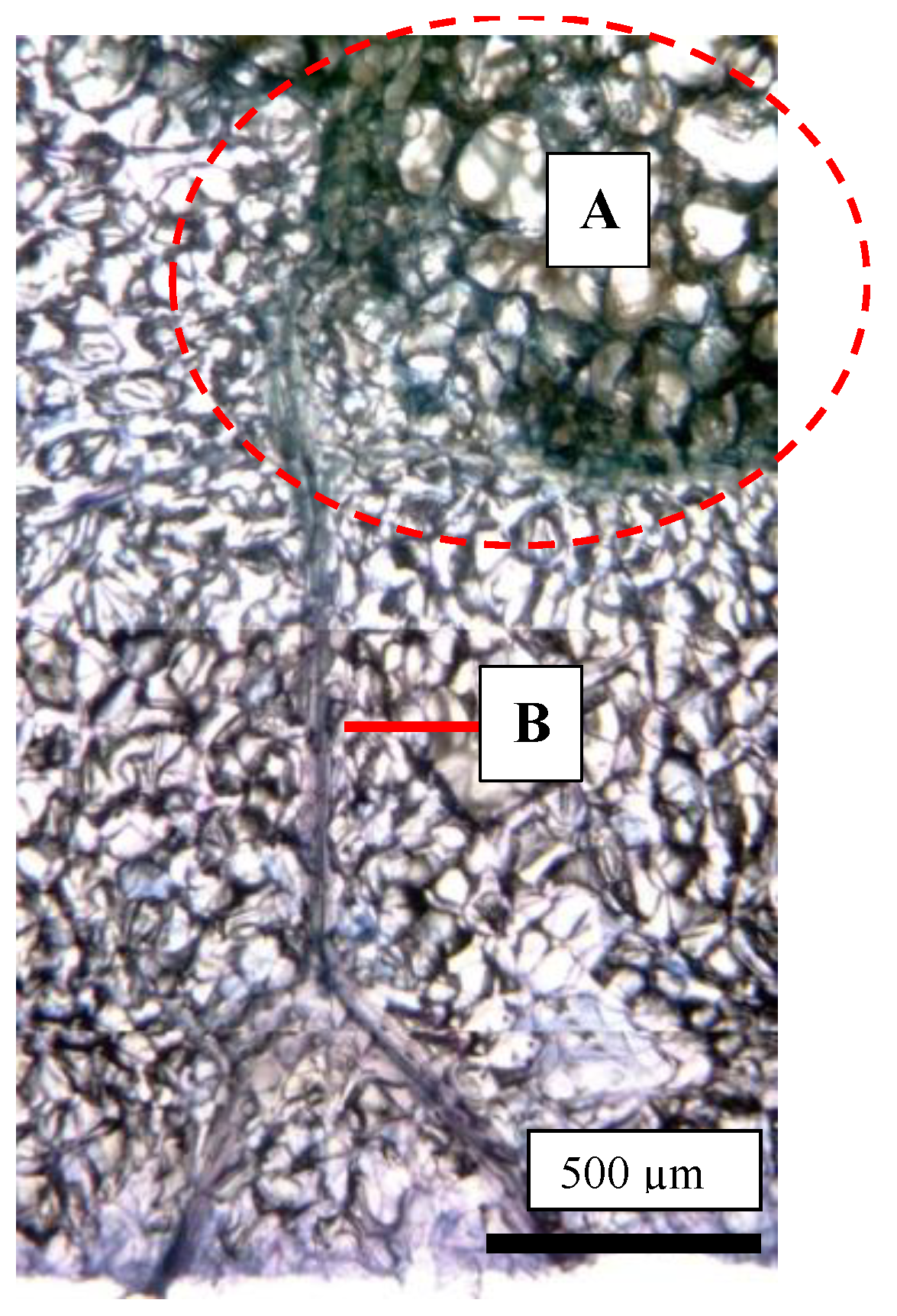
2.4. Size Distribution of CSPD and the Dimensions of Cuticular and Cellular Components in the Affected Area
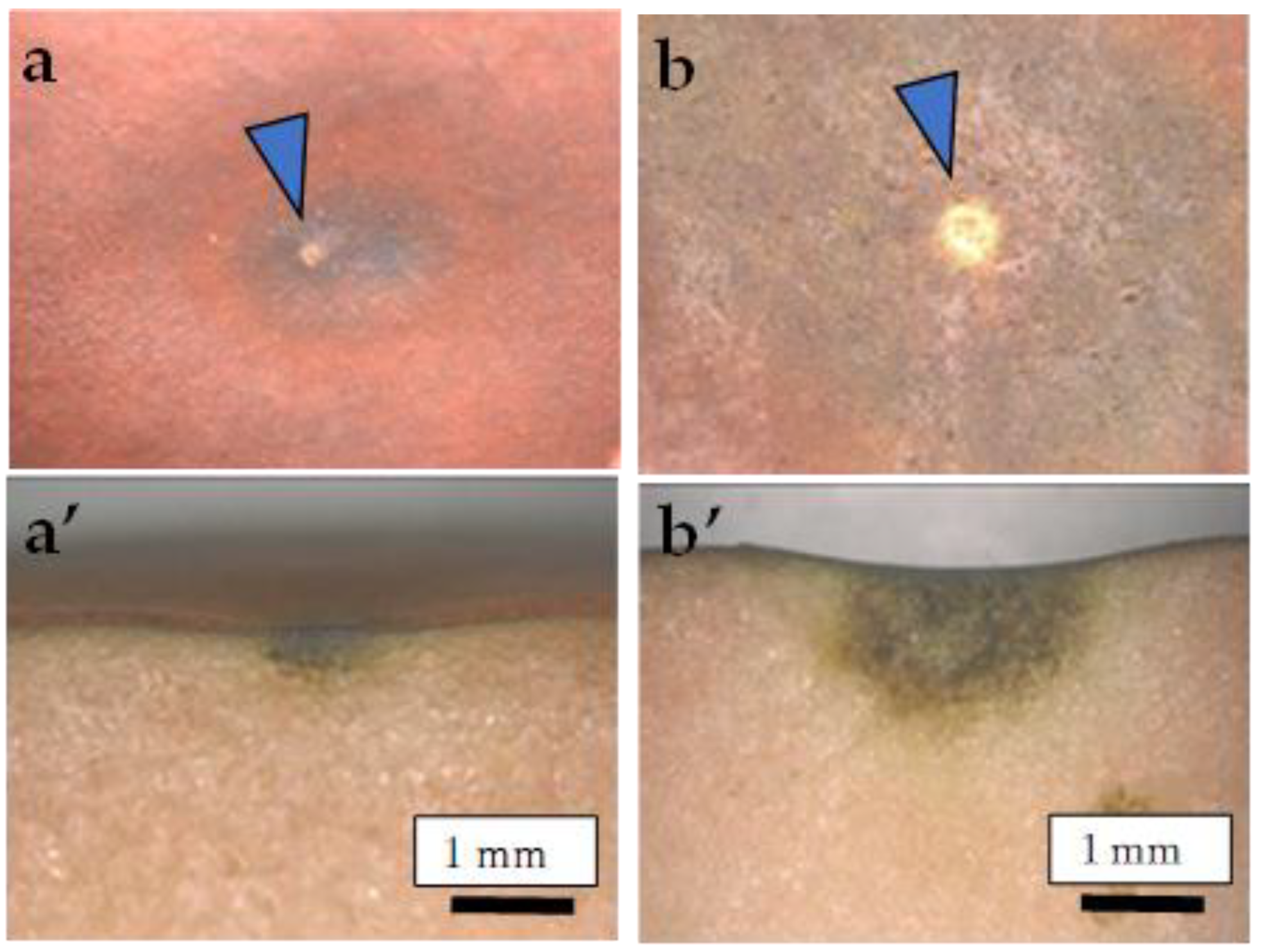
2.5. Destructive Observation of the Sliced Sections and the CSPD Spots of Non-Bagged ‘Kurenainoyume’ Fruit
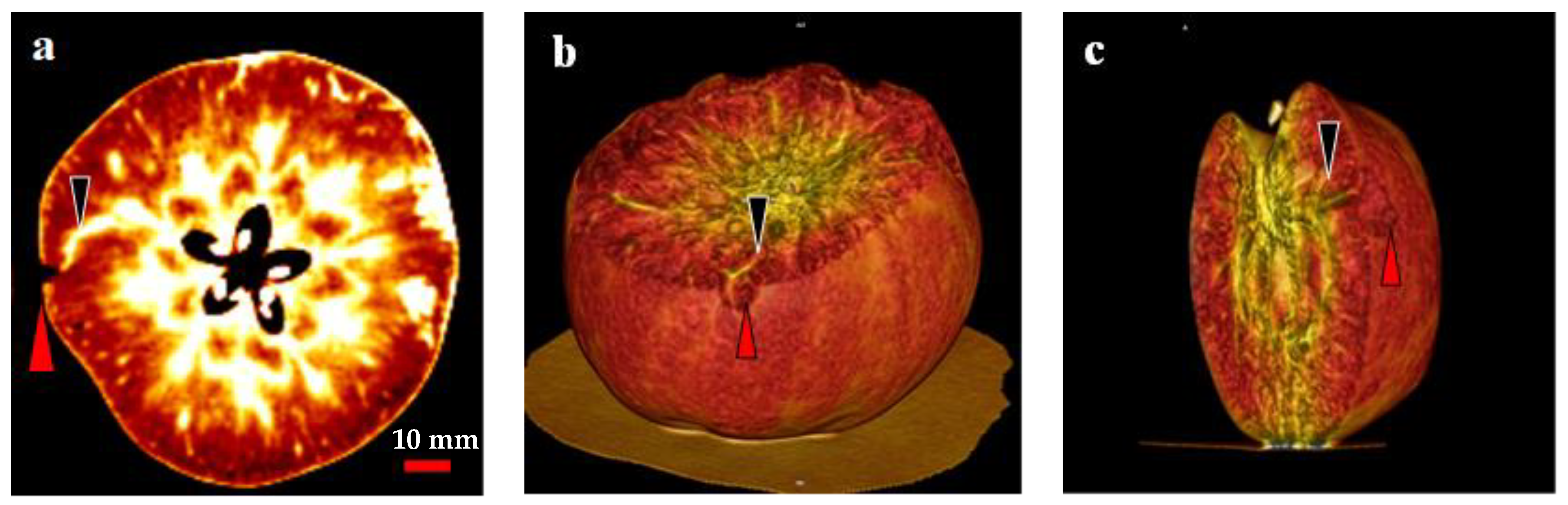
2.6. Analysis of the Relationship between the CSPD Spots and the Vascular Bundles Using CT Scanning
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Fruit Development Analysis and Quality Measurement
4.3. Nondestructive Observation of Number and Structure of the CSPD
4.4. Observation of the Number and Structure of the Lenticels
4.5. Destructive Observation of CSPD, Cuticular Thickness, and Fresh Cell Size
4.6. Nondestructive Image Scanning by CT
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kellerhals, M. Introduction to Apple (Malus × Domestica). In Genetics and Genomics of Rosaceae; Plant Genetics and Genomics: Crops and Models; Folta, K.M., Gardiner, S.E., Eds.; Springer: New York, NY, USA, 2009; pp. 73–84. [Google Scholar]
- Pereira-Lorenzo, S.; Ramos-Cabrer, A.M.; Fischer, M. Breeding Apple (Malus x Domestica Borkh). In Breeding Plantation Tree Crops: Temperate Species; Gradziel, T.M., Ed.; Springer: New York, NY, USA, 2009; pp. 33–81. [Google Scholar] [CrossRef]
- Honda, C.; Iwanami, H.; Naramoto, K.; Maejima, T.; Kanamaru, K.; Moriya-Tanaka, Y.; Hanada, T.; Wada, M. Thinning and Bagging Treatments and the Growing Region Influence Anthocyanin Accumulation in Red-Fleshed Apple Fruit. Hortic. J. 2017, 86, 291–299. [Google Scholar] [CrossRef]
- Bars-Cortina, D.; Macià, A.; Iglesias, I.; Romero, M.P.; Motilva, M.J. Phytochemical Profiles of New Red-Fleshed Apple Varieties Compared with Traditional and New White-Fleshed Varieties. J. Agric. Food Chem. 2017, 65, 1684–1696. [Google Scholar] [CrossRef]
- Silvestri, C.; Cirilli, M.; Zecchini, M.; Muleo, R.; Ruggieri, A. Consumer Acceptance of the New Red-Fleshed Apple Variety. J. Food Prod. Mark. 2018, 24, 1–21. [Google Scholar] [CrossRef]
- Abe, K.; Soejima, J.; Iwanami, H.; Kotoda, N.; Moriya, S.; Bessho, H.; Komori, S.; Ito, Y.; Takahashi, S.; Okada, K.; et al. New Apple Cultivar ‘Rose Pearl’. Bull. NARO Fruit Tree Tea Sci. 2017, 1, 9–17. [Google Scholar] [CrossRef]
- Abe, K.; Soejima, J.; Iwanami, H.; Kotoda, N.; Moriya, S.; Takahashi, S.; Ito, Y.; Bessho, H.; Okada, K.; Kato, H.; et al. New Apple Cultivar ‘Ruby Sweet’. Bull. NARO Fruit Tree Tea Sci. 2018, 2, 9–17. [Google Scholar] [CrossRef]
- Matsumoto, K.; Maeda, H.; Fujita, T.; Sato, S.; Shiozaki, Y. Apple Breeding Programs at Hirosaki University, Japan: Yellow Skin, Red Flesh, and Large Size. Acta Hortic. 2016, 29–34. [Google Scholar] [CrossRef]
- Banno, K. Red-Fleshed Apple Cultivars Bred at Shinshu University, ‘Honey Rouge’ and ‘Red Sensation’. Kajitsu Nippon 2017, 72, 8–12. [Google Scholar]
- Moriya, S. Deployment Status of Promising Cultivars in Apple and Current Status of Apple Breeding. Kajitsu Nippon 2014, 69, 45–49. [Google Scholar]
- Yoshiie, K. Newly Registered Varieties of Fruit Trees Under the Seeds and Seedlings Law. Kaju Shubyo 2018, 151, 17. [Google Scholar]
- Umemura, H.; Otagaki, S.; Wada, M.; Kondo, S.; Matsumoto, S. Expression and Functional Analysis of a Novel MYB Gene, MdMYB110a_JP, Responsible for Red Flesh, Not Skin Color in Apple Fruit. Planta 2013, 238, 65–76. [Google Scholar] [CrossRef]
- Espley, R.V.; Hellens, R.P.; Putterill, J.; Stevenson, D.E.; Kutty-Amma, S.; Allan, A.C. Red Coloration in Apple Fruit Is Due to the Activity of the MYB Transcription Factor, MdMYB10. Plant J. 2007, 49, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Espley, R.V.; Brendolise, C.; Chagne, D.; Kutty-Amma, S.; Green, S.; Volz, R.; Putterill, J.; Schouten, H.J.; Gardiner, S.E.; Hellens, R.P. Multiple Repeats of a Promoter Segment Causes Transcription Factor Autoregulation in Red Apples. Plant Cell 2009, 21, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Kobayashi, T.; Kougo, T.; Fujita, T.; Sato, S.; Moriguchi, T. Prevention of New Cork Spot-like Physiological Disorder in ‘Kurenainoyume’ Apples by Pre-Harvest Fruit Bagging. Hortic. J. 2018, 87, 174–183. [Google Scholar] [CrossRef]
- Matsumoto, K.; Fujita, T.; Sato, S.; Moriguchi, T. Effects of Low Temperature, Shading, Defoliation, and Crop Load on the Flesh Coloration of the Type 2 Red-Fleshed Apple ‘Kurenainoyume’. Hortic. J. 2018, 87, 452–461. [Google Scholar] [CrossRef]
- Fukuda, H.; Masuda, T. Saving of Labor Inputs to Grow Apple Fruit. Bull. Natl. Inst. Fruit Tree Sci. 2006, 5, 1–13. [Google Scholar] [CrossRef]
- Gago, C.M.; Guerreiro, A.C.; Miguel, G.; Panagopoulos, T.; da Silva, M.M.; Antunes, M.D. Effect of Calcium Chloride and 1-MCP (SmartfreshTM) Postharvest Treatment on ‘Golden Delicious’ Apple Cold Storage Physiological Disorders. Sci. Hortic. 2016, 211, 440–448. [Google Scholar] [CrossRef]
- Matsumoto, K.; Fujita, T.; Sato, S. Effects of 1-MCP and Pre-Harvest Fruit Bagging Treatments on Cold Storability of the Red-Fleshed Apple ‘Kurenainoyume’. Hortic. J. 2018, 87, 443–451. [Google Scholar] [CrossRef]
- Matsumoto, K.; Chun, J.; Nakata, N.; Tamura, F. Rapid mesocarp cell elongation enhances gumming syndrome in Japanese apricot (Prunus mume Sieb. et Zucc.) fruit. J. Food Qual. 2008, 31, 205–215. [Google Scholar] [CrossRef]
- Hayama, H.; Mitani, N.; Yamane, T.; Inoue, H.; Kusaba, S. Characteristics of Cork Spot like Disorder in Japanese Pear ‘Akizuki’ and ‘Oushuu’. Hortic. Res Jpn. 2017, 16, 79–87. [Google Scholar] [CrossRef][Green Version]
- Reid, M.; Kalcsits, L. Water Deficit Timing Affects Physiological Drought Response, Fruit Size, and Bitter Pit Development for ‘Honeycrisp’ Apple. Plants 2020, 9, 874. [Google Scholar] [CrossRef] [PubMed]
- Clements, H.F. Morphology and Physiology of the Pome Lenticels of Pyrus malus. Bot. Gaz. 1935, 97, 101–117. [Google Scholar] [CrossRef]
- Khanal, B.P.; Si, Y.; Knoche, M. Lenticels and Apple Fruit Transpiration. Postharvest Biol. Technol. 2020, 167, 111221. [Google Scholar] [CrossRef]
- Maguire, K.M.; Lang, A.; Banks, N.H.; Hall, A.; Hopcroft, D.; Bennett, R. Relationship between Water Vapour Permeance of Apples and Micro-Cracking of the Cuticle. Postharvest Biol. Technol. 1999, 17, 89–96. [Google Scholar] [CrossRef]
- Veraverbeke, E.A.; Verboven, P.; Van Oostveldt, P.; Nicolai, B.M. Prediction of Moisture Loss Across the Cuticle of Apple (Malus sylvestris subsp. Mitis (Wallr.)) during Storage: Part 1. Model Development and Determination of Diffusion Coefficients. Postharvest Biol. Technol. 2003, 30, 75–88. [Google Scholar] [CrossRef]
- Tomana, T. Histological and Physiological Studies on the Cause of the Jonathan Spot in Apples. I Varietal Difference in Skin Structure of Fruit in Relation to the Spot-Development. J. Jpn. Soc. Hortic. Sci. 1960, 29, 273–280. [Google Scholar] [CrossRef]
- Endo, M. Studies on the Daily Change of Fruit Size of the Japanese Pear VII. Effect of Bagging with Different Paper Bag Types on Fruit Growth and Quality with Special Reference to Diurnal Fluctuation in Fruit Size. J. Jpn. Soc. Hortic. Sci. 1976, 44, 381–394. [Google Scholar] [CrossRef][Green Version]
- Harada, T.; Kurahashi, W.; Yanai, M.; Wakasa, Y.; Satoh, T. Involvement of Cell Proliferation and Cell Enlargement in Increasing the Fruit Size of Malus Species. Sci. Hortic. 2005, 105, 447–456. [Google Scholar] [CrossRef]
- Herremans, E.; Melado-Herreros, A.; Defraeye, T.; Verlinden, B.; Hertog, M.; Verboven, P.; Val, J.; Fernández-Valle, M.E.; Bongaers, E.; Estrade, P. Comparison of X-Ray CT and MRI of Watercore Disorder of Different Apple Cultivars. Postharvest Biol. Technol. 2014, 87, 42–50. [Google Scholar] [CrossRef]
- Lammertyn, J.; Dresselaers, T.; Van Hecke, P.; Jancsók, P.; Wevers, M.; Nicolai, B.M. MRI and X-Ray CT Study of Spatial Distribution of Core Breakdown in ‘Conference’ Pears. Magn. Reson. Imaging 2003, 21, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Arendse, E.; Fawole, O.A.; Magwaza, L.S.; Opara, U.L. Non-Destructive Characterization and Volume Estimation of Pomegranate Fruit External and Internal Morphological Fractions Using X-Ray Computed Tomography. J. Food Eng. 2016, 186, 42–49. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kondo, N.; Shibusawa, S. Internal Quality Evaluation of Fruit with X-ray CT. J. Soc. High Technol. Agric. 2005, 17, 75–83. [Google Scholar] [CrossRef]
- Ogawa, Y.; Morita, K.; Tanaka, S.; Setoguchi, M.; Thai, C.N. Application of X-ray CT for Detection of Physical Foreign Materials in Foods. Trans. ASAE 1998, 41, 157–162. [Google Scholar] [CrossRef]

| Treatment | CSPD | Fresh Weight (g) | Length (mm) | Diameter (mm) | Firmness (N) | Soluble Solid Contents (Brix°) | Titratable Acidity (%) |
|---|---|---|---|---|---|---|---|
| Non-bagged | Yes | 302.1 | 73.2 | 92.1 | 72.2 | 11.3 | 0.87 |
| Bagged | No | 260.3 | 71.5 | 85.9 | 77.5 | 10.0 | 0.85 |
| Significant | NS | NS | NS | NS | * | NS |
| Treatment | Number of Lenticels (/10 mm2) | Diameter of Lenticels (mm) | Opened Lenticel Rate (%) |
|---|---|---|---|
| Non-bagged | 4.5 | 0.50 | 52.0 |
| Bagged | 5.4 | 0.38 | 17.9 |
| Significant | NS | * | ** |
| Date | 2018 | 2019 |
|---|---|---|
| Full bloom | 7 May | 10 May |
| Bagging | 6 July (59 *) | 27 June (48) |
| Bag removal | 25 September (141) | 24 September (137) |
| Maturity | 24 October (170) | 23 October (166) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imura, E.; Nakagomi, M.; Hayashida, T.; Fujita, T.; Sato, S.; Matsumoto, K. Unraveling the Mechanism of Cork Spot-like Physiological Disorders in ‘Kurenainoyume’ Apples Based on Occurrence Location. Plants 2024, 13, 381. https://doi.org/10.3390/plants13030381
Imura E, Nakagomi M, Hayashida T, Fujita T, Sato S, Matsumoto K. Unraveling the Mechanism of Cork Spot-like Physiological Disorders in ‘Kurenainoyume’ Apples Based on Occurrence Location. Plants. 2024; 13(3):381. https://doi.org/10.3390/plants13030381
Chicago/Turabian StyleImura, Eichi, Mitsuho Nakagomi, Taishi Hayashida, Tomomichi Fujita, Saki Sato, and Kazuhiro Matsumoto. 2024. "Unraveling the Mechanism of Cork Spot-like Physiological Disorders in ‘Kurenainoyume’ Apples Based on Occurrence Location" Plants 13, no. 3: 381. https://doi.org/10.3390/plants13030381
APA StyleImura, E., Nakagomi, M., Hayashida, T., Fujita, T., Sato, S., & Matsumoto, K. (2024). Unraveling the Mechanism of Cork Spot-like Physiological Disorders in ‘Kurenainoyume’ Apples Based on Occurrence Location. Plants, 13(3), 381. https://doi.org/10.3390/plants13030381