New Prospects for Improving Microspore Embryogenesis Induction in Highly Recalcitrant Winter Wheat Lines
Abstract
1. Introduction
2. Results
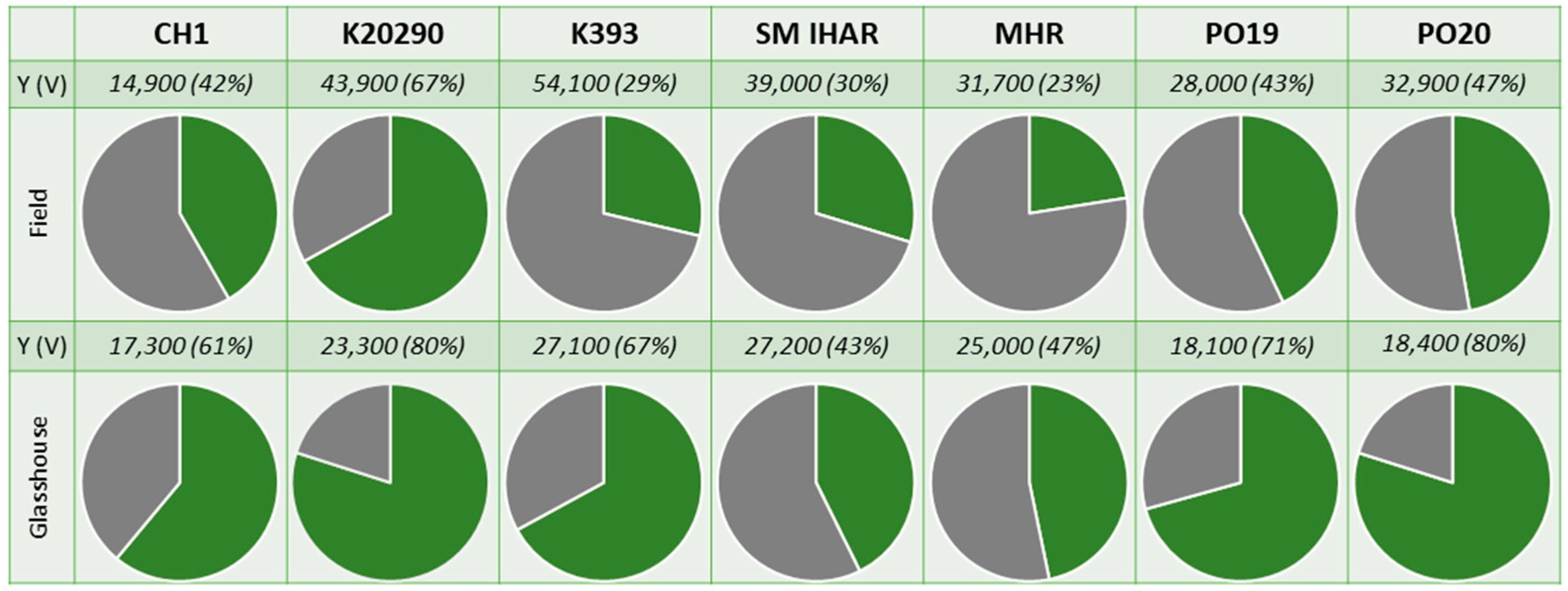
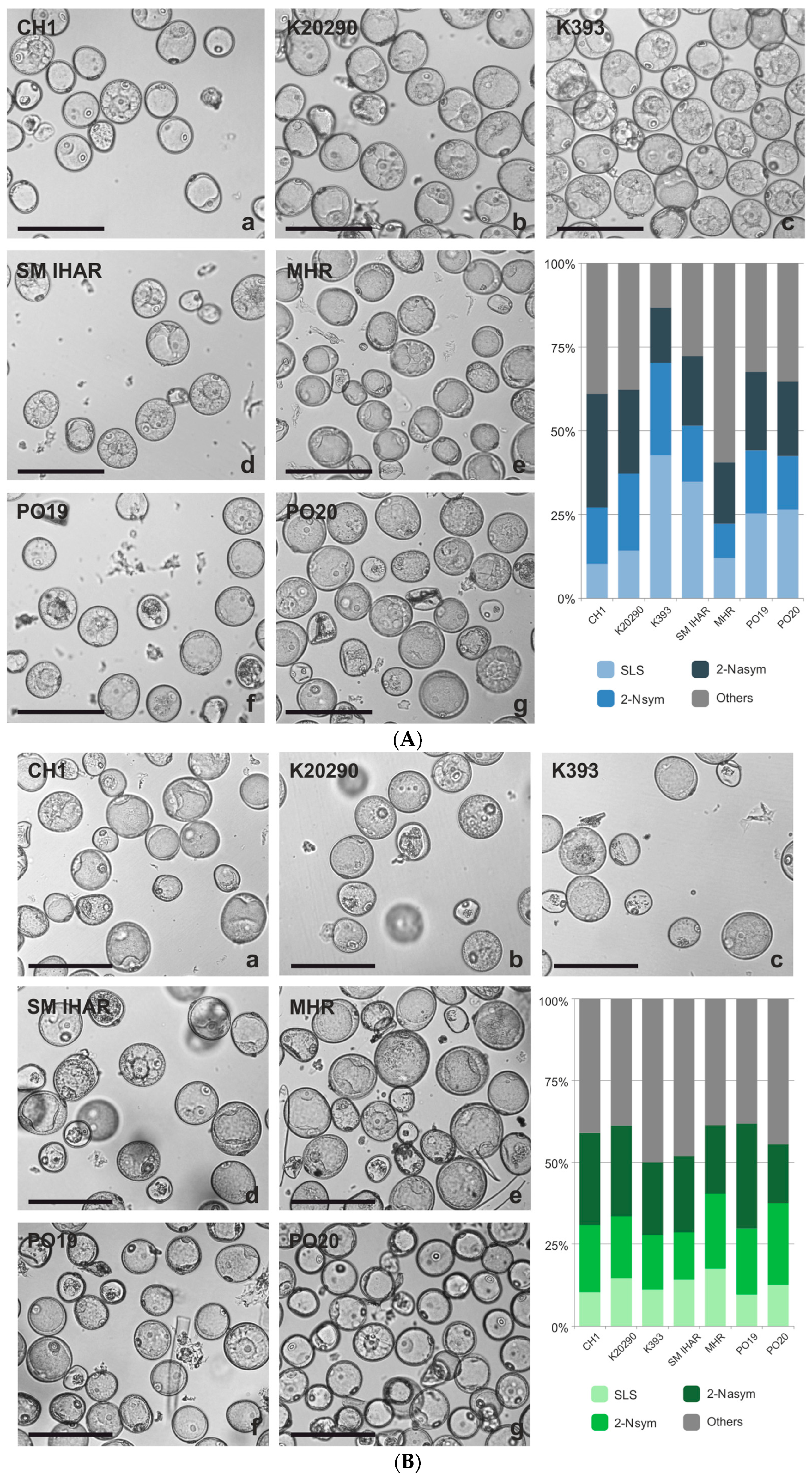
2.1. Microspore Yield, Viability and Cytological Characteristic (Experiment 2020/2021)
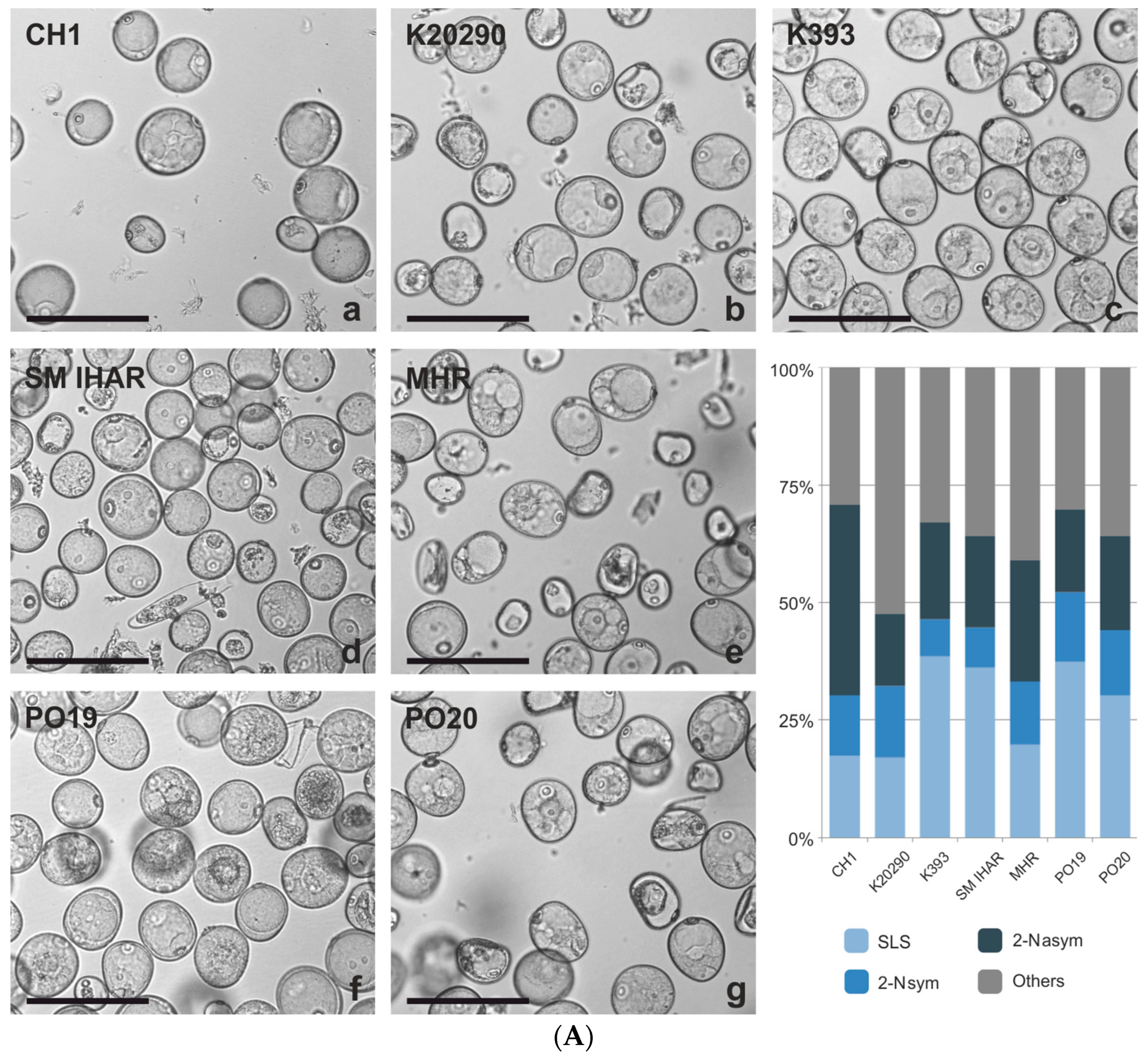
2.2. Effectiveness of ME Induction in Isolated Microspore and Anther Cultures (Experiment 2020/2021)
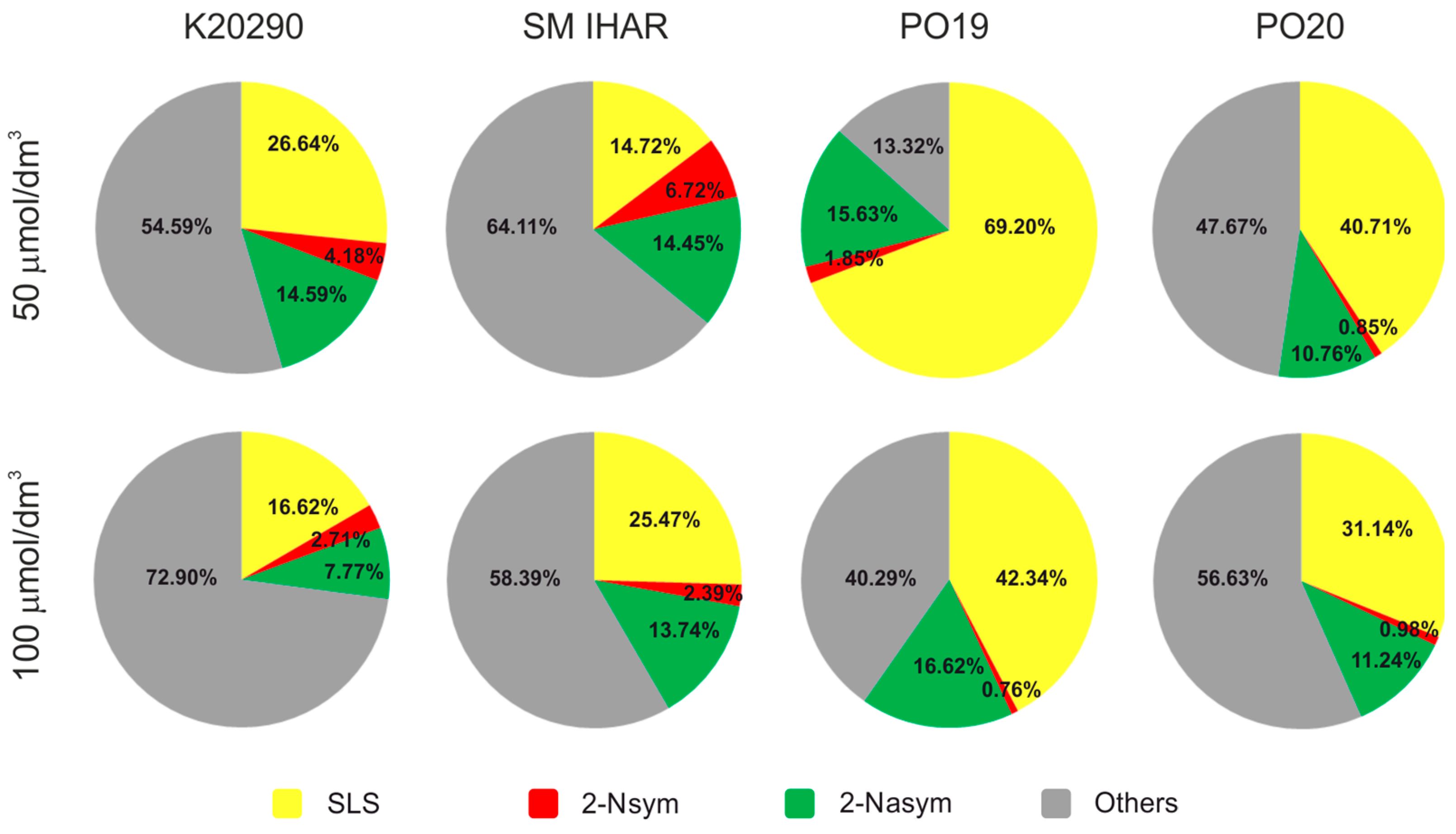
2.3. Effect of Spike Pre-Treatment Modifications on Microspore Yield, Viability and Cytological Characteristics (Experiment 2021/2022)
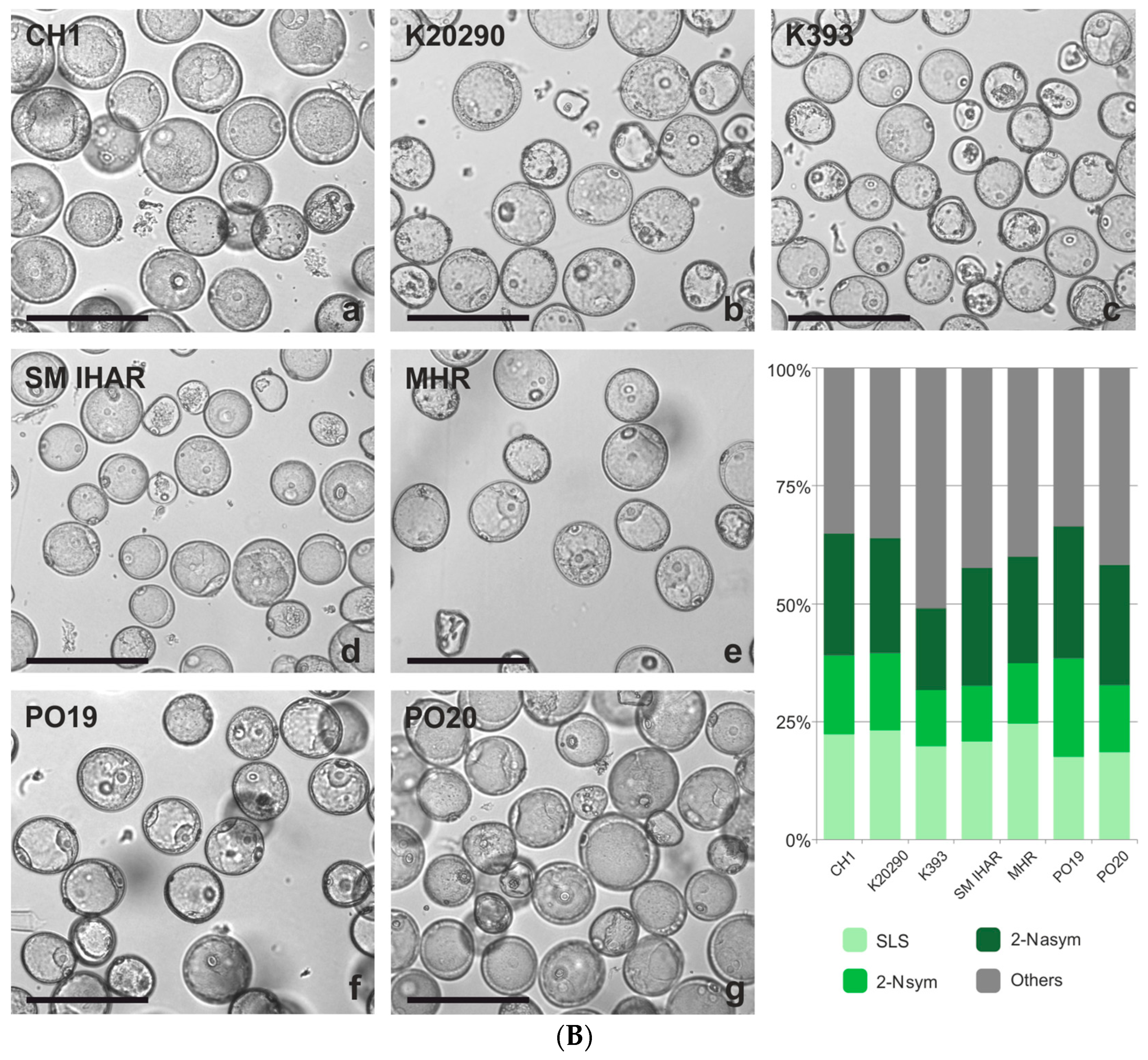
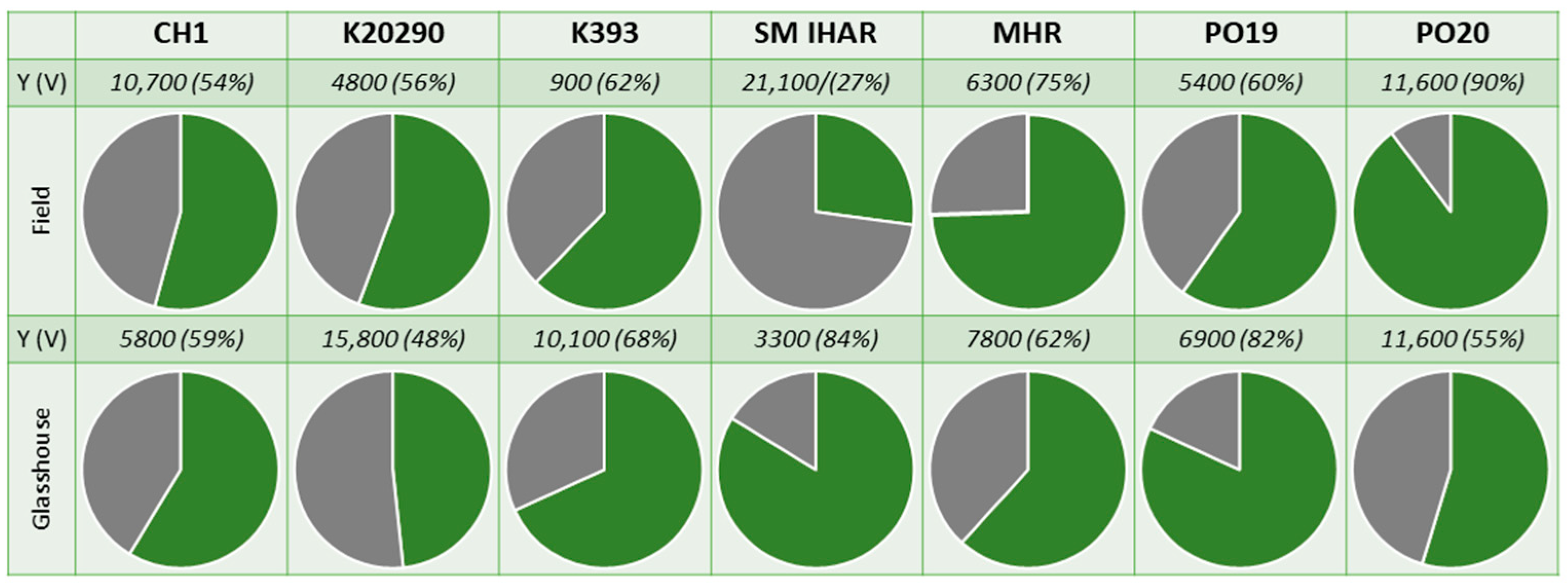
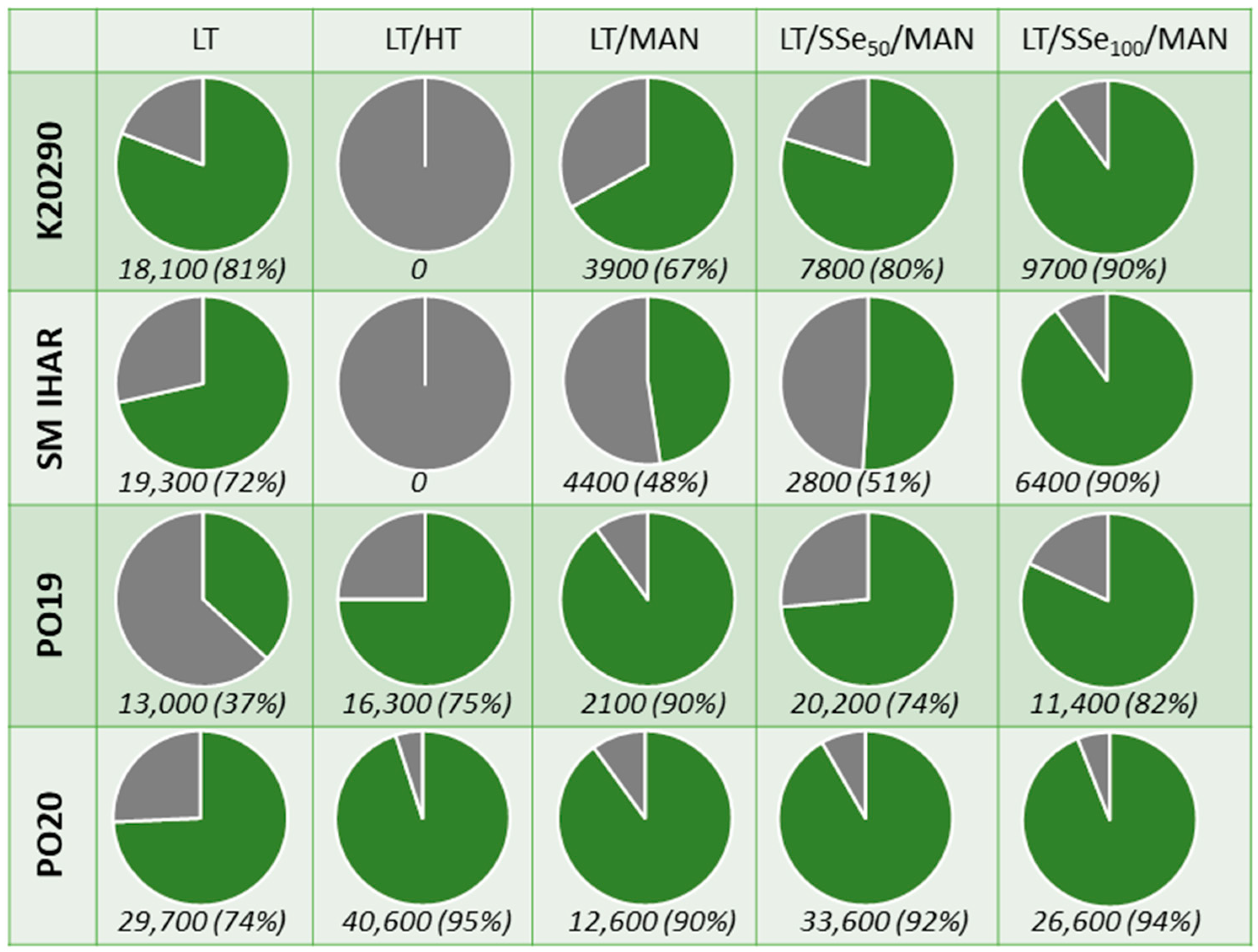
2.4. Effectiveness of ME Induction in Isolated Microspore and Anther Cultures (Experiment 2021/2022)
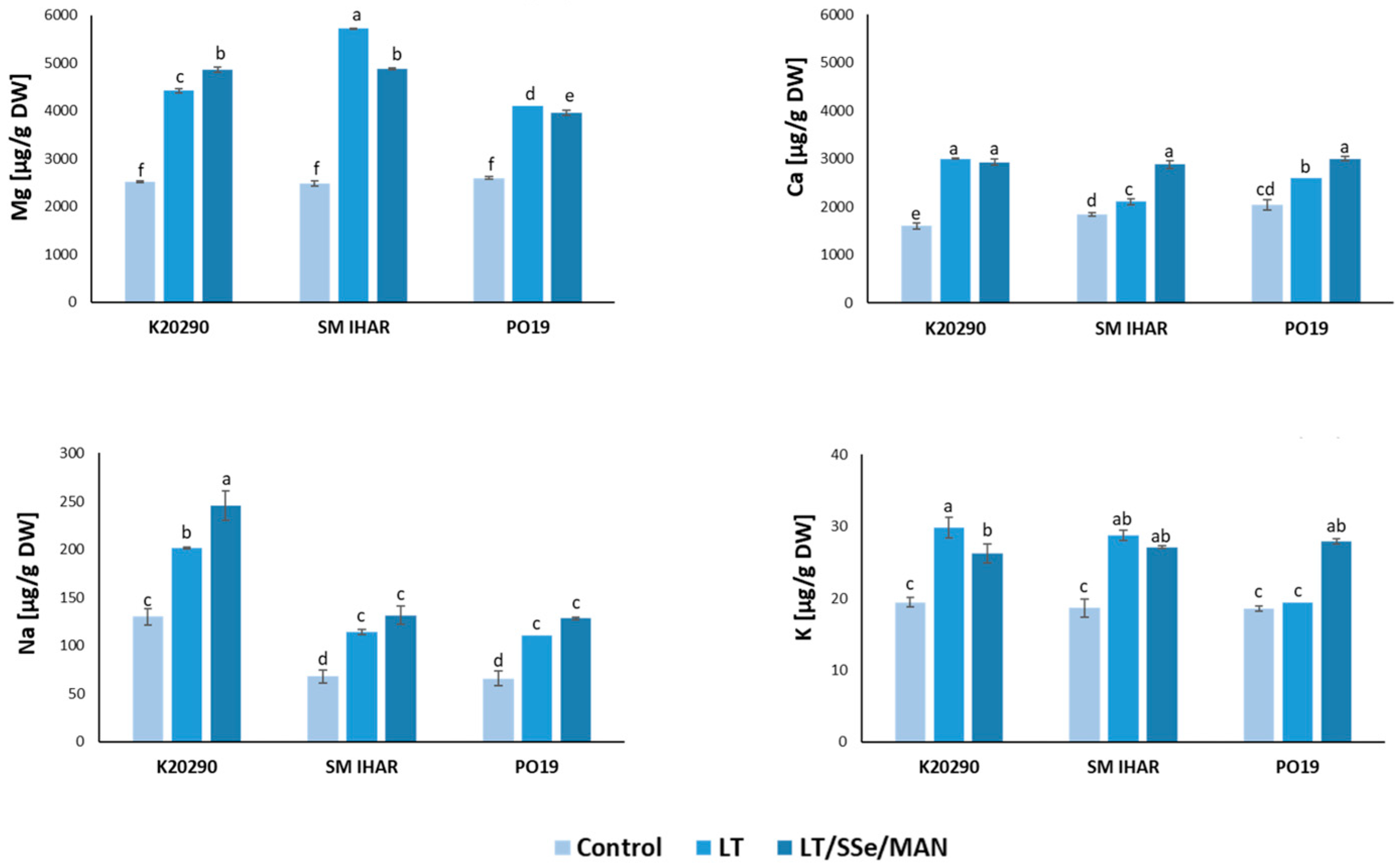
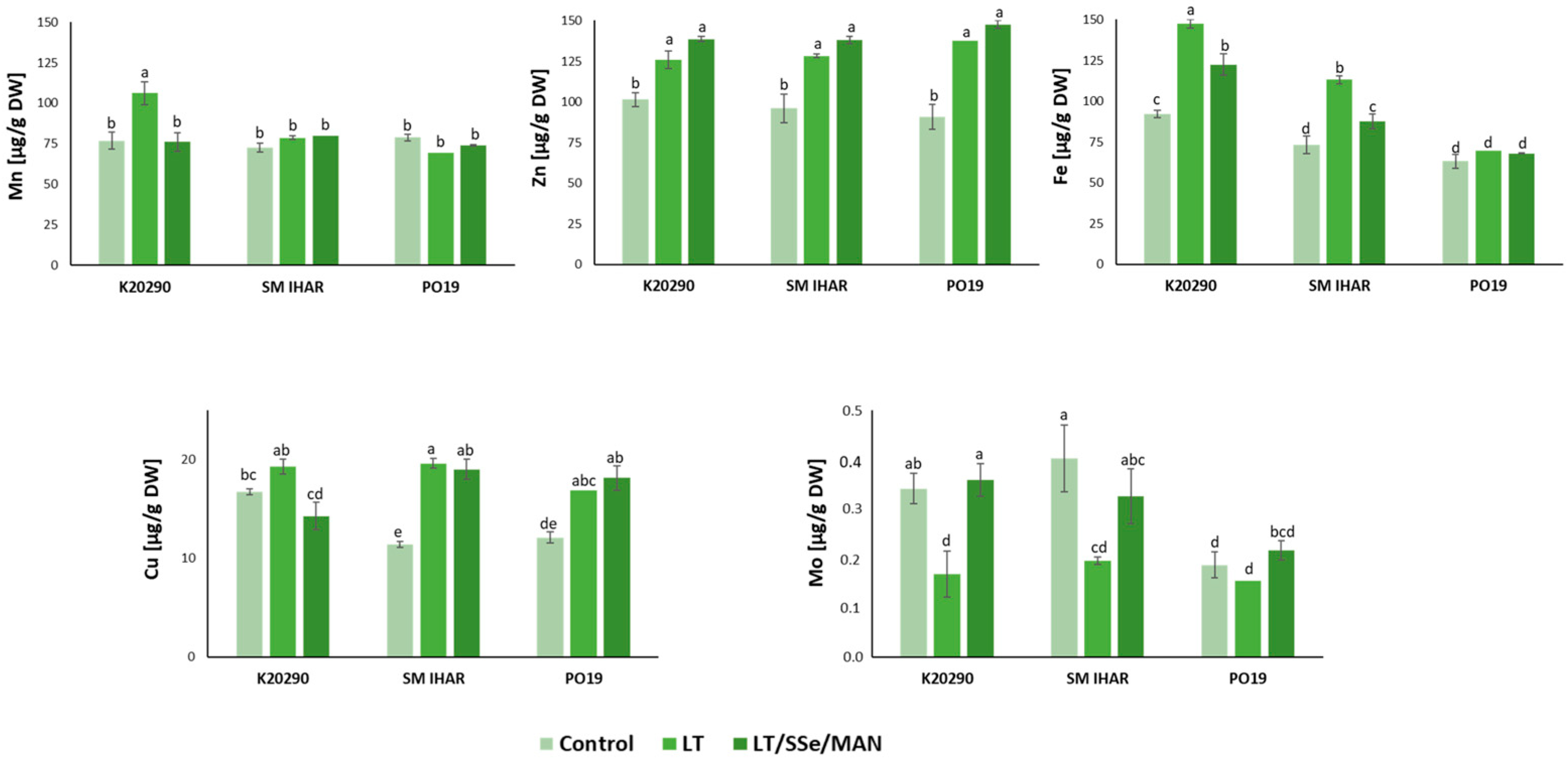
2.5. Macro- and Micronutrient Contents in Wheat Anthers and Their Changes Induced by Modified ME Induction Procedure
2.6. Glutathione and Ascorbate Levels and Redox Balance in Wheat Anthers and Changes in These Parameters Induced by the ME Induction Procedure
2.7. Total Activity of Low-Molecular-Weight Antioxidants in Wheat Anthers and Alterations in This Activity Stimulated by the ME Induction Procedure
2.8. H2O2 Generation in Wheat Anthers and Changes in this Parameter Caused by the ME Induction Procedure
3. Discussion
3.1. Influence of Donor Plant Growth Conditions, Tiller Pre-Treatment and In Vitro Culture Technique on the Efficiency of Wheat ME
3.2. Effect of ME Induction Procedure on Micro- and Macronutrient Levels, Intensity of Oxidative Stress and Non-Enzymatic Antioxidant Defense in Wheat Anthers
4. Materials and Methods
4.1. Plant Material
4.2. Plant Growth
4.3. Tiller Pre-Treatment for Microspore Embryogenesis Induction
4.4. Tiller Pre-Treatment Modifications
- (i)
- Low-temperature tiller pre-treatment for 21–28 days at 4 °C;
- (ii)
- Low/high-temperature tiller pre-treatment for 21–28 days at 4 °C, followed by 24 h at 30 °C;
- (iii)
- Low-temperature/osmotic/starvation tiller pre-treatment for 21–28 days at 4 °C, followed by 4 days in 0.4 mol/dm3 mannitol at 20 °C;
- (iv)
- Low-temperature/osmotic/starvation/selenium tiller pre-treatment for 21–28 days at 4 °C with the last three days incubated in 50 µmol/dm3 or 100 µmol/dm3 sodium selenate (Na2SeO4; SSe) solution, followed by 4 days in 0.7 mol/dm3 mannitol at 20 °C.
4.5. Isolated Microspore Culture
4.6. Cytological Analyses
4.7. Anther Culture
4.8. Sampling for Biochemical Analyses
4.9. Macro- and Microelement Analyses
4.10. Total Low-Molecular-Weight Antioxidant Activity
4.11. Sampling and Detecting Ascorbate and Glutathione
4.12. Reduced and Oxidized Glutathione Assay
4.13. Reduced and Oxidized Ascorbate Assay
4.14. Hydrogene Peroxide (H2O2)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venske, E.; dos Santos, R.S.; Busanello, C.; Gustafson, P.; de Oliveira, A.C. Bread wheat: A role model for plant domestication and breeding. Hereditas 2019, 156, 16. [Google Scholar] [CrossRef]
- Fischer, T. History of wheat breeding: A personal view. In Wheat Improvement: Food Security in a Changing Climate; Reynolds, M.P., Braun, H.J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 17–30. [Google Scholar]
- FAO; OECD. Cereals. In OECD-FAO Agricultural Outlook 2022–2031; 2022; pp. 154–167. Available online: https://www.fao.org/3/CC0308EN/Cereals.pdf (accessed on 29 June 2022).
- Kasha, K.; Maluszynski, M. Production of doubled haploids in crop plants. An introduction. In Doubled Haploid Production in Crop Plants: A Manual; Maluszynski, M., Kasha, K.J., Forster, B.R., Szarejko, I., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 2003; pp. 1–4. [Google Scholar]
- Konieczny, R.; Czaplicki, A.Z.; Golczyk, H.; Przywara, L. Two pathways of plant regeneration in wheat anther culture. PCTOC 2003, 73, 177–187. [Google Scholar] [CrossRef]
- Weigt, D.; Kiel, A.; Siatkowski, I.; Zyprych-Walczak, J.; Tomkowiak, A.; Kwiatek, M. Comparison of the androgenic response of spring and winter wheat (Triticum aestivum L.). Plants 2020, 9, 49. [Google Scholar] [CrossRef]
- Jacquard, C.; Mazeyrat-Gourbeyre, F.; Devaux, P.; Boutilier, K.; Baillieul, F.; Clément, C. Microspore embryogenesis in barley: Anther pre-treatment stimulates plant defence gene expression. Planta 2009, 229, 393–402. [Google Scholar] [CrossRef]
- Żur, I.; Dubas, E.; Golemiec, E.; Szechyńska-Hebda, M.; Gołębiowska, G.; Wędzony, M. Stress-related variation in antioxidative enzymes activity and cell metabolism efficiency associated with embryogenesis induction in isolated microspore culture of triticale (x Triticosecale Wittm.). Plant Cell Rep. 2009, 28, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Żur, I.; Dubas, E.; Krzewska, M.; Janowiak, F.; Hura, K.; Pociecha, E.; Bączek-Kwinta, R.; Płażek, A. Antioxidant activity and ROS tolerance in triticale (×Triticosecale Wittm.) anthers affect the efficiency of microspore embryogenesis. PCTOC 2014, 119, 79–94. [Google Scholar] [CrossRef]
- Żur, I.; Dubas, E.; Krzewska, M.; Zieliński, K.; Fodor, J.; Janowiak, F. Glutathione provides antioxidative defence and promotes microspore-derived embryo development in isolated microspore cultures of triticale (× Triticosecale Wittm.). Plant Cell Rep. 2019, 38, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Żur, I.; Dubas, E.; Krzewska, M.; Kopeć, P.; Nowicka, A.; Surówka, E.; Gawrońska, K.; Gołębiowska, G.; Juzoń, K.; Malaga, S. Triticale and barley microspore embryogenesis induction requires both reactive oxygen species generation and efficient system of antioxidative defence. PCTOC 2021, 145, 347–366. [Google Scholar] [CrossRef]
- Rodríguez-Serrano, M.; Bárány, I.; Prem, D.; Coronado, M.J.; Risueño, M.C.; Testillano, P.S. NO, ROS, and cell death associated with caspase-like activity increase in stress-induced microspore embryogenesis of barley. J. Exp. Bot. 2012, 63, 2007–2024. [Google Scholar] [CrossRef] [PubMed]
- Żur, I.; Kopeć, P.; Surówka, E.; Dubas, E.; Krzewska, M.; Nowicka, A.; Janowiak, F.; Juzoń, K.; Janas, A.; Barna, B.; et al. Impact of ascorbate-glutathione cycle components on the effectiveness of embryogenesis induction in isolated microspore cultures of barley and triticale. Antioxidants 2021, 10, 1254. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.J.; Desikan, R.; Clarke, A.; Hurst, R.D.; Hancock, J.T. Hydrogen peroxide and nitric oxide as signalling molecules in plants. J. Exp. Bot. 2002, 53, 1237–1247. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Touraev, A.; Indrianto, A.; Wratschko, I.; Vicente, O.; HeberleBors, E. Efficient microspore embryogenesis in wheat (Triticum aestivum L.) induced by starvation at high temperature. Sex. Plant Reprod. 1996, 9, 209–215. [Google Scholar] [CrossRef]
- Kasha, K.; Simion, E.; Miner, M.; Letarte, J.; Hu, T. Haploid wheat isolated microspore culture protocol. In Doubled Haploid Production in Crop Plants: A Manual; Maluszynski, M., Kasha, K.J., Forster, B.R., Szarejko, I., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 77–81. [Google Scholar]
- Pauk, J.; Mihály, R.; Puolimatka, M. Protocol of wheat (Triticum aestivum L.) anther culture. In Doubled Haploid Production in Crop Plants: A Manual; Maluszynski, M., Kasha, K.J., Forster, B.R., Szarejko, I., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 2003; pp. 59–64. [Google Scholar]
- Tuvesson, S.; von Post, R.; Ljungberg, A. Wheat anther culture. In Doubled Haploid Production in Crop Plants: A Manual; Maluszynski, M., Kasha, K.J., Forster, B.P., Szarejko, I., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 71–76. [Google Scholar] [CrossRef]
- Cistué, L.; Soriano, M.; Castillo, A.M.; Vallés, M.P.; Sanz, J.M.; Echávarri, B. Production of doubled haploids in durum wheat (Triticum turgidum L.) through isolated microspore culture. Plant Cell Rep. 2006, 25, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Cistué, L.; Romagosa, I.; Batlle, F.; Echávarri, B. Improvements in the production of doubled haploids in durum wheat (Triticum turgidum L.) through isolated microspore culture. Plant Cell Rep. 2009, 28, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Lantos, C.; Bóna, L.; Nagy, É.; Békés, F.; Pauk, J. Induction of in vitro androgenesis in anther and isolated microspore culture of different spelt wheat (Triticum spelta L.) genotypes. PCTOC 2018, 133, 385–393. [Google Scholar] [CrossRef]
- Saini, H.S.; Sedgley, M.; Aspinall, D. Developmental anatomy in wheat of mail sterility induced by heat stress, water deficit or abscisic acid. Aust. J. Plant Physiol. 1984, 11, 243–253. [Google Scholar] [CrossRef]
- Gustafson, V.D.; Baenziger, P.S.; Wright, M.S.; Stroup, W.W.; Yen, Y. Isolated wheat microspore culture. PCTOC 1995, 42, 207–213. [Google Scholar] [CrossRef]
- De Storme, N.; Geelen, D. The impact of environmental stress on male reproductive development in plants: Biological processes and molecular mechanisms. Plant Cell Environ. 2014, 37, 1–18. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Muller, F.; Rieu, I.; Winter, P. Epigenetic events in plant male germ cell heat stress responses. Plant Reprod. 2016, 29, 21–29. [Google Scholar] [CrossRef]
- Dogramaci-Altuntepe, M.; Peterson, T.S.; Jauhar, P.P. Anther culture-derived regenerants of durum wheat and their cytological characterization. J. Hered. 2001, 92, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Indrianto, A.; Heberle-Bors, E.; Touraev, A. Assessment of various stresses and carbohydrates for their effect on the induction of embryogenesis in isolated wheat microspores. Plant Sci. 1999, 143, 71–79. [Google Scholar] [CrossRef]
- Hu, T.C.; Kasha, K.J. A cytological study of pretreatments used to improve isolated microspore cultures of wheat (Triticum aestivum L.) cv. Chris. Genome 1999, 42, 432–441. [Google Scholar] [CrossRef]
- Zoriniants, S.; Tashpulatov, A.S.; Heberle-Bors, E.; Touraev, A. The role of stress in the induction of haploid microspore embryogenesis. In Haploids in Crop Improvement II; Don Palmer, C.E., Keller, W.A., Kasha, K.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 35–52. [Google Scholar] [CrossRef]
- Żur, I.; Dubas, E.; Golemiec, E.; Szechyńska-Hebda, M.; Janowiak, F.; Wędzony, M. Stress-induced changes important for effective androgenic induction in isolated microspore culture of triticale (×Triticosecale Wittm.). PCTOC 2008, 94, 319–328. [Google Scholar] [CrossRef]
- Lei, X.N.; Liu, B. Tapetum-dependent male meiosis progression in plants: Increasing evidence emerges. Front. Plant Sci. 2020, 10, 1667. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Suen, D.F.; Huang, C.Y.; Kung, S.Y.; Huang, A.H.C. The maize tapetum employs diverse mechanisms to synthesize and store proteins and flavonoids and transfer them to the pollen surface. Plant Physiol. 2012, 158, 1548–1561. [Google Scholar] [CrossRef]
- Tariq, N.; Yaseen, M.; Xu, D.; Rehman, H.M.; Bibi, M.; Uzair, M. Rice anther tapetum: A vital reproductive cell layer for sporopollenin biosynthesis and pollen exine patterning. Plant Biol. 2023, 25, 233–245. [Google Scholar] [CrossRef]
- Zur, I.; Dubas, E.; Krzewska, M.; Waligorski, P.; Dziurka, M.; Janowiak, F. Hormonal requirements for effective induction of microspore embryogenesis in triticale (×Triticosecale Wittm.) anther cultures. Plant Cell Rep. 2015, 34, 47–62. [Google Scholar] [CrossRef]
- Zieliński, K.; Krzewska, M.; Nowicka, A.; Żur, I.; Fodor, J.; Dubas, E. Redox regulation of androgenesis in rye (Secale cereale L.). Acta Physiol. Plant. 2018, 40, 82. [Google Scholar]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Guignardi, Z.; Schiavon, M. Biochemistry of plant selenium uptake and metabolism. In Selenium in Plants: Molecular, Physiological, Ecological and Evolutionary Aspects; Pilon-Smits, E.A.H., Winkel, L.H.E., Lin, Z.-Q., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 21–34. [Google Scholar] [CrossRef]
- Lanza, M.; dos Reis, A.R. Roles of selenium in mineral plant nutrition: ROS scavenging responses against abiotic stresses. Plant Physiol. Biochem. 2021, 164, 27–43. [Google Scholar] [CrossRef]
- Hartikainen, H.; Xue, T.L.; Piironen, V. Selenium as an anti-oxidant and pro-oxidant in ryegrass. Plant Soil 2000, 225, 193–200. [Google Scholar] [CrossRef]
- Silva, V.M.; Tavanti, R.F.R.; Gratao, P.L.; Alcock, T.D.; dos Reis, A.R. Selenate and selenite affect photosynthetic pigments and ROS scavenging through distinct mechanisms in cowpea (Vigna unguiculata (L.) walp) plants. Ecotox. Environ. Saf. 2020, 201, 110777. [Google Scholar] [CrossRef]
- Dubas, E.; Castillo, A.M.; Żur, I.; Krzewska, M.; Vallés, M.P. Microtubule organization changes severely after mannitol and n-butanol treatments inducing microspore embryogenesis in bread wheat. BMC Plant Biol. 2021, 21, 586. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, B.N.; Gridley, K.L.; Brady, J.N.; Phillips, T.; Tyerman, S.D. The role of molybdenum in agricultural plant production. Ann. Bot. 2005, 96, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Tejada-Jimenez, M.; Chamizo-Ampudia, A.; Llamas, A.; Galvan, A.; Fernandez, E. Roles of molybdenum in plants and improvement of its acquisition and use efficiency. In Plant Micronutrient Use Efficiency; Hossain, M.A., Kamiya, T., Burritt, D.J., Phan Tran, L.-S., Fujiwara, T., Eds.; Elsevier Academic Press: London Wall, UK; San Diego, CA, USA; Cambridge, UK; Oxford, UK, 2018; pp. 137–159. [Google Scholar]
- Zhong, R.; Thompson, J.; Ottesen, E.; Lamppa, G.K. A forward genetic screen to explore chloroplast protein import in vivo identifies Moco sulfurase, pivotal for ABA and IAA biosynthesis and purine turnover. Plant J. 2010, 63, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-A potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Dorion, S.; Ouellet, J.C.; Rivoal, J. Glutathione metabolism in plants under stress: Beyond reactive oxygen species detoxification. Metabolites 2021, 11, 641. [Google Scholar] [CrossRef]
- Xiao, M.G.; Li, Z.X.; Zhu, L.; Wang, J.Y.; Zhang, B.; Zheng, F.Y.; Zhao, B.P.; Zhang, H.W.; Wang, Y.J.; Zhang, Z.J. The multiple roles of ascorbate in the abiotic stress response of plants: Antioxidant, cofactor, and regulator. Front. Plant Sci. 2021, 12, 598173. [Google Scholar] [CrossRef] [PubMed]
- Kocsy, G.; Tari, I.; Vanková, R.; Zechmann, B.; Gulyás, Z.; Poór, P.; Galiba, G. Redox control of plant growth and development. Plant Sci. 2013, 211, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Bilska, K.; Wojciechowska, N.; Alipour, S.; Kalemba, E.M. Ascorbic acid-The little-known antioxidant in woody plants. Antioxidants 2019, 8, 645. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Molec. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Belmonte, M.F.; Ambrose, S.J.; Ross, A.R.S.; Abrams, S.R.; Stasolla, C. Improved development of microspore-derived embryo cultures of Brassica napus cv Topaz following changes in glutathione metabolism. Physiol. Plant. 2006, 127, 690–700. [Google Scholar] [CrossRef]
- Elhiti, M.; Yang, C.C.; Belmonte, M.F.; Gulden, R.H.; Stasolla, C. Transcriptional changes of antioxidant responses, hormone signalling and developmental processes evoked by the Brassica napus SHOOTMERISTEMLESS during in vitro embryogenesis. Plant Physiol. Biochem. 2012, 58, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, M.; Ghadimzadeh, M.; Ahmadi, B.; Silva, J.A.T. Effects of ascorbic acid, alpha-tocopherol, and glutathione on microspore embryogenesis in Brassica napus L. In Vitro Cell. Dev. Biol.-Plant 2014, 50, 26–35. [Google Scholar] [CrossRef]
- Yerzhebayeva, R.S.; Abekova, A.M.; Ainebekova, B.A.; Urazaliyev, K.R.; Bazylova, T.A.; Daniyarova, A.K.; Bersimbayeva, G.K. Influence of different concentrations of ascorbic and gibberellic acids and pH of medium on embryogenesis and regeneration in anther culture of spring triticale. Cytol. Genet. 2017, 51, 448–454. [Google Scholar] [CrossRef]
- Heidari-Zefreh, A.A.; Shariatpanahi, M.E.; Mousavi, A.; Kalatejari, S. Enhancement of microspore embryogenesis induction and plantlet regeneration of sweet pepper (Capsicum annuum L.) using putrescine and ascorbic acid. Protoplasma 2019, 256, 13–24. [Google Scholar] [CrossRef]
- Zieliński, K.; Krzewska, M.; Żur, I.; Juzoń, K.; Kopeć, P.; Nowicka, A.; Moravcikova, J.; Skrzypek, E.; Dubas, E. The effect of glutathione and mannitol on androgenesis in anther and isolated microspore cultures of rye (Secale cereale L.). PCTOC 2020, 140, 577–592. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Noctor, G.; Reichheld, J.P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.B.; Hu, X.Y.; Kang, M.; Qiu, X.L.; Liu, B.; Tang, L.; Zhu, Y.; Cao, W.X.; Liu, L.L. Effects of pre-anthesis low-temperature stress on the mineral components in wheat grains. Front. Plant Sci. 2023, 14, 1221466. [Google Scholar] [CrossRef]
- Kalisz, A.; Sekara, A.; Smolen, S.; Grabowska, A.; Gil, J.; Cebula, S. Mineral composition of cauliflowers with differently coloured curds modified by the chilling of juvenile plants. Sci. Hortic. 2018, 232, 216–225. [Google Scholar] [CrossRef]
- Ankit, A.; Kamali, S.; Singh, A. Genomic & structural diversity and functional role of potassium (K+) transport proteins in plants. Int. J. Biol. Macromol. 2022, 208, 844–857. [Google Scholar] [CrossRef]
- Ragel, P.; Raddatz, N.; Leidi, E.O.; Quintero, F.J.; Pardo, J.M. Regulation of K+ Nutrition in Plants. Front. Plant Sci. 2019, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J. Potassium control of plant functions: Ecological and agricultural implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef]
- Sieprawska, A.; Filek, M.; Walas, S.; Tobiasz, A.; Mrowiec, H.; Miszalski, Z. Does micro- and macroelement content differentiate grains of sensitive and tolerant wheat varieties? Acta Physiol. Plant. 2014, 36, 3095–3100. [Google Scholar] [CrossRef]
- Kleiner, F.H.; Helliwell, K.E.; Chrachri, A.; Hopes, A.; Parry-Wilson, H.; Gaikwad, T.; Mieszkowska, N.; Mock, T.; Wheeler, G.L.; Brownlee, C. Cold-induced Ca2+cyt elevations function to support osmoregulation in marine diatoms. Plant Physiol. 2022, 190, 1384–1399. [Google Scholar] [CrossRef]
- Zhang, X.X.; Zhang, D.; Sun, W.; Wang, T.Z. The adaptive mechanism of plants to iron deficiency via iron uptake, transport, and homeostasis. Int. J. Mol. Sci. 2019, 20, 2424. [Google Scholar] [CrossRef]
- Pennanen, A.; Xue, T.L.; Hartikainen, H. Protective role of selenium in plant subjected to severe UV irradiation stress. J. Appl. Bot.-Angew. Bot. 2002, 76, 66–76. [Google Scholar]
- Seppänen, M.; Turakainen, M.; Hartikainen, H. Selenium effects on oxidative stress in potato. Free Radic. Res. 2003, 37, 46. [Google Scholar] [CrossRef]
- Filek, M.; Keskinen, R.; Hartikainen, H.; Szarejko, I.; Janiak, A.; Miszalski, Z.; Golda, A. The protective role of selenium in rape seedlings subjected to cadmium stress. J. Plant Physiol. 2008, 165, 833–844. [Google Scholar] [CrossRef]
- Tobiasz, A.; Walas, S.; Filek, M.; Mrowiec, H.; Samsel, K.; Sieprawska, A.; Hartikainen, H. Effect of selenium on distribution of macro- and micro-elements to different tissues during wheat ontogeny. Biol. Plant. 2014, 58, 370–374. [Google Scholar] [CrossRef]
- Filek, M.; Zembala, M.; Kornas, A.; Walas, S.; Mrowiec, H.; Hartikainen, H. The uptake and translocation of macro- and microelements in rape and wheat seedlings as affected by selenium supply level. Plant Soil 2010, 336, 303–312. [Google Scholar] [CrossRef]
- Filek, M.; Kościelniak, J.; Labanowska, M.; Bednarska, E.; Bidzińska, E. Selenium-induced protection of photosynthesis activity in rape (Brassica napus) seedlings subjected to cadmium stress. Fluorescence and EPR measurements. Photosynth. Res. 2010, 105, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Sieprawska, A.; Kornaś, A.; Filek, M. Involvement of selenium in protective mechanisms of plants under environmental stress conditions—Review. Acta Biol. Crac. Ser. Bot. 2015, 57, 9–20. [Google Scholar] [CrossRef]
- Tamaoki, M.; Maruyama-Nakashita, A. Molecular mechanisms of selenium responses and resistance in plants. In Selenium in Plants. Molecular, Physiological, Ecological and Evolutionary Aspects; Pilon-Smits, E.A.H., Winkel, L.H.E., Lin, Z.-Q., Eds.; Springer: Cham, Switzerland, 2017; pp. 35–51. [Google Scholar]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.T.; Sun, J.W.; Sui, X.Y.; Yu, Y.C.; Dong, C.H. Generation of high iron content wheat mutants and expression analysis of the genes involved in iron accumulation and ethylene response. Plant Growth Regul. 2023, 100, 509–518. [Google Scholar] [CrossRef]
- Kumlehn, J.; Serazetdinova, L.; Hensel, G.; Becker, D.; Loerz, H. Genetic transformation of barley (Hordeum vulgare L.) via infection of androgenetic pollen cultures with Agrobacterium tumefaciens. Plant Biotechnol. J. 2006, 4, 251–261. [Google Scholar] [CrossRef]
- Heslop-Harrison, J.; Heslop-Harrison, Y. Evaluation of pollen viability by enzymatically induced fluorescence—Intracellular hydrolysis of fluorescein diacetate. Stain Technol. 1970, 45, 115–120. [Google Scholar] [CrossRef]
- Barnabás, B.; Szakács, É.; Karsai, I.; Bedö, Z. In vitro androgenesis of wheat: From fundamentals to practical application. Euphytica 2001, 119, 211–216. [Google Scholar] [CrossRef]
- Wang, P.; Chen, Y. Preliminary study on prediction of height of pollen H2 generation in winter wheat grown in the field. Acta Agron. Sin. 1983, 9, 283–284. [Google Scholar]
- Murashige, T.C.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Zembala, M.; Filek, M.; Walas, S.; Mrowiec, H.; Kornas, A.; Miszalski, Z.; Hartikainen, H. Effect of selenium on macro- and microelement distribution and physiological parameters of rape and wheat seedlings exposed to cadmium stress. Plant Soil 2010, 329, 457–468. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free-radical method to evaluate antioxidant activity. Food Sci. Technol.-Lebensm.-Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Law, M.Y.; Charles, S.A.; Halliwell, B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of paraquat. Biochem. J. 1983, 210, 899–903. [Google Scholar] [CrossRef]
- Foyer, C.; Rowell, J.; Walker, D. Measurements of the ascorbate content of spinach leaf protoplasts and chloroplasts during illumination. Planta 1983, 157, 239–244. [Google Scholar] [CrossRef]
- Harrach, B.D.; Fodor, J.; Pogány, M.; Preuss, J.; Barna, B. Antioxidant, ethylene and membrane leakage responses to powdery mildew infection of near-isogenic barley lines with various types of resistance. Eur. J. Plant Pathol. 2008, 121, 21–33. [Google Scholar] [CrossRef]

| Wheat Line | Treatment | GSH + GSSG | GSH | ASC + DHA | ASC |
|---|---|---|---|---|---|
| GSH + GSSG | ASC + DHA | ||||
| K20290 | Control | 697.5 ± 12.3 b | 0.72 | 1770.7 ± 3.4 d | 0.24 |
| LT | 651.7 ± 8.8 c | 0.82 | 1608.2 ± 5.9 e | 0.38 | |
| LT/SSe/MAN | 398.6 ± 9.8 f | 0.96 | 924.3 ± 3.9 g | 0.33 | |
| SM IHAR | Control | 510.4 ± 4.3 d | 0.85 | 1946.7 ± 5.9 c | 0.05 |
| LT | 885.1 ± 11.7 a | 0.26 | 1955.8 ± 3.4 c | 0.76 | |
| LT/SSe/MAN | 440.8 ± 7.0 e | 0.95 | 797.5 ± 3.8 h | 0.43 | |
| PO19 | Control | 350.2 ± 3.0 g | 0.72 | 2014.4 ± 3.9 b | 0.66 |
| LT | 377.2 ± 3.6 f | 0.41 | 2161.1 ± 12.0 a | 0.59 | |
| LT/SSe/MAN | 256.2 ± 5.4 h | 0.87 | 1199.6 ± 5.9 f | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubas, E.; Krzewska, M.; Surówka, E.; Kopeć, P.; Springer, A.; Janowiak, F.; Weigt, D.; Mikołajczyk, S.K.; Telk, A.; Żur, I. New Prospects for Improving Microspore Embryogenesis Induction in Highly Recalcitrant Winter Wheat Lines. Plants 2024, 13, 363. https://doi.org/10.3390/plants13030363
Dubas E, Krzewska M, Surówka E, Kopeć P, Springer A, Janowiak F, Weigt D, Mikołajczyk SK, Telk A, Żur I. New Prospects for Improving Microspore Embryogenesis Induction in Highly Recalcitrant Winter Wheat Lines. Plants. 2024; 13(3):363. https://doi.org/10.3390/plants13030363
Chicago/Turabian StyleDubas, Ewa, Monika Krzewska, Ewa Surówka, Przemysław Kopeć, Agnieszka Springer, Franciszek Janowiak, Dorota Weigt, Sylwia Katarzyna Mikołajczyk, Anna Telk, and Iwona Żur. 2024. "New Prospects for Improving Microspore Embryogenesis Induction in Highly Recalcitrant Winter Wheat Lines" Plants 13, no. 3: 363. https://doi.org/10.3390/plants13030363
APA StyleDubas, E., Krzewska, M., Surówka, E., Kopeć, P., Springer, A., Janowiak, F., Weigt, D., Mikołajczyk, S. K., Telk, A., & Żur, I. (2024). New Prospects for Improving Microspore Embryogenesis Induction in Highly Recalcitrant Winter Wheat Lines. Plants, 13(3), 363. https://doi.org/10.3390/plants13030363










