Patterns of Genetic and Morphological Variability of Teucrium montanum sensu lato (Lamiaceae) on the Balkan Peninsula
Abstract
1. Introduction
2. Results
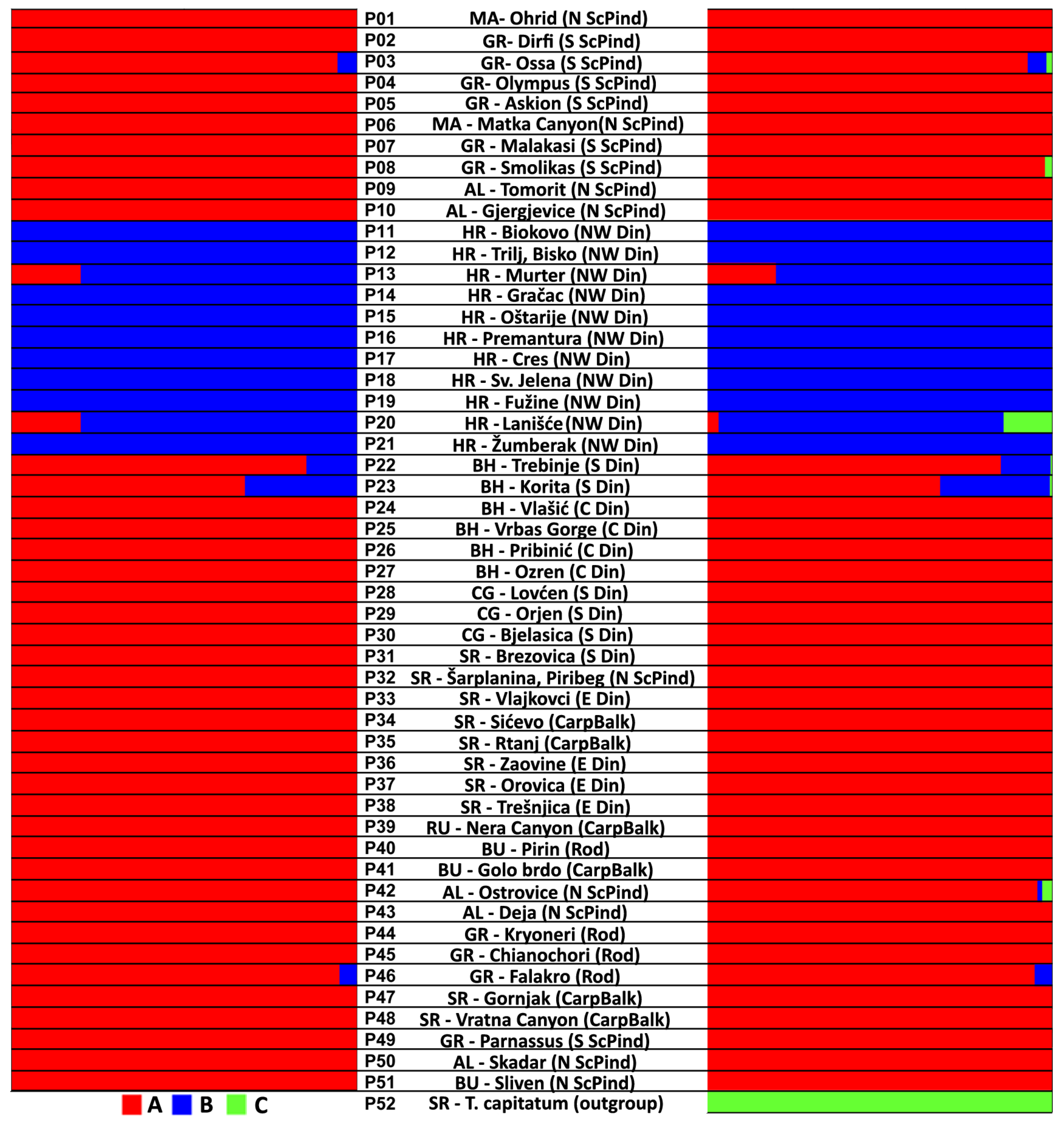
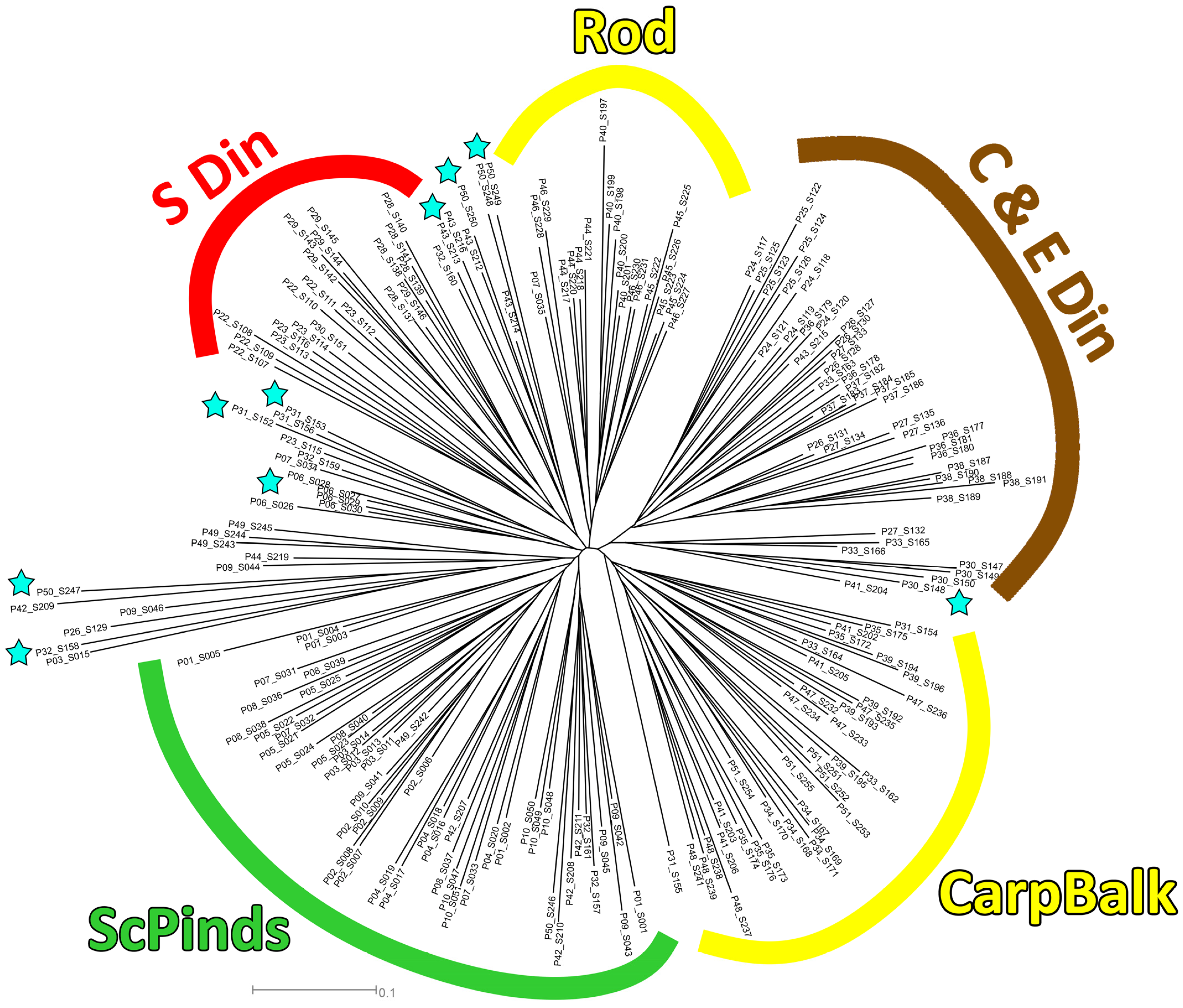
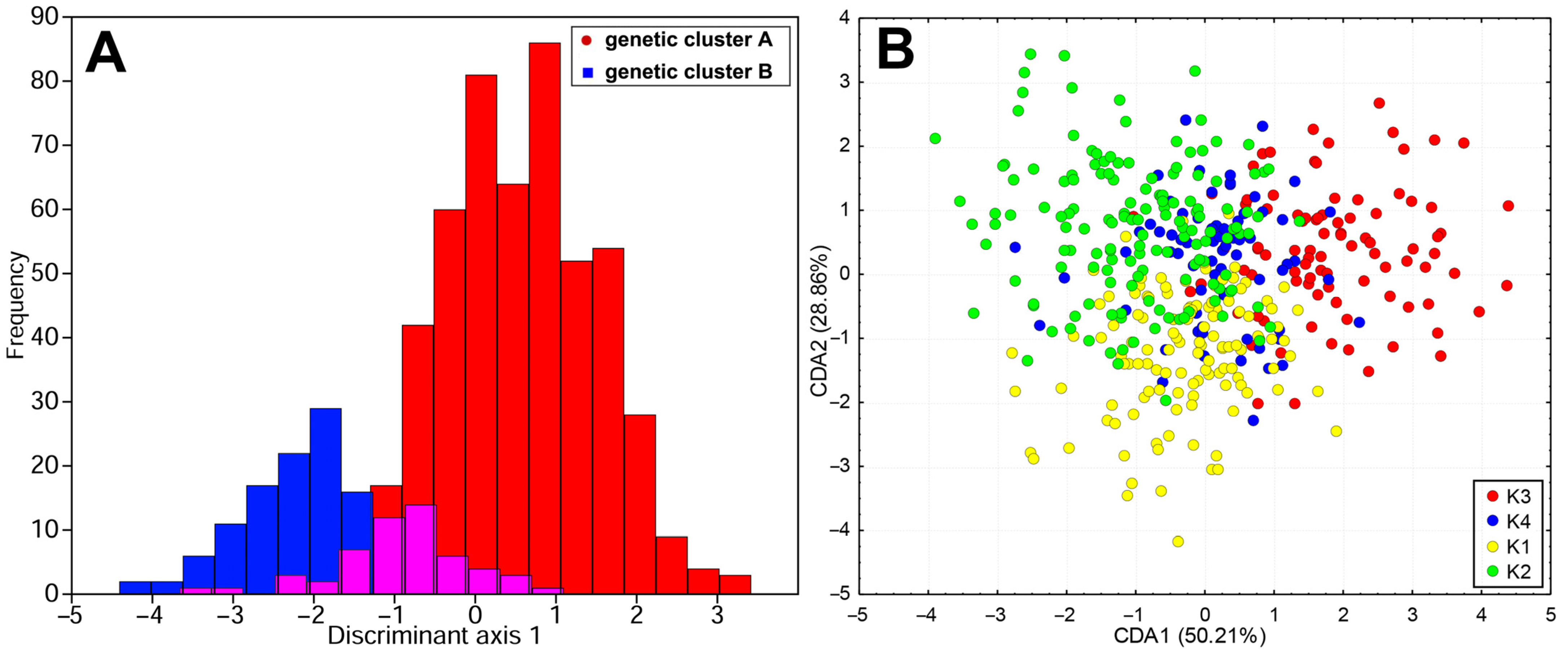
2.1. Genetic Diversification of Teucrium montanum sensu lato on the Balkan Peninsula
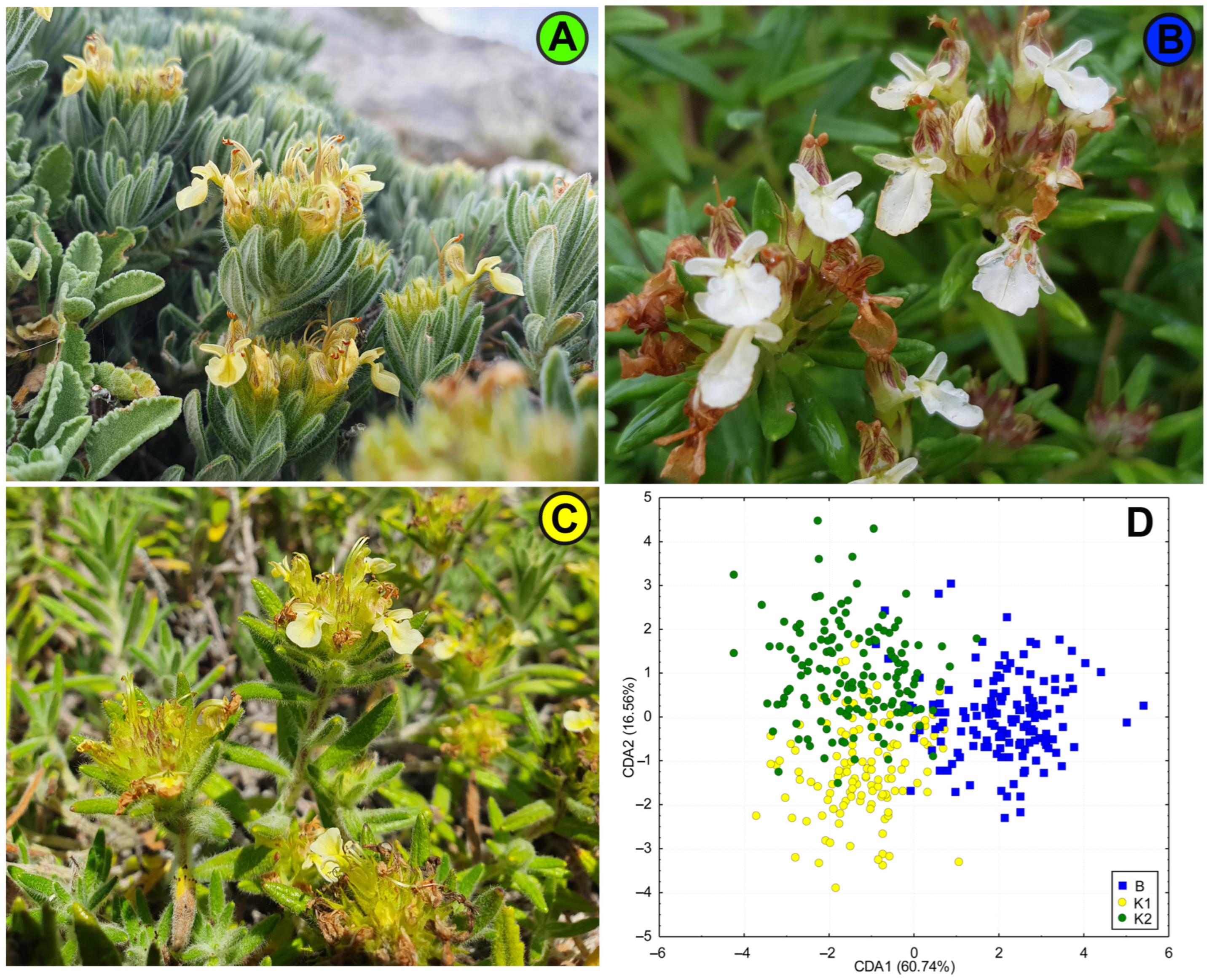
2.2. Morphological Diversification of Genetic Groups
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. DNA Isolation and AFLP Analysis
4.3. Morpho-Anatomical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayek, A. Prodromus Florae Peninsulae Balcanicae. Repert. Specierum Nov. Regni Veg. 1927, 30, 1–472. [Google Scholar]
- A.B.R. The Plant-Life of the Balkan Peninsula: A Phytogeographical Study. Nature 1929, 124, 6. [Google Scholar] [CrossRef]
- Španiel, S.; Rešetnik, I. Plant Phylogeography of the Balkan Peninsula: Spatiotemporal Patterns and Processes. Plant Syst. Evol. 2022, 308, 38. [Google Scholar] [CrossRef]
- Stevanović, V.; Tan, K.; Petrova, A. Mapping the Endemic Flora of the Balkans–a Progress Report. Bocconea 2007, 21, 131–137. [Google Scholar]
- Tomović, G.; Niketić, M.; Lakušić, D.; Ranđelović, V.; Stevanović, V. Balkan Endemic Plants in Central Serbia and Kosovo Regions: Distribution Patterns, Ecological Characteristics, and Centres of Diversity. Bot. J. Linn. Soc. 2014, 176, 173–202. [Google Scholar] [CrossRef]
- Vukojičić, S.; Jakovljević, K.; Matevski, V.; Randjelović, V.; Niketić, M.; Lakušić, D. Distribution, Diversity and Conservation of Boreo-Montane Plant Species in the Central Part of the Balkan Peninsula and the Southern Part of the Pannonian Plain. Folia Geobot. 2014, 49, 487–505. [Google Scholar] [CrossRef]
- Stevanović, B.; Stevanović, V. Morpho-Anatomical Characteristics of the Species Teucrium montanum L. from Different Habitats. Bot. Serb. 1985, 9, 73–88. [Google Scholar]
- Lakušić, D.; Lakušić, B. Morpho-Anatomical Differentiation of the Species Teucrium montanum (Lamiaceae) in the Central Balkan Peninsula. Bot. Serb. 2014, 38, 109–120. [Google Scholar]
- Zbiljić, M.; Lakušić, B.; Kuzmanović, N.; Stojanović, D.; Lakušić, D. Morphological Diversification of Teucrium montanum sensu lato on the Balkan Peninsula. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2023, 157, 670–687. [Google Scholar] [CrossRef]
- Tutin, T.G.; Wood, D. Teucrium. Flora Eur. 1972, 3, 129–135. [Google Scholar]
- Lakušić, B.; Stevanović, B.; Jančić, R.; Lakušić, D. Habitat-Related Adaptations in Morphology and Anatomy of Teucrium (Lamiaceae) Species from the Balkan Peninsula (Serbia and Montenegro). Flora-Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 633–646. [Google Scholar] [CrossRef]
- Zlatić, N.; Budečević, S.; Stanković, M. Geological Substrate Effects on Teucrium montanum L. (Lamiaceae) Morphological Traits: Geometric Morphometrics Approach. Plants 2023, 12, 2381. [Google Scholar] [CrossRef]
- Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. 2023. Available online: https://powo.science.kew.org (accessed on 25 November 2024).
- Euro+Med. Euro+Med PlantBase: The Information Resource for Euro-Mediterranean Plant Diversity. Continuously Updated. Available online: http://www.europlusmed.org (accessed on 25 November 2024).
- Zbiljić, M.; Lakušić, D.; Stevanoski, I.; Kuzmanović, N. Lectotypification of Names Related to Teucrium montanum L. (Lamiaceae) Reported for the Balkan Peninsula. Phytotaxa 2022, 530, 198–204. [Google Scholar] [CrossRef]
- Diklić, N. Teucrium. In Flora of the Socialist Republic of Serbia; Serbian Academy of Sciences and Arts: Belgrade, Serbia, 1974; pp. 349–356. [Google Scholar]
- Peev, D. Flora of the People’s Republic of Bulgaria; Bulgarian Academy of Sciences Publishing House: Sofia, Bulgaria, 1989; Volume 9. [Google Scholar]
- Mártonfi, P. Teucrium Montanum (Lamiaceae) in the Czech and Slovak Republics. Preslia Praha 1995, 66, 289–304. [Google Scholar]
- Herrmann, D.; Poncet, B.N.; Manel, S.; Rioux, D.; Gielly, L.; Taberlet, P.; Gugerli, F. Selection Criteria for Scoring Amplified Fragment Length Polymorphisms (AFLPs) Positively Affect the Reliability of Population Genetic Parameter Estimates. Genome 2010, 53, 302–310. [Google Scholar] [CrossRef]
- Lakušić, D.; Liber, Z.; Nikolić, T.; Surina, B.; Kovačić, S.; Bogdanović, S.; Stefanović, S. Molecular Phylogeny of the Campanula pyramidalis Species Complex (Campanulaceae) Inferred from Chloroplast and Nuclear Non-Coding Sequences and Its Taxonomic Implications. Taxon 2013, 62, 505–524. [Google Scholar] [CrossRef]
- Surina, B.; Schönswetter, P.; Schneeweiss, G.M. Quaternary Range Dynamics of Ecologically Divergent Species (Edraianthus serpyllifolius and E. tenuifolius, Campanulaceae) within the Balkan Refugium: Quaternary Range Dynamics within the Balkan Refugium. J. Biogeogr. 2011, 38, 1381–1393. [Google Scholar] [CrossRef]
- Mereda, P.; Hodálová, I.; Kučera, J.; Zozomová-Lihová, J.; Letz, D.R.; Slovák, M. Genetic and Morphological Variation in Viola suavis s.l. (Violaceae) in the Western Balkan Peninsula: Two Endemic Subspecies Revealed. Syst. Biodivers. 2011, 9, 211–231. [Google Scholar] [CrossRef]
- Surina, B.; Schneeweiss, G.M.; Glasnović, P.; Schönswetter, P. Testing the Efficiency of Nested Barriers to Dispersal in the Mediterranean High Mountain Plant Edraianthus graminifolius (Campanulaceae). Mol. Ecol. 2014, 23, 2861–2875. [Google Scholar] [CrossRef] [PubMed]
- Rešetnik, I.; Baričevič, D.; Batîr Rusu, D.; Carović-Stanko, K.; Chatzopoulou, P.; Dajić-Stevanović, Z.; Gonceariuc, M.; Grdiša, M.; Greguraš, D.; Ibraliu, A. Genetic Diversity and Demographic History of Wild and Cultivated/Naturalised Plant Populations: Evidence from Dalmatian Sage (Salvia officinalis L., Lamiaceae). PLoS ONE 2016, 11, e0159545. [Google Scholar] [CrossRef] [PubMed]
- Kuzmanović, N.; Comanescu, P.; Frajman, B.; Lazarević, M.; Paun, O.; Schönswetter, P.; Lakušić, D. Genetic, Cytological and Morphological Differentiation within the Balkan-Carpathian Sesleria rigida Sensu Fl. Eur. (Poaceae): A Taxonomically Intricate Tetraploid-octoploid Complex. Taxon 2013, 62, 458–472. [Google Scholar] [CrossRef]
- Škondrić, S.; Aleksić, J.M.; Lakušić, D. Campanula cichoracea (Campanulaceae), a Neglected Species from the Balkan-Carpathian C. lingulata Complex as Inferred from Molecular and Morphological Characters. Willdenowia 2014, 44, 77–96. [Google Scholar] [CrossRef]
- Janković, I.; Šatović, Z.; Liber, Z.; Kuzmanović, N.; Radosavljević, I.; Lakušić, D. Genetic Diversity and Morphological Variability in the Balkan Endemic Campanula secundiflora s.l. (Campanulaceae). Bot. J. Linn. Soc. 2016, 180, 64–88. [Google Scholar] [CrossRef]
- Đurovic, S.; Schönswetter, P.; Niketic, M.; Tomovic, G.; Frajman, B. Disentangling Relationships among the Members of the Silene saxífraga Alliance (Caryophyllaceae): Phylogenetic Structure Is Geographically Rather than Taxonomically Segregated. Taxon 2017, 66, 343–364. [Google Scholar] [CrossRef]
- Španiel, S.; Zozomová-Lihová, J.; Marhold, K. Revised Taxonomic Treatment of the Alyssum montanum—A. repens Complex in the Balkans: A Multivariate Morphometric Analysis. Plant Syst. Evol. 2017, 303, 1413–1442. [Google Scholar] [CrossRef]
- Carnicero, P.; Garcia-Jacas, N.; Sáez, L.; Constantinidis, T.; Galbany-Casals, M. Disentangling Relationships among Eastern Mediterranean Cymbalaria Including Description of a Novel Species from the Southern Peloponnese (Greece). Plant Syst. Evol. 2021, 307, 13. [Google Scholar] [CrossRef]
- Varga, F.; Liber, Z.; Turudić, A.; Jakše, J.; Juzbašić, L.; Jeran, N.; Grdiša, M.; Zbiljić, M.; Šatović, Z. Joint Identification and Application of Microsatellite Markers in Genetic Diversity Study of Closely Related Species Teucrium montanum, T. capitatum and Their Natural Hybrid. Diversity 2024, 16, 206. [Google Scholar] [CrossRef]
- Lakušić, D.; Zbiljić, M.; Šatović, Z.; Kuzmanović, N.; Liber, Z. Asymmetric Introgression and Cryptic Natural Hybridization between Two Species of Teucrium Section Polium (Lamiaceae) on the Balkan Peninsula. Plants 2024, 13, 1617. [Google Scholar] [CrossRef] [PubMed]
- Riseberg, L.H.; Wendel, J.F. Introgression and Its Consequences. In Hybrid Zones and the Evolutionary Process; Oxford University Press: New York, NY, USA, 1993; pp. 70–109. [Google Scholar]
- Rhymer, J.M.; Simberloff, D. Extinction by Hybridization and Introgression. Annu. Rev. Ecol. Syst. 1996, 27, 83–109. [Google Scholar] [CrossRef]
- Salmaki, Y.; Kattari, S.; Heubl, G.; Bräuchler, C. Phylogeny of Non-monophyletic Teucrium (Lamiaceae: Ajugoideae): Implications for Character Evolution and Taxonomy. Taxon 2016, 65, 805–822. [Google Scholar] [CrossRef]
- Zbiljić, M. Morfoanatomska i Genotipska Diverzifikacija Teucrium montanum sensu lato Na Prostoru Balkanskog Poluostrva. Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 2023. [Google Scholar]
- Carović-Stanko, K.; Liber, Z.; Politeo, O.; Strikić, F.; Kolak, I.; Milos, M.; Satovic, Z. Molecular and Chemical Characterization of the Most Widespread Ocimum Species. Plant Syst. Evol. 2011, 294, 253–262. [Google Scholar] [CrossRef]
- Schönswetter, P.; Tribsch, A. Vicariance and Dispersal in the Alpine Perennial Bupleurum stellatum L. (Apiaceae). Taxon 2005, 54, 725–732. [Google Scholar] [CrossRef]
- Ehrich, D. aflpdat: A Collection of r Functions for Convenient Handling of AFLP Data. Mol. Ecol. Notes 2006, 6, 603–604. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; The University of Illinois Press: Champaign, IL, USA, 1949. [Google Scholar]
- Lewontin, R.C. The Apportionment of Human Diversity. In The Concept of Race in Natural and Social Science; Routledge: London, UK, 2014; pp. 7–24. [Google Scholar]
- Zhivotovsky, L.A. Estimating Population Structure in Diploids with Multilocus Dominant DNA Markers. Mol. Ecol. 1999, 8, 907–913. [Google Scholar] [CrossRef]
- Vekemans, X.; Beauwens, T.; Lemaire, M.; Roldán-Ruiz, I. Data from Amplified Fragment Length Polymorphism (AFLP) Markers Show Indication of Size Homoplasy and of a Relationship between Degree of Homoplasy and Fragment Size. Mol. Ecol. 2002, 11, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Nei, M. Genetic Distance between Populations. Am. Nat. 1972, 106, 283–292. [Google Scholar] [CrossRef]
- Felsenstein, J. PHYLIP (Phylogeny Inference Package), Version 3.6; Distributed by the Author; Department of Genome Sciences, University of Washington: Seattle, WA, USA, 2005.
- Bryant, D.; Moulton, V. Neighbor-Net: An Agglomerative Method for the Construction of Phylogenetic Networks. Mol. Biol. Evol. 2004, 21, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Huson, D.H.; Bryant, D. Application of Phylogenetic Networks in Evolutionary Studies. Mol. Biol. Evol. 2006, 23, 254–267. [Google Scholar] [CrossRef]
- Corander, J.; Waldmann, P.; Sillanpää, M.J. Bayesian Analysis of Genetic Differentiation between Populations. Genetics 2003, 163, 367–374. [Google Scholar] [CrossRef]
- Corander, J.; Marttinen, P. Bayesian Identification of Admixture Events Using Multilocus Molecular Markers. Mol. Ecol. 2006, 15, 2833–2843. [Google Scholar] [CrossRef]
- Hartigan, J.A.; Wong, M.A. Algorithm AS 136: A k-Means Clustering Algorithm. J. R. Stat. Soc. Ser. C Appl. Stat. 1979, 28, 100–108. [Google Scholar] [CrossRef]
- Arrigo, N.; Tuszynski, J.W.; Ehrich, D.; Gerdes, T.; Alvarez, N. Evaluating the Impact of Scoring Parameters on the Structure of Intra-Specific Genetic Variation Using RawGeno, an R Package for Automating AFLP Scoring. BMC Bioinform. 2009, 10, 33. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the Number of Clusters of Individuals Using the Software structure: A Simulation Study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- STATISTICA (Data Analysis Software System), Version 8; Statsoft, Inc.: Tulsa, OK, USA, 2007.
- Hammer, Ø.; Harper, D.A. Past: Paleontological Statistics Software Package for Educaton and Data Anlysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zbiljić, M.; Lakušić, D.; Šatović, Z.; Liber, Z.; Kuzmanović, N. Patterns of Genetic and Morphological Variability of Teucrium montanum sensu lato (Lamiaceae) on the Balkan Peninsula. Plants 2024, 13, 3596. https://doi.org/10.3390/plants13243596
Zbiljić M, Lakušić D, Šatović Z, Liber Z, Kuzmanović N. Patterns of Genetic and Morphological Variability of Teucrium montanum sensu lato (Lamiaceae) on the Balkan Peninsula. Plants. 2024; 13(24):3596. https://doi.org/10.3390/plants13243596
Chicago/Turabian StyleZbiljić, Miloš, Dmitar Lakušić, Zlatko Šatović, Zlatko Liber, and Nevena Kuzmanović. 2024. "Patterns of Genetic and Morphological Variability of Teucrium montanum sensu lato (Lamiaceae) on the Balkan Peninsula" Plants 13, no. 24: 3596. https://doi.org/10.3390/plants13243596
APA StyleZbiljić, M., Lakušić, D., Šatović, Z., Liber, Z., & Kuzmanović, N. (2024). Patterns of Genetic and Morphological Variability of Teucrium montanum sensu lato (Lamiaceae) on the Balkan Peninsula. Plants, 13(24), 3596. https://doi.org/10.3390/plants13243596







