Optimization of γ-Aminobutyric Acid Production in Brown Rice via Prolonged Seed Priming
Abstract
1. Introduction
2. Results
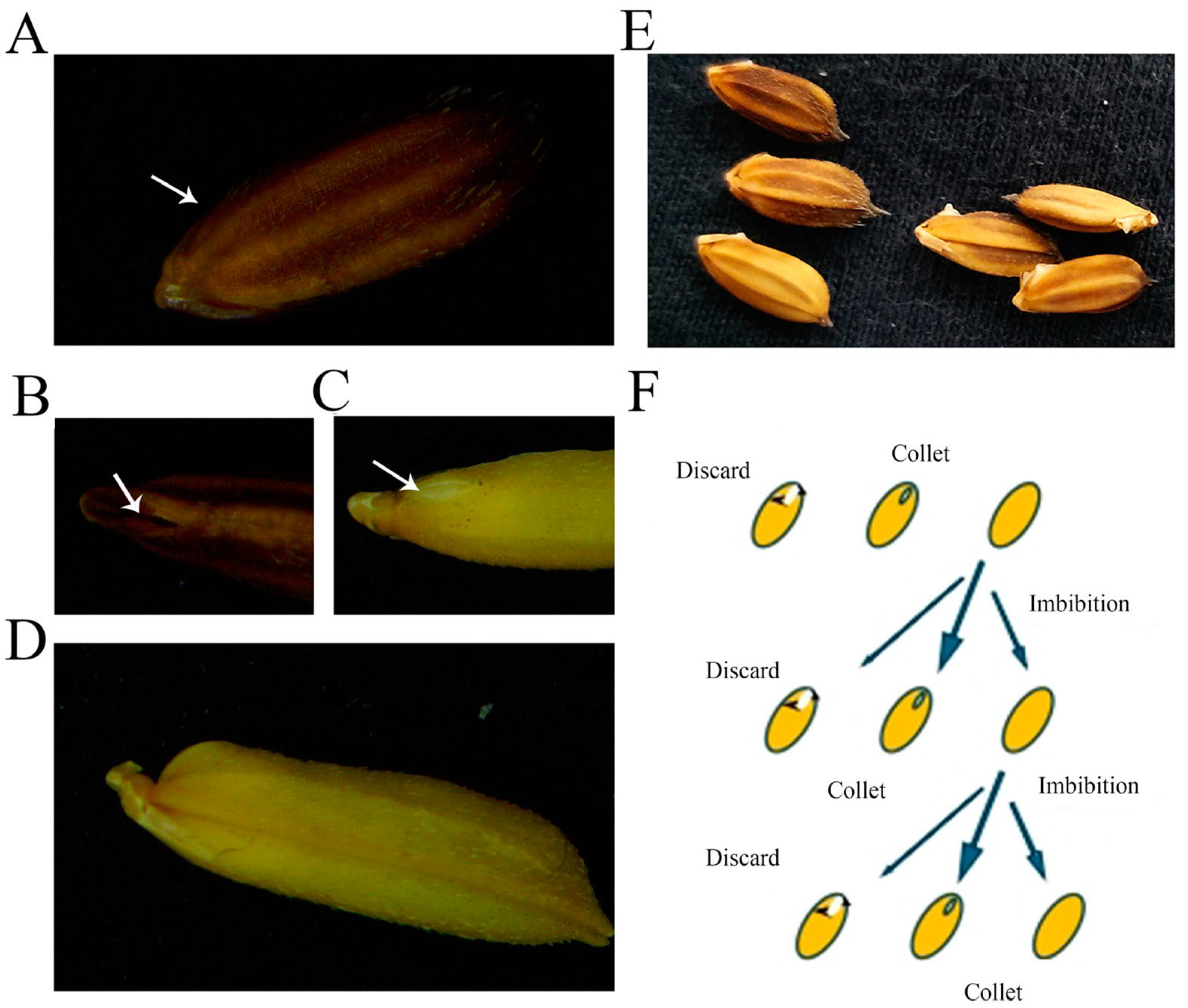
2.1. PLP Demonstrated Effective Embryo Exposure
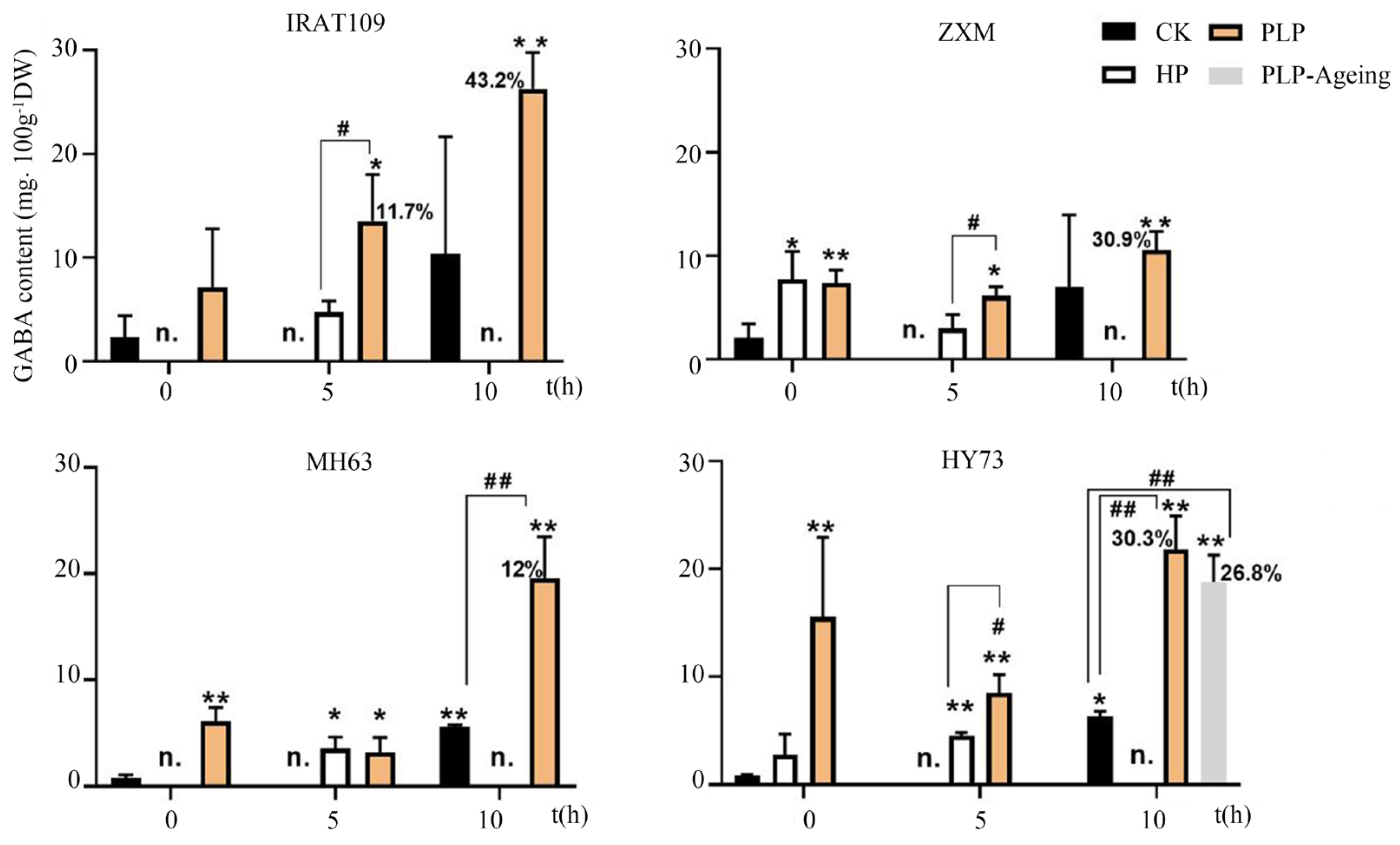
2.2. PLP Increased GABA Synthesis
2.3. PLP Preserved Better Grain Wholeness and Bran Color than HP
2.4. Benefits of PLP on Nutrient Levels
3. Discussion
3.1. Feasibility of PLP and Short Germination for GBR Production
3.2. PLP Induces Multiple Bioactive Nutrients
3.3. PLP Requires a Desiccation-Tolerant Variety
4. Material and Methods
4.1. Materials and Processing
4.2. Evalution of Embryo Exposure and DT
4.3. Examination of Brown Rice’s Physical Characteristics
4.4. Analysis of Brown Rice’s Bioactive Nutrients
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PLP | prolonged priming |
| DT | desiccation tolerance |
| GABA | γ-aminobutyric acid |
| GBR | germinated brown rice |
| HY73 | Hanyou73 |
| MH63 | MingHui63 |
| ZXM | ZhaXiMa |
| MDA | malondialdehyde |
| OPA | o-phthalaldehyde |
| CK | control |
References
- Guo, S.; Ge, Y.; Na, J.K. A review of phytochemistry, metabolite changes, and medicinal uses of the common sunflower seed and sprouts (Helianthus annuus L.). Chem. Cent. J. 2017, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Ikram, A.; Saeed, F.; Afzaal, M.; Imran, A.; Niaz, B.; Tufail, T.; Hussain, M.; Anjum, F.M. Nutritional and end-use perspectives of sprouted grains: A comprehensive review. Food Sci. Nutr. 2021, 9, 4617–4628. [Google Scholar] [CrossRef]
- Idowu, A.T.; Olatunde, O.O.; Adekoya, A.E.; Idowu, S. Germination: An alternative source to promote phytonutrients in edible seeds. Food Qual. Saf. 2020, 4, 129–133. [Google Scholar] [CrossRef]
- Garg, M.; Sharma, A.; Vats, S.; Tiwari, V.; Kumari, A.; Mishra, V.; Krishania, M. Vitamins in Cereals: A Critical Review of Content, Health Effects, Processing Losses, Bioaccessibility, Fortification, and Biofortification Strategies for Their Improvement. Front. Nutr. 2021, 8, 586815. [Google Scholar] [CrossRef]
- Beaulieu, J.C.; Boue, S.M.; Goufo, P. Health-promoting germinated rice and value-added foods: A comprehensive and systematic review of germination effects on brown rice. Crit. Rev. Food Sci. Nutr. 2023, 63, 11570–11603. [Google Scholar] [CrossRef] [PubMed]
- Sitanggang, A.B.; Joshua, M.; Munarko, H.; Kusnandar, F.; Budijanto, S. Increased γ-Aminobutyric Acid Content of Germinated Brown Rice Produced in Membrane Reactor. Food Technol. Biotechnol. 2021, 59, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Kamjijam, B.; Suwannaporn, P.; Bednarz, H.; Na, J.K.; Niehaus, K. Elevation of gamma-aminobutyric acid (GABA) and essential amino acids in vacuum impregnation mediated germinated rice traced by MALDI imaging. Food Chem. 2021, 365, 130399. [Google Scholar] [CrossRef]
- Soi-ampornkul, R.; Kanyok, S.; Junnu, S.; Liammongkolkul, S.; Katunyoo, W.; Umpornsirirat, S.; Soi-ampornkul, P. Potent antioxidant and anti-apoptotic activity of pre-germinated brown rice extract against hydrogen peroxide in neuronal SK-N-SH cells: A model of Alzheimer’s disease. Alzheimer’s Dement. 2012, 45, 503. [Google Scholar] [CrossRef]
- Jabeen, R.; Jan, N.; Naseer, B.; Sarangi, P.K.; Sridhar, K.; Dikkala, P.K.; Bhaswant, M.; Hussain, S.Z.; Inbaraj, B.S. Development of Germinated-Brown-Rice-Based Novel Functional Beverage Enriched with γ-Aminobutyric Acid: Nutritional and Bio-Functional Characterization. Foods 2024, 13, 1282. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Kaufman, D.L. The GABA and GABA-Receptor System in Inflammation, Anti-Tumor Immune Responses, and COVID-19. Biomedicines 2023, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.L.; Chen, F.S.; Yan Zhao, Y.; Gu, Z.X.; Yang, H.S. Selenium accumulation in protein fractions during germination of Se-enriched brown rice and molecular weights distribution of Se-containing proteins. Food Chem. 2011, 127, 1526–1531. [Google Scholar] [CrossRef]
- Liu, K.; Ning, M. Antioxidant activity stability and digestibility of protein from Se-enriched germinated brown rice. LWT 2021, 142, 111032. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Yu, R.; Huang, D.; Chen, S.; Zhu, S. Effects of Different Drying Methods on the Selenium Bioaccessibility and Antioxidant Activity of Cardamine violifolia. Foods 2023, 12, 758. [Google Scholar] [CrossRef]
- Jeevan Dananjaya Liyanaarachchi, G.V.V.; Mahanama, K.R.R.; Somasiri, S.; Punyasiri, N.; Gunawardhana, K.V.T.; Kottawa-Arachchi, J.D. Impact of parboiling and cultivars on the free and total amino acid composition of rice (Oryza sativa L.). J. Food Process Preserv. 2021, 45, e15763. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, Y.; Fang, W.; Yang, Z. Potential of germinated brown rice in beer brewing. J. Cereal Sci. 2023, 114, 103792. [Google Scholar] [CrossRef]
- Yu, S.M.; Lee, H.T.; Lo, S.F.; Ho, T.D. How does rice cope with too little oxygen during its early life? New Phytol. 2021, 229, 36–41. [Google Scholar] [CrossRef]
- Ren, M.; Tan, B.; Xu, J.; Yang, Z.; Zheng, H.; Tang, Q.; Zhang, X.; Wang, W. Priming methods affected deterioration speed of primed rice seeds by regulating reactive oxygen species accumulation, seed respiration and starch degradation. Front. Plant Sci. 2023, 14, 1267103. [Google Scholar] [CrossRef]
- Xu, L.X.; Xin, X.; Yin, G.K.; Zhou, J.; Zhou, Y.C.; Lu, X.X. Timing for antioxidant-priming against rice seed ageing: Optimal only in non-resistant stage. Sci. Rep. 2020, 10, 13294. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, R.; Caballero, I.; Nimubona, D.; Blanco, C.A. Brewing with Starchy Adjuncts: Its Inffuence on the Sensory and Nutritional Properties of Beer. Foods 2021, 10, 1726. [Google Scholar] [CrossRef] [PubMed]
- Kamjijam, B.; Bednarz, H.; Suwannaporn, P.; Jom, K.N.; Niehaus, K. Localization of amino acids in germinated rice grain: Gamma-aminobutyric acid and essential amino acids production approach. J. Cereal Sci. 2020, 93, 102958. [Google Scholar] [CrossRef]
- Tomé-Sánchez, I.; Martín-Diana, A.B.; Peñas, E.; Bautista-Expósito, S.; Frias, J.; Rico, D.; González-Maillo, L.; Martinez-Villaluenga, C. Soluble Phenolic Composition Tailored by Germination Conditions Accompany Antioxidant and Anti-inflammatory Properties of Wheat. Antioxidants 2020, 9, 426. [Google Scholar] [CrossRef]
- Xie, Y.N.; Qi, Q.Q.; Li, W.H.; Li, Y.L.; Zhang, Y.; Wang, H.M.; Zhang, Y.F.; Ye, Z.H.; Guo, D.P.; Qian, Q.; et al. Domestication, breeding, omics research, and important genes of Zizania latifolia and Zizania palustris. Front. Plant Sci. 2023, 14, 1183739. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, H.S.; Lim, S.T.; Reddy, C.K. Enhanced accumulation of gamma-aminobutyric acid in rice bran using anaerobic incubation with various additives. Food Chem. 2019, 271, 187–192. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, E.J.; Lim, S.T.; Han, J.A. Self-enhancement of GABA in rice bran using various stress treatments. Food Chem. 2015, 172, 657–662. [Google Scholar] [CrossRef]
- Huang, Y.; Lei, N.; Xiong, Y.; Liu, Y.; Tong, L.; Wang, F.; Fan, B.; Maesen, P.; Blecker, C. Influence of Selenium Biofortification of Soybeans on Speciation and Transformation during Seed Germination and Sprouts Quality. Foods 2022, 11, 1200. [Google Scholar] [CrossRef] [PubMed]
- Rico, D.; Peñas, E.; Del Carmen García, M.; Rai, D.K.; Martínez-Villaluenga, C.; Frias, J.; Martín-Diana, A.B. Development of Antioxidant and Nutritious Lentil (Lens culinaris) Flour Using Controlled Optimized Germination as a Bioprocess. Foods 2021, 10, 2924. [Google Scholar] [CrossRef] [PubMed]
- Yodpitak, S.; Mahatheeranont, S.; Boonyawan, D.; Sookwong, P.; Roytrakul, S.; Norkaew, O. Cold plasma treatment to improve germination and enhance the bioactive phytochemical content of germinated brown rice. Food Chem. 2019, 289, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Zarei, I.; Brown, D.G.; Nealon, N.J.; Ryan, E.P. Rice Bran Metabolome Contains Amino Acids, Vitamins & Cofactors, and Phytochemicals with Medicinal and Nutritional Properties. Rice 2017, 10, 24. [Google Scholar] [CrossRef]
- Chen, R.; Yan, X.; Cai, M.; Cai, J.; Dai, T.; Liu, Y.; Wu, J. Impact of Germination on the Edible Quality and Nutritional Properties of Brown Rice Noodles. Foods 2024, 13, 2152. [Google Scholar] [CrossRef] [PubMed]
- Shanghai Academy of Agricultural Sciences. “Hanyou 73” Won the Second National Gold Medal for Taste-Quality Among Rice Varieties. 2019. Available online: https://www.saas.sh.cn/xwzx/ztzl/xczx/dxal/content_24235 (accessed on 26 June 2024).
- Morita, N.; Maeda, T.; Watanabe, M.; Yano, S. Pre-germinated brown rice substituted bread: Dough characteristics and bread structure. Int. J. Food Prop. 2007, 10, 779–789. [Google Scholar] [CrossRef]
- Yang, S.Y.; Lu, Z.X.; Jiao, Y.; Ai, J.; Lu, F.X.; Bie, X.M. Determination of γ-aminobutyric acid in broth of fermentation by high-performance liquid chromatograph. J. Guangxi Agric. Biol. Sci. 2007, 4, 331–334. [Google Scholar]




| Indica | Germination Percentage | Standard Error | Japonica | Germination Percentage | Standard Error |
|---|---|---|---|---|---|
| HY73 | 72.97% | 2.77% | IRAT109 | 81.97% | 4.48% |
| MH63 | 61.88% | 9.82% | ZXM | 50.45% | 8.46% |
| Nagina22 | 98.35% | 0.84% | YunLu8 | 81.92% | 9.32% |
| 9311 | 94.96% | 0.99% | LongHuaMaoHu | 95.11% | 0.83% |
| ZhenShan97B | 95.45% | 1.66% | NanGeng46 | 11.74% | 5.48% |
| HanHui3 | 93.62% | 8.33% | Nipponbare | 39.14% | 11.53% |
| HanHui15 | 85.76% | 3.91% | WuYuGeng39 | 9.07% | 3.58% |
| HuangHuaZhan | 76.14% | 4.95% | NanGeng9108 | 20.22% | 2.99% |
| HuHan1B | 22.93% | 6.00% |
| Round of Collection | 1st | 2nd | 3rd | 4th |
|---|---|---|---|---|
| IRAT109 | 24 h | 30 h | 36 h | |
| ZXM | 30 h | 36 h | 42 h | 48 h |
| MH63 | 27 h | 36 h | 42 h | |
| HY73 | 28 h | 36 h | 42 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Wang, X.; Li, Q.; Niu, Y.; Ding, G.; He, J.; Chen, W.; Tian, D. Optimization of γ-Aminobutyric Acid Production in Brown Rice via Prolonged Seed Priming. Plants 2024, 13, 3594. https://doi.org/10.3390/plants13243594
Xu L, Wang X, Li Q, Niu Y, Ding G, He J, Chen W, Tian D. Optimization of γ-Aminobutyric Acid Production in Brown Rice via Prolonged Seed Priming. Plants. 2024; 13(24):3594. https://doi.org/10.3390/plants13243594
Chicago/Turabian StyleXu, Lingxiang, Xiaoan Wang, Qixiang Li, Yuqing Niu, Guohui Ding, Jiawei He, Weiping Chen, and Dagang Tian. 2024. "Optimization of γ-Aminobutyric Acid Production in Brown Rice via Prolonged Seed Priming" Plants 13, no. 24: 3594. https://doi.org/10.3390/plants13243594
APA StyleXu, L., Wang, X., Li, Q., Niu, Y., Ding, G., He, J., Chen, W., & Tian, D. (2024). Optimization of γ-Aminobutyric Acid Production in Brown Rice via Prolonged Seed Priming. Plants, 13(24), 3594. https://doi.org/10.3390/plants13243594





