A Study on the Effect of Indirect Nitrate Supply on the Nitrogen Fixation Capacity of Soybean Nodules
Abstract
1. Introduction
2. Results
2.1. Soybean Nodule N Fixation Ability Affected by Nitrate
2.2. Soybean Nodule Carbohydrate Concentration Affected by Nitrate
2.3. Soybean Nodule Microstructure Affected by Nitrate
3. Discussion
3.1. Effect of Nitrate on Nodulation and N Fixation of Soybean
3.2. The Relationship Between Nitrate, Carbohydrate, and Nodulation N Fixation
4. Materials and Methods
4.1. Experimental Design
4.2. Sampling and Measurement
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sulieman, S.; Abdelrahman, M.; Tran, L.-S. Carbon metabolic adjustment in soybean nodules in response to phosphate limitation: A metabolite perspective. Environ. Exp. Bot. 2022, 196, 104810. [Google Scholar] [CrossRef]
- Woodall, J.; Forde, B. Glutamine synthetase polypeptides in the roots of 55 legume species in relation to their climatic origin and the partitioning of nitrate assimilation. Plant Cell Environ. 2006, 19, 848–858. [Google Scholar] [CrossRef]
- Fujikake, H.; Yamazaki, A.; Ohtake, N.; Sueyoshi, K.; Matsubayashi, S.; Ito, T.; Mizuniwa, C.; Kume, T.; Hashimoto, S.; Ishioka, N.; et al. Quick and reversible inhibition of soybean root nodule growth by nitrate involves a decrease in sucrose supply to nodules. J. Exp. Bot. 2003, 54, 1379–1388. [Google Scholar] [CrossRef]
- Li, S.; Lyu, X.; Wang, X.; Zhao, S.; Ma, C.; Yan, C.; Gong, Z. Assimilation of nitrate into asparagine for transport in soybeans. Agronomy-Basel 2023, 13, 2767. [Google Scholar] [CrossRef]
- Sulieman, S.; Fischinger, S.; Gresshoff, P.; Schulze, J. Asparagine as a major factor in the N-feedback regulation of N2 fixation in Medicago truncatula. Physiol. Plant. 2010, 140, 21–31. [Google Scholar] [CrossRef]
- Matamoros, M.; Baird, L.; Escuredo, K.; Dalton, D.; Minchin, F.; Iturbe-Ormaetxe, I.; Rubio, M.; Moran, J.; Gordon, A.; Becana, M. Stress-induced legume root nodule senescence. physiological, biochemical, and structural alterations. Plant Physiol. 1999, 121, 97–112. [Google Scholar] [CrossRef]
- Wasfi, M.; Prioul, J. A comparison of inhibition of French-bean and soybean nitrogen fixation by nitrate, 1% oxygen or direct assimilate deprivation. Physiol. Plant. 1986, 66, 481–490. [Google Scholar] [CrossRef]
- Fujikake, H.; Yashima, H.; Sato, T.; Norikuni, O.; Sueyoshi, K.; Ohyama, T. Rapid and reversible nitrate inhibition of nodule growth and N2 fixation activity in soybean (Glycine max (L.) Merr.). Soil Sci. Plant Nutr. 2002, 48, 211–217. [Google Scholar] [CrossRef]
- Lyu, X.; Xia, X.; Wang, C.; Ma, C.; Dong, S.; Gong, Z. Effects of changes in applied nitrogen concentrations on nodulation, nitrogen fixation and nitrogen accumulation during the soybean growth period. Soil Sci. Plant Nutr. 2019, 65, 479–489. [Google Scholar] [CrossRef]
- Li, S.; Xiao, F.; Yang, D.; Lyu, X.; Ma, C.; Dong, S.; Yan, C.; Gong, Z. Nitrate transport and distribution in soybean plants with dual-root systems. Front. Plant Sci. 2021, 12, 661054. [Google Scholar] [CrossRef]
- Saito, A.; Tanabata, S.; Tanabata, T.; Tajima, S.; Ueno, M.; Ishikawa, S.; Ohtake, N.; Sueyoshi, K.; Ohyama, T. Effect of nitrate on nodule and root growth of soybean (Glycine max (L.) Merr.). Int. J. Mol. Sci. 2014, 15, 4464–4480. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Ono, Y.; Ohtake, N.; Sueyoshi, K.; Tanabata, S.; Ohyama, T. Transcriptome and metabolome analyses reveal that nitrate strongly promotes nitrogen and carbon metabolism in soybean roots, but tends to repress it in nodules. Plants 2018, 7, 32. [Google Scholar] [CrossRef]
- Gordon, A.; Skøt, L.; James, C.; Minchin, F. Short-term metabolic responses of soybean root nodules to nitrate. J. Exp. Bot. 2002, 53, 423–428. [Google Scholar] [CrossRef]
- Paau, A.; Cowles, J. Bacteroid distribution in alfalfa nodules upon dark-induced senescence and subsequent partial rejuvenation. Physiol. Plant. 1981, 52, 43–46. [Google Scholar] [CrossRef]
- Vauclare, P.; Bligny, R.; Gout, E.; De Meuron, V.; Widmer, F. Metabolic and structural rearrangement during dark-induced autophagy in soybean (Glycine max L.) nodules: An electron microscopy and 31P and 13C nuclear magnetic resonance study. Planta 2010, 231, 1495–1504. [Google Scholar] [CrossRef]
- Selker, J.; Newcomb, E. Spatial relationships between uninfected and infected cells in root nodules of soybean. Planta 1985, 165, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, Y.; Ren, Z.; Zhang, X.; Ren, J.; Su, J.; Zhang, C.; Tian, J.; Yu, Y.; Gao, G.; et al. Transfer cells mediate nitrate uptake to control root nodule symbiosis. Nat. Plants 2020, 6, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Streeter, J. Nitrate inhibition of legume nodule growth and activity: II. Short term studies with high nitrate supply. Plant Physiol. 1985, 77, 325–328. [Google Scholar] [CrossRef]
- Du, M.; Gao, Z.; Li, X.; Liao, H. Excess nitrate induces nodule greening and reduces transcript and protein expression levels of soybean leghaemoglobins. Ann. Bot. 2020, 126, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Izmailov, S.; Bruskova, R.; Chechetka, S.; Kirnos, S.; Nikiforova, T.; Satskaya, M. Nitrate assimilation function of yellow lupine root nodules? Biol. Bull. 2003, 30, 140–148. [Google Scholar] [CrossRef]
- Kanayama, Y.; Watanabe, I.; Yamamoto, Y. Inhibition of nitrogen fixation in soybean plants supplied with nitrate I. Nitrite accumulation and formation of nitrosylleghemoglobin in nodules. Plant Cell Physiol. 1990, 31, 341–346. [Google Scholar]
- Minchin, F.; Becana, M.; Sprent, J. Short-term inhibition of legume N2 fixation by nitrate: II. Nitrate effects on nodule oxygen diffusion. Planta 1989, 180, 46–52. [Google Scholar] [CrossRef]
- Lucinski, R.; Polcyn, W.; Ratajczak, L. Nitrate reduction and nitrogen fixation in symbiotic association rhizobium-legumes. Acta Biochim. Pol. 2002, 49, 537–546. [Google Scholar] [CrossRef]
- Kato, K.; Kanahama, K.; Kanayama, Y. Involvement of nitric oxide in the inhibition of nitrogenase activity by nitrate in Lotus root nodules. J. Plant Physiol. 2010, 167, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Streeter, J. Effect of nitrate in the rooting medium on carbohydrate composition of soybean nodules. Plant Physiol. 1981, 68, 840–844. [Google Scholar] [CrossRef]
- Hacin, J.; Bohlool, B.; Singleton, P. Partitioning of 14C-labelled photosynthate to developing nodules and roots of soybean (Glycine max). New Phytol. 1997, 137, 257–265. [Google Scholar] [CrossRef]
- Daimon, H.; Yoshioka, M. Responses of root nodule formation and nitrogen fixation activity to nitrate in a split-Root system in peanut (Arachis hypogaea L.). J. Agron. Crop Sci. 2001, 187, 89–95. [Google Scholar] [CrossRef]
- Yashima, H.; Fujikake, H.; Sato, T.; Ohtake, N.; Sueyoshi, K.; Ohyama, T. Systemic and local effects of long-term application of nitrate on nodule growth and N2 fixation in soybean (Glycine max [L.] Merr.). Soil. Sci. Plant Nutr. 2003, 49, 825–834. [Google Scholar] [CrossRef]
- Lyu, X.; Li, M.; Li, X.; Li, S.; Yan, C.; Ma, C.; Gong, Z. Assessing the systematic effects of the concentration of nitrogen supplied to dual-root systems of soybean plants on nodulation and nitrogen fixation. Agronomy 2020, 10, 763. [Google Scholar] [CrossRef]
- Li, S.; Wu, C.; Liu, H.; Lyu, X.; Xiao, F.; Zhao, S.; Ma, C.; Yan, C.; Liu, Z.; Li, H.; et al. Systemic regulation of nodule structure and assimilated carbon distribution by nitrate in soybean. Front. Plant Sci. 2023, 14, 1101074. [Google Scholar] [CrossRef]
- Newcomb, W. Nodule morphogenesis and differentiation. In Biology of the Rhizobiaceae; Giles, K., Atherly, A., Eds.; Academic Press: Berkeley, CA, USA, 1981; pp. 247–298. [Google Scholar]
- Gordon, A.; Thomas, B.; Reynolds, P. Localization of sucrose synthase in soybean root nodules. New Phytol. 1992, 122, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Truchet, G.; Coulomb, P. Mise en évidence et évolution du système Phytolysosomal dans les cellules des différentes zones de nodules radiculaires de Pois (Pisum sativum L.). Notion d’hétérophagie. J. Ultrastruct. 1973, 43, 36–57. [Google Scholar] [CrossRef]
- Sujkowska, M.; Górska-Czekaj, M.; Bederska, M.; Borucki, W. Vacuolar organization in the nodule parenchyma is important for the functioning of pea root nodules. Symbiosis 2011, 54, 1–16. [Google Scholar] [CrossRef][Green Version]
- Gogorcena, Y.; Gordon, A.; Escuredo, P.; Minchin, F.; Witty, J.; Moran, J.; Becana, M. N2 fixation, carbon metabolism, and oxidative damage in nodules of dark-stressed common bean plants. Plant Physiol. 1997, 113, 1193–1201. [Google Scholar] [CrossRef]
- Vance, C.; Johnson, L.; Miller, S.; Groat, R. Birdsfoot trefoil (Lotus corniculatus) root nodules: Morphogenesis and the effect of forage harvest on structure and function. Can. J. Bot. 1982, 60, 505–518. [Google Scholar] [CrossRef]
- Pfeiffer, N.; Malik, N.; Wagner, F. Reversible dark-induced senescence of soybean root nodules. Plant Physiol. 1983, 71, 393–399. [Google Scholar] [CrossRef]
- Cohen, H.; Sarath, G.; Lee, K.; Wagner, F. Soybean root nodule ultrastructure during dark-induced stress and recovery. Protoplasma 1986, 132, 69–75. [Google Scholar] [CrossRef]
- Vance, C.; Johnson, L.; Halvorsen, A.; Heichel, G.; Barnes, D. Histological and ultrastructural observations of Medicago sativa root nodule senescence after foliage removal. Can. J. Bot. 1980, 58, 295–309. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, C.; Teng, W.; Wang, J.; Lyu, X.; Dong, S.; Kang, S.; Gong, Z.; Ma, C. Accumulation and distribution of fertilizer nitrogen and nodule-fixed nitrogen in soybeans with dual root systems. Agronomy 2020, 10, 397. [Google Scholar] [CrossRef]
- Xia, X.; Ma, C.; Dong, S.; Xu, Y.; Gong, Z. Effects of nitrogen concentrations on nodulation and nitrogenase activity in dual root systems of soybean plants. Soil Sci. Plant Nutr. 2017, 63, 470–482. [Google Scholar] [CrossRef]
- Tiwari, M.; Mhatre, S.; Vyas, T.; Bapna, A.; Raghavan, G. A validated HPLC-RID method for quantification and optimization of total sugars: Fructose, glucose, sucrose, and lactose in eggless mayonnaise. Separations 2023, 10, 199. [Google Scholar] [CrossRef]
- Bacanamwo, M.; Harper, J. Regulation of nitrogenase activity in Bradyrhizobium japonicum/soybean symbiosis by plant N status as determined by shoot C:N ratio. Physiol. Plant. 1996, 98, 529–538. [Google Scholar] [CrossRef]
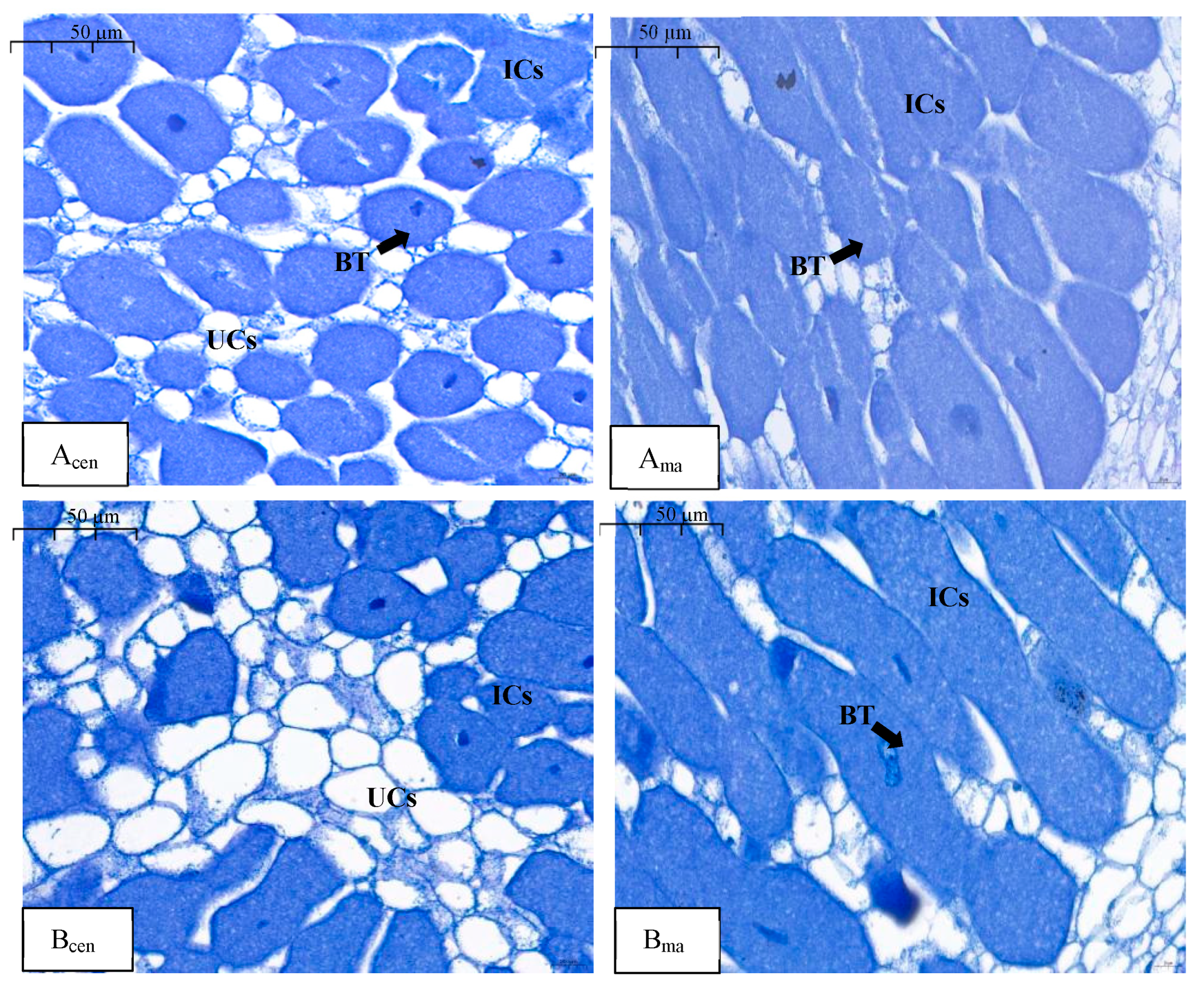

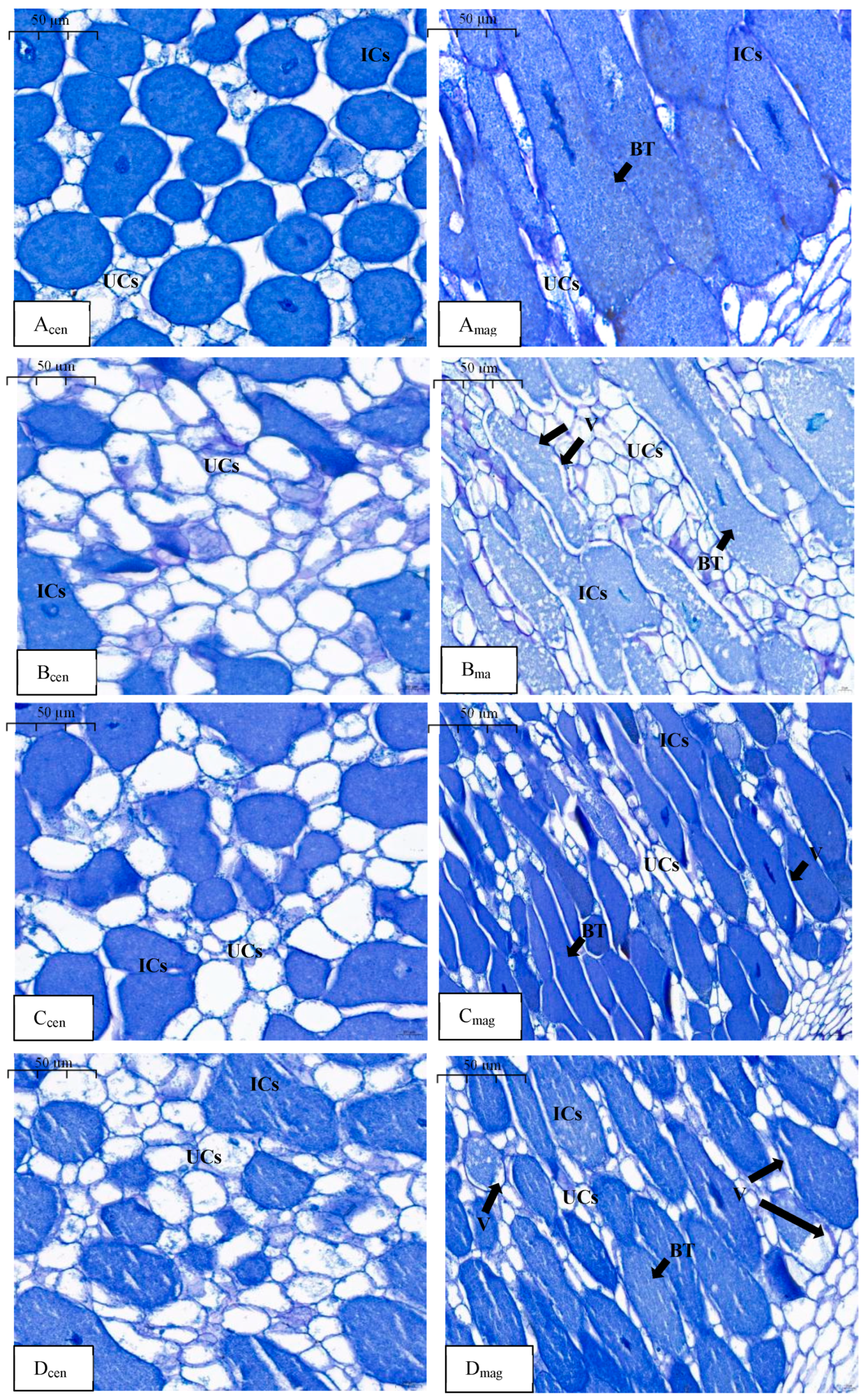
| Treatments | N Concentration (mg·L−1) | Phase I | Phase II | Phase III | |
|---|---|---|---|---|---|
| Nodule Number (per plant) | NLLL | 0-0-0 | 380.0 ± 14.48 a | 458.8 ± 4.87 a | 481.5 ± 5.89 a |
| NHHH | 200-200-200 | 343.3 ± 14.73 a | 372.0 ± 4.88 b | 370.8 ± 2.98 c | |
| NHLL | 200-0-0 | 408.0 ± 14.20 ab | 423.3 ± 2.42 b | ||
| NHLH | 200-0-200 | 382.5 ± 11.57 bc | |||
| Dry Weight (g·plant−1) | NLLL | 0-0-0 | 0.99 ± 0.07 a | 1.01 ± 0.03 a | 1.11 ± 0.07 a |
| NHHH | 200-200-200 | 0.84 ± 0.06 a | 0.85 ± 0.04 b | 0.76 ± 0.05 b | |
| NHLL | 200-0-0 | 0.90 ± 0.03 ab | 1.08 ± 0.05 a | ||
| NHLH | 200-0-200 | 0.89 ± 0.07 b |
| Treatments | N Concentration (mg·L−1) | Phase I | Phase II | Phase III | |
|---|---|---|---|---|---|
| ARA (C2H4 µmol·h−1·plant−1) | NLLL | 0-0-0 | 42.71 ± 3.35 a | 47.69 ± 2.24 a | 42.34 ± 3.37 a |
| NHHH | 200-200-200 | 24.72 ± 1.73 b | 25.17 ± 1.47 b | 18.61 ± 2.93 b | |
| NHLL | 200-0-0 | 29.34 ± 1.12 b | 40.23 ± 2.34 a | ||
| NHLH | 200-0-200 | 21.81 ± 3.16 b | |||
| SNA (C2H4 µmol g−1·nodule dry mass·h−1) | NLLL | 0-0-0 | 42.95 ± 0.60 a | 47.26 ± 1.31 a | 44.67 ± 4.46 a |
| NHHH | 200-200-200 | 30.01 ± 3.34 b | 29.88 ± 1.38 b | 25.49 ± 5.05 b | |
| NHLL | 200-0-0 | 33.06 ± 2.01 b | 37.19 ± 0.61 a | ||
| NHLH | 200-0-200 | 24.18 ± 1.77 b |
| Treatments | N Concentration (mg·L−1) | Phase I | Phase II | Phase III | |
|---|---|---|---|---|---|
| Sucrose | NLLL | 0-0-0 | 8.90 ± 0.34 a | 10.53 ± 0.21 a | 10.88 ± 0.29 a |
| NHHH | 200-200-200 | 9.64 ± 0.03 a | 9.59 ± 0.08 b | 9.77 ± 0.17 b | |
| NHLL | 200-0-0 | 9.90 ± 0.30 ab | 10.81 ± 0.18 a | ||
| NHLH | 200-0-200 | 10.50 ± 0.25 ab | |||
| Starch | NLLL | 0-0-0 | 7.07 ± 0.18 a | 7.51 ± 0.17 a | 9.94 ± 0.30 a |
| NHHH | 200-200-200 | 6.80 ± 0.23 a | 6.94 ± 0.34 a | 8.53 ± 0.67 b | |
| NHLL | 200-0-0 | 7.39 ± 0.41 a | 10.95 ± 0.80 a | ||
| NHLH | 200-0-200 | 8.97 ± 0.45 ab | |||
| Soluble sugar | NLLL | 0-0-0 | 11.8 ± 0.44 a | 14.47 ± 0.29 a | 15.21 ± 0.24 a |
| NHHH | 200-200-200 | 9.98 ± 0.54 a | 12.82 ± 0.26 b | 12.92 ± 0.36 b | |
| NHLL | 200-0-0 | 13.64 ± 0.60 ab | 15.89 ± 0.59 a | ||
| NHLH | 200-0-200 | 12.52 ± 0.39 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Hu, H.; Yu, B.; Han, L.; Li, W.; Liu, Z.; Liu, X.; Lyu, X.; Gong, Z.; Ma, C. A Study on the Effect of Indirect Nitrate Supply on the Nitrogen Fixation Capacity of Soybean Nodules. Plants 2024, 13, 3571. https://doi.org/10.3390/plants13243571
Li S, Hu H, Yu B, Han L, Li W, Liu Z, Liu X, Lyu X, Gong Z, Ma C. A Study on the Effect of Indirect Nitrate Supply on the Nitrogen Fixation Capacity of Soybean Nodules. Plants. 2024; 13(24):3571. https://doi.org/10.3390/plants13243571
Chicago/Turabian StyleLi, Sha, Huidi Hu, Baiyang Yu, Liwen Han, Wei Li, Zhilei Liu, Xuesheng Liu, Xiaochen Lyu, Zhenping Gong, and Chunmei Ma. 2024. "A Study on the Effect of Indirect Nitrate Supply on the Nitrogen Fixation Capacity of Soybean Nodules" Plants 13, no. 24: 3571. https://doi.org/10.3390/plants13243571
APA StyleLi, S., Hu, H., Yu, B., Han, L., Li, W., Liu, Z., Liu, X., Lyu, X., Gong, Z., & Ma, C. (2024). A Study on the Effect of Indirect Nitrate Supply on the Nitrogen Fixation Capacity of Soybean Nodules. Plants, 13(24), 3571. https://doi.org/10.3390/plants13243571






