Expression and Characterization of Alkaline Phosphatase from Cobetia amphilecti KMM 296 in Transiently Transformed Tobacco Leaves and Transgenic Calli
Abstract
1. Introduction
2. Results
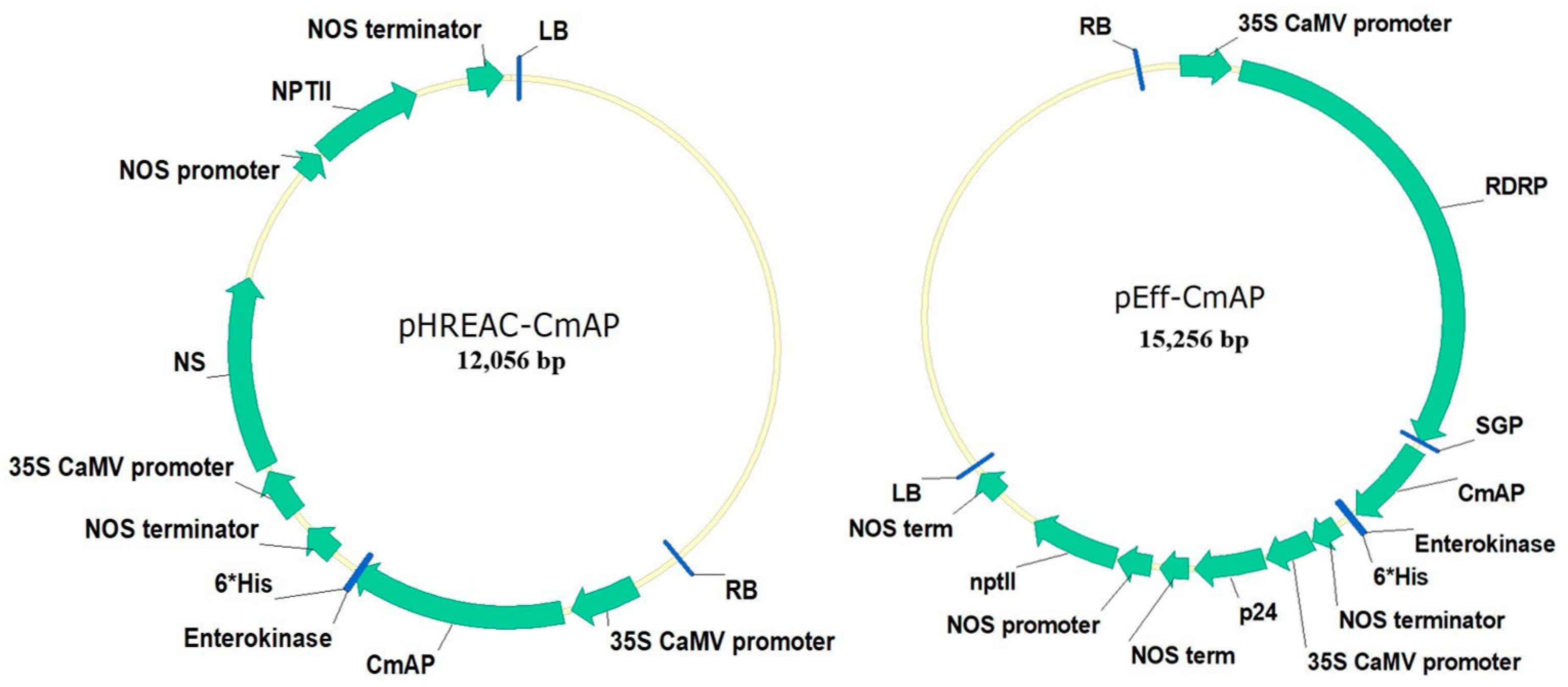
2.1. Genetic Constructs and Molecular Characterization of Recombinant Plant-Based Bacterial Alkaline Phosphatase CmAP
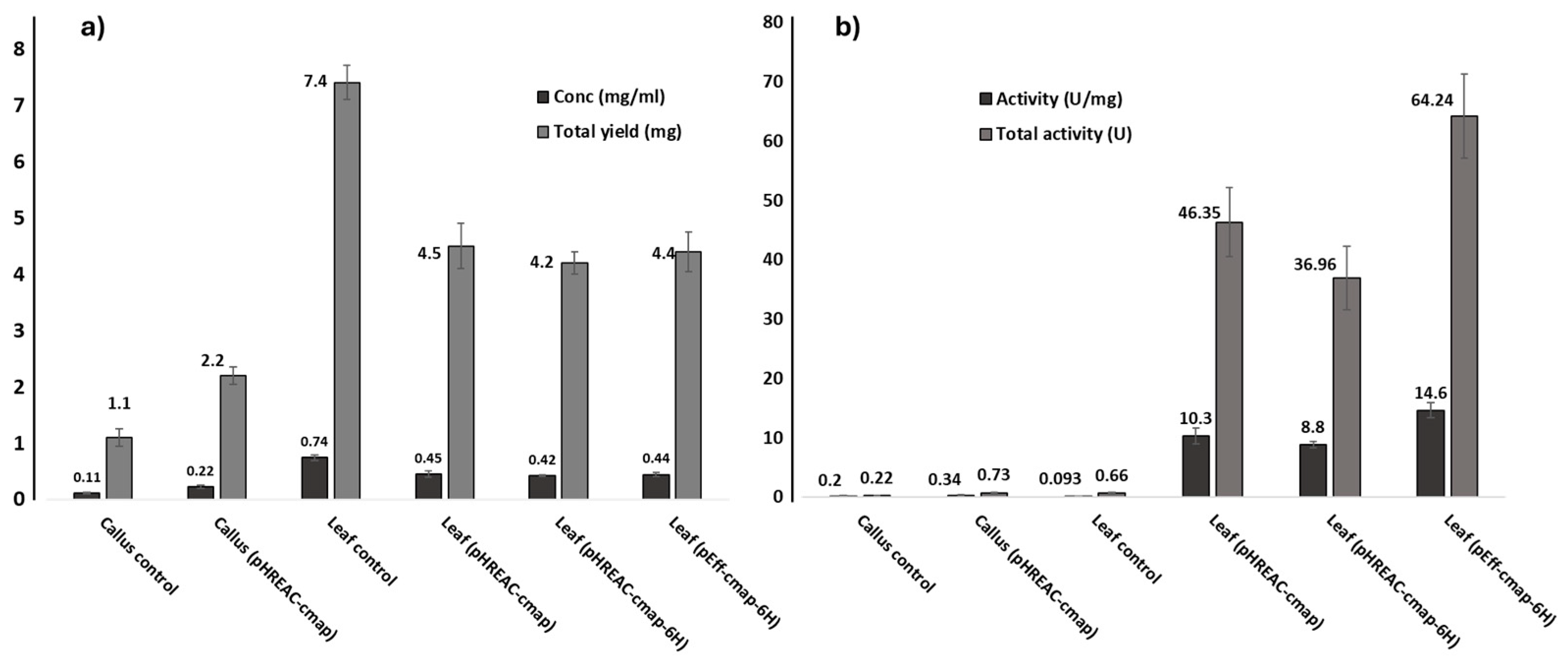
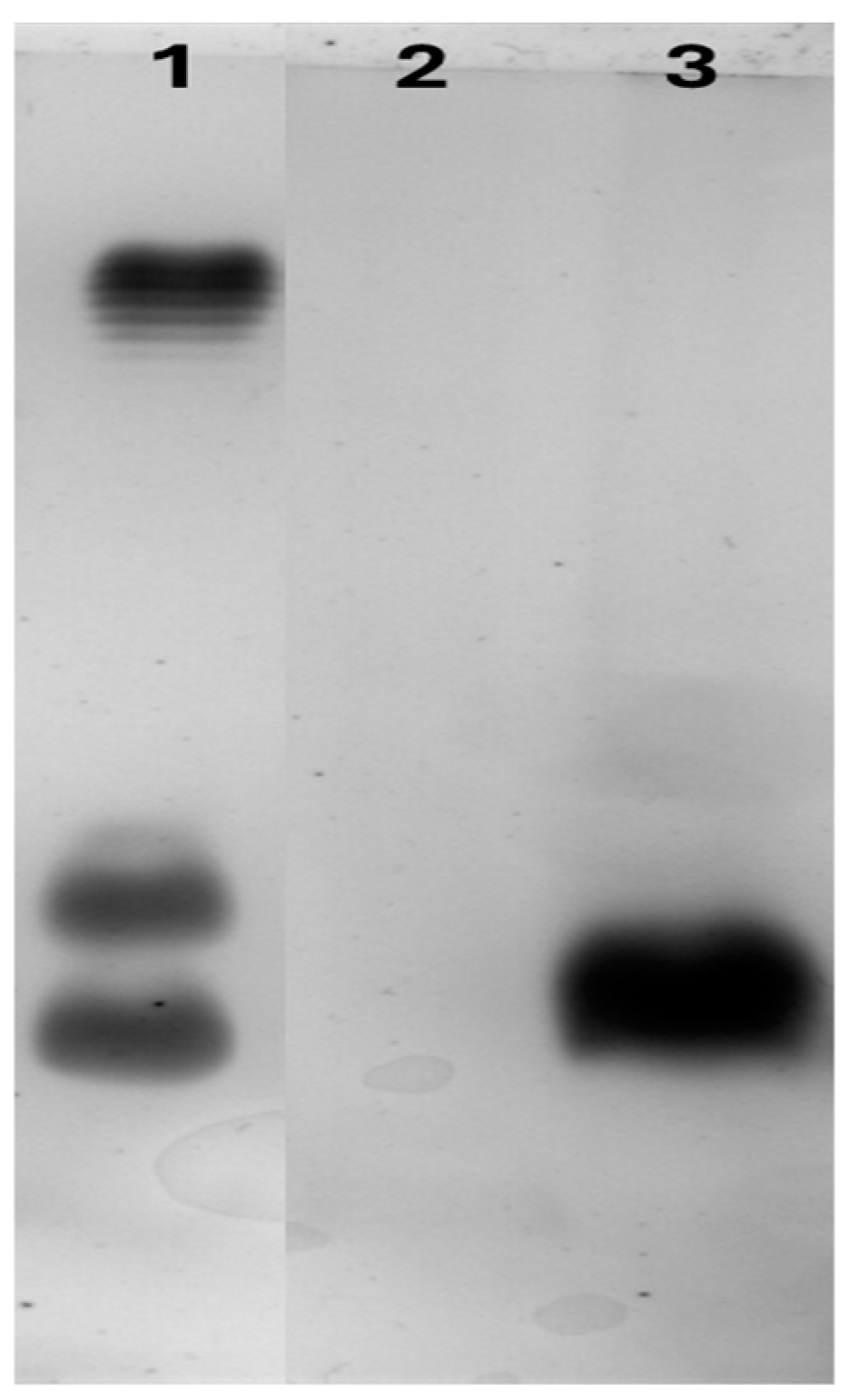
2.2. Recombinant Gene Expression for rCmAP Production
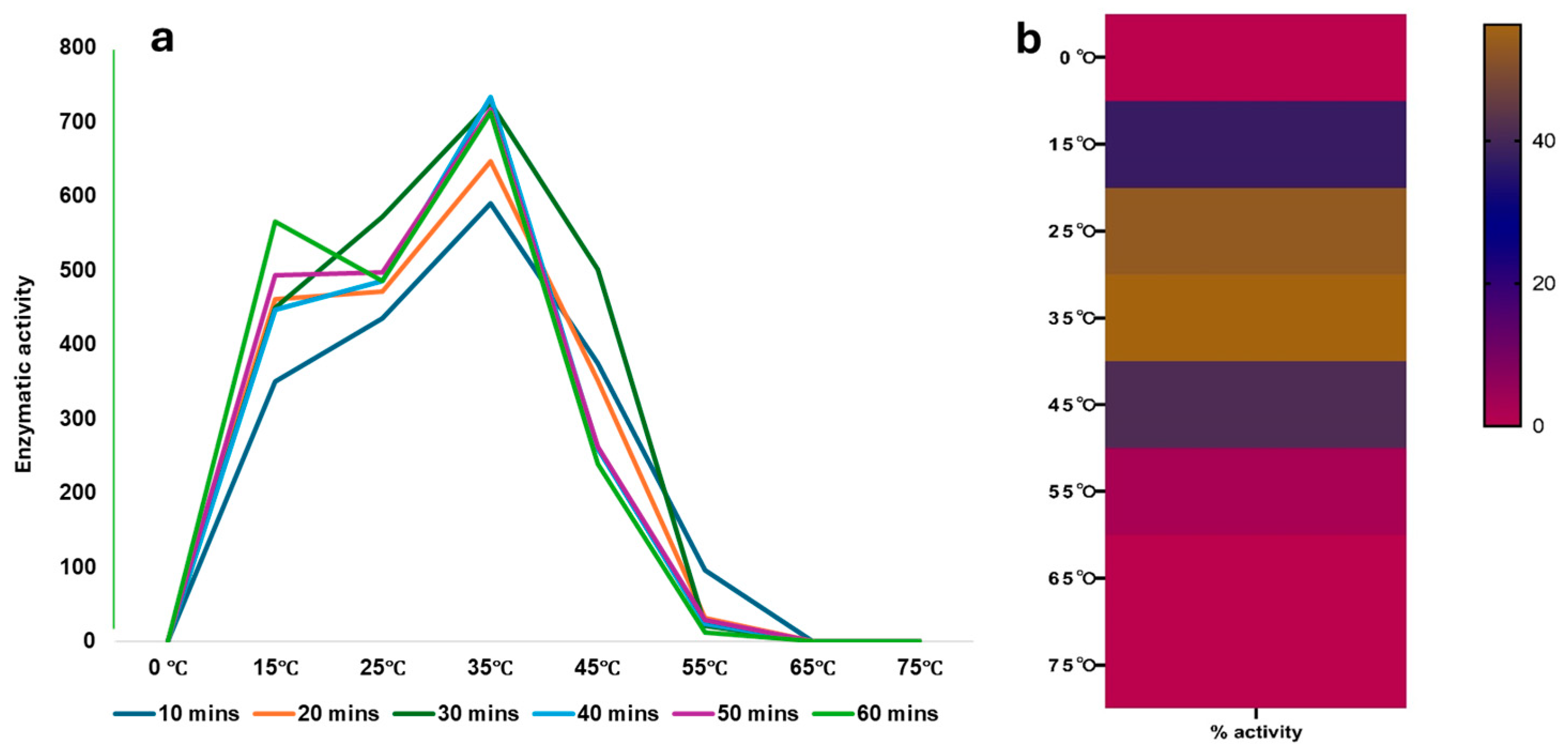
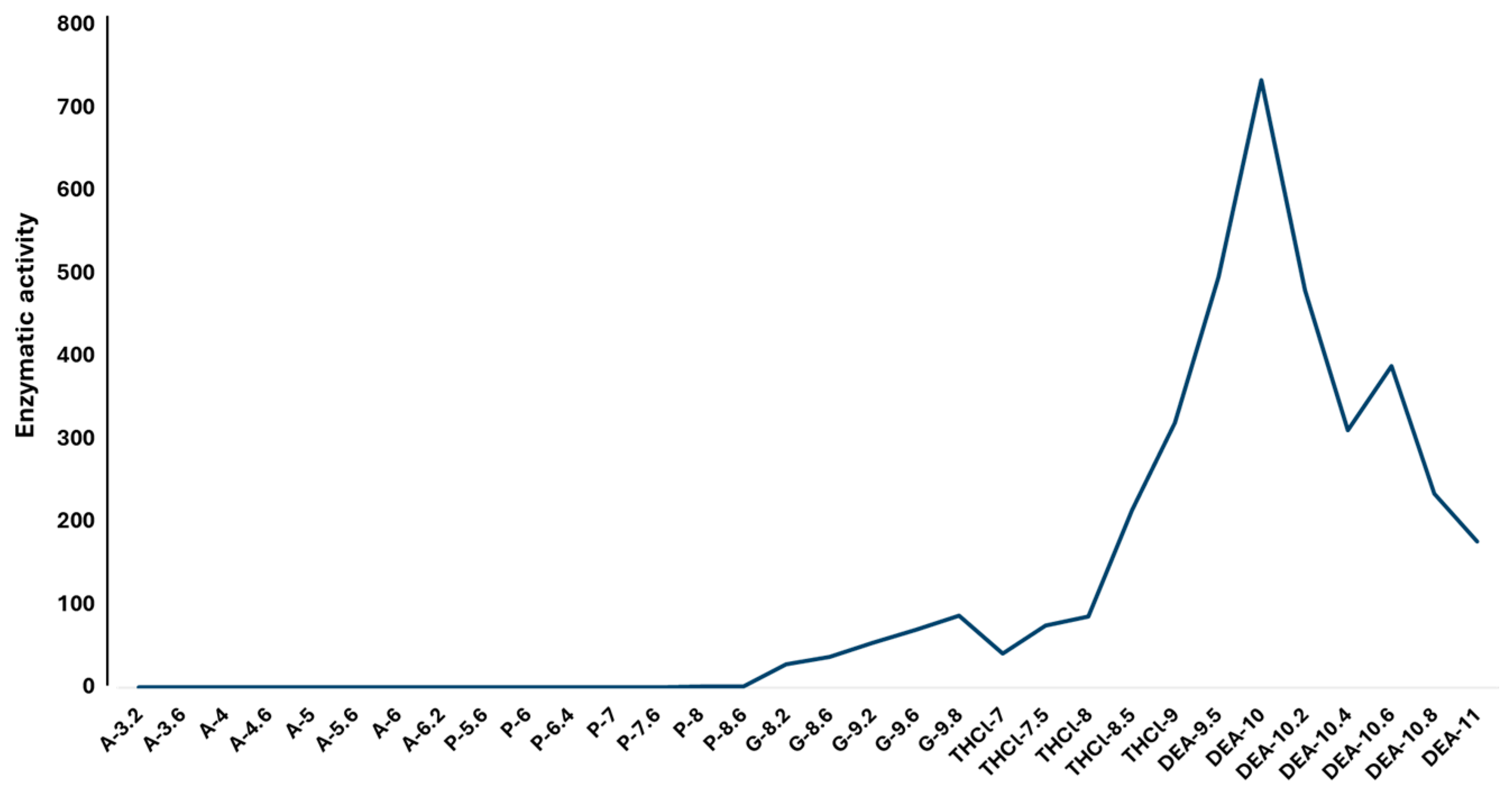
2.3. Effect of Temperature and pH on rCmAP Activity
2.4. Effect of Salt Concentration on rCmAP Activity
2.5. Effect of Bivalent Metal Ions on rCmAP Activity
2.6. Screening rCmAP Preparation for LPS Contamination
3. Discussion
3.1. Differential Expression of rCmAP in Calli and Leaves
3.2. Expression Vectors Influence on rCmAP Yield
3.3. A Putative Influence of Post-Translational Modification on rCmAP Purification
3.4. Recombinant CmAP Characterization
3.5. Tobacco Expression System Yields LPS-Free Recombinant CmAP
4. Materials and Methods
4.1. Gene Cloning and Vector Construction
4.2. Preparation of Agrobacterium tumefaciens Strains
4.3. Nicotiana tabacum Transient Transformation and Callus Induction
4.4. Recombinant Protein Purification
4.4.1. Nickel-Sepharose Affinity Chromatography
4.4.2. MonoQ Anion Exchange Chromatography
4.5. Protein Quantification and Enzyme Activity Assay
4.6. Enzymatic Temperature and Optimum Temperature
4.7. Effects of Salts, Buffers, and pH on CmAP Activity
4.8. Effect of Bivalent Metal Ions on CmAP Activity
4.9. Screening CmAP for Glycosylation Sites
4.10. Screening Recombinant Protein for LPS Contamination
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nedashkovskaya, O.; Balabanova, L.; Otstavnykh, N.; Zhukova, N.; Detkova, E.; Seitkalieva, A.; Bystritskaya, E.; Noskova, Y.; Tekutyeva, L.; Isaeva, M. In-Depth Genome Characterization and Pan-Genome Analysis of Strain KMM 296, a Producer of Highly Active Alkaline Phosphatase; Proposal for the Reclassification of Cobetia litoralis and Cobetia pacifica as the Later Heterotypic Synonyms of Cobetia amphilecti and Cobetia marina, and Emended Description of the Species Cobetia amphilecti and Cobetia marina. Biomolecules 2024, 14, 196. [Google Scholar] [CrossRef] [PubMed]
- Balabanova, L.; Nedashkovskaya, O.; Podvolotskaya, A.; Slepchenko, L.; Golotin, V.; Belik, A.; Shevchenko, L.; Son, O.; Rasskazov, V. Data Supporting Functional Diversity of the Marine Bacterium Cobetia Amphilecti KMM 296. Data Brief 2016, 8, 726–732. [Google Scholar] [CrossRef]
- Romanenko, L.A.; Tanaka, N.; Svetashev, V.I.; Falsen, E. Description of Cobetia Amphilecti Sp. Nov., Cobetia Litoralis Sp. Nov. and Cobetia Pacifica Sp. Nov., Classification of Halomonas halodurans as a Later Heterotypic Synonym of Cobetia Marina and Emended Descriptions of the Genus Cobetia and Cobetia Marina. Int. J. Syst. Evol. Microbiol. 2013, 63, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Balabanova, L.; Bakholdina, S.; Buinovskaya, N.; Noskova, Y.; Kolpakova, O.; Vlasova, V.; Bondarev, G.; Seitkalieva, A.; Son, O.; Tekutyeva, L. LPS-Dephosphorylating Cobetia amphilecti Alkaline Phosphatase of PhoA Family Divergent from the Multiple Homologues of Cobetia Spp. Microorganisms 2024, 12, 631. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Q.; Shao, Z.; Liu, Q.; He, Y.; Ren, D.; Yang, H.; Li, X. Purification and Characterization of the Enzyme Fucoidanase from Cobetia amphilecti Utilizing Fucoidan from Undaria Pinnatifida. Foods 2023, 12, 1555. [Google Scholar] [CrossRef]
- Noskova, Y.; Likhatskaya, G.; Terentieva, N.; Son, O.; Tekutyeva, L.; Balabanova, L. A Novel Alkaline Phosphatase/Phosphodiesterase, CamPhoD, from Marine Bacterium Cobetia amphilecti KMM 296. Mar. Drugs 2019, 17, 657. [Google Scholar] [CrossRef]
- Yu Plisova, E.; Balabanova, L.A.; Ivanova, E.P.; Kozhemyako, V.B.; Mikhailov, V.V.; Agafonova, E.V.; Rasskazov, V.A. A Highly Active Alkaline Phosphatase from the Marine Bacterium Cobetia. Mar. Biotechnol. 2005, 7, 173–178. [Google Scholar] [CrossRef]
- Golotin, V.; Balabanova, L.; Likhatskaya, G.; Rasskazov, V. Recombinant Production and Characterization of a Highly Active Alkaline Phosphatase from Marine Bacterium Cobetia marina. Mar. Biotechnol. 2014, 17, 130–143. [Google Scholar] [CrossRef]
- Balabanova, L.; Podvolotskaya, A.; Slepchenko, L.; Eliseikina, M.; Noskova, Y.; Nedashkovskaya, O.; Son, O.; Tekutyeva, L.; Rasskazov, V. Nucleolytic Enzymes from the Marine Bacterium Cobetia Amphilecti KMM 296 with Antibiofilm Activity and Biopreservative Effect on Meat Products. Food Control 2017, 78, 270–278. [Google Scholar] [CrossRef]
- Lidbury, I.D.E.A.; Scanlan, D.J.; Murphy, A.R.J.; Christie-Oleza, J.A.; Aguilo-Ferretjans, M.M.; Hitchcock, A.; Daniell, T.J. A Widely Distributed Phosphate-Insensitive Phosphatase Presents a Route for Rapid Organophosphorus Remineralization in the Biosphere. Proc. Natl. Acad. Sci. USA 2022, 119, e2118122119. [Google Scholar] [CrossRef] [PubMed]
- Balabanova, L.; Bondarev, G.; Seitkalieva, A.; Son, O.; Tekutyeva, L. Insights into Alkaline Phosphatase Anti-Inflammatory Mechanisms. Biomedicines 2024, 12, 2502. [Google Scholar] [CrossRef] [PubMed]
- Kühn, F.; Adiliaghdam, F.; Cavallaro, P.M.; Hamarneh, S.R.; Tsurumi, A.; Hoda, R.S.; Munoz, A.R.; Dhole, Y.; Ramirez, J.M.; Liu, E.; et al. Intestinal Alkaline Phosphatase Targets the Gut Barrier to Prevent Aging. JCI Insight 2020, 5, e134049. [Google Scholar] [CrossRef] [PubMed]
- Detel, D.; Baticic, L.; Varljen, J. The Influence of Age on Intestinal Dipeptidyl Peptidase IV (DPP IV/CD26), Disaccharidases, and Alkaline Phosphatase Enzyme Activity in C57BL/6 Mice. Exp. Aging Res. 2007, 34, 49–62. [Google Scholar] [CrossRef]
- Pickkers, P.; Angus, D.; Bass, K.; Bellomo, R.; van den Berg, E.; Bernholz, J.; Bestle, M.H.; Doi, K.; Doig, C.; Ferrer, R.; et al. Phase 3 Trial of Recombinant Human Alkaline Phosphatase for Patients with Sepsis-Associated Acute Kidney Injury (REVIVAL). Intensive Care Med. 2024, 50, 68–78. [Google Scholar] [CrossRef]
- Rosin, D.L.; Hall, J.P.; Zheng, S.; Huang, L.; Campos-Bilderback, S.; Sandoval, R.; Bree, A.; Beaumont, K.; Miller, E.; Larsen, J.; et al. Human Recombinant Alkaline Phosphatase (Ilofotase Alfa) Protects against Kidney Ischemia-Reperfusion Injury in Mice and Rats through Adenosine Receptors. Front. Med. 2022, 9, 931293. [Google Scholar] [CrossRef] [PubMed]
- Nasu, E.; Ichiyanagi, A.; Gomi, K. Cloning and Expression of a Highly Active Recombinant Alkaline Phosphatase from Psychrotrophic Cobetia Marina. Biotechnol. Lett. 2011, 34, 321–328. [Google Scholar] [CrossRef]
- Valkova, R.; Apostolova, E.; Naimov, S. Plant Molecular Farming: Opportunities and Challenges. J. Serbian Chem. Soc. 2013, 78, 407–415. [Google Scholar] [CrossRef]
- Ku, P.; Sharma, D.; Singh, S.; Sahu, P. Molecular Farming: A biotechnological approach in agriculture for production of useful metabolites. Int. J. Res. Biotechnol. Biochem. 2014, 4, 23–30. [Google Scholar]
- Joensuu, J.J.; Conley, A.J.; Lienemann, M.; Brandle, J.E.; Linder, M.B.; Menassa, R. Hydrophobin Fusions for High-Level Transient Protein Expression and Purification in Nicotiana benthamiana. Plant Physiol. 2009, 152, 622–633. [Google Scholar] [CrossRef]
- Conley, A.J.; Zhu, H.; Le, L.C.; Jevnikar, A.M.; Lee, B.H.; Brandle, J.E.; Menassa, R. Recombinant Protein Production in a Variety of Nicotiana Hosts: A Comparative Analysis. Plant Biotechnol. J. 2010, 9, 434–444. [Google Scholar] [CrossRef]
- Yin, J.-L.; Wong, W.-S. Production of Santalenes and Bergamotene in Nicotiana Tabacum Plants. PLoS ONE 2019, 14, e0203249. [Google Scholar] [CrossRef]
- Peyret, H.; Brown, J.K.M.; Lomonossoff, G.P. Improving Plant Transient Expression through the Rational Design of Synthetic 5′ and 3′ Untranslated Regions. Plant Methods 2019, 15, 108. [Google Scholar] [CrossRef] [PubMed]
- Mardanova, E.S.; Blokhina, E.A.; Tsybalova, L.M.; Peyret, H.; Lomonossoff, G.P.; Ravin, N.V. Efficient Transient Expression of Recombinant Proteins in Plants by the Novel pEff Vector Based on the Genome of Potato Virus X. Front. Plant Sci. 2017, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, M.; Ammerman, J.W. The Alkaline Phosphatase PhoX Is More Widely Distributed in Marine Bacteria than the Classical PhoA. ISME J. 2009, 3, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Saavedra, D.E.M.; Thomson, B.; García, J.A.L.; Zhao, Z.; Patrick, W.M.; Herndl, G.J.; Baltar, F. Enzyme Promiscuity in Natural Environments: Alkaline Phosphatase in the Ocean. ISME J. 2021, 15, 3375–3383. [Google Scholar] [CrossRef]
- Wan, B.; Huang, R.; Diaz, J.M.; Tang, Y. Rethinking the Biotic and Abiotic Remineralization of Complex Phosphate Molecules in Soils and Sediments. Sci. Total Environ. 2022, 833, 155187. [Google Scholar] [CrossRef] [PubMed]
- Nedashkovskaya, O.I.; Balabanova, L.A.; Otstavnykh, N.Y.; Zhukova, N.V.; Seitkalieva, A.V.; Noskova, Y.A.; Tekutyeva, L.A. Pangenome- and Genome-Based Taxonomic Classification Inference for the Marine Bacterial Strain KMM 296 Producing a Highly Active PhoA Alkaline Phosphatase and Closely Related Cobetiaspecies; Cold Spring Harbor Laboratory: New York, NY, USA, 2023. [Google Scholar]
- Pandeya, D.; López-Arredondo, D.L.; Janga, M.R.; Campbell, L.M.; Estrella-Hernández, P.; Bagavathiannan, M.V.; Herrera-Estrella, L.; Rathore, K.S. Selective Fertilization with Phosphite Allows Unhindered Growth of Cotton Plants Expressing the ptxD Gene While Suppressing Weeds. Proc. Natl. Acad. Sci. USA 2018, 115, E6946–E6955. [Google Scholar] [CrossRef]
- Ram, B.; Fartyal, D.; Sheri, V.; Varakumar, P.; Borphukan, B.; James, D.; Yadav, R.; Bhatt, A.; Agrawal, P.K.; Achary, V.M.M.; et al. Characterization of phoA, a Bacterial Alkaline Phosphatase for Phi Use Efficiency in Rice Plant. Front. Plant Sci. 2019, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Michoux, F.; Ahmad, N.; McCarthy, J.; Nixon, P.J. Contained and High-level Production of Recombinant Protein in Plant Chloroplasts Using a Temporary Immersion Bioreactor. Plant Biotechnol. J. 2010, 9, 575–584. [Google Scholar] [CrossRef]
- Feng, Z.; Li, X.; Fan, B.; Zhu, C.; Chen, Z. Maximizing the Production of Recombinant Proteins in Plants: From Transcription to Protein Stability. Int. J. Mol. Sci. 2022, 23, 13516. [Google Scholar] [CrossRef]
- Lozano Terol, G.; Gallego-Jara, J.; Sola Martínez, R.A.; Martínez Vivancos, A.; Cánovas Díaz, M.; de Diego Puente, T. Impact of the Expression System on Recombinant Protein Production in Escherichia coli BL21. Front. Microbiol. 2021, 12, 682001. [Google Scholar] [CrossRef]
- Strasser, R. Plant Protein Glycosylation. Glycobiology 2016, 26, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Jeon, J.-H.; Lee, K.J.; Ko, K. N-Glycosylation Modification of Plant-Derived Virus-Like Particles: An Application in Vaccines. BioMed Res. Int. 2014, 2014, 249519. [Google Scholar] [CrossRef]
- Strasser, R. Plant Glycoengineering for Designing Next-Generation Vaccines and Therapeutic Proteins. Biotechnol. Adv. 2023, 67, 108197. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R. Biological Significance of Complex N-Glycans in Plants and Their Impact on Plant Physiology. Front. Plant Sci. 2014, 5, 363. [Google Scholar] [CrossRef]
- Mamedov, T.; Cicek, K.; Gulec, B.; Ungor, R.; Hasanova, G. In Vivo Production of Non-Glycosylated Recombinant Proteins in Nicotiana Benthamiana Plants by Co-Expression with Endo-β-N-Acetylglucosaminidase H (Endo H) of Streptomyces plicatus. PLoS ONE 2017, 12, e0183589. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, J.S.; Bhat, A.H.; Raghava, G.P.S.; Rao, A. GlycoPP: A Webserver for Prediction of N- and o-Glycosites in Prokaryotic Protein Sequences. PLoS ONE 2012, 7, e40155. [Google Scholar] [CrossRef]
- Gupta, R.; Brunak, S. Prediction of Glycosylation across the Human Proteome and the Correlation to Protein Function. Pac. Symp. Biocomput. 2001, 7, 310–322. [Google Scholar]
- Kao, M.-R.; Karmarkar Saldivar, R.; Hsieh, Y.S.Y. Production of Therapeutic Glycoproteins in Glycoengineered Plant: Old Farm for New Crops. Curr. Opin. Biotechnol. 2024, 87, 103145. [Google Scholar] [CrossRef]
- Bennett, L.; Yang, Q.; Berquist, B.; Giddens, J.; Ren, Z.; Kommineni, V.; Murray, R.; White, E.; Holtz, B.; Wang, L.-X.; et al. Implementation of Glycan Remodeling to Plant-Made Therapeutic Antibodies. Int. J. Mol. Sci. 2018, 19, 421. [Google Scholar] [CrossRef] [PubMed]
- Ruginescu, R.; Enache, M.; Popescu, O.; Gomoiu, I.; Cojoc, R.; Batrinescu-Moteau, C.; Maria, G.; Dumbravician, M.; Neagu, S. Characterization of Some Salt-Tolerant Bacterial Hydrolases with Potential Utility in Cultural Heritage Bio-Cleaning. Microorganisms 2022, 10, 644. [Google Scholar] [CrossRef] [PubMed]
- Wackett, L.P. Industrial Applications of Microbial Salt-tolerant Enzymes. Microb. Biotechnol. 2012, 5, 668–669. [Google Scholar] [CrossRef]
- Jia, B.; Jeon, C.O. High-Throughput Recombinant Protein Expression in Escherichia coli: Current Status and Future Perspectives. Open Biol. 2016, 6, 160196. [Google Scholar] [CrossRef]
- Niazi, S.K.; Magoola, M. Advances in Escherichia coli-Based Therapeutic Protein Expression: Mammalian Conversion, Continuous Manufacturing, and Cell-Free Production. Biologics 2023, 3, 380–401. [Google Scholar] [CrossRef]
- Virzì, G.M.; Mattiotti, M.; de Cal, M.; Ronco, C.; Zanella, M.; De Rosa, S. Endotoxin in Sepsis: Methods for LPS Detection and the Use of Omics Techniques. Diagnostics 2022, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Thiem, J.; Braulke, T. Glycostructures in Biological Systems—Synthesis and Function. Eur. J. Cell Biol. 2010, 89, 1. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, P.; Dhakal, S.; Dozois, C.M. The Diversity of Escherichia coli Pathotypes and Vaccination Strategies against This Versatile Bacterial Pathogen. Microorganisms 2023, 11, 344. [Google Scholar] [CrossRef]
- Cong, M.; Tan, S.; Li, S.; Gao, L.; Huang, L.; Zhang, H.-G.; Qiao, H. Technology Insight: Plant-Derived Vesicles—How Far from the Clinical Biotherapeutics and Therapeutic Drug Carriers? Adv. Drug Deliv. Rev. 2022, 182, 114108. [Google Scholar] [CrossRef] [PubMed]
- Burnett, M.J.B.; Burnett, A.C. Therapeutic Recombinant Protein Production in Plants: Challenges and Opportunities. Plants People Planet 2019, 2, 121–132. [Google Scholar] [CrossRef]
- Shkryl, Y.; Yugay, Y.; Avramenko, T.; Grigorchuk, V.; Gorpenchenko, T.; Grischenko, O.; Bulgakov, V. CRISPR/Cas9-Mediated Knockout of HOS1 Reveals Its Role in the Regulation of Secondary Metabolism in Arabidopsis Thaliana. Plants 2021, 10, 104. [Google Scholar] [CrossRef]
- Hood, E.E.; Gelvin, S.B.; Melchers, L.S.; Hoekema, A. NewAgrobacterium Helper Plasmids for Gene Transfer to Plants. Transgenic Res. 1993, 2, 208–218. [Google Scholar] [CrossRef]
- Degtyarenko, A.I.; Gorpenchenko, T.Y.; Grigorchuk, V.P.; Bulgakov, V.P.; Shkryl, Y.N. Optimization of the Transient Agrobacterium-Mediated Transformation of Panax Ginseng Shoots and Its Use to Change the Profile of Ginsenoside Production. Plant Cell Tissue Organ Cult. PCTOC 2021, 146, 357–373. [Google Scholar] [CrossRef]
- Horsch, R.B.; Fry, J.; Hoffmann, N.; Neidermeyer, J.; Rogers, S.G.; Fraley, R.T. Leaf Disc Transformation. In Plant Molecular Biology Manual; Springer: Dordrecht, The Netherlands, 1989; pp. 63–71. [Google Scholar]
- Gallois, P.; Marinho, P. Leaf Disk Transformation Using Agrobacterium Tumefaciens-Expression of Heterologous Genes in Tobacco. In Plant Gene Transfer and Expression Protocols; Humana Press: Totowa, NJ, USA, 1995; pp. 39–48. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]



| N. tabacum/E. coli | Total Protein (mg) | Specific Activity (U/mg) | Total Activity (U) | |||
|---|---|---|---|---|---|---|
| Homogenate | 4.40 ± 0.29 | 378.00 ± 8.34 | 14.60 ± 0.88 | 101.60 ± 5.10 | 64.24 ± 3.48 | 38,404.80 ± 212.30 |
| Ni-Sepharose elute | 1.06 ± 0.20 | 108.40 ± 4.45 | 113.20 ± 2.89 | 787.50 ± 12.23 | 119.90 ± 4.41 | 85,365.00 ± 182.65 |
| Dialysis | 1.15 ± 0.15 | 11.50 ± 1.20 | 136.84 ± 5.77 | 853.20 ± 9.10 | 157.09 ± 4.62 | 9811.80 ± 75.20 |
| HisTrap | 0.69 ± 0.12 | 3.10 ± 0.23 | 318.84 ± 8.82 | 1984.60 ± 21.12 | 219.36 ± 5.01 | 6152.30 ± 89.08 |
| Mono-Q elute | 0.23 ± 0.04 | 1.40 ± 0.12 | 733.30 ± 15.28 | 4052.60 ± 32.87 | 166.50 ± 5.77 | 5673.60 ± 76.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adedibu, P.A.; Noskova, Y.A.; Yugay, Y.A.; Ovsiannikova, D.M.; Vasyutkina, E.A.; Kudinova, O.D.; Grigorchuk, V.P.; Shkryl, Y.N.; Tekutyeva, L.A.; Balabanova, L.A. Expression and Characterization of Alkaline Phosphatase from Cobetia amphilecti KMM 296 in Transiently Transformed Tobacco Leaves and Transgenic Calli. Plants 2024, 13, 3570. https://doi.org/10.3390/plants13243570
Adedibu PA, Noskova YA, Yugay YA, Ovsiannikova DM, Vasyutkina EA, Kudinova OD, Grigorchuk VP, Shkryl YN, Tekutyeva LA, Balabanova LA. Expression and Characterization of Alkaline Phosphatase from Cobetia amphilecti KMM 296 in Transiently Transformed Tobacco Leaves and Transgenic Calli. Plants. 2024; 13(24):3570. https://doi.org/10.3390/plants13243570
Chicago/Turabian StyleAdedibu, Peter Adeolu, Yulia Aleksandrovna Noskova, Yulia Anatolievna Yugay, Daria Mikhailovna Ovsiannikova, Elena Anatolievna Vasyutkina, Olesya Dmitrievna Kudinova, Valeria Petrovna Grigorchuk, Yury Nikolaevich Shkryl, Liudmila Aleksandrovna Tekutyeva, and Larissa Anatolievna Balabanova. 2024. "Expression and Characterization of Alkaline Phosphatase from Cobetia amphilecti KMM 296 in Transiently Transformed Tobacco Leaves and Transgenic Calli" Plants 13, no. 24: 3570. https://doi.org/10.3390/plants13243570
APA StyleAdedibu, P. A., Noskova, Y. A., Yugay, Y. A., Ovsiannikova, D. M., Vasyutkina, E. A., Kudinova, O. D., Grigorchuk, V. P., Shkryl, Y. N., Tekutyeva, L. A., & Balabanova, L. A. (2024). Expression and Characterization of Alkaline Phosphatase from Cobetia amphilecti KMM 296 in Transiently Transformed Tobacco Leaves and Transgenic Calli. Plants, 13(24), 3570. https://doi.org/10.3390/plants13243570






