Heterogeneity in Mechanical Properties of Plant Cell Walls
Abstract
1. Introduction
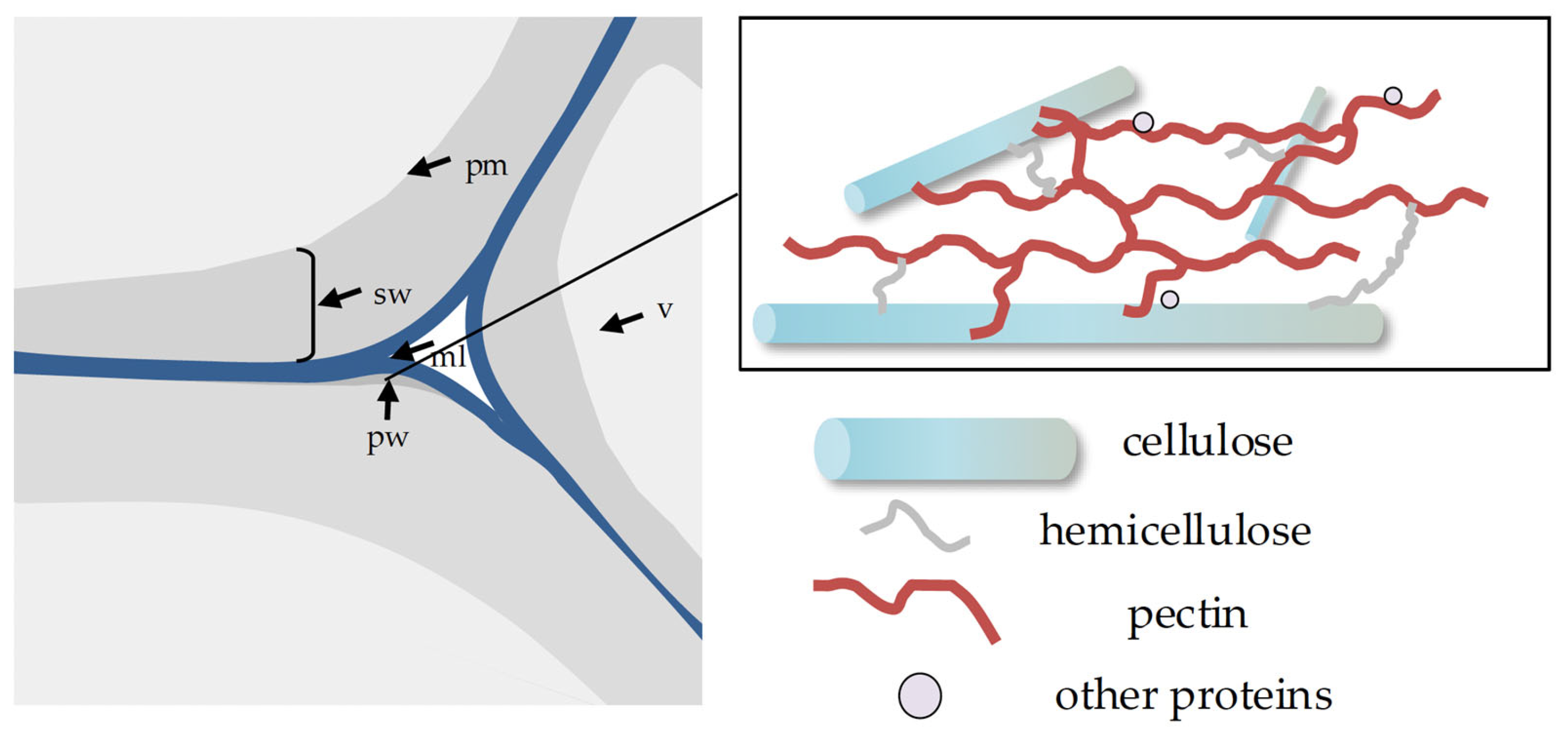
2. Cell Wall Components and Dynamics
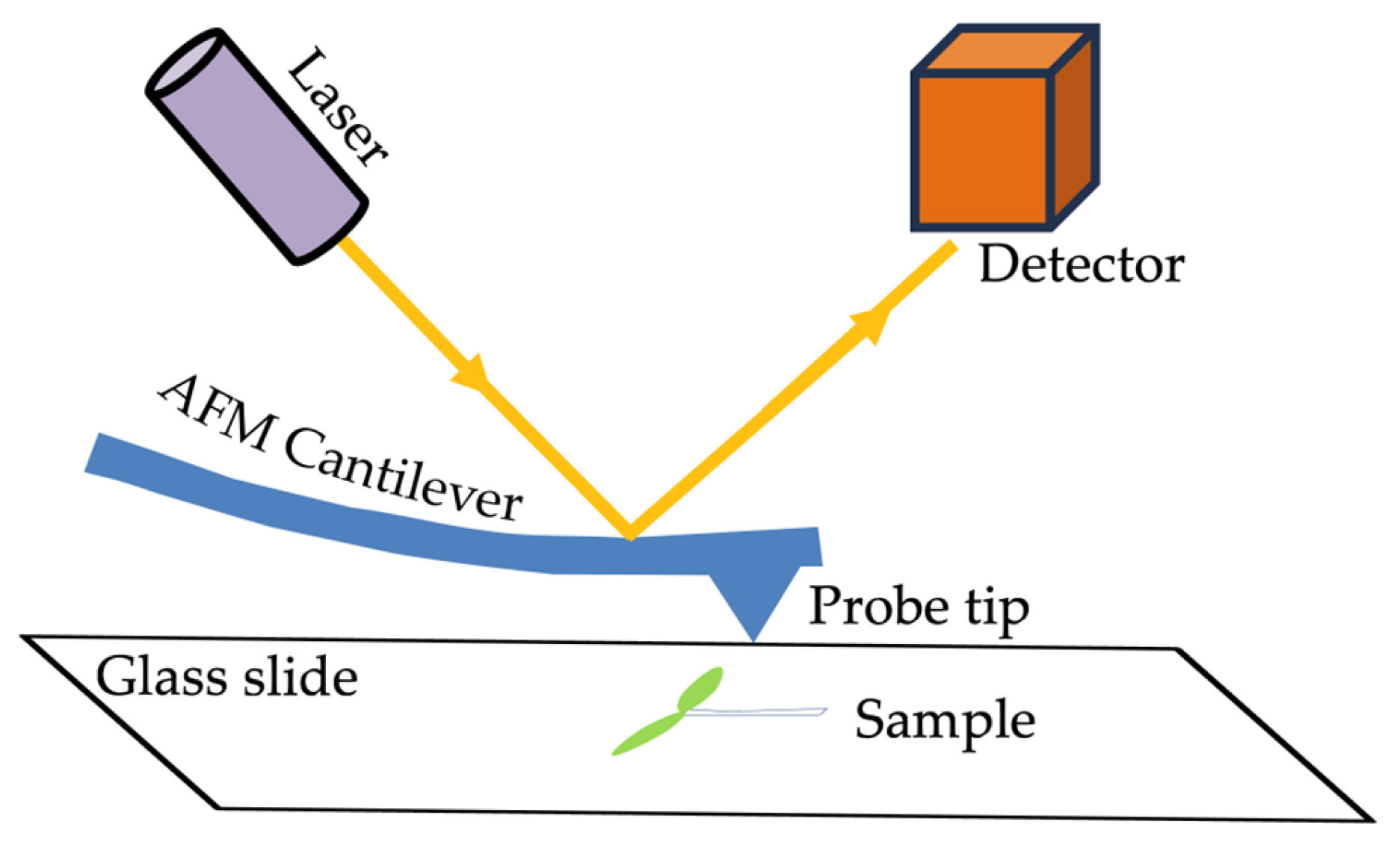
3. Techniques and Tools for Probing Biophysical and Biochemical Properties of Cell Walls
4. Advanced Applications of AFM in Plant Cell Walls
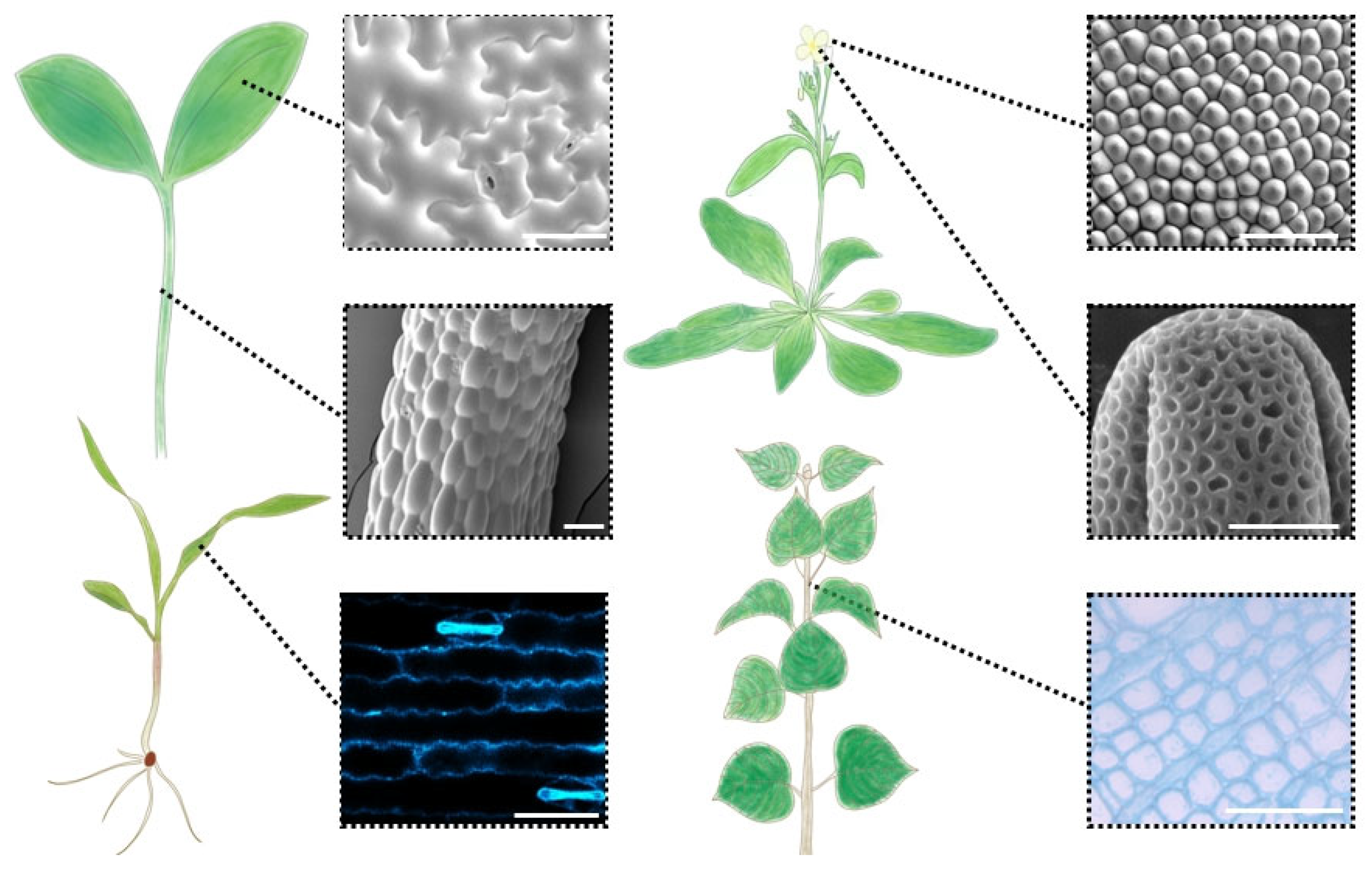
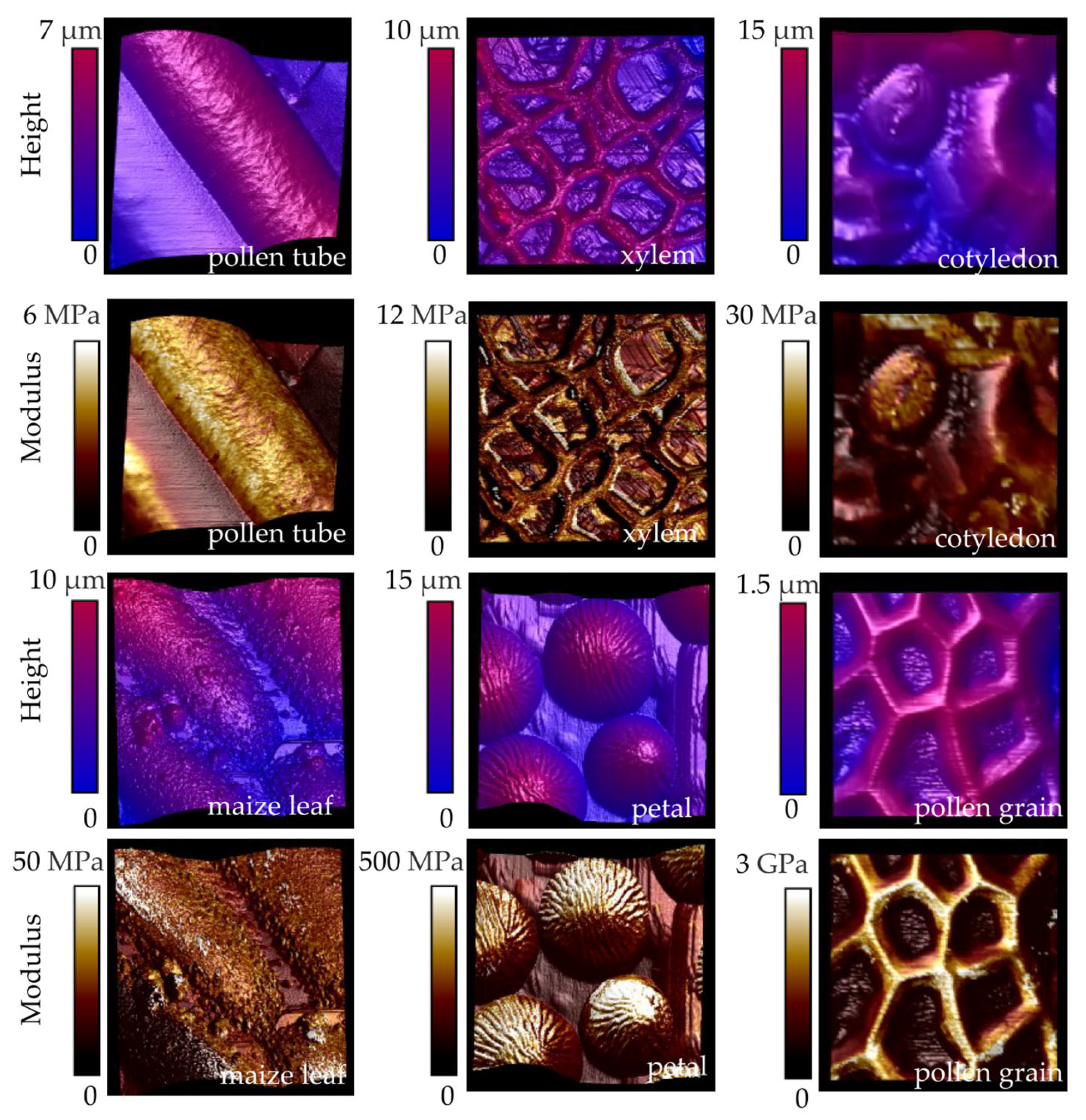
5. Heterogeneity in Mechanical Properties of Plant Cell Walls
6. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Szymanski, D.B.; Cosgrove, D.J. Dynamic coordination of cytoskeletal and cell wall systems during plant cell morphogenesis. Curr. Biol. 2009, 19, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Kurepin, L.V.; Pharis, R.P. Light signaling and the phytohormonal regulation of shoot growth. Plant Sci. 2014, 229, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S. Cell Wall Signaling in Plant Development and Defense. Annu. Rev. Plant Biol. 2022, 73, 323–353. [Google Scholar] [CrossRef] [PubMed]
- Gigli-Bisceglia, N.; Engelsdorf, T.; Hamann, T. Plant cell wall integrity maintenance in model plants and crop species-relevant cell wall components and underlying guiding principles. Cell. Mol. Life Sci. 2020, 77, 2049–2077. [Google Scholar] [CrossRef]
- Nelissen, H.; Gonzalez, N. Understanding plant organ growth: A multidisciplinary field. J. Exp. Bot. 2020, 71, 7–10. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Structure and growth of plant cell walls. Nat. Rev. Mol. Cell Biol. 2024, 25, 340–358. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef]
- Lin, W.; Yang, Z. Unlocking the mechanisms behind the formation of interlocking pavement cells. Curr. Opin. Plant Biol. 2020, 57, 142–154. [Google Scholar] [CrossRef]
- Liu, S.; Jobert, F.; Rahneshan, Z.; Doyle, S.M.; Robert, S. Solving the Puzzle of Shape Regulation in Plant Epidermal Pavement Cells. Annu. Rev. Plant Biol. 2021, 72, 525–550. [Google Scholar] [CrossRef]
- Whitney, H.M.; Bennett, K.M.V.; Dorling, M.; Sandbach, L.; Prince, D.; Chittka, L.; Glover, B.J. Why do so many petals have conical epidermal cells? Ann. Bot. 2011, 108, 609–616. [Google Scholar] [CrossRef]
- Gendreau, E.; Traas, J.; Desnos, T.; Grandjean, O.; Caboche, M.; Hofte, H. Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol. 1997, 114, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Radja, A.; Horsley, E.M.; Lavrentovich, M.O.; Sweeney, A.M. Pollen Cell Wall Patterns Form from Modulated Phases. Cell 2019, 176, 856–868.e10. [Google Scholar] [CrossRef] [PubMed]
- Decou, R.; Labrousse, P.; Bere, E.; Fleurat-Lessard, P.; Krausz, P. Structural features in tension wood and distribution of wall polymers in the G-layer of in vitro grown poplars. Protoplasma 2020, 257, 13–29. [Google Scholar] [CrossRef]
- Ali, O.; Traas, J. Force-Driven Polymerization and Turgor-Induced Wall Expansion. Trends Plant Sci. 2016, 21, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Geitmann, A.; Ortega, J.K. Mechanics and modeling of plant cell growth. Trends Plant Sci. 2009, 14, 467–478. [Google Scholar] [CrossRef]
- Anderson, C.T.; Kieber, J.J. Dynamic Construction, Perception, and Remodeling of Plant Cell Walls. Annu. Rev. Plant Biol. 2020, 71, 39–69. [Google Scholar] [CrossRef]
- Liu, M.C.J.; Yeh, F.L.J.; Yvon, R.; Simpson, K.; Jordan, S.; Chambers, J.; Wu, H.M.; Cheung, A.Y. Extracellular pectin-RALF phase separation mediates FERONIA global signaling function. Cell 2024, 187, 312–330.e22. [Google Scholar] [CrossRef]
- Gorelova, V.; Sprakel, J.; Weijers, D. Plant cell polarity as the nexus of tissue mechanics and morphogenesis. Nat. Plants 2021, 7, 1548–1559. [Google Scholar] [CrossRef]
- Vogler, H.; Felekis, D.; Nelson, B.J.; Grossniklaus, U. Measuring the Mechanical Properties of Plant Cell Walls. Plants 2015, 4, 167–182. [Google Scholar] [CrossRef]
- Pauly, M.; Keegstra, K. Plant cell wall polymers as precursors for biofuels. Curr. Opin. Plant Biol. 2010, 13, 305–312. [Google Scholar] [CrossRef]
- Höfte, H.; Voxeur, A. Plant cell walls. Curr. Biol. 2017, 27, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Lampugnani, E.R.; Khan, G.A.; Somssich, M.; Persson, S. Building a plant cell wall at a glance. J. Cell Sci. 2018, 131, jcs207373. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Nanoscale structure, mechanics and growth of epidermal cell walls. Curr. Opin. Plant Biol. 2018, 46, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Bidhendi, A.J.; Geitmann, A. Relating the mechanics of the primary plant cell wall to morphogenesis. J. Exp. Bot. 2016, 67, 449–461. [Google Scholar] [CrossRef]
- Somerville, C.; Bauer, S.; Brininstool, G.; Facette, M.; Hamann, T.; Milne, J.; Osborne, E.; Paredez, A.; Persson, S.; Raab, T.; et al. Toward a systems approach to understanding plant-cell walls. Science 2004, 306, 2206–2211. [Google Scholar] [CrossRef]
- De Lorenzo, G.; Ferrari, S.; Giovannoni, M.; Mattei, B.; Cervone, F. Cell wall traits that influence plant development, immunity, and bioconversion. Plant J. 2019, 97, 134–147. [Google Scholar] [CrossRef]
- Barnes, W.J.; Anderson, C.T. Cytosolic invertases contribute to cellulose biosynthesis and influence carbon partitioning in seedlings of Arabidopsis thaliana. Plant J. 2018, 94, 956–974. [Google Scholar] [CrossRef]
- Wolf, S.; Hematy, K.; Hofte, H. Growth control and cell wall signaling in plants. Annu. Rev. Plant Biol. 2012, 63, 381–407. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Plant cell wall extensibility: Connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J. Exp. Bot. 2016, 67, 463–476. [Google Scholar] [CrossRef]
- Oliveri, H.; Traas, J.; Godin, C.; Ali, O. Regulation of plant cell wall stiffness by mechanical stress: A mesoscale physical model. J. Math. Biol. 2019, 78, 625–653. [Google Scholar] [CrossRef]
- Hill, J.L.; Hammudi, M.B.; Tien, M. The Arabidopsis Cellulose Synthase Complex: A Proposed Hexamer of CESA Trimers in an Equimolar Stoichiometry. Plant Cell 2014, 26, 4834–4842. [Google Scholar] [CrossRef] [PubMed]
- Polko, J.K.; Kieber, J.J. The Regulation of Cellulose Biosynthesis in Plants. Plant Cell 2019, 31, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Purushotham, P.; Fang, C.; Maranas, C.; Díaz-Moreno, S.M.; Bulone, V.; Zimmer, J.; Kumar, M.; Nixon, B.T. Synthesis and Self-Assembly of Cellulose Microfibrils from Reconstituted Cellulose Synthase. Plant Physiol. 2017, 175, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.W.; Cai, C.; Staiger, C.J. Myosins XI Are Involved in Exocytosis of Cellulose Synthase Complexes. Plant Physiol. 2019, 179, 1537–1555. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Park, Y.B.; Cosgrove, D.J. Xyloglucan and its Interactions with Other Components of the Growing Cell Wall. Plant Cell Physiol. 2015, 56, 180–194. [Google Scholar] [CrossRef]
- Culbertson, A.T.; Ehrlich, J.J.; Choe, J.Y.; Honzatko, R.B.; Zabotina, O.A. Structure of xyloglucan xylosyltransferase 1 reveals simple steric rules that define biological patterns of xyloglucan polymers. Proc. Natl. Acad. Sci. USA 2018, 115, 6064–6069. [Google Scholar] [CrossRef]
- Anders, N.; Wilkinson, M.D.; Lovegrove, A.; Freeman, J.; Tryfona, T.; Pellny, T.K.; Weimar, T.; Mortimer, J.C.; Stott, K.; Baker, J.M.; et al. Glycosyl transferases in family 61 mediate arabinofuranosyl transfer onto xylan in grasses. Proc. Natl. Acad. Sci. USA 2012, 109, 989–993. [Google Scholar] [CrossRef]
- Cavalier, D.M.; Lerouxel, O.; Neumetzler, L.; Yamauchi, K.; Reinecke, A.; Freshour, G.; Zabotina, O.A.; Hahn, M.G.; Burgert, I.; Pauly, M.; et al. Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. Plant Cell 2008, 20, 1519–1537. [Google Scholar] [CrossRef]
- Chou, Y.H.; Pogorelko, G.; Zabotina, O.A. Xyloglucan Xylosyltransferases XXT1, XXT2, and XXT5 and the Glucan Synthase CSLC4 Form Golgi-Localized Multiprotein Complexes. Plant Physiol. 2012, 159, 1355–1366. [Google Scholar] [CrossRef][Green Version]
- Derbyshire, P.; Ménard, D.; Green, P.; Saalbach, G.; Buschmann, H.; Lloyd, C.W.; Pesquet, E. Proteomic Analysis of Microtubule Interacting Proteins over the Course of Xylem Tracheary Element Formation in Arabidopsis. Plant Cell 2015, 27, 2709–2726. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.B.; Cosgrove, D.J. Changes in cell wall biomechanical properties in the xyloglucan-deficient xxt1/xxt2 mutant of Arabidopsis. Plant Physiol. 2012, 158, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Zhang, T.; Zheng, Y.; Cosgrove, D.J.; Anderson, C.T. Xyloglucan Deficiency Disrupts Microtubule Stability and Cellulose Biosynthesis in Arabidopsis, Altering Cell Growth and Morphogenesis. Plant Physiol. 2016, 170, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Dama, M.; Pauly, M. Identification of an arabinopyranosyltransferase from Physcomitrella patens involved in the synthesis of the hemicellulose xyloglucan. Plant Direct 2018, 2, e00046. [Google Scholar] [CrossRef]
- Grantham, N.J.; Wurman-Rodrich, J.; Terrett, O.M.; Lyczakowski, J.J.; Stott, K.; Iuga, D.; Simmons, T.J.; Durand-Tardif, M.; Brown, S.P.; Dupree, R.; et al. An even pattern of xylan substitution is critical for interaction with cellulose in plant cell walls. Nat. Plants 2017, 3, 859–865. [Google Scholar] [CrossRef]
- Zhu, X.; Xin, X.; Gu, Y. Cellulose and hemicellulose synthesis and their regulation in plant cells. In Extracellular Sugar-Based Biopolymers Matrices; Cohen, E., Merzendorfer, H., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 303–353. [Google Scholar] [CrossRef]
- Anderson, C.T. We be jammin’: An update on pectin biosynthesis, trafficking and dynamics. J. Exp. Bot. 2016, 67, 495–502. [Google Scholar] [CrossRef]
- Sterling, J.D.; Atmodjo, M.A.; Inwood, S.E.; Kumar Kolli, V.S.; Quigley, H.F.; Hahn, M.G.; Mohnen, D. Functional identification of an Arabidopsis pectin biosynthetic homogalacturonan galacturonosyltransferase. Proc. Natl. Acad. Sci. USA 2006, 103, 5236–5241. [Google Scholar] [CrossRef]
- Saffer, A.M. Expanding roles for pectins in plant development. J. Integr. Plant Biol. 2018, 60, 910–923. [Google Scholar] [CrossRef]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D.A. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef]
- Rui, Y.; Chen, Y.; Yi, H.; Purzycki, T.; Puri, V.M.; Anderson, C.T. Synergistic Pectin Degradation and Guard Cell Pressurization Underlie Stomatal Pore Formation. Plant Physiol. 2019, 180, 66–77. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Peaucelle, A.; Braybrook, S.; Hofte, H. Cell wall mechanics and growth control in plants: The role of pectins revisited. Front. Plant Sci. 2012, 3, 121. [Google Scholar] [CrossRef] [PubMed]
- Atmodjo, M.A.; Sakuragi, Y.; Zhu, X.; Burrell, A.J.; Mohanty, S.S.; Atwood III, J.A.; Orlando, R.; Scheller, H.V.; Mohnen, D. Galacturonosyltransferase (GAUT)1 and GAUT7 are the core of a plant cell wall pectin biosynthetic homogalacturonan: Galacturonosyltransferase complex. Proc. Natl. Acad. Sci. USA 2011, 108, 20225–20230. [Google Scholar] [CrossRef] [PubMed]
- Atmodjo, M.A.; Hao, Z.; Mohnen, D. Evolving views of pectin biosynthesis. Annu. Rev. Plant Biol. 2013, 64, 747–779. [Google Scholar] [CrossRef]
- Rui, Y.; Xiao, C.; Yi, H.; Kandemir, B.; Wang, J.Z.; Puri, V.M.; Anderson, C.T. POLYGALACTURONASE INVOLVED IN EXPANSION3 Functions in Seedling Development, Rosette Growth, and Stomatal Dynamics in Arabidopsis thaliana. Plant Cell 2017, 29, 2413–2432. [Google Scholar] [CrossRef]
- Zykwinska, A.W.; Ralet, M.C.; Garnier, C.D.; Thibault, J.F. Evidence for in vitro binding of pectin side chains to cellulose. Plant Physiol. 2005, 139, 397–407. [Google Scholar] [CrossRef]
- Anderson, C.T. Pectic polysaccharides in plants: Structure, biosynthesis, functions, and applications. In Extracellular Sugar-Based Biopolymers Matrices; Cohen, E., Merzendorfer, H., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 487–514. [Google Scholar] [CrossRef]
- Saffer, A.M.; Carpita, N.C.; Irish, V.F. Rhamnose-Containing Cell Wall Polymers Suppress Helical Plant Growth Independently of Microtubule Orientation. Curr. Biol. 2017, 27, 2248–2259.e4. [Google Scholar] [CrossRef]
- Du, J.; Kirui, A.; Huang, S.; Wang, L.; Barnes, W.J.; Kiemle, S.N.; Zheng, Y.; Rui, Y.; Ruan, M.; Qi, S.; et al. Mutations in the Pectin Methyltransferase QUASIMODO2 Influence Cellulose Biosynthesis and Wall Integrity in Arabidopsis. Plant Cell 2020, 32, 3576–3597. [Google Scholar] [CrossRef]
- Daher, F.B.; Chen, Y.; Bozorg, B.; Clough, J.; Jönsson, H.; Braybrook, S.A. Anisotropic growth is achieved through the additive mechanical effect of material anisotropy and elastic asymmetry. eLife 2018, 7, e38161. [Google Scholar] [CrossRef]
- Peaucelle, A.; Wightman, R.; Hofte, H. The Control of Growth Symmetry Breaking in the Arabidopsis Hypocotyl. Curr. Biol. 2015, 25, 1746–1752. [Google Scholar] [CrossRef]
- Velasquez, S.M.; Ricardi, M.M.; Dorosz, J.G.; Fernandez, P.V.; Nadra, A.D.; Pol-Fachin, L.; Egelund, J.; Gille, S.; Harlolt, J.; Ciancia, M.; et al. O-Glycosylated Cell Wall Proteins Are Essential in Root Hair Growth. Science 2011, 332, 1401–1403. [Google Scholar] [CrossRef] [PubMed]
- Braybrook, S.A. Measuring the elasticity of plant cells with atomic force microscopy. Methods Cell Biol. 2015, 125, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Zhao, H.; Liu, B.; Günther-Pomorski, T.; Chen, S.; Liesche, J. Novel tool to quantify cell wall porosity relates wall structure to cell growth and drug uptake. J. Cell Biol. 2019, 218, 1408–1421. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.J.; Blyth, M. How water flow, geometry, and material properties drive plant movements. J. Exp. Bot. 2019, 70, 3549–3560. [Google Scholar] [CrossRef]
- Kutschera, U.; Niklas, K.J. Cell division and turgor-driven stem elongation in juvenile plants: A synthesis. Plant Sci. 2013, 207, 45–56. [Google Scholar] [CrossRef]
- Bassel, G.W. Multicellular Systems Biology: Quantifying Cellular Patterning and Function in Plant Organs Using Network Science. Mol. Plant 2019, 12, 731–742. [Google Scholar] [CrossRef]
- Zhao, Y.; Man, Y.; Wen, J.; Guo, Y.; Lin, J. Advances in Imaging Plant Cell Walls. Trends Plant Sci. 2019, 24, 867–878. [Google Scholar] [CrossRef]
- Vaahtera, L.; Schulz, J.; Hamann, T. Cell wall integrity maintenance during plant development and interaction with the environment. Nat. Plants 2019, 5, 924–932. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Ischebeck, T.; Stenzel, I.; Heilmann, I. Type B phosphatidylinositol-4-phosphate 5-kinases mediate Arabidopsis and Nicotiana tabacum pollen tube growth by regulating apical pectin secretion. Plant Cell 2008, 20, 3312–3330. [Google Scholar] [CrossRef]
- Voiniciuc, C.; Pauly, M.; Usadel, B. Monitoring Polysaccharide Dynamics in the Plant Cell Wall. Plant Physiol. 2018, 176, 2590–2600. [Google Scholar] [CrossRef]
- Sala, K.; Potocka, I.; Kurczynska, E. Spatio-temporal distribution and methyl-esterification of pectic epitopes provide evidence of developmental regulation of pectins during somatic embryogenesis in Arabidopsis thaliana. Biol. Plant. 2013, 57, 410–416. [Google Scholar] [CrossRef]
- Jupa, R.; Didi, V.; Hejatko, J.; Gloser, V. An improved method for the visualization of conductive vessels in Arabidopsis thaliana inflorescence stems. Front. Plant Sci. 2015, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.T.; Carroll, A.; Akhmetova, L.; Somerville, C. Real-time imaging of cellulose reorientation during cell wall expansion in Arabidopsis roots. Plant Physiol. 2010, 152, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, Z.; Zhuang, Y.; Suo, Y.; Du, J.; Gao, Z.; Pan, J.; Li, L.; Wang, T.; Xiao, L.; et al. MicroRNA775 regulates intrinsic leaf size and reduces cell wall pectin levels by targeting a galactosyltransferase gene in Arabidopsis. Plant Cell 2021, 33, 581–602. [Google Scholar] [CrossRef]
- Guo, H.; Xiao, C.; Liu, Q.; Li, R.; Yan, Z.; Yao, X.; Hu, H. Two galacturonosyltransferases function in plant growth, stomatal development, and dynamics. Plant Physiol. 2021, 187, 2820–2836. [Google Scholar] [CrossRef]
- Allen, P.J.; Napoli, R.S.; Parish, R.W.; Li, S.F. MYB-bHLH-TTG1 in a Multi-tiered Pathway Regulates Arabidopsis Seed Coat Mucilage Biosynthesis Genes Including PECTIN METHYLESTERASE INHIBITOR14 Required for Homogalacturonan Demethylesterification. Plant Cell Physiol. 2023, 64, 906–919. [Google Scholar] [CrossRef]
- Kim, Y.J.; Jeong, H.Y.; Kang, S.Y.; Silva, J.; Kim, E.J.; Park, S.K.; Jung, K.H.; Lee, C. Physiological Importance of Pectin Modifying Genes During Rice Pollen Development. Int. J. Mol. Sci. 2020, 21, 4840. [Google Scholar] [CrossRef]
- Villouta, C.; Workmaster, B.A.; Livingston, D.P.; Atucha, A. Acquisition of Freezing Tolerance in Vaccinium macrocarpon Ait. Is a Multi-Factor Process Involving the Presence of an Ice Barrier at the Bud Base. Front. Plant Sci. 2022, 13, 891488. [Google Scholar] [CrossRef]
- Piccinini, L.; Ramamonjy, F.N.; Ursache, R. Imaging plant cell walls using fluorescent stains: The beauty is in the details. J. Microsc. 2024, 295, 102–120. [Google Scholar] [CrossRef]
- Zárský, V.; Cvrčková, F. Plant Cell Morphogenesis: Methods and Protocols. Methods in Molecular Biology; Walker, J.M., Ed.; Humana Press: New York, NY, USA, 2014; Volume 1080. [Google Scholar]
- Zhang, T.; Zheng, Y.; Cosgrove, D.J. Spatial organization of cellulose microfibrils and matrix polysaccharides in primary plant cell walls as imaged by multichannel atomic force microscopy. Plant J. 2016, 85, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.M.; Bjurhager, I.; Gerber, L.; Sundberg, B.; Salmen, L. Ultra-structural organisation of cell wall polymers in normal and tension wood of aspen revealed by polarisation FTIR microspectroscopy. Planta 2011, 233, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Yang, Z.L.; Han, L.J.; Jiang, X.P.; Ji, G.Y. Study on in situ analysis of cellulose, hemicelluloses and lignin distribution linked to tissue structure of crop stalk internodal transverse section based on FTIR microspectroscopic imaging. Cellulose 2015, 22, 139–149. [Google Scholar] [CrossRef]
- Xu, S.Y.; Camp, C.H.; Lee, Y.J. Coherent anti-Stokes Raman scattering microscopy for polymers. J. Polym. Sci. 2022, 60, 1244–1265. [Google Scholar] [CrossRef]
- Krafft, C.; Dietzek, B.; Schmitt, M.; Popp, J. Raman and coherent anti-Stokes Raman scattering microspectroscopy for biomedical applications. J. Biomed. Opt. 2012, 17, 040801. [Google Scholar] [CrossRef]
- Li, Y.P.; Shen, B.; Li, S.; Zhao, Y.; Qu, J.; Liu, L. Review of Stimulated Raman Scattering Microscopy Techniques and Applications in the Biosciences. Adv. Biol.-Ger. 2021, 5, 2000184. [Google Scholar] [CrossRef]
- Rongpipi, S.; Ye, D.; Gomez, E.D.; Gomez, E.W. Progress and Opportunities in the Characterization of Cellulose—An Important Regulator of Cell Wall Growth and Mechanics. Front. Plant Sci. 2019, 9, 1894. [Google Scholar] [CrossRef]
- Verbelen, J.P.; Vissenberg, K. The Expanding Cell; Springer: Berlin/Heidelberg, Germany, 2007; 297p. [Google Scholar]
- Milani, P.; Braybrook, S.A.; Boudaoud, A. Shrinking the hammer: Micromechanical approaches to morphogenesis. J. Exp. Bot. 2013, 64, 4651–4662. [Google Scholar] [CrossRef]
- Vogler, H.; Draeger, C.; Weber, A.; Felekis, D.; Eichenberger, C.; Routier-Kierzkowska, A.L.; Boisson-Dernier, A.; Ringli, C.; Nelson, B.J.; Smith, R.S.; et al. The pollen tube: A soft shell with a hard core. Plant J. 2013, 73, 617–627. [Google Scholar] [CrossRef]
- Braybrook, S.A.; Hofte, H.; Peaucelle, A. Probing the mechanical contributions of the pectin matrix: Insights for cell growth. Plant Signal Behav. 2012, 7, 1037–1041. [Google Scholar] [CrossRef]
- Majda, M.; Grones, P.; Sintorn, I.-M.; Vain, T.; Milani, P.; Krupinski, P.; Zagórska-Marek, B.; Viotti, C.; Jönsson, H.; Mellerowicz, E.J.; et al. Mechanochemical Polarization of Contiguous Cell Walls Shapes Plant Pavement Cells. Dev. Cell 2017, 43, 290–304.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Vavylonis, D.; Durachko, D.M.; Cosgrove, D.J. Nanoscale movements of cellulose microfibrils in primary cell walls. Nat. Plants 2017, 3, 17056. [Google Scholar] [CrossRef] [PubMed]
- Routier-Kierzkowska, A.L.; Smith, R.S. Measuring the mechanics of morphogenesis. Curr. Opin. Plant Biol. 2013, 16, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Farahi, R.H.; Charrier, A.M.; Tolbert, A.; Lereu, A.L.; Ragauskas, A.; Davison, B.H.; Passian, A. Plasticity, elasticity, and adhesion energy of plant cell walls: Nanometrology of lignin loss using atomic force microscopy. Sci. Rep. 2017, 7, 152. [Google Scholar] [CrossRef]
- Routier-Kierzkowska, A.L.; Weber, A.; Kochova, P.; Felekis, D.; Nelson, B.J.; Kuhlemeier, C.; Smith, R.S. Cellular Force Microscopy for in Vivo Measurements of Plant Tissue Mechanics. Plant Physiol. 2012, 158, 1514–1522. [Google Scholar] [CrossRef]
- Geitmann, A. Experimental approaches used to quantify physical parameters at cellular and subcellular levels. Am. J. Bot. 2006, 93, 1380–1390. [Google Scholar] [CrossRef]
- Krieg, M.; Fläschner, G.; Alsteens, D.; Gaub, B.M.; Roos, W.H.; Wuite, G.J.; Gaub, H.E.; Gerber, G.; Dufrêne, Y.F.; Müller, D.J. Atomic force microscopy-based mechanobiology. Nat. Rev. Phys. 2019, 1, 41–57. [Google Scholar] [CrossRef]
- Qi, J.; Wu, B.; Feng, S.; Lü, S.; Guan, C.; Zhang, X.; Qiu, D.; Hu, Y.; Zhou, Y.; Li, C.; et al. Mechanical regulation of organ asymmetry in leaves. Nat. Plants 2017, 3, 724–733. [Google Scholar] [CrossRef]
- Dang, J.M.C.; Copeland, L. Imaging rice grains using atomic force microscopy. J. Cereal Sci. 2003, 37, 165–170. [Google Scholar] [CrossRef]
- Chakraborty, I.; Pallen, S.; Shetty, Y.; Roy, N.; Mazumder, N. Advanced microscopy techniques for revealing molecular structure of starch granules. Biophys. Rev. 2020, 12, 105–122. [Google Scholar] [CrossRef]
- Wang, M.M.; Zhu, X.; Peng, G.; Liu, M.; Zhang, S.; Chen, M.; Liao, S.; Wei, X.; Xu, P.; Tan, X.; et al. Methylesterification of cell-wall pectin controls the diurnal flower-opening times in rice. Mol. Plant 2022, 15, 956–972. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, L.; Petrova, A.; Ananchenko, B.; Gorshkova, T. Assessment of Primary Cell Wall Nanomechanical Properties in Internal Cells of Non-Fixed Maize Roots. Plants 2019, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Fang, Y.; Zhang, H.; Quan, M.; Zhou, J.; Li, P.; Wang, D.; Ji, L.; Ingvarsson, P.K.; Wu, H.X.; et al. Natural variation in the prolyl 4-hydroxylase gene PtoP4H9 contributes to perennial stem growth in Populus. Plant Cell 2023, 35, 4046–4065. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.Y.; Liu, Y.S.; Zeng, Y.; Himmel, M.E.; Baker, J.O.; Bayer, E.A. How Does Plant Cell Wall Nanoscale Architecture Correlate with Enzymatic Digestibility? Science 2012, 338, 1055–1060. [Google Scholar] [CrossRef]
- Fan, T.F.; Park, S.; Shi, Q.; Zhang, X.; Liu, Q.; Song, Y.; Chin, H.; Ibrahim, M.S.B.; Mokrzecka, N.; Yang, Y.; et al. Transformation of hard pollen into soft matter. Nat. Commun. 2020, 11, 1449. [Google Scholar] [CrossRef]
- Cojocaru, R.; Mannix, O.; Capron, M.; Miller, C.G.; Jouneau, P.H.; Gallet, B.; Falconet, D.; Pacureanu, A.; Stukins, S. A biological nanofoam: The wall of coniferous bisaccate pollen. Sci. Adv. 2022, 8, eabd0892. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Diffuse Growth of Plant Cell Walls. Plant Physiol. 2018, 176, 16–27. [Google Scholar] [CrossRef]
- Robinson, S.; Huflejt, M.; Barbier de Reuille, P.; Braybrook, S.A.; Schorderet, M.; Reinhardt, D.; Kuhlemeier, C. An Automated Confocal Micro-Extensometer Enables in Vivo Quantification of Mechanical Properties with Cellular Resolution. Plant Cell 2017, 29, 2959–2973. [Google Scholar] [CrossRef]
- Chickarmane, V.; Roeder, A.H.K.; Tarr, P.T.; Cunha, A.; Tobin, C.; Meyerowitz, E.M. Computational morphodynamics: A modeling framework to understand plant growth. Annu. Rev. Plant Biol. 2010, 61, 65–87. [Google Scholar] [CrossRef]
- Andriankaja, M.; Dhondt, S.; Bodt, S.D.; Vanhaeren, H.; Coppens, F.; Milde, L.D.; Mühlenbock, P.; Skirycz, A.; Gonzalez, N.; Beemster, G.T.S.; et al. Exit from Proliferation during Leaf Development in Arabidopsis thaliana: A Not-So-Gradual Process. Dev. Cell 2012, 22, 64–78. [Google Scholar] [CrossRef]
- Boudaoud, A. An introduction to the mechanics of morphogenesis for plant biologists. Trends Plant Sci. 2010, 15, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Braybrook, S.A.; Jonsson, H. Shifting foundations: The mechanical cell wall and development. Curr. Opin. Plant Biol. 2016, 29, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Sambade, A.; Pratap, A.; Buschmann, H.; Morris, R.J.; Lloyd, C. The Influence of Light on Microtubule Dynamics and Alignment in the Arabidopsis Hypocotyl. Plant Cell 2012, 24, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Ivakov, A.; Flis, A.; Apelt, F.; Fünfgeld, M.; Scherer, U.; Stitt, M.; Kragler, F.; Vissenberg, K.; Persson, S.; Suslov, D. Cellulose Synthesis and Cell Expansion Are Regulated by Different Mechanisms in Growing Arabidopsis Hypocotyls. Plant Cell 2017, 29, 1305–1315. [Google Scholar] [CrossRef]
- Eng, R.C.; Sampathkumar, A. Getting into shape: The mechanics behind plant morphogenesis. Curr. Opin. Plant Biol. 2018, 46, 25–31. [Google Scholar] [CrossRef]
- Wong, J.H.; Kato, T.; Belteton, S.A.; Shimizu, R.; Kinoshita, N.; Higaki, T.; Sakumura, Y.; Szymanski, D.B.; Hashimoto, T. Basic Proline-Rich Protein-Mediated Microtubules Are Essential for Lobe Growth and Flattened Cell Geometry. Plant Physiol. 2019, 181, 1535–1551. [Google Scholar] [CrossRef]
- Bidhendi, A.J.; Altartouri, B.; Gosselin, F.P.; Geitmann, A. Mechanical Stress Initiates and Sustains the Morphogenesis of Wavy Leaf Epidermal Cells. Cell Rep. 2019, 28, 1237–1250.E6. [Google Scholar] [CrossRef]
- Yu, X.C.; Zhao, J.; Xu, Z.; Wei, J.; Wang, Q.; Shen, F.; Yang, X.; Guo, Z.L. AIpollen: An Analytic Website for Pollen Identification Through Convolutional Neural Networks. Plants 2024, 13, 3118. [Google Scholar] [CrossRef]
- Qu, Z.; Meredith, J.C. The atypically high modulus of pollen exine. J. R. Soc. Interface 2018, 15, 20180533. [Google Scholar] [CrossRef]
- Fayant, P.; Girlanda, O.; Chebli, Y.; Aubin, C.E.; Villemure, I.; Geitmann, A. Finite element model of polar growth in pollen tubes. Plant Cell 2010, 22, 2579–2593. [Google Scholar] [CrossRef]
- Kierzkowski, D.; Nakayama, N.; Routier-Kierzkowska, A.L.; Weber, A.; Bayer, E.; Schorderet, M.; Reinhardt, D.; Kuhlemeier, C.; Smith, R.S. Elastic domains regulate growth and organogenesis in the plant shoot apical meristem. Science 2012, 335, 1096–1099. [Google Scholar] [CrossRef] [PubMed]
- York, W.S.; Darvill, A.G.; Mcneil, M.; Stevenson, T.T.; Albersheim, P. Isolation and Characterization of Plant-Cell Walls and Cell-Wall Components. Methods Enzymol. 1986, 118, 3–40. [Google Scholar] [CrossRef]
- Parsons, H.T.; Christiansen, K.; Knierim, B.; Carroll, A.; Ito, J.; Batth, T.S.; Smith-Moritz, A.M.; Morrison, S.; McInerney, P.; Hadi, M.Z.; et al. Isolation and Proteomic Characterization of the Arabidopsis Golgi Defines Functional and Novel Components Involved in Plant Cell Wall Biosynthesis. Plant Physiol. 2012, 159, 12–26. [Google Scholar] [CrossRef] [PubMed]
- McNeil, M.; Darvill, A.G.; Fry, S.C.; Albersheim, P. Structure and function of the primary cell walls of plants. Annu. Rev. Biochem. 1984, 53, 625–663. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.A.; Gidley, M.J.; Fincher, G.B. Heterogeneity in the chemistry, structure and function of plant cell walls. Nat. Chem. Biol. 2010, 6, 724–732. [Google Scholar] [CrossRef]
- DeVree, B.T.; Steiner, L.M.; Głazowska, S.; Ruhnow, F.; Herburger, K.; Persson, S.; Mravec, J. Current and future advances in fluorescence-based visualization of plant cell wall components and cell wall biosynthetic machineries. Biotechnol. Biofuels 2021, 14, 78. [Google Scholar] [CrossRef]
- Verhertbruggen, Y.; Marcus, S.E.; Haeger, A.; Ordaz-Ortiz, J.J.; Knox, J.P. An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydr. Res. 2009, 344, 1858–1862. [Google Scholar] [CrossRef]
- Gierlinger, N.; Keplinger, T.; Harrington, M. Imaging of plant cell walls by confocal Raman microscopy. Nat. Protoc. 2012, 7, 1694–1708. [Google Scholar] [CrossRef]
- Trinh, D.C.; Alonso-Serra, S.; Asaoka, M.; Colin, L.; Cortes, M.; Malivert, A.; Takatani, S.A.; Zhao, F.; Traas, J.; Trehin, C.; et al. How Mechanical Forces Shape Plant Organs. Curr. Biol. 2021, 31, 143–159. [Google Scholar] [CrossRef]
- Hamant, O.; Inoue, D.; Bouchez, D.; Dumais, J.; Mjolsness, E. Are microtubules tension sensors? Nat. Commun. 2019, 10, 2360. [Google Scholar] [CrossRef]
- Landrein, B.; Hamant, O. How mechanical stress controls microtubule behavior and morphogenesis in plants: History, experiments and revisited theories. Plant J. 2013, 75, 324–338. [Google Scholar] [CrossRef]
- Boyer, J.S. Enzyme-Less Growth in Chara and Terrestrial Plants. Front. Plant Sci. 2016, 7, 866. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Xiao, L.; Qin, S.; Kuang, Z.; Wan, M.; Li, Z.; Li, L. Heterogeneity in Mechanical Properties of Plant Cell Walls. Plants 2024, 13, 3561. https://doi.org/10.3390/plants13243561
Zhang H, Xiao L, Qin S, Kuang Z, Wan M, Li Z, Li L. Heterogeneity in Mechanical Properties of Plant Cell Walls. Plants. 2024; 13(24):3561. https://doi.org/10.3390/plants13243561
Chicago/Turabian StyleZhang, He, Liang Xiao, Siying Qin, Zheng Kuang, Miaomiao Wan, Zhan Li, and Lei Li. 2024. "Heterogeneity in Mechanical Properties of Plant Cell Walls" Plants 13, no. 24: 3561. https://doi.org/10.3390/plants13243561
APA StyleZhang, H., Xiao, L., Qin, S., Kuang, Z., Wan, M., Li, Z., & Li, L. (2024). Heterogeneity in Mechanical Properties of Plant Cell Walls. Plants, 13(24), 3561. https://doi.org/10.3390/plants13243561






