Genetic Analyses of Flower, Fruit, and Stem Traits of Intergeneric Hybrids Between ‘Honghuagqinglong’ and ‘Heilong’ Pitayas
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Acquirement of Hybrids
2.3. Authenticity Identification of Hybrid Offspring
2.4. Observation and Measurement of Flower, Fruit, and Stem Traits
2.5. Determination of Betalain Content in Petal
2.6. Inheritance Analyses of Pitaya Main Traits
2.7. Data Analyses
3. Results
3.1. Identification of Hybridization Offspring
3.2. Flower Traits
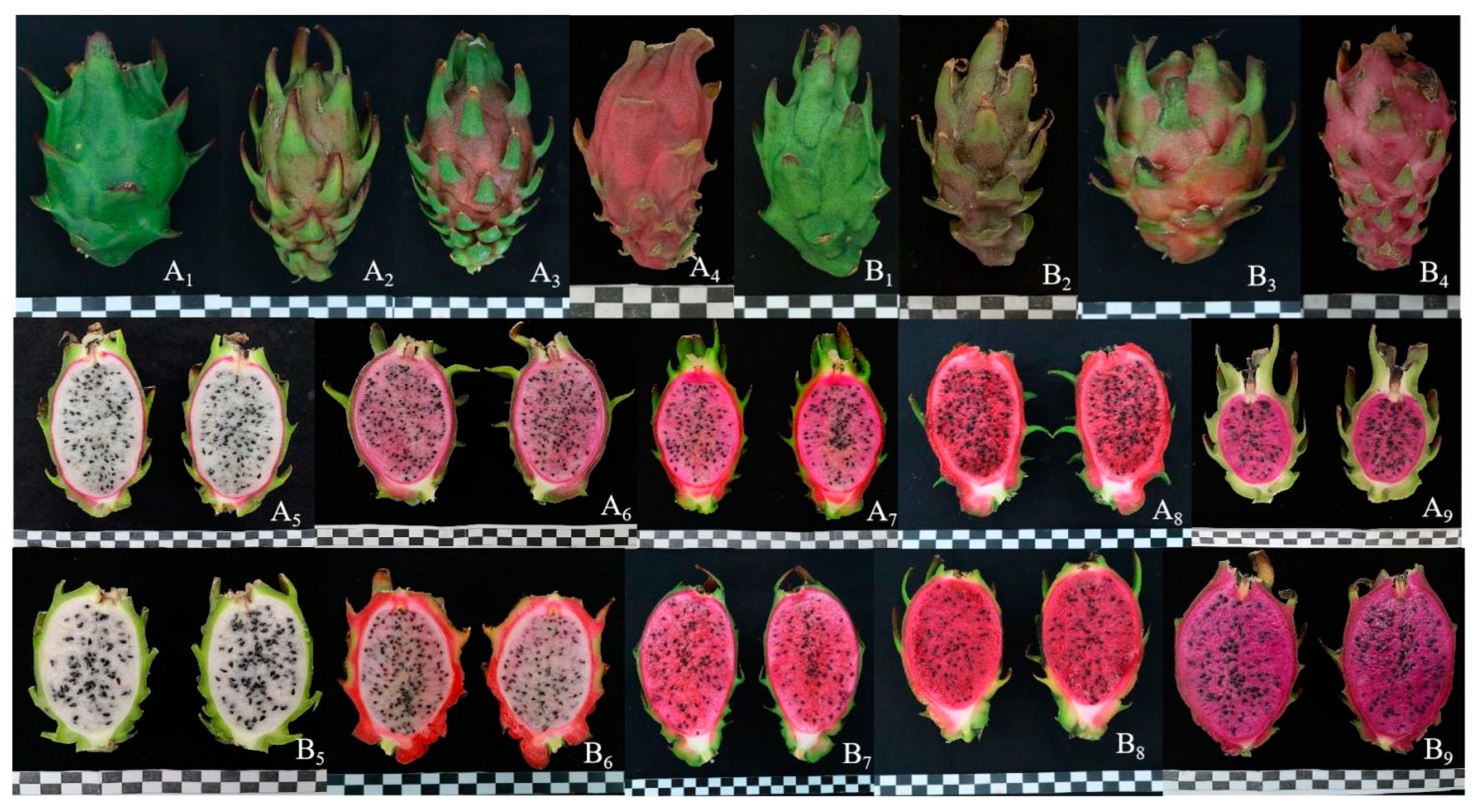
3.2.1. Petal Color and Stigma Bifurcates Traits
3.2.2. Distributions of Main Flower Traits
3.2.3. Genetic Analyses of Flower Traits
3.2.4. Genetic Analyses of Main Genes and Multigene of Flower Traits
3.3. Fruit Traits
3.3.1. Fruit Weight, TSS Content, Peel and Pulp Color
3.3.2. Distributions of Main Fruit Traits
3.3.3. Genetic Analyses of Fruit Traits
3.3.4. Genetic Analyses of Main Genes and Multigene of Fruit Traits
3.4. Stem Traits
3.4.1. Distributions of Stem Traits
3.4.2. Genetic Analyses of Stem Traits
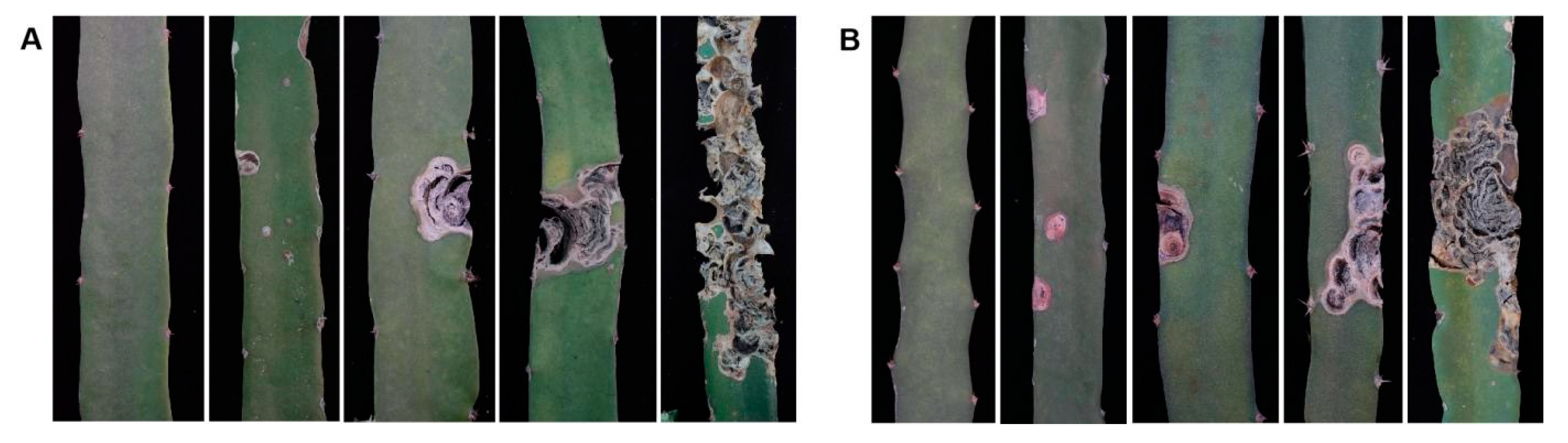
3.4.3. Some Pseudo Qualitative Traits and the Infection Status of Canker Disease
3.4.4. Genetic Analyses of Main Genes and Multigene of Stem Traits
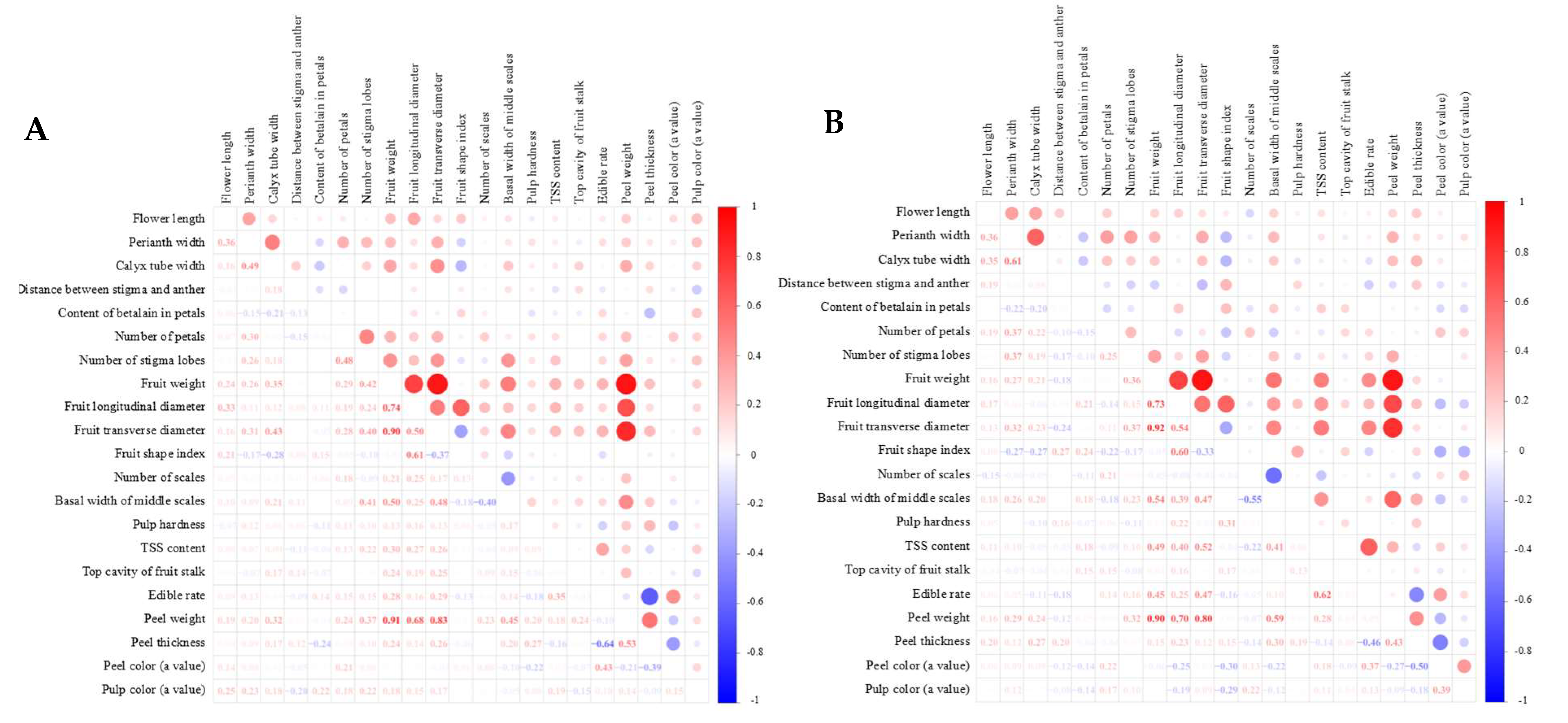
3.5. Correlation Analysis of Flower and Fruit Traits
3.6. Screening of New Germplasm
4. Discussion
4.1. The Genetic Effect of Flower Color
4.2. The Genetic Effect of Flower Types
4.3. The Genetic Effect of Stigmas
4.4. Genetic Analysis of Fruit Traits
4.5. The Appearance Quality of the Fruit
4.6. The Genetic Effect of TSS Content, Flesh Hardness and Color
4.7. Genetic Analyses of Stem Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nerd, A.; Mizrahi, Y. Reproductive biology of cactus fruit crops. Hortic. Rev. 1997, 18, 321–346. [Google Scholar]
- Mizrahi, Y. Vine-cati pitayas-the new crops of the world. Hortic. Rev. 1997, 18, 291–319. [Google Scholar]
- Tel-Zur, N.; Dudai, M.; Raveh, E.; Mizrahi, Y. In situ induction of chromosome doubling in vine cacti (Cactaceae). Sci. Hortic. 2011, 129, 570–576. [Google Scholar] [CrossRef]
- Tel-Zur, N. Breeding an underutilized fruit crop: A long-term program for Hylocereus. Hortic. Res. 2022, 9, 078. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; He, Y.Y.; Li, J.; Liu, R.; Li, X.J. Research progress on comprehensive processing of dragon fruit. Grain Food Ind. 2022, 29, 30–32. [Google Scholar]
- Shah, K.; Chen, J.Y.; Chen, J.X.; Qin, Y.H. Pitaya nutrition, biology, and biotechnology: A review. Int. J. Mol. Sci. 2023, 24, 13986. [Google Scholar] [CrossRef]
- Nizamlıoğlu, N.M.; Ünver, A.; Kadakal, C. Mineral content of pitaya (Hylocereus polyrhizus and Hylocereus undatus) seeds grown in turkey. Erwerbs Obstbau 2021, 63, 209–213. [Google Scholar] [CrossRef]
- Huang, Y.; Brennan, M.A.; Kasapis, S.; Richardson, S.J.; Brennan, C.S. Maturation process, nutritional profile, bioactivities and utilisation in food products of red pitaya fruits: A review. Foods 2021, 10, 2862. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Hernández, Y.D.; Carrillo-Salazar, J.A. Pitahaya (Hylocereus spp.): A short review. Comun. Sci. 2012, 3, 220–237. [Google Scholar]
- Bellec, F.L.; Vaillant, F.; Imbert, E. Pitahaya (Hylocereus spp.): A new fruit crop, a market with a future. Int. Rev. Red Cross 2006, 61, 237–250. [Google Scholar]
- Sun, Q.M.; Li, C.Y.; Liu, Y.Q.; Yi, G.J. Breeding of a new variety of pitaya ‘Yuehong No. 3’. J. Fruit Sci. 2017, 34, 778–780. [Google Scholar]
- Huang, L.F.; Deng, H.Y.; Wang, X.M.; Ren, H.; Liu, Y.Q.; Lin, H.W.; Liang, G.D. New variety of red-fleshed pitaya ‘Meilong 1’. Acta Hortic. Sin. 2021, 48, 1267–1268. [Google Scholar]
- Ye, Y.X.; Hu, G.B.; Li, J.Q.; Qin, Y.H.; Zhou, Y.W.; Zhai, X.; Huang, Q.L.; Liu, Z.X.; Li, J.X. New variety of pitaya ‘Guanhuabai’. Acta Hortic. Sin. 2018, 45, 2739–2740. [Google Scholar]
- Ye, Y.X.; Hu, G.B.; Li, J.Q.; Qin, Y.H.; Gu, W.Q.; Zhai, X.; Liu, Z.X.; Li, J.X. New variety of pitaya ‘Guanhuahong’. Acta Hortic. Sin. 2018, 45, 2267–2268. [Google Scholar]
- Chen, C.; Wu, P.; Xie, F.; Sun, L.; Xing, Y.; Hua, Q.; Zhang, Z.; Chen, J.; Zhao, J.; Hu, G.; et al. Breeding of ‘Hongguan No. 1’ and ‘Shuangse No. 1’ pitayas with superior quality. HortScience 2018, 53, 404–409. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Wang, L.; Zhao, X.Z.; Yuan, Q.F.; Xiao, T.J.; Ma, Y.H. Breeding of a new variety of pitaya ‘Qianhong’. Chin. Fruit Trees 2020, 2, 105–106+141. [Google Scholar]
- Zhang, Z.K.; Ma, M.B.; Hu, G.B.; Yan, C.D.; Ye, Y.X.; Zhang, R.; Qin, Y.H. Breeding of a new variety of dragon fruit ‘Fenhong No. 1’. J. Fruit Sci. 2023, 40, 822–826. [Google Scholar]
- Ma, M.B.; Zhang, Z.K.; Hu, G.B.; Zhang, R.; Yan, C.D.; Ye, Y.X.; Qin, Y.H. Breeding of a new variety of dragon fruit, ‘Hongguan No. 2’. J. Fruit Sci. 2023, 40, 1497–1502. [Google Scholar]
- Ning, F.N.; Liang, G.D.; Pang, Y.G.; Xu, C.W. Characteristics and key cultivation techniques for high yield of the ‘Guihonglong No. 1’ red dragon fruit. Chin. Trop. Agric. 2014, 65–67. [Google Scholar]
- Wang, B.; Zheng, W.; Cai, Y.Q.; Yuan, J.Q.; Zhang, Y.Z.; Pan, X.; Jiang, P.; Su, Z.; Jian, X.Q. New variety of dragon fruit ‘Qianguo No. 1’. Acta Hortic. Sin. 2016, 43, 605–606. [Google Scholar]
- Zheng, W.; Wang, B.; Cai, Y.Q.; Pan, X.; Zhang, Y.Z.; Su, Z.; Jiang, P.; Jian, X.Q. New variety of dragon fruit ‘Qianguo No. 2’. Acta Hortic. Sin. 2016, 43, 2285–2286. [Google Scholar]
- Xue, X.; Shi, S.Y.; Hou, S.K. Analysis of fruit quality traits and comprehensive evaluation of diverse traits in offspring of longan ‘Shuguan’ × ‘Dawuyuan’ hybrid. Acta Hortic. Sin. 2020, 47, 827–836. [Google Scholar]
- Yang, C.; Sha, G.; Wei, T.; Ma, B.; Li, C.; Li, P.; Zou, Y.; Xu, L.; Ma, F. Linkage map and QTL mapping of red flesh locus in apple using a ‘R1R1’ × ‘R6R6’ population. Hortic. Plant J. 2021, 7, 393–400. [Google Scholar] [CrossRef]
- Wu, H.X.; Li, X.; Xu, W.T.; Zheng, B.; Ma, X.W.; Su, M.Q.; Liang, Q.Z.; Yao, Q.S.; Wang, S.B. Genetic analysis of fruit traits in the progeny of mango ‘Jinhuang’ × ‘Renong No. 1’. Acta Hortic. Sin. 2022, 49, 416–426. [Google Scholar]
- Liu, Y.C.; Li, J.Q.; Liu, C. A study on the genetic laws of fruit size in the cowberry and its correlation with floral corolla and leaf area. J. Fruit Sci. 2023, 40, 2050–2060. [Google Scholar]
- Zhang, Y.; Cao, Y.F.; Huo, H.L.; Tian, L.M.; Dong, X.G.; Qi, D.; Zhang, X.S. Research on the diversity of pear germplasm resources based on floral phenotypic traits. Acta Hortic. Sin. 2016, 43, 1245–1256. [Google Scholar]
- Yin, M.Y.; Zhu, X.C.; Liu, H.M.; Pan, J.Q.; Wu, Y.T.N. Floral phenotypic variation of Siberian apricot germplasm resources. J. Northwest AF Univ. Nat. Sci. Ed. 2018, 46, 92–103. [Google Scholar]
- Wei, X.X.; Lai, R.L.; Chen, J.; Pan, S.L.; Wu, R.J. Study on the genetic diversity of inflorescence phenotypic traits in olive germplasm resources. J. Trop. Subtrop. Bot. 2019, 27, 1–10. [Google Scholar]
- Wang, Y.; Liu, Z.S.; Zhang, J.; Yang, P.F.; Ma, L.; Wang, Z.Y.; Tu, H.X.; Yang, S.F.; Wang, H.; Chen, T.; et al. Analysis of genetic inclination of several floral and fruit traits in the F1 generation of hybrid Chinese cherries. Acta Hortic. Sin. 2022, 49, 1853–1865. [Google Scholar]
- Yang, Z.; Zhang, C.J.; Wang, Z.L.; Yan, F.F.; Wu, C.Y.; Wang, J.R.; Liu, M.J.; Lin, M.J. Floral trait segregation patterns and mixed inheritance analysis in the F1 generation of jujube and sour jujube hybrids. Mol. Plant Breed. 2023, 21, 231–242. [Google Scholar]
- NY/T 4211-2022; Qin, Y.H.; Hu, G.B.; Yang, X.H.; Rao, D.H.; Sun, Q.M.; Wu, P.Y.; Zhang, Z.K.; Sun, L.Y.; Xie, F.F.; Wang, Y. Guidelines for the conduct of tests for distinctness, uniformity and stability-Hylocereus (Berger) Britt. et Rose. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2022. [Google Scholar]
- Hua, Q.Z.; Chen, C.J.; Chen, Z.; Chen, P.K.; Ma, Y.W.; Wu, J.Y.; Zheng, J.; Hu, G.B.; Zhao, J.T.; Qin, Y.H. Transcriptomic analysis reveals key genes related to betalain biosynthesis in pulp coloration of Hylocereus polyrhizus. Front. Plant Sci. 2016, 6, 1179. [Google Scholar]
- Zhang, T.; Xing, K.; Chen, J.; Sabir, I.A.; Shah, K.; Chen, J.; Zhang, Z.; Zhao, J.; Hu, G.; Qin, Y. Genetic analyses of flower main traits from two pitayas and their progenies: A cactus plant. Plants 2024, 13, 699. [Google Scholar] [CrossRef]
- Song, X.B.; Zhao, X.G.; Fan, G.X.; Gao, K.; Dai, S.L.; Zhang, M.M. Genetic analysis of the corolla tube merged degree and the relative number of ray florets in chrysanthemum (Chrysanthemum × morifolium Ramat.). Sci. Hortic. 2018, 242, 214–224. [Google Scholar] [CrossRef]
- Zeng, T.; Li, J.W.; Zhou, L.; Li, J.J.; Shi, A.Q.; Fu, H.S.; Luo, J.; Zheng, R.R.; Wang, Y.Y.; Wang, C.Y. Research progress on the co-adaptation relationship between flower color of ornamental plants and pollinating insects. Acta Hortic. Sin. 2021, 48, 2001–2017. [Google Scholar]
- Ge, C.C.; Wang, Y.N.; Li, L.X.; Zhang, Y.Q.; Yuan, X.M.; Huang, G.X. Analysis of representative phenotypes and genetic variations of the progeny of ‘Perfume Lemon’ × ‘White Flower Lemon’. J. Fruit Sci. 2022, 39, 169–183. [Google Scholar]
- Zhou, Y.W.; Xu, G.Y.; Wang, Q.; Yan, F.L.; Yu, Y.Y.; Yu, R.C.; Fan, Y.P. Genetic analysis of flower color inheritance in the F1 generation of the cross ‘Baijianghua’ × ‘Jinjianghua’ and development of related SSR molecular markers. Acta Hortic. Sin. 2021, 48, 1921–1933. [Google Scholar]
- Zhu, J.; Kong, W.G.; Ma, L.; Lv, X.H.; Chen, G.X.; Pan, H.J.; Zhang, J.H.; Dong, D.F. Study on the separation rules of ornamental traits in the hybrids of cattleya hybrids with red moth orchid flowers. Chin. Agric. Sci. Bull. 2018, 34, 70–77. [Google Scholar]
- Jiang, S.; Yi, X.W.; Xu, T.L.; Yang, Y.; Yu, C.; Luo, L.; Cheng, T.R.; Wang, J.; Zhang, Q.X.; Pan, H.T. Genetic analysis of petal number in roses. J. Plant Sci. 2021, 39, 142–151. [Google Scholar]
- Zhang, L.; Guo, L.L.; Guo, D.L.; Hou, X.G. Separation analysis and mixed genetic analysis of phenotype traits in F1 progenies of tree peony. J. Nanjing For. Univ. Nat. Sci. Ed. 2018, 42, 51–60. [Google Scholar]
- Fu, H.S.; Zhang, Y.W.; Zhao, Y.Y.; Luo, T.T.; Deng, H.J.; Meng, C.Y.; Wang, C.Y. Analysis of hybrid vigor and mixed inheritance of floral traits of ‘Lvdingdang’ × ‘Maohuaju’ chrysanthemum offspring populations. Acta Hortic. Sin. 2021, 48, 96–106. [Google Scholar]
- Tang, H.Q.; Zhang, F.; Chen, F.D.; Fang, W.M.; Wang, C.C.; Chen, S.M. Heterosis and mixed genetic analysis of inflorescence traits of anemone typed chrysanthemum. Acta Hortic. Sin. 2015, 42, 907–916. [Google Scholar]
- Huang, F.Z.; Lu, G.F.; Wei, S.T.; Wu, Z.J.; Li, Z.Y.; Deng, H.Y.; Huang, L.F.; Liang, G.D. Phenotypic diversity of pitaya flowers and their correlation with fruit traits. Chin. J. Trop. Agric. 2021, 4, 24–29. [Google Scholar]
- Guo, K.B.; Li, S.; Qiao, G. Research progress on the physiological characteristics and regulatory mechanisms of flower and fruit drop in sweet cherries. Seed 2018, 37, 50–56. [Google Scholar]
- Liang, G.D.; Hu, Z.Y.; Li, L.Z.; Tang, Z.P.; Deng, J.Y.; Chanthavilath, S. The impact of artificial pollination on the fruit development of pitaya. Guangxi Plants 2011, 31, 813–817. [Google Scholar]
- Liu, J.C.; Zhang, Q.P.; Niu, T.Q.; Liu, N.; Zhang, Y.P.; Xu, M.; Ma, X.X.; Zhang, Y.J.; Liu, S.; Liu, W. Analyzing the genetic tendency of fruit traits in ‘Chuanzhihong’ and ‘Saimaiti’ apricot offspring after reciprocal crossing. J. Fruit Trees 2020, 37, 625–634. [Google Scholar]
- Lu, M.Y.; Wang, Q.; Yan, X.K.; Wu, C.H.; Zhao, Y.; Zhang, M.J. Analysis of genetic tendency of fruit traits in pear hybrid F1. J. Plant Genet. Resour. 2024, 25, 294–302. [Google Scholar]
- Liu, Y.; Liu, H.N.; Deng, Y.; Liu, Y.S.; Yin, X.Y.; Sun, H.Y.; Li, Y.D. Genetic tendencies of partial traits in the offspring of cranberry reciprocal crosses. J. Jilin Agric. Univ. 2019, 41, 35–41. [Google Scholar]
- Wu, X.H.; Jing, C.J.; Chen, X.F.; Zhao, X.P.; Yuan, L.Y.; Liang, S.; Zhang, X.C.; Tang, H.Y.; Li, L.Y. A study on the genetic tendency of fruit traits in offspring of reciprocal crosses between ‘Jintaiyang’ and ‘Chuanzhihong’ apricots. Jiangxi Agric. Sci. 2018, 30, 13–18. [Google Scholar]
- Wei, S.T.; Lu, G.F.; Li, Z.Y.; Liang, G.D.; Huang, L.F.; Deng, H.Y.; Wu, Z.J.; Huang, F.Z. Preliminary study on the inheritance tendency of main fruit characteristics of offspring of ‘Zilong’ and ‘Wuming No. 4’ pitaya hybrid. Chin. J. South. Fruit Trees 2021, 50, 40–44. [Google Scholar]
- Wu, Z.J.; Huang, F.Z.; Wei, S.T.; Huang, L.F.; Deng, H.Y.; Ye, X.Y.; Liang, G.D.; Li, Z.Y.; Liu, C.A.; Lu, G.F. Analysis of the relationship between fruit traits and fruit cracking in pitaya germplasm resources. J. Plant Genet. Resour. 2024, 25, 52–63. [Google Scholar]
- Zhao, Y.N.; Ou, C.Q.; Wang, F.; Zhang, Y.J.; Ma, L.; Jiang, S.C. Genetic analysis of fruit traits and correlation study in the F1 generation of pear. J. Northwest AF Univ. 2018, 27, 1811–1818. [Google Scholar]
- Wei, J.L.; Chen, Y.L.; Li, C.J.; Li, J.C.; Huang, X.Y. Correlation analysis of dragon fruit fruit cracking and main fruit traits. South. Agric. 2022, 16, 46–49. [Google Scholar]
- Lei, L.; Xu, G.H.; Wang, L.; Du, Q.H.; Li, Y.F.; Wang, H.X. Analysis of the inheritance tendency of main economic traits in the offspring of reciprocal crosses between blueberry cultivars ‘Senmao No. 1’ and ‘Lvbaoshi’. J. Fruit Trees 2022, 39, 1573–1586. [Google Scholar]


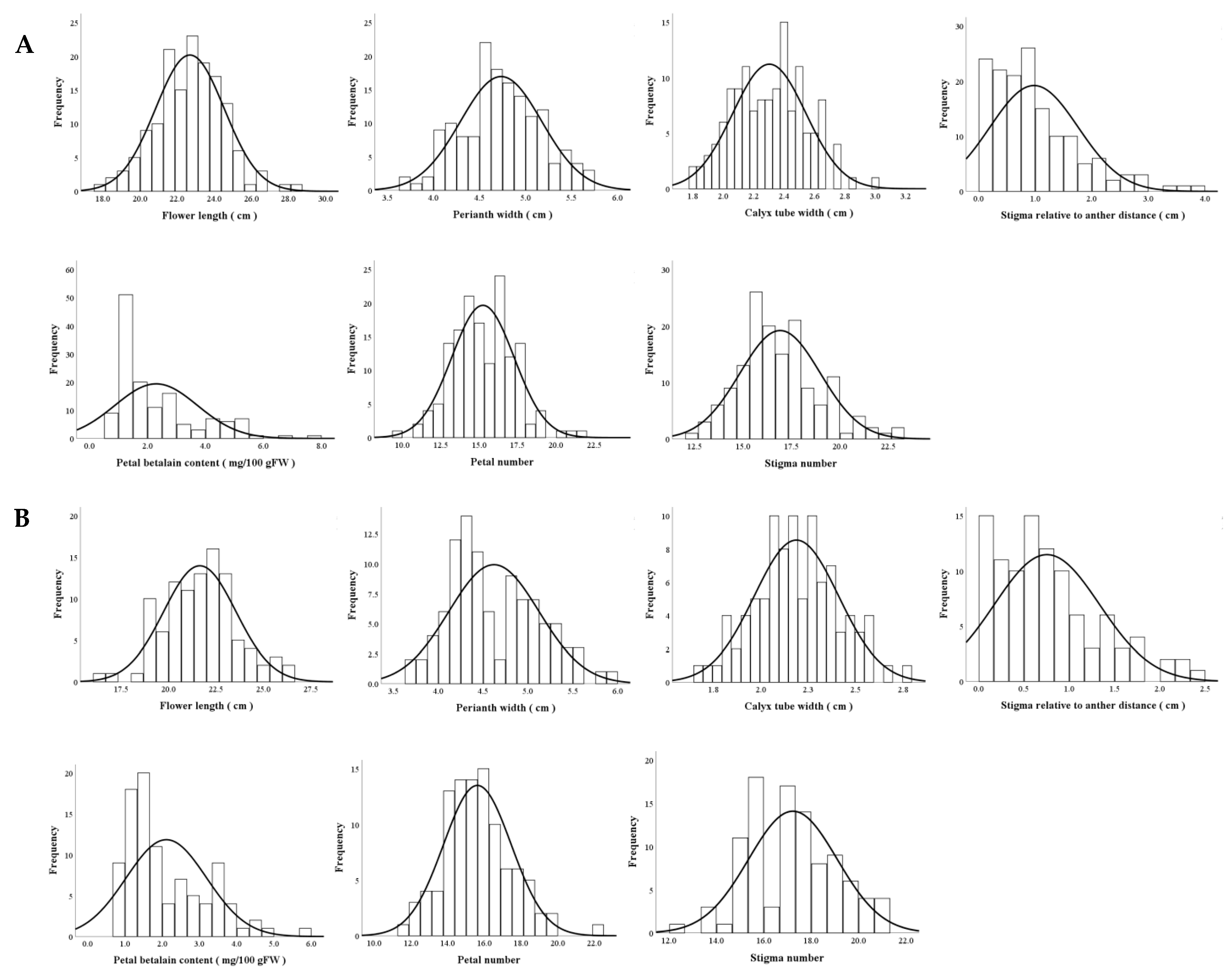
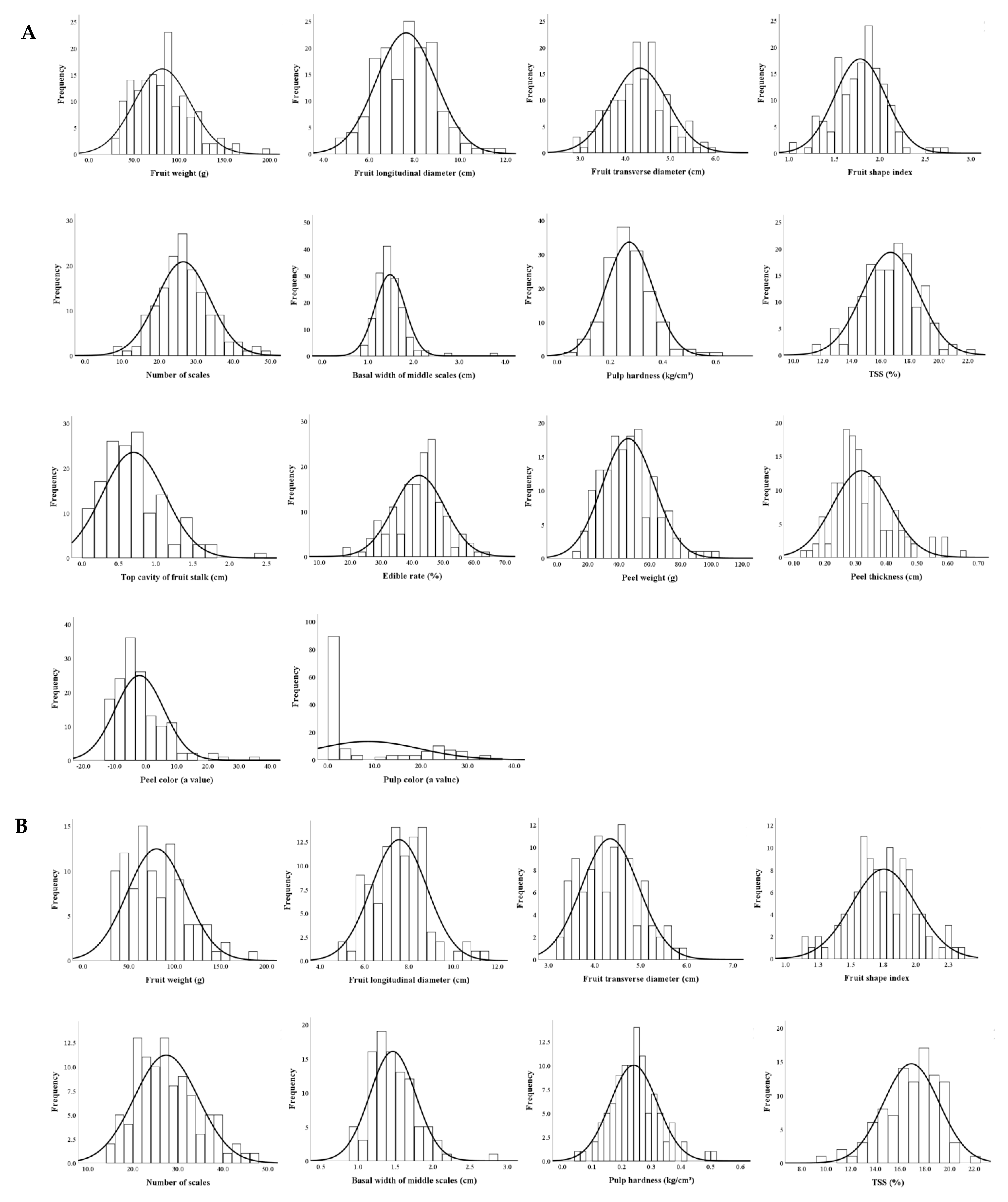
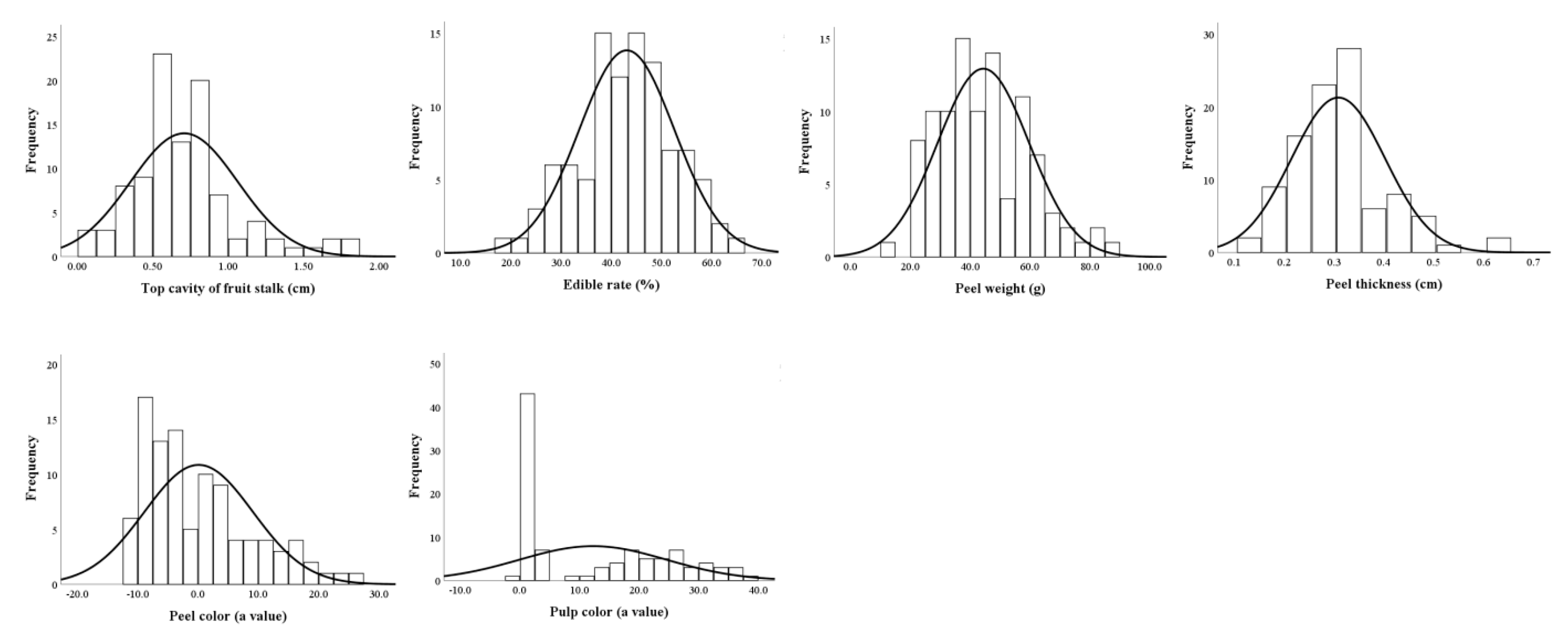
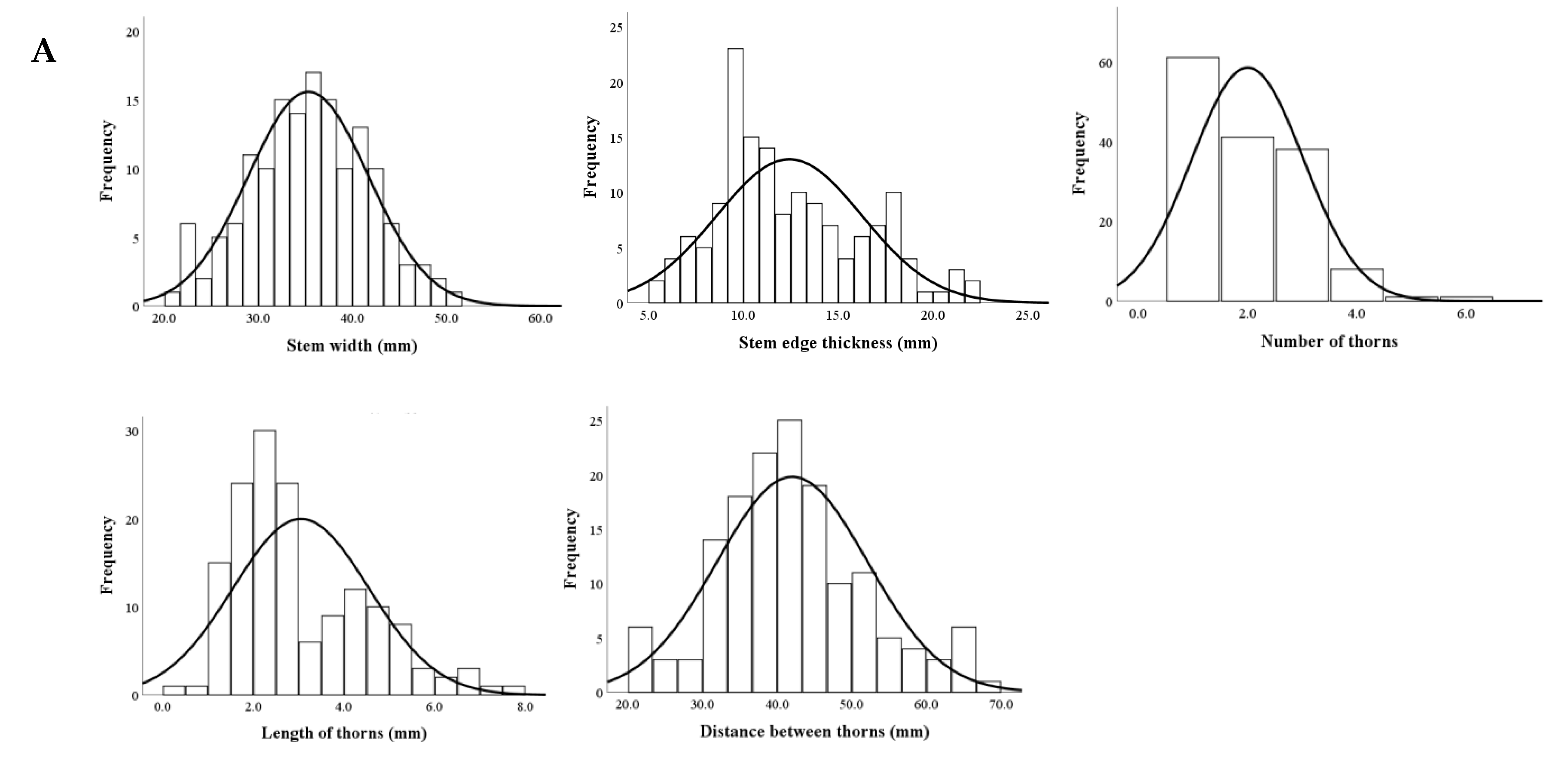
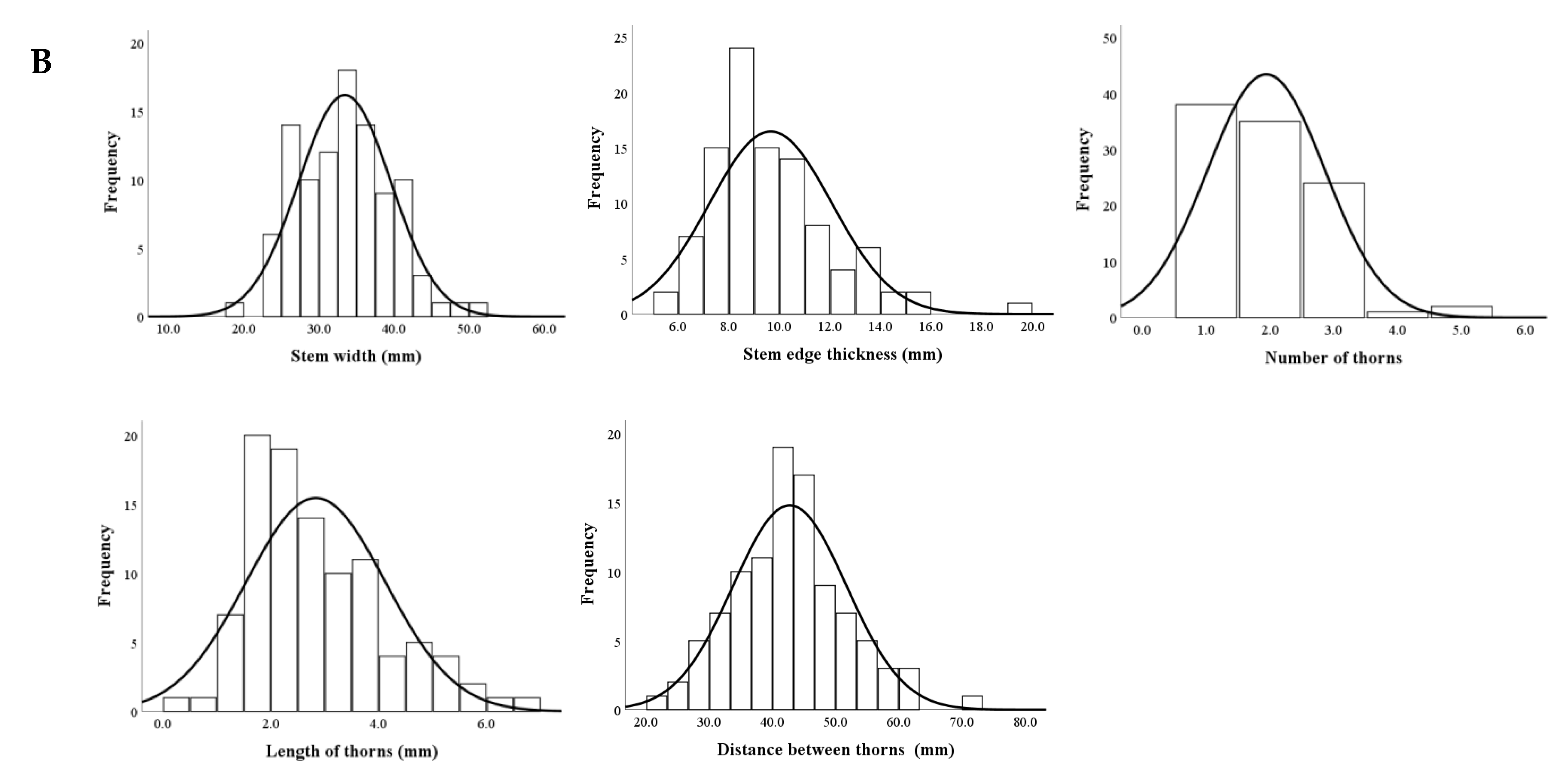
| Flower Traits | Cross Combinations | Parents | F1 Progenies | |||||
|---|---|---|---|---|---|---|---|---|
| HHQL | HL | MP | F ± S | CV (%) | Ta (%) | Ha (%) | ||
| Flower length (cm) | Q × H | 20.86 | 26.53 | 23.70 | 22.68 ± 1.85 | 8.17 | 95.71 | −4.29 |
| H × Q | 23.70 | 21.64 ± 1.9 | 8.80 | 91.32 | −8.68 | |||
| Perianth width (cm) | Q × H | 3.79 | 4.90 | 4.35 | 4.73 ± 0.44 | 9.33 | 108.90 | 8.90 |
| H × Q | 4.35 | 4.61 ± 0.5 | 10.83 | 106.14 | 6.14 | |||
| Calyx tube width (cm) | Q × H | 2.25 | 2.36 | 2.30 | 2.3 ± 0.24 | 10.42 | 99.94 | −0.06 |
| H × Q | 2.30 | 2.19 ± 0.22 | 10.15 | 94.90 | −5.10 | |||
| Distance between stigma and anther (cm) | Q × H | 0.94 | 0.00 | 0.47 | 0.98 ± 0.78 | 79.77 | 208.06 | 108.06 |
| H × Q | 0.47 | 0.75 ± 0.58 | 77.02 | 160.20 | 60.20 | |||
| Content of betalain in petals (mg/100 g FW) | Q × H | 5.77 | 1.00 | 3.38 | 2.3 ± 1.42 | 61.87 | 67.86 | −32.14 |
| H × Q | 3.38 | 2.11 ± 1.08 | 51.12 | 62.33 | −37.67 | |||
| No. of petals | Q × H | 13.71 | 18.50 | 16.11 | 15.23 ± 2.03 | 13.33 | 94.58 | −5.42 |
| H × Q | 16.11 | 15.61 ± 1.85 | 11.84 | 96.89 | −3.11 | |||
| No. of stigma lobes | Q × H | 19.57 | 16.75 | 18.16 | 16.91 ± 2.08 | 12.30 | 93.09 | −6.91 |
| H × Q | 18.16 | 17.22 ± 1.87 | 10.85 | 94.84 | −5.16 | |||
| Traits | Cross Combination | Model | m | da | db | ha | hb | i | jab | jba | l | pσ2mg | Hmg2 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flower length | Q × H | 2MG-EA | 22.6873 | 1.5358 | 0.5471 | 16.1798 | |||||||
| H × Q | 2MG-AD | 21.0861 | 1.7029 | 0.4173 | −0.79 | 2.1996 | 3.429 | 99.0503 | |||||
| Perianth width | Q × H | 2MG-EA | 4.7558 | 0.3138 | 0.1382 | 69.5537 | |||||||
| H × Q | 2MG-EA | 4.6074 | 0.3262 | 0.2187 | 87.5941 | ||||||||
| Calyx tube width | Q × H | 2MG-EA | 2.3024 | 0.2027 | 0.026 | 45.9066 | |||||||
| H × Q | 2MG-EA | 2.193 | 0.181 | 0.0278 | 62.2776 | ||||||||
| Distance between stigma and anther | Q × H | 2MG-AD | 1.149 | 0.8179 | 0.3312 | −0.2547 | −0.0661 | 0 | 0 | ||||
| H × Q | 2MG-EA | 0.779 | 0.3895 | 0.0996 | 29.6612 | ||||||||
| Petal color | Q × H | 2MG-AD | 2.8421 | 1.6813 | 0.1343 | −0.8732 | 0.035 | 0 | 0 | ||||
| H × Q | 2MG-AD | 2.4486 | 1.2516 | 0.2333 | −0.5821 | −0.0839 | 0 | 0 | |||||
| No. of petals | Q × H | 2MG-EA | 15.2361 | 0.8835 | 0 | 0 | |||||||
| H × Q | 2MG-EA | 15.6832 | 1.5893 | 0 | 0 | ||||||||
| No. of stigma lobes | Q × H | 2MG-EA | 16.9195 | 1.1422 | 0 | 0 | |||||||
| H × Q | 2MG-AD | 17.7536 | 1.8213 | 1.0014 | −0.9967 | 0.0076 | 3.3319 | 95.331 |
| Fruit Traits | Cross Combinations | Parents | F1 | |||||
|---|---|---|---|---|---|---|---|---|
| ‘HHQL’ | ‘HL’ | MP | F ± S | CV (%) | Ta (%) | Ha (%) | ||
| Fruit weight (%) | Q × H | 155.69 | 68.92 | 112.30 | 80.88 ± 30.95 | 38.27 | 72.02 | −27.98 |
| H × Q | 79.99 ± 32.03 | 40.04 | 71.23 | −28.77 | ||||
| Fruit longitudinal diameter (cm) | Q × H | 9.27 | 5.90 | 7.59 | 7.62 ± 1.31 | 17.22 | 100.44 | 0.44 |
| H × Q | 7.55 ± 1.25 | 16.61 | 99.56 | −0.44 | ||||
| Fruit transverse diameter (cm) | Q × H | 5.25 | 4.65 | 4.95 | 4.32 ± 0.62 | 14.39 | 87.14 | −12.86 |
| H × Q | 4.33 ± 0.62 | 14.26 | 87.52 | −12.48 | ||||
| Fruit shape index | Q × H | 1.76 | 1.27 | 1.52 | 1.78 ± 0.28 | 15.76 | 117.4 | 17.4 |
| H × Q | 1.75 ± 0.25 | 14.11 | 115.62 | 15.62 | ||||
| Number of scales | Q × H | 22.76 | 36.18 | 29.47 | 26.45 ± 7.19 | 27.17 | 89.75 | −10.25 |
| H × Q | 27.39 ± 7.12 | 25.99 | 92.95 | −7.05 | ||||
| Basal width of middle scales (cm) | Q × H | 2.53 | 0.53 | 1.53 | 1.48 ± 0.33 | 22.16 | 96.69 | −3.31 |
| H × Q | 1.46 ± 0.31 | 21.01 | 95.51 | −4.49 | ||||
| Pulp hardness (kg/cm3) | Q × H | 0.24 | 0.12 | 0.18 | 0.27 ± 0.09 | 32.51 | 149.75 | 49.75 |
| H × Q | 0.24 ± 0.08 | 33.08 | 132.26 | 32.26 | ||||
| TSS (%) | Q × H | 19.39 | 16.32 | 17.85 | 16.64 ± 1.96 | 11.75 | 93.18 | −6.82 |
| H × Q | 16.89 ± 2.26 | 13.4 | 94.61 | −5.39 | ||||
| Top cavity of fruit (cm) | Q × H | 0.86 | 0.43 | 0.64 | 0.7 ± 0.42 | 60.77 | 108.67 | 8.67 |
| H × Q | 0.71 ± 0.36 | 50.39 | 110.12 | 10.12 | ||||
| Edible rate (%) | Q × H | 43.87 | 57.71 | 50.79 | 42.18 ± 8.32 | 19.72 | 83.05 | −16.95 |
| H × Q | 43.07 ± 9.52 | 22.1 | 84.8 | −15.2 | ||||
| Peel weight (g) | Q × H | 87.59 | 29.08 | 58.34 | 46.05 ± 16.95 | 36.8 | 78.94 | −21.06 |
| H × Q | 43.07 ± 9.52 | 22.1 | 84.8 | −15.2 | ||||
| Peel thickness (cm) | Q × H | 0.41 | 0.23 | 0.32 | 0.32 ± 0.09 | 29.23 | 99.41 | −0.59 |
| H × Q | 0.31 ± 0.09 | 30.39 | 96.2 | −3.8 | ||||
| Peel color (a value) | Q × H | −11.26 | 24.74 | 6.74 | −2.02 ± 7.79 | −385.32 | −30 | −130 |
| H × Q | 0.08 ± 9.01 | 10,632.86 | 1.26 | −98.74 | ||||
| Pulp color (a value) | Q × H | 1.18 | 29.56 | 15.37 | 8.63 ± 10.98 | 127.21 | 56.17 | −43.83 |
| H × Q | 12.24 ± 12.34 | 100.83 | 79.64 | −20.36 | ||||
| Fruit Traits | Cross Combinations | Model | m | da | db | ha | hb | i | jab | jba | l | pσ2mg | Hmg2 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fruit weight | Q × H | 2MG-A | 81.4138 | −5.2296 | 32.4847 | 0 | 0 | ||||||
| H × Q | 2MG-AD | 89.3111 | 37.3628 | 13.7251 | −7.7391 | −8.0902 | 328.9568 | 32.0647 | |||||
| Fruit longitudinal diameter | Q × H | 2MG-EA | 7.6034 | 0.7411 | 0 | 0 | |||||||
| H × Q | 2MG-AD | 7.6645 | 0.8943 | 0.8661 | 0.0599 | −0.1903 | 0 | 0 | |||||
| Fruit transverse diameter | Q × H | 2MG-EA | 4.3152 | 0.1616 | 0 | 0 | |||||||
| H × Q | 2MG-AD | 4.5945 | 0.5498 | 0.4488 | 0.0343 | −0.6234 | 0.3536 | 92.5371 | |||||
| Fruit shape index | Q × H | 2MG-A | 1.7872 | 0.0728 | 0.2888 | 0 | 0 | ||||||
| H × Q | 2MG-A | 1.7485 | −0.1325 | 0.0048 | 0 | 0 | |||||||
| Number of scales | Q × H | 1MG-A | 26.4444 | 7.0627 | 3.4187 | 6.6203 | |||||||
| H × Q | 2MG-EA | 27.2588 | 5.8099 | 32.7702 | 64.6687 | ||||||||
| Basal width of middle scales | Q × H | 2MG-A | 1.5046 | 0.0771 | 0.2648 | 0 | 0 | ||||||
| H × Q | 2MG-EA | 1.4625 | 0.2609 | 0 | 0 | ||||||||
| Flesh hardness | Q × H | 1MG-A | 0.2724 | 0.0576 | 0 | 0 | |||||||
| H × Q | 1MG-A | 0.2405 | 0.0516 | 0 | 0 | ||||||||
| TSS content | Q × H | 2MG-EA | 16.6353 | 0.6598 | 2.7346 | 71.5213 | |||||||
| H × Q | 2MG-EA | 16.967 | 1.3149 | 4.9581 | 96.7798 | ||||||||
| Top cavity of fruit | Q × H | 2MG-AD | 0.6663 | 0.4386 | 0.2277 | −0.1472 | 0.201 | 0 | 0 | ||||
| H × Q | 2MG-EA | 0.7287 | 0.2484 | 0 | 0 | ||||||||
| Edible rate | Q × H | 2MG-AD | 0.3497 | 0.0592 | 0.0186 | 0.0968 | 0.0482 | 0.0069 | 99.5973 | ||||
| H × Q | 2MG-EA | 0.4336 | 0.0685 | 0.0086 | 95.0738 | ||||||||
| Peel weight | Q × H | 2MG-AD | 50.1727 | 12.7099 | 16.1696 | 0.4114 | −8.9628 | 11.5839 | 4.0332 | ||||
| H × Q | 2MG-AD | 50.6289 | 15.1547 | 9.873 | −3.1703 | −8.7904 | 176.0511 | 75.4654 | |||||
| Peel thickness | Q × H | 2MG-A | 0.315 | 0.0289 | 0.0849 | 0 | 0 | ||||||
| H × Q | 2MG-AD | 0.3331 | 0.069 | 0.0659 | 0.0118 | −0.0618 | 0 | 0 | |||||
| Peel color | Q × H | 2MG-AD | 0.5249 | 6.1507 | 5.2031 | −1.4405 | −3.5765 | 0 | 0 | ||||
| H × Q | 2MG-AD | 2.8634 | 10.5269 | 2.4549 | −4.01 | −0.6129 | 50.9704 | 62.8422 | |||||
| Flesh color | Q × H | 1MG-AD | 11.8026 | 10.9527 | −10.0023 | 65.8128 | 54.552 | ||||||
| H × Q | 1MG-AD | 16.3114 | 14.8952 | 3.9782 | 136.5994 | 89.6459 |
| Traits | Cross Combinations | Parents | F1 | |||||
|---|---|---|---|---|---|---|---|---|
| ‘HHQL’ | ‘HL’ | MP | F ± S | CV (%) | Ta (%) | Ha (%) | ||
| Stem width | Q × H | 39.91 | 23.65 | 31.78 | 35.37 ± 6.8 | 19.22 | 111.30 | 11.30 |
| H × Q | 33.62 ± 6.14 | 18.27 | 105.78 | 5.78 | ||||
| Stem edge thickness | Q × H | 4.85 | 10.79 | 7.82 | 12.26 ± 3.66 | 29.86 | 156.83 | 56.83 |
| H × Q | 9.78 ± 2.83 | 28.88 | 125.12 | 25.12 | ||||
| No. of thorns | Q × H | 1.68 | 1.80 | 1.74 | 2.0 ± 1.01 | 49.95 | 116.67 | 16.67 |
| H × Q | 1.93 ± 0.9 | 46.94 | 110.75 | 10.75 | ||||
| Length of thorns | Q × H | 4.16 | 1.50 | 2.83 | 2.98 ± 1.44 | 48.26 | 105.43 | 5.43 |
| H × Q | 2.84 ± 1.26 | 44.29 | 100.51 | 0.51 | ||||
| Distance between thorns | Q × H | 56.84 | 34.24 | 45.54 | 42.44 ± 10.06 | 23.71 | 93.18 | −6.82 |
| H × Q | 42.47 ± 8.55 | 20.12 | 93.25 | −6.75 | ||||
| Infection Degree of Canker Disease | Number (Proportion%) | |
|---|---|---|
| 0 | 23 (15.4) | 10 (10.0) |
| 1 | 42 (28.0) | 28 (28.0) |
| 2 | 62 (41.3) | 33 (33.0) |
| 3 | 17 (11.3) | 20 (20.0) |
| 4 | 6 (4.0) | 9 (9.0) |
| Traits | Cross Combinations | Model | m | da | db | ha | hb | i | jab | jba | l | pσ2mg | Hmg2(%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stem width | Q × H | 2MG-EA | 35.1176 | 6.5151 | 0 | 0 | |||||||
| H × Q | 2MG-AD | 37.8574 | 4.4315 | 4.0126 | −1.1909 | −6.6436 | 12.9563 | 34.3389 | |||||
| Stem edge thickness | Q × H | 2MG-AD | 13.9416 | 4.5009 | −0.3552 | −1.1979 | −2.1556 | 9.5687 | 71.3481 | ||||
| H × Q | 2MG-EA | 9.7159 | 1.7433 | 0 | 0 | ||||||||
| No. of thorns | Q × H | 2MG-EA | 2.4408 | 0.7204 | 0 | 0 | |||||||
| H × Q | 2MG-A | 2.1072 | 0.8603 | −0.14 | 0 | 0 | |||||||
| Length of thorns | Q × H | 2MG-AD | 3.4052 | 1.5201 | 0.5609 | −0.882 | 0.0047 | 0.529 | 25.1501 | ||||
| H × Q | 1MG-AD | 3.0022 | 1.1478 | −0.4084 | 0 | 0 | |||||||
| Distance between thorns | Q × H | 1MG-AD | 43.0431 | 11.1056 | −1.1581 | 37.498 | 37.0371 | ||||||
| H × Q | 2MG-AD | 46.8981 | 6.7489 | 1.9961 | −0.3493 | −8.0521 | 22.0752 | 30.484 |
| Cross Combination Traits | Early Flowering * | Plant with Short Thorns | Plant Without Thorns | Weak of Infection Canker Disease * | Early Flowering with Weak Infection of Canker Disease |
|---|---|---|---|---|---|
| ‘HHQL’ × ‘HL’ | 5 | 2 | 0 | 2 | 3 |
| ‘HL’ × ‘HHQL’ | 2 | 3 | 3 | 0 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, X.; Khan, I.; Zhang, T.; Huang, G.; Chen, J.; Ding, Y.; Ji, X.; Zhang, Z.; Zhao, J.; Hu, G.; et al. Genetic Analyses of Flower, Fruit, and Stem Traits of Intergeneric Hybrids Between ‘Honghuagqinglong’ and ‘Heilong’ Pitayas. Plants 2024, 13, 3546. https://doi.org/10.3390/plants13243546
Pu X, Khan I, Zhang T, Huang G, Chen J, Ding Y, Ji X, Zhang Z, Zhao J, Hu G, et al. Genetic Analyses of Flower, Fruit, and Stem Traits of Intergeneric Hybrids Between ‘Honghuagqinglong’ and ‘Heilong’ Pitayas. Plants. 2024; 13(24):3546. https://doi.org/10.3390/plants13243546
Chicago/Turabian StylePu, Xinyue, Imran Khan, Tiantian Zhang, Guohua Huang, Jiayi Chen, Yu Ding, Xuewu Ji, Zhike Zhang, Jietang Zhao, Guibing Hu, and et al. 2024. "Genetic Analyses of Flower, Fruit, and Stem Traits of Intergeneric Hybrids Between ‘Honghuagqinglong’ and ‘Heilong’ Pitayas" Plants 13, no. 24: 3546. https://doi.org/10.3390/plants13243546
APA StylePu, X., Khan, I., Zhang, T., Huang, G., Chen, J., Ding, Y., Ji, X., Zhang, Z., Zhao, J., Hu, G., Sabir, I. A., & Qin, Y. (2024). Genetic Analyses of Flower, Fruit, and Stem Traits of Intergeneric Hybrids Between ‘Honghuagqinglong’ and ‘Heilong’ Pitayas. Plants, 13(24), 3546. https://doi.org/10.3390/plants13243546







