Abstract
Mandarin, one of the winter fruits commonly used in the preparation of foods and juices, is a fruit native to China and Southeast Asia. In this work, essential oils (EOs) obtained from by-products of the Citrus reticulata Blanco flavedo of five cultivars present and cultivated within the Botanical Garden of Palermo were chemically and biologically studied: C. reticulata ‘Avana’ (C1), C. reticulata ‘Tardivo di Ciaculli’ (C2), C. reticulata ‘Bombajensis’ (C3), C. reticulata ‘Aurantifolia’ (C4), and C. reticulata ‘Padre Bernardino’ (C5). The GC and GC-MS analysis performed on all the extracted samples clearly highlighted the notable presence of limonene, a characteristic hydrocarbon monoterpene of EOs of the Citrus genus. C1, C2, C3, C4, and C5 were tested in relation to their possible antibacterial and allelopathic activity, also highlighting the activity of limonene, the main compound. For the antibacterial activity, eight different bacterial strains were used, both Gram-positive and Gram-negative (Staphylococcus aureus, Proteus vulgaris, Klebsiella pneumoniae, Enterobacter cloacae, Pseudomonas aeruginosa, Escherichia coli, Salmonella typhi, and Enterobacter aerogens). For the allelopathic effect, two model systems were chosen: the germination of radish seeds (Raphanus sativus L.) and of spores in the moss Tortula muralis (Hedw.). The EOs from all cultivars showed pronounced antibacterial effects against all strains with an MIC comprised in the range of 16–256 μg/mL. Limonene showed the highest activity with an MIC between 4 and 16. The allelopathic effects showed a decrease in the percentage of seed germination, root, and epicotyl growth on Raphanus and a strong reduction in the germination of Tortula spores with an alteration in the development of the protonema. Limonene showed the same but more intense allelopathic activity.
1. Introduction
Citrus is one of the most important horticultural crops, with a worldwide production of over 100 million tonnes per year [1]. Their fruits, largely consumed around the world, contain different types of phytochemicals such as carotenoids and flavonoids, and they are an excellent source of vitamin C, which is a powerful natural antioxidant. Moreover, they are a good source of dietary fibres that improve human health, reducing the risk of several chronic degenerative diseases [2,3,4].
Mandarin (Citrus reticulata Blanco) is native to China and south-eastern Asia, and it is one of the basic taxa of Citrus, together with pummelo [C. maxima (Burm.) Merr.], citron (C. medica L.), and a wild Papeda species (C. micrantha Wester, sin. Citrus hystrix DC.) based on biochemical polymorphism, karyotype investigations, and especially on molecular markers and genome sequencing [5,6,7,8]. All cultivated Citrus fruits originated from these species. Recent genomic and molecular marker studies revealed that several modern mandarins are not pure C. reticulata but are introgressed by C. maxima genome fragments [9,10,11].
According to Webber [12], the first appearance of mandarin in Europe dates back to the introduction of two cultivars from Canton (China) to England in 1805.
In Italy, the largest Citrus fruit cultivations are in Sicily, where the production is almost 4 million tons per year, mostly oranges, lemons, mandarins, and grapefruits. The Citrus crop could be considered, in fact, a major part of the Sicilian economy. In particular, the production of mandarins was about 60,000 t in 2023 [13].
As Lo Piccolo reports [14], the mandarin was introduced to Palermo in 1810 when Louis Philippe d’Orleans sent two plants to Ferdinand of Bourbon. In the following years, it was cultivated at the “Real Tenuta della Favorita”, and in 1817, the botanist Giovanni Gussone introduced ten saplings, purchased in Malta, to the experimental and acclimatisation garden of Boccadifalco. The mandarin arrived at the Botanical Garden of Palermo probably around 1820 and subsequently spread to the countryside around the town and, in particular, to the “Conca d’Oro” area [14].
At a phytochemical level, EOs of the Citrus genus are characterised by the high presence of limonene, a hydrocarbon monoterpene, and by small quantities of various compounds such as β-myrcene, linalool, and α-terpineol [15].
The particular abundance of this compound has allowed several studies to be carried out both in vitro and in vivo. In fact, the biological properties of the Citrus EOs obtained from different parts of the plant, such as fruits, seeds, leaves, and flowers, as well as by-products such as flavedo (coloured outer layer) and albedo (inner layer), have been investigated. Activities such as antioxidant [16,17], antidiabetic [18], antimicrobial [19], anti-obesity [20], anti-inflammatory [21], cytotoxic [22], and anxiolytic [23] have been extensively investigated. The particular richness of healthy, natural compounds allows the use of this species for the treatment of various pathologies related to the heart, liver, and intestinal system, and against obesity [21,22,23,24].
The by-products, primarily the peels obtained from the agri-food industry that processes Citrus fruits, have an important socio-economic interest. Several studies, in fact, have shown that from the recycling of this waste, biologically active compounds such as flavones, flavonols, chalcones, terpenes, vitamins, and limonoids can be obtained [16,25,26].
Finally, the large-scale use of EOs is noteworthy, both for the production of perfumes (the cosmetic field) and for use in the healthcare and culinary fields [27]. In fact, Citrus species have an important economic–commercial value. These fruits are mainly consumed fresh, but they are also used for the production of drinks and fruit juices. The by-products, i.e., the dehydrated pulp, peels, leaves, and aerial parts, are instead used for their bioactive potential to obtain products that can be used for animal feed or even for healthcare [28]. Among the main producing countries are Italy, especially in Sicily, some member states of the USA, and several countries in Southeast Asia. EOs obtained mainly from the flavedo of these citrus fruits are widely used as flavourings and accepted by the Food and Drug Administration in the form of additives for food products, especially thanks to the excellent antimicrobial properties they possess [29]. Finally, there is a very important industrial application with the use of EOs in the formulation of hygiene products as alternatives to the use of traditional solvents in the production of cosmetics and in perfumes [30].
Among the many activities studied so far for the essential oils of Citrus reticulata Blanco, allelopathic activity does not appear, which could represent an interesting possibility for using the waste products of fruit processing.
In this context, the EOs of five Sicilian cultivars grown within the Palermo Botanical Garden, including C. reticulata ‘Avana’ (C1), C. reticulata ‘Tardivo di Ciaculli’ (C2), C. reticulata ‘Bombajensis’ (C3), C. reticulata ‘Aurantifolia’ (C4), and C. reticulata ‘Padre Bernardino’ (C5) (Figure 1), were chemically analysed by GC and GC-MS analyses, and were tested for their antibacterial and allelopathic activity.

Figure 1.
The five Citrus reticulata Blanco cultivars collected in the Palermo Botanical Garden: C. reticulata ‘Avana’ (C1), C. reticulata ‘Tardivo di Ciaculli’ (C2), C. reticulata ‘Bombajensis’ (C3), C. reticulata ‘Aurantifolia’ (C4), and C. reticulata ‘Padre Bernardino’ (C5).
In relation to the first, eight strains were used, both Gram-positive and Gram-negative (Staphylococcus aureus, Proteus vulgaris, Klebsiella pneumoniae, Enterobacter cloacae, Pseudomonas aeruginosa, Escherichia coli, Salmonella typhi, and Enterobacter aerogens). For the second, two model systems, the germination of radish seeds (Raphanus sativus L.) and of spores in the moss Tortula muralis (Hedw.), were chosen. In addition to the inhibitory activity on germination in both systems, the effect on morphology was evaluated with respect to the development of radish seedlings in R. sativus, as root and epicotyl growth, and as an extension of the hairy area, and to the alteration in the development of the protonema and brood and tmema cells in T. muralis, specific resistance cells produced by the protonema of mosses in response to various stressors.
2. Results and Discussion
2.1. Chemical Composition of EOs (C1–C5)
The hydrodistillates (EOs) obtained from the flavedos of the different Sicilian cultivars of C. reticulata (C1–C5) were all yellow oils. In total, thirty-six metabolites were identified by GC and GC-MS analysis and tabulated in Table 1 based on retention times, linear retention indices on the apolar DB-5MS column, and clearly clustered into different chemical classes, including monoterpene hydrocarbons, oxygenated monoterpenes, sesquiterpene hydrocarbons, oxygenated sesquiterpenes, and other compounds.

Table 1.
Chemical composition flavedo EOs of the five Sicilian cultivars of C. reticulata collected in the Palermo Botanical Garden.
An examination of the results (Table 1) revealed how the five different EOs (C1–C5) were characterised by the massive presence of hydrocarbon monoterpene compounds with a percentage range varying from 96.69% to 98.78%. Figure S1 shows a histogram highlighting the chemical differences of samples C1–C5. All the different samples (C1–C5) are characterised by the abundance of limonene (70.74–86.80%), with a variable that may have depended on the vegetative season and the ripening of the harvested fruits [31].
α-Pinene, β-pinene, β-myrcene, γ-terpinene, and terpinolene were other metabolites present in all the EOs examined and also contributed moderately to the percentage increase in the class of hydrocarbon monoterpenes. Moderate amounts of oxygenated monoterpenes (0.37–1.04%) were detected in all samples, while only sample C2 presented oxygenated sesquiterpene compounds (0.25%) and other metabolites (others class) (0.12%).
From research conducted on the major scientific information systems such as Scopus, SciFinder, and Google Scholar, the presence of chemical information and the biological applicability of the EOs for the varieties ‘Avana’, ‘Tardivo di Ciaculli’, ‘Bombajensis’, ‘Aurantifolia’, and ‘Padre Bernardino’ was not evident. However, there are several works that have reported the chemical composition of EOs obtained from waste, such as C. reticulata flavedo. By evaluating the chemical composition of C. reticulata EOs [32], a high percentage of limonene (60.74%) and the moderate presence of monoterpene compounds, such as terpinene and myrcene, were found. High amounts of limonene have also been found in recent studies on EOs obtained from C. reticulata samples collected and cultivated in China [33], India [34], and Brazil [35], underlining that there is no geographic difference resulting in true chemical variation.
2.2. Antibacterial Activity
C1–C5 showed inhibition activity against all the bacterial strains tested (MIC between 64 and 256 μg/mL). Staphylococcus aureus was the most sensitive bacterium (MIC between 16 and 32 μg/mL), followed by Proteus vulgaris (MIC between 32 and 128 μg/mL), while Pseudomonas aeruginosa and Salmonella typhi were the least sensitive (MIC between 128 and 256 μg/mL). EOs with an MIC of 256 μg/mL were inactive. (Table 2).

Table 2.
Antibacterial activity (MIC values are expressed as μg/mL) of C1–C5 against several bacterial strains.
All EOs showed inhibitory activity against all bacterial strains tested, especially with S. aureus, the most sensitive bacterium, while Salmonella was the least sensitive. Among the numerous compounds present in EOs, it was decided to test for limonene because it is present in considerable quantities and because its antibacterial activity and its ability to interact with membranes, a possible target for an allelopathic activity, are known [36,37]. Furthermore, since it has been demonstrated that limonene acts in S. aureus on numerous essential cellular activities (destruction of the cellular morphology and integrity of the cell wall), loss of biological macromolecules (nucleic acids and proteins, damage to the cell membrane with increased permeability and reduction of its potential, reduction of respiratory metabolic activity, and a slowing down of metabolism with metabolic dysfunction) [37], it is possible that all these effects may be responsible for the antibacterial activity demonstrated to us both for EOs and for limonene. Furthermore, these same effects, if demonstrated in plant cells, could give an idea of the possible mechanism of action of the allelopathic effect shown by us. The sometimes-close value of pure limonene and EOs, in which limonene represents only a good percentage, may be due to the effect of the other oils present and their possible synergistic effect. Finally, it is intriguing to consider that in C3 and C4, the percentage of limonene is the highest.
2.3. Allelopathic Activity
2.3.1. Raphanus Sativus Seed Germination
Regarding the germination of R. sativus seeds, EOs at 10 and 1 μg/mL completely inhibited seed germination, while the other concentrations inhibited the percentage of seed germination, the length of the hypocotyl-root axis, and hair growth in a dose-dependent manner (Figure 2 and Table 3). In the treated samples, the most evident morphological alteration was the thickening of the root apex. Furthermore, the length of the hair zone and hair growth were reduced, corresponding to early developmental stages (e.g., a 2-day-old root was comparable to a 12-h-old root).
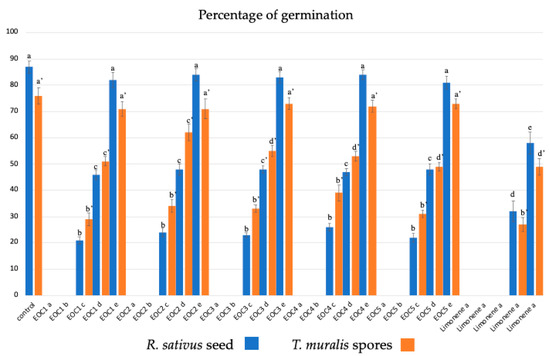
Figure 2.
Effect of C1–C5 and limonene on the percentage of germination of the Raphnus sativus seed and Tortula muralis spores a = 10 µg/mL; b = 1 µg/mL; c = 0.1 µg/mL; d = 0.01 µg/mL; e = 0.001 µg/mL. Data were presented as mean and standard error, and they were analysed with a paired t-test. Bars not accompanied by the same letter were significantly different at p < 0.05.

Table 3.
Effect of C1–C5 and limonene on the R. sativus hypocotyl-root axis length (cm) and on the cell number of main protonemata filaments, number of brood cells, and number of tmema cells of the T. muralis. Data were presented as mean and standard error, and they were analysed with a paired t-test. Bars not accompanied by the same letter were significantly different at p < 0.05.
As can be seen from studies carried out using SEM (Scanning Electron Microscopy, Cambridge 250 Mark 3) in the control sample (A), an extensive hairy area is evident, while in the sample treated with C1 (0.1 mM) (B), only a few short hairs appear to form on the rhizoderm (Figure 3).
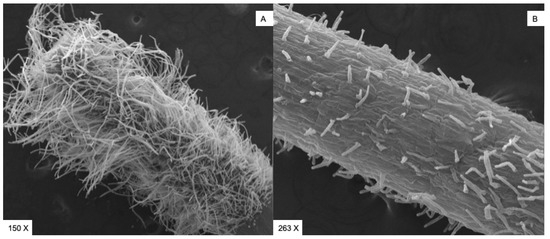
Figure 3.
(A) SEM micrograph of the control samples after 7 days of culture showing a large hairy area densely populated by root hairs. (B) SEM micrograph of the samples treated with C1 at 0.1 µg/mL after 7 days of culture showing only a few short hairs.
The potential impact of different EOs of C. genuson and limonene on plant growth may occur through several mechanisms. They may inhibit seed germination by disrupting cell membranes or altering the hormonal balance required for germination, or, even at non-toxic concentrations, they may suppress root elongation and shoot development, thereby reducing overall plant growth.
High concentrations of limonene may cause a significant decrease in the percentage of germinated seeds. This may be due to the potential toxicity of the chemical to the embryo or its interference with biochemical processes required for germination. Even at lower concentrations, limonene may delay the time it takes for seeds to germinate, thereby inhibiting early growth.
2.3.2. Moss Spore Germination
Under control conditions, the moss T. muralis began to germinate already after 3 days and reached its maximum percentage of germination (80–90%) after 14 days. The germination pattern, unipolar or bipolar, showed chloronemata (with transverse septa and numerous chloroplasts) as the first type of filament originating from the spore. After 21 days, chloronemata and caulonemata (with oblique septa, a few plastids arranged in longitudinal rows, and an apical exclusion zone) were evident, while brood and tmema cells appeared after 30 days. C1–C5 at 10 and 1 µg/mL inhibited spore germination completely (Figure 2 and Table 3). The other concentrations inhibited both germination percentage and protonemata development in a dose-dependent manner, showing the highest inhibition values for C1 and C5. In the moss, the main morphological changes were swollen and shorter cells and early development of brood and tmema cells after 14 days in culture, with higher concentrations of both cell types for C1- and C5-treated samples. Limonene showed the same but more intense activity.
Finally, also with regard to allelopathic activity, C1 and C5 showed the highest inhibition values both for seed germination and plant development in Raphanus and for spore germination and protonema development in Tortula.
Brood cells are resistant structures due to their thick walls, abundant nutrient reserves, and a low surface/volume ratio that reduces exchanges [38,39,40]. They are produced in response to flavonoids and to heavy metal (lead) stress [41]. The frequent occurrence of these diaspores after exposure to toxic substances confirms that they are a common stress response of moss protonemata. Also, in this case, the dose-dependent production of brood cells in response to treatment with essential oils could indicate an increase in the resistance of the moss model, thanks to the production of these forms of resistance.
Abscission, or tmema, cells are specialised cells causing protonemata breakage and liberation of short filaments [40,41,42,43]. Our findings regarding both tmema cell formation and toxic effects after treatment with C. reticulata Blanco essential oil are consistent with the literature data regarding these cells as a means of propagation of mosses in adverse conditions, such as scant nourishment, low Caþ2 concentration, and ageing and heavy metals [41,44]. Just like the brood cells, the tmema cells, which we highlighted as a dose-dependent increase after treatment with EOs, can be considered a defence and resistance mechanism of the moss protonema.
The allelopathic activity of Citrus reticulata essential oils has been shown in previous works and has been demonstrated against seed germination and seedling growth of Heliantus annus (sunflower), Portulaca oleracea (purslane), Lupinus albus (field lupine), and Malva parviflora (Egyptian mallow), and showing complete inhibition of seed germination and seedling growth of H. annus and M. parviflora at all concentrations [45].
Furthermore, it has been reported that EOs from flowering aerial parts of Dracocephalum kotschyi that showed a high amount of EOs with a significant percentage of limonene-10-al and limonene exerts inhibitory effects on seed germination and seedling growth of Amaranthus retroflexus L. and Chenopodium album L. [46].
Finally, Xanthium sibiricum EOs and two of its constituents (including limonene) showed allelopathic activity on the germination of Amaranthus retroflexus L. and Poa annua L., demonstrating a strong inhibitory activity on seedling growth and root elongation in both species [47].
The contemporary occurrence of different bioactivities (allelopathic and antibacterial) is shared by other plant-derived substances [48,49]. S. italica EOs and flavonoids from Castanea sativa, for example, have been observed to inhibit both bacterial and R. sativus root growth, and flavonoids from mosses inhibited spore germination and protonemal growth in T. muralis and seed germination and epicotyl growth in R. sativus [50].
Like seeds, spores may show reduced germination under the influence of different EOs of C. genuson and limonene, especially if the compound directly interferes with physiological processes involved in spore activation. Furthermore, it may also affect the growth rate of spores once they begin to germinate, potentially reducing the establishment of new plants. Limonene inhibits germination and growth, which would suggest that citrus plants may use allelopathy as a competitive mechanism, potentially influencing plant community dynamics in their natural habitats.
3. Materials and Methods
3.1. Plant Materials
The five cultivars of Citrus reticulata analysed, grown in the Botanical Garden of Palermo (38°06′48.39″ N; 13°22′21.68″ E), Sicily (Italy), were harvested between December 2022 and February 2023. In the present work, fruits belonging to the following five cultivars were examined: C. reticulata ‘Avana’ (C1), C. reticulata ‘Tardivo di Ciaculli’ (C2), C. reticulata ‘Bombajensis’ (C3), C. reticulata ‘Aurantifolia’ (C4), and C. reticulata ‘Padre Bernardino’ (C5). The samples, identified by Prof. Rosario Schicchi and Prof. Anna Geraci, were stored in the Herbarium Mediterraneum of the Botanical Garden at the University of Palermo (PAL). The voucher number is reported for each cultivar.
C. reticulata ‘Avana’ (C1) (Voucher No. 109766) is characterised by medium-sized, slightly flattened, globular fruits with a smooth, thin epicarp and a sweet, juicy endocarp (pulp) consisting of easily divisible, orange-coloured segments with few seeds. The leaves are ovate, with a short petiole. It ripens between mid-November and January.
C. reticulata ‘Tardivo di Ciaculli’ (C2) (Voucher No. 109767) is characterised by a slightly smaller fruit than the ‘Avana’ cv. from which it is derived by bud mutation. It takes its name from Ciaculli, a locality situated in Palermo’s famous Conca d’Oro. The fruit is globular in shape, slightly flattened, and has a smooth, thin, orange epicarp. The flesh is juicy and very sweet, consisting of easily divisible segments with few seeds. It ripens between mid-February and March.
C. reticulata ‘Bombajensis’ (C3) (Voucher No. 109768) is characterised by medium-sized fruits, slightly flattened and depressed in the distal part, with an irregular, moderately thick, reddish-orange epicarp. The mesocarp is well represented, and the flesh is sweet and juicy, consisting of divisible segments with few seeds. The leaves are ovate-oblong, with a slightly winged petiole. It ripens between mid-January and February.
C. reticulata ‘Aurantifolius’ (C4) (Voucher No. 109770) is characterised by medium-sized fruit, tending to be globular in shape, with a slightly wrinkled epicarp, a deep orange colour and sweet flesh consisting of easily divisible segments. The leaves are ovate-oblong, wider than in previous cultivars and are similar in shape, width, and length to those of the orange. It ripens between December and January.
C. reticulata ‘Padre Bernardino’ (C5) (Voucher No. 109769) is characterised by medium-small, slightly flattened fruits with a smooth, thin epicarp and a slightly orange colour. The juicy flesh is slightly sour and consists of easily divisible segments with several seeds. The leaves are elliptical, with a short petiole. It ripens between January and February.
3.2. Extraction of EOs
EOs extraction was performed following a reported method [51]. The orange part (flavedo), without the albedo, was obtained using an electric peeler (093209-006-BLCK, 770 Boulevard Guimond, Longueuil Quebec, Canada), was weighed, and subjected to hydro-distillation using Clevenger’s apparatus [52]. Amounts used were 300 g, 212 g, 260 g, 299 g, and 173 g for C1, C2, C3, C4, and C5, respectively. The oils’ yields were 0.69%, 1.01%, 0.89%, 0.58%, and 0.66% (v/w) for C1, C2, C3, C4, and C5, respectively. The obtained EOs were dried with Na2SO4, stored in appropriate vials, and placed in the freezer at −20 °C until the time of analysis.
3.3. GC and GC-MS Analyses
Analysis of EOs was carried out according to the procedure already reported [53]. GC-MS analysis was performed using a Shimadzu QP 2010 plus equipped with an AOC-20i autoinjector (Shimadzu, Kyoto, Japan) gas chromatograph equipped with a FID, a capillary column (DB-5MS) 30 m × 0.25 mm i.d., a film thickness 0.25 μm, and a data processor. The oven program was as follows: the temperature was held at 40 °C for 5 min, then increased at a rate of 2 °C/min up to 260 °C, then isothermal for 20 min. Helium was used as carrier gas (1 mL min−1). The injector and detector temperatures were set at 250 and 290 °C, respectively. One μL of EO solution (3% EO/hexane v/v) was injected in split mode 1:50; MS range 40–600. The settings were as follows: ionisation voltage, 70 eV; electron multiplier energy, 2000 V; transfer line temperature, 295 °C; and solvent delay, 3 min.
Linear retention indices (LRIs) were calculated on DB-5MS retention indices using a mixture of pure n-alkanes (C8–C40), and all the peaks’ compounds were identified by comparison with MS and by comparison to their relative retention indices with WILEY275, NIST 17, ADAMS, and FFNSC2 libraries. The analyses were performed in triplicate, and the results are expressed as the average of three measurements ± standard deviation.
3.4. Antimicrobial Assay
The following eight bacterial strains were obtained from the American Type Culture Collection (ATCC; Rockville, MD, USA): Staphylococcus aureus (ATCC 13709), (Gram-positive), and Proteus vulgaris (ATCC 12454), Klebsiella pneumoniae (ATCC 10031), Enterobacter cloacae (ATCC 10699), Pseudomonas aeruginosa (ATCC 27853), Escherichia coli (ATCC 11229), Salmonella typhi (ATCC 10699), and Enterobacter aerogens (ATCC 13048) (all Gram-negative). Bacterial strains were grown on MH agar plates (DIFCO, Detroit, MI, USA) and suspended in MH broth (DIFCO). The minimum inhibitory concentration (MIC) values were determined using the MH broth-dilution method [54]. The inoculum suspensions were prepared from 6-h broth cultures and adjusted to a 0.5 McFarland standard turbidity. The extracts, sterilised by 0.45-μm Millipore filters, were added to the MH broth medium. Serial 10-fold dilutions were made that furnished a concentration range from 0.01 to 1000 μg/mL for the plant extract. The lowest concentrations of the extract with activity underwent two-fold dilutions for a more accurate measurement of the MIC. The bacterial suspensions were aerobically incubated for 24 h at 37 °C. The MIC was defined as the lowest concentration able to inhibit any visible bacterial growth. Control cultures containing only sterile physiological Tris buffer were also prepared. In addition, MIC values for tetracycline hydrochloride (Pharmacia, Milano, Italy), benzylpenicillin sodium (Cynamid, Catania, Italy) and cefotaxime sodium (Roussel Pharmacia, Milano, Italy) were also determined.
3.5. Allelopathic Test
Tests on the allelopathic effects of EOs of the C. genuson and limonene on the germination percentage of R. sativus seeds (2 days after sowing) and of T. muralis spores (14 days after sowing) were conducted.
All tested plants were grown on a modified Mohr medium, as reported in Basile et al. [49], used as a control, and on the same medium with the addition of serial 10-fold dilutions of essential oils of the Citrus genuson and limonene to obtain the concentration range (from 0.001 to 10 µg/mL). EOs from the flowerheads of Sideritis italica (Miller) Greuter et Burdet, a widespread Mediterranean Lamiaceae, was used as a positive control (Figure S2) because it demonstrated efficacy in inhibiting germination of the plants chosen as “models”, as R. sativus and T. muralis had already been confirmed in previous experiments [46].
The media were sterilised by filtration through Millipore filters (0.45 mm). The culture solutions were replaced every 2 days. The cultures were kept in a growth chamber with a temperature ranging from 13 °C at night to 20 °C by day, 70% constant relative humidity, and a 16-h light (45 mE m−2 s−1)/8 h dark photoperiod. The plants were maintained in the growth chamber for 30 days. The experiments were carried out in triplicate.
3.6. Seed Germination Tests
Seeds of R. sativus L. were surface-sterilised [49] and subsequently washed (10 min) with sterile distilled water. Then, they were put in Petri dishes (5 cm diameter), with 20 seeds per dish, on fine granular washed quartz (Merck, Germany) (10 g), with 10 mL of sterile culture medium.
3.7. Moss Spore Germination Test
Mature capsules of T. muralis (Hedw.) were surface-sterilised in 70% ethanol (2 min) and 2% NaClO with the addition of a few drops of Triton X-100 (Sigma-Aldrich, USA) (5 min). Subsequently, they were washed (10 min) with sterile distilled water, and the contents of 10 capsules were suspended in 10 mL of sterile distilled water. Aliquots (200 mL) of spore suspension were inoculated in Petri dishes (5 cm diameter) with 10 mL of sterile liquid medium to test C1–C5.
3.8. Evaluation of Oil Effects
Observations were made, and photographs were taken using a Leitz Aristoplan microscope (Leica, Wetzlar, Germany) equipped with differential interference contrast optics (Nomarski) and a Wild Heerbrugg M3Z binocular. Gemmalings were measured using a calibrated, square graticule eyepiece. R. sativus seed germination percentages were evaluated by examining 20 seeds per replicate at 2 days, and hypocotyl-root length was measured on 15 roots per replicate at 3 days after inoculation. As for the mosses, T. muralis, we monitored spore germination percentage, cell number in the main filament, length of newly formed filaments, type of filaments, order of branch formation, and presence of brood and tmema cells (see below). The moss was visually evaluated every 2 days.
3.9. Scanning Electron Microscopy
For scanning electron microscopy (SEM), small pieces of control and EO1 0.1 mM exposed samples were cut from the hairy area using a sharp razor blade. The samples were fixed in 3% glutaraldehyde in a phosphate buffer (65 mM, pH 7.2–7.4) for 2 h at room temperature, post-fixed with 1% osmium tetroxide in the same phosphate buffer for 1.5 h at room temperature and dehydrated with ethanol and critical point drying. The samples were then mounted on aluminium stubs, coated with a thin gold film using an Edward E306 Evaporator, and observed with an FEI (Hillsboro, OR, USA) Quanta 200 ESEM (FEI Company, Hillsboro, OR) in high vacuum mode at 30 kV voltage.
3.10. Statistical Analysis
The data are mean values of the three experiments. A one-way ANOVA test was performed. The significance of differences between means was checked by Student’s t-test (p < 0.05).
4. Conclusions
The EOs obtained from flavedo waste of the Citrus reticulata Blanco cultivars (C1–C5) were investigated both chemically and biologically. The analyses carried out by GC-MS highlighted the class of hydrocarbon monoterpenes as the main class, with quantities of limonene varying from 70.83 to 86.73%. Small amounts of oxygenated monoterpenes were found in all samples, while C2 was the most chemically diverse sample. All EOs, together with the limonene standard, were tested for antibacterial and allelopathic activity and showed pronounced antibacterial effects against all strains with an MIC in the range of 16–256 µg/mL, a strong reduction in the germination of Tortula spores with an alteration in the development of the protonema, and a decrease in the percentage of seed germination, root, and epicotyl growth in Raphanus. Fairly modest, if not minimal, quantities of sesquiterpene compounds were detected in all samples except for C3. EOs of Citrus reticulata Blanco can perform a multifaceted defensive action against a wide range of competitors and/or stressors and could be used as a natural base for formulations in the pharmaceutical and/or agricultural fields, avoiding or reducing the use of synthetic compounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13243527/s1, Figure S1. Percentages of chemical classes in the different samples C1–C5. Figure S2. Effects of S. italica leaf oil on the germination percentage of R. sativus seeds of T. muralis (positive control).
Author Contributions
Conceptualization, N.B. and V.M.; methodology, N.B. and V.M.; software, N.B. and V.M.; validation, N.B., A.P., M.D. and V.M.; formal analysis, N.B. and V.M.; investigation, N.B., F.S., A.G., A.P. and V.M.; resources, N.B. and M.B.; data curation, N.B. and V.M.; writing-original draft preparation, A.G., N.B., A.P. and V.M.; writing—review and editing, N.B., M.B., A.B. and V.M.; visualization, R.S. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the PNRR Spoke 6 Activity 2: “Bio-prospecting and bioactivity, Task 2.2: Sustainability of extraction processes from biological matrices and scalability”, National Biodiversity Future Center-NBFC (Cod. ID. CN00000033, CUP B73C22000790001 of the University of Palermo). This work was supported by a grant from “Progetto Finanziato da Next Generation EU PNRR–Missione 4 “Istruzione e Ricerca”–Componente C2 -investimento 1.1 (PNRR M4.C2.1.1), Fondo per il Programma Nazionale di Ricerca e Progetti di Rilevante Interesse Nazionale (PRIN)–codice P2022CKMPW_002–CUP B53D23025620001”.


Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Moulehi, I.; Bourgou, S.; Ourghemmi, I.; Tounsi, M.S. Variety and ripening impact on phenolic composition and antioxidant activity of mandarin (Citrus reticulate Blanco) and bitter orange (Citrus aurantium L.) seeds extracts. Ind. Crops Prod. 2012, 39, 74–80. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Heying, E.; Tanumihardjo, S.A. History, Global Distribution, and Nutritional Importance of Citrus Fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- Costanzo, G.; Vitale, E.; Iesce, M.R.; Naviglio, D.; Amoresano, A.; Fontanarosa, C.; Spinelli, M.; Ciaravolo, M.; Arena, C. Antioxidant Properties of Pulp, Peel and Seeds of Phlegrean Mandarin (Citrus reticulata Blanco) at Different Stages of Fruit Ripening. Antioxidants 2022, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Bentahar, A.; Bouaziz, A.; Djidel, S.; Khennouf, S. Phenolic content and antioxidant activity of ethanolic extracts from Citrus sinensis L. and Citrus reticulata L. fruits. J. Drug Deliv. Therap. 2020, 10, 308–313. [Google Scholar] [CrossRef]
- Nicolosi, E. Origin and taxonomy. In Citrus Genetics, Breeding and Biotechnology; Khan, I.A., Ed.; CAB International: Oxfordshire, UK, 2007; pp. 19–44. [Google Scholar]
- Luro, F.; Curk, F.; Froelicher, Y.; Ollitrault, P. Recent insights on Citrus diversity and phylogeny. In AGRUMED: Archaeology and History of Citrus Fruit in the Mediterranean: Acclimatization, Diversifications, Uses [Online]; Publications du Centre Jean Bérard: Naples, Italy, 2017; ISBN 9782918887775. [Google Scholar] [CrossRef]
- Ollitrault, P.; Curk, F.; Krueger, R. The Genus Citrus. In Citrus Taxonomy; Manuel Talon, M., Marco Caruso, M., Fred, G., Gmitter, F.G., Jr., Eds.; Woodhead Publishing: Cambridge, UK, 2020. [Google Scholar] [CrossRef]
- Badalamenti, N.; Bruno, M.; Schicchi, R.; Geraci, A.; Leporini, M.; Tundis, R.; Loizzo, M.R. Reuse of Food Waste: The Chemical Composition and Health Properties of Pomelo (Citrus maxima) Cultivar Essential Oils. Molecules 2022, 27, 3273. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.A.; Prochnik, S.; Jenkins, J.; Salse, J.; Hellsten, U.; Murat, F.; Perrier, X.; Ruiz, M.; Scalabrin, S.; Terol, J.; et al. Sequencing of diverse mandarin, pummelo and orange genomes reveals complex history of admixture during citrus domestication. Nat. Biotechnol. 2014, 32, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Curk, F.; Ancillo, G.; Ollitrault, F.; Perrier, X.; Jacquemoud-Collet, J.-P.; Garcia-Lor, A.; Navarro, L.; Ollitrault, P. Nuclear Species-Diagnostic SNP Markers Mined from 454 Amplicon Sequencing Reveal Admixture Genomic Structure of Modern Citrus Varieties. PLoS ONE 2015, 10, e0125628. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lor, A.; Luro, F.; Ollitrault, P.; Navarro, L. Genetic diversity and population-structure analysis of mandarin germplasm by nuclear, chloroplastic and mitochondrial markers. Tree Genet. Genomes 2015, 11, 123. [Google Scholar] [CrossRef]
- Webber, H.J. History and development of the citrus industry. In The Citrus Industry; Batchelor, L.D., Webber, H.J., Eds.; University of California Press: Berkeley, CA, USA, 1943. [Google Scholar]
- Ciriminna, R.; Angellotti, G.; Luque, R.; Pagliaro, M. The citrus economy in Sicily in the early bioeconomy era: A case study for bioeconomy practitioners. Biofuels Bioprod. Bioref. 2024, 18, 356–364. [Google Scholar] [CrossRef]
- Lo Piccolo, F. Palermo Dominante. Il soggiorno dei Borbone alla Favorita e a Boccadifalco (1798–1820); 40due Edizioni: Palermo, Italy, 2024; p. 368. ISBN ISBN 88-98115-84-9. EAN13: 9788898115846. [Google Scholar]
- Radan, M.; Parcina, A.; Burkul, F. Chemical composition and antioxidant activity of essential oil obtained from bitter orange peel (Citrus aurantium L.) using two methods. Croat. Chem. Acta 2018, 91, 125–128. [Google Scholar] [CrossRef]
- Bonesi, M.; Loizzo, M.R.; Leporini, M.; Tenuta, M.C.; Passalacqua, N.G.; Tundis, R. Comparative evaluation of petitgrain oils from six Citrus species alone and in combination as potential functional anti-radicals and antioxidant agents. Plant Biosyst. 2018, 152, 986–993. [Google Scholar] [CrossRef]
- Badalamenti, N.; Bruno, M.; Schicchi, R.; Geraci, A.; Leporini, M.; Gervasi, L.; Tundis, R.; Loizzo, M.R. Chemical Compositions and Antioxidant Activities of Essential Oils, and Their Combinations, Obtained from Flavedo By-Product of Seven Cultivars of Sicilian Citrus aurantium L. Molecules 2022, 27, 1580. [Google Scholar] [CrossRef]
- Maksoud, S.; Abdel-Massih, R.M.; Rajha, H.N.; Louka, N.; Chemat, F.; Barba, F.J.; Debs, E. Citrus aurantium L. active constituents, biological effects and extraction methods. an updated review. Molecules 2021, 26, 5832. [Google Scholar] [CrossRef] [PubMed]
- Ben Hsouna, A.; Hamdi, N.; Ben Halima, N.; Abdelkafi, S. Characterization of essential oil of Citrus aurantium L. flowers: Antimicrobial and antioxidant activities. J. Oleo Sci. 2013, 62, 763–772. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Biological activities and safety of Citrus spp. Essential Oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef] [PubMed]
- Moraes, T.M.; Kushima, H.; Moleiro, F.C.; Santos, R.C.; Machado Rocha, L.R.; Marques, M.O.; Vilegas, W.; Hiruma-Lima, C.A. Effects of limonene and essential oil from Citrus aurantium on gastric mucosa: Role of prostaglandins and gastric mucus secretion. Chem. Biol. Interact. 2009, 180, 499–505. [Google Scholar] [CrossRef]
- Narang, N.; Jiraungkoorskul, W. Anticancer Activity of Key Lime, Citrus aurantifolia. Pharmacogn. Rev. 2016, 10, 118–122. [Google Scholar]
- de Moraes Pultrini, A.; Almeida Galindo, L.; Costa, M. Effects of the essential oil from Citrus aurantium L. in experimental anxiety models in mice. Life Sci. 2006, 78, 1720–1725. [Google Scholar] [CrossRef]
- Fugh-Berman, A.; Myers, A. Citrus aurantium, an ingredient of dietary supplements marketed for weight loss: Current status of clinical and basic research. Exp. Biol. Med. 2004, 229, 698–704. [Google Scholar] [CrossRef]
- Russo, C.; Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Barreca, D.; Rapisarda, A.; Cirmi, S.; Navarra, M. The second life of Citrus fruit waste: A valuable source of bioactive compounds. Molecules 2021, 26, 5991. [Google Scholar] [CrossRef]
- Nieto, G.; Fernández-López, J.; Pérez-Álvarez, J.A.; Peñalver, R.; Ros-Berruezo, G.; Viuda-Martos, M. Valorization of Citrus co-products: Recovery of bioactive compounds and application in meat and meat products. Plants 2021, 10, 1069. [Google Scholar] [CrossRef]
- FAOSTAT Database. Available online: http://faostat3.fao.org/home/index.html (accessed on 25 November 2024).
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; GarcíaViguera, C. Natural bioactive compounds of Citrus limon for food and health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Settanni, L.; Palazzolo, E.; Guarrasi, V.; Aleo, A.; Mammina, C.; Moschetti, G.; Germanà, M.A. Inhibition of foodborne pathogen bacteria by essential oils extracted from Citrus fruits cultivated in Sicily. Food Contr. 2012, 26, 326–330. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A. Lipids and Essential Oils as Antimicrobial Agents; John Wiley & Sons, Ltd.: Sessex, UK, 2011; pp. 203–238. [Google Scholar]
- Luro, F.; Garcia Neves, C.; Costantino, G.; da Silva Gesteira, A.; Paoli, M.; Ollitrault, P.; Tomi, F.; Micheli, F.; Gibernau, M. Effect of Environmental Conditions on the Yield of Peel and Composition of Essential Oils from Citrus Cultivated in Bahia (Brazil) and Corsica (France). Agronomy 2020, 10, 1256. [Google Scholar] [CrossRef]
- Tao, N.; Jia, L.; Zhou, H. Anti-fungal activity of Citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum. Food Chem. 2014, 153, 265–271. [Google Scholar] [CrossRef]
- Li, C.; Cai, Q.; Wu, X.; Tan, Z.; Huang, S.; Wei, C.; Zhang, W.; Chen, Z.; Zhang, L.; Xiang, X. Variation in Compositions and Biological Activities of Essential Oils from Four Citrus Species: Citrus limon, Citrus sinensis, Citrus paradisi, and Citrus reticulata. Chem. Biodivers. 2022, 19, e202100910. [Google Scholar] [CrossRef] [PubMed]
- Chutia, M.; Deka Bhuyan, P.; Pathak, M.G.; Sarma, T.C.; Boruah, P. Antifungal activity and chemical composition of Citrus reticulata Blanco essential oil against phytopathogens from North-East India. LWT 2009, 42, 777–780. [Google Scholar] [CrossRef]
- Fouad, H.A.; da Camara, C.A.G. Chemical composition and bioactivity of peel oils from Citrus aurantiifolia and Citrus reticulata and enantiomers of their major constituent against Sitophilus zeamais (Coleoptera: Curculionidae). J. Stored Prod. Res. 2017, 73, 30–36. [Google Scholar] [CrossRef]
- Hąc-Wydro, K.; Flasiński, M.; Romańczuk, K. Essential oils as food eco-preservatives: Model system studies on the effect of temperature on limonene antibacterial activity. Food Chem. 2017, 235, 127–135. [Google Scholar] [CrossRef]
- Han, Y.; Chen, W.; Sun, Z. Antimicrobial activity and mechanism of limonene against Staphylococcus aureus. J. Food Saf. 2021, 41, e12918. [Google Scholar] [CrossRef]
- Duckett, J.G.; Ligrone, R. A survey of diaspore liberation mechanism and germination patterns in mosses. J. Bryol. 1992, 17, 335–354. [Google Scholar] [CrossRef]
- Goode, J.A.; Stead, A.D.; Ligrone, R.; Duckett, J.G. Studies of protonemal morphogenesis in mosses IV. Aloina (Pottiales). J. Bryol. 1994, 18, 27–41. [Google Scholar] [CrossRef]
- Goode, J.A.; Stead, A.D.; Duckett, J.G. Redifferentiation of moss protonemata: An experimental and immunofluorescence study of brood cell formation. Can. J. Bot. 1993, 71, 1510–1519. [Google Scholar] [CrossRef]
- Basile, A.; Giordano, S.; Spagnuolo, V.; Alfano, F.; Castaldo Cobianchi, R. Effect of lead and colchicine on morphogenesis in proto- nemata of the moss Funaria hygrometrica. Ann. Bot. 1995, 76, 597–606. [Google Scholar] [CrossRef]
- Bopp, M.; Quader, H.; Thoni, C.; Sawidis, T.; Schnepf, E. Filament disruption in Funaria protonemata: Formation and disintegration of tmema cells. J. Plant Physiol. 1991, 137, 273–284. [Google Scholar] [CrossRef]
- Schnepf, E. Structure and development of tmema cells in protonemata of Funaria hygrometrica (Bryophyta). Criptogam. Bot. 1992, 3, 35–39. [Google Scholar]
- Rao, D.N. Response of bryophytes to air pollution. In Bryophyte Ecology; Smith, A.J., Ed.; Chapman & Hall: London, UK, 1982; pp. 445–471. [Google Scholar]
- El Sawi, S.A.; Ibrahim, M.E.; El-Rokiek, K.G.; El-Din, S.A.S. Allelopathic potential of essential oils isolated from peels of three citrus species. Ann. Agric. Sci. 2019, 64, 89–94. [Google Scholar] [CrossRef]
- Jalaei, Z.; Fattahi, M.; Aramideh, S. Allelopathic and insecticidal activities of essential oil of Dracocephalum kotschyi Boiss. from Iran: A new chemotype with highest limonene-10-al and limonene. Ind. Crops Prod. 2015, 73, 109–117. [Google Scholar] [CrossRef]
- Tang, J.S.; Jiang, C.Y.; Liu, Y.; Zhang, X.Y.; Shao, H.; Zhang, C. Allelopathic potential of volatile organic compounds released by Xanthium sibiricum Patrin ex Widder. Allelopath. J. 2019, 47, 233–242. [Google Scholar] [CrossRef]
- Vaughn, S.F. Phytotoxic and antimicrobial activity of 5,7-dihydroxychromone from Peanut shells. J. Chem. Ecol. 1995, 21, 107–115. [Google Scholar] [CrossRef]
- Basile, A.; Cobianchi, R.C.; Rigano, D.; Senatore, F.; Bruno, M.; Rosselli, S.; Conte, B.; Sorbo, S. Potential allelopathic activity of Sideritis italica (Miller) Greuter et Burdet essential oil. Plant Biosyst. 2011, 145, 241–247. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Giordano, S.; Ricciardi, L.; Ferrara, S.; Montesano, D.; Castaldo Cobianchi, R.; Vuotto, M.L.; Ferrara, L. Antibacterial and allelopathic activity of Castanea sativa Mill. leaves. Fitoterapia 2000, 71, S110–S116. [Google Scholar] [CrossRef]
- Gagliano Candela, R.; Ilardi, V.; Badalamenti, N.; Bruno, M.; Rosselli, S.; Maggi, F. Essential oil compositions of Teucrium fruticans, T. scordium subsp. scordioides and T. siculum growing in Sicily and Malta. Nat. Prod. Res. 2021, 35, 3460–3469. [Google Scholar] [CrossRef]
- Council of Europe (EDQM). European Pharmacopoeia, 6th ed.; EDQM: Strasbourg, France, 2008. [Google Scholar]
- Rigano, D.; Formisano, C.; Rosselli, S.; Badalamenti, N.; Bruno, M. GC and GC-MS Analysis of Volatile Compounds from Ballota nigra subsp. uncinata collected in Aeolian Islands, Sicily (Southern Italy). Nat. Prod. Commun. 2020, 15, 1934578X2092048. [Google Scholar] [CrossRef]
- Erickson, H.H.; Sherris, J.C. Antibiotic sensitivity testing report of international collaborative study. Acta Pathol. Microbiol. Scand. 1971, 217B, 1–9. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).