Physiological and Biochemical Responses of Pseudocereals with C3 and C4 Photosynthetic Metabolism in an Environment with Elevated CO2
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions
2.1.1. Description of the Experimental Site (OTCs)
2.1.2. Elevated CO2 Treatment
2.2. Growth Parameters
2.3. Physiological and Biochemical Parameters
2.4. Yield Components
2.5. Experimental Design and Data Analyses
3. Results
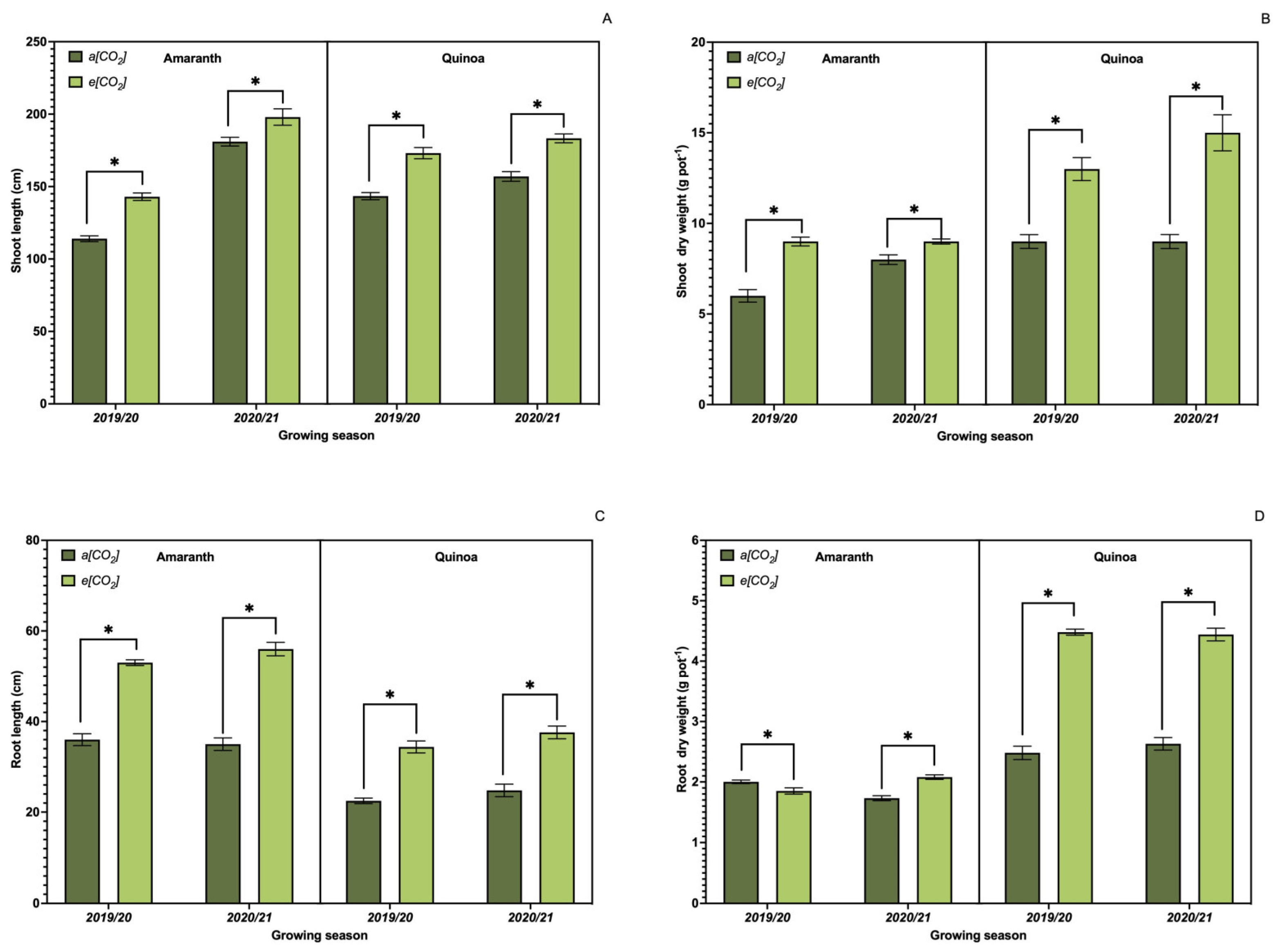
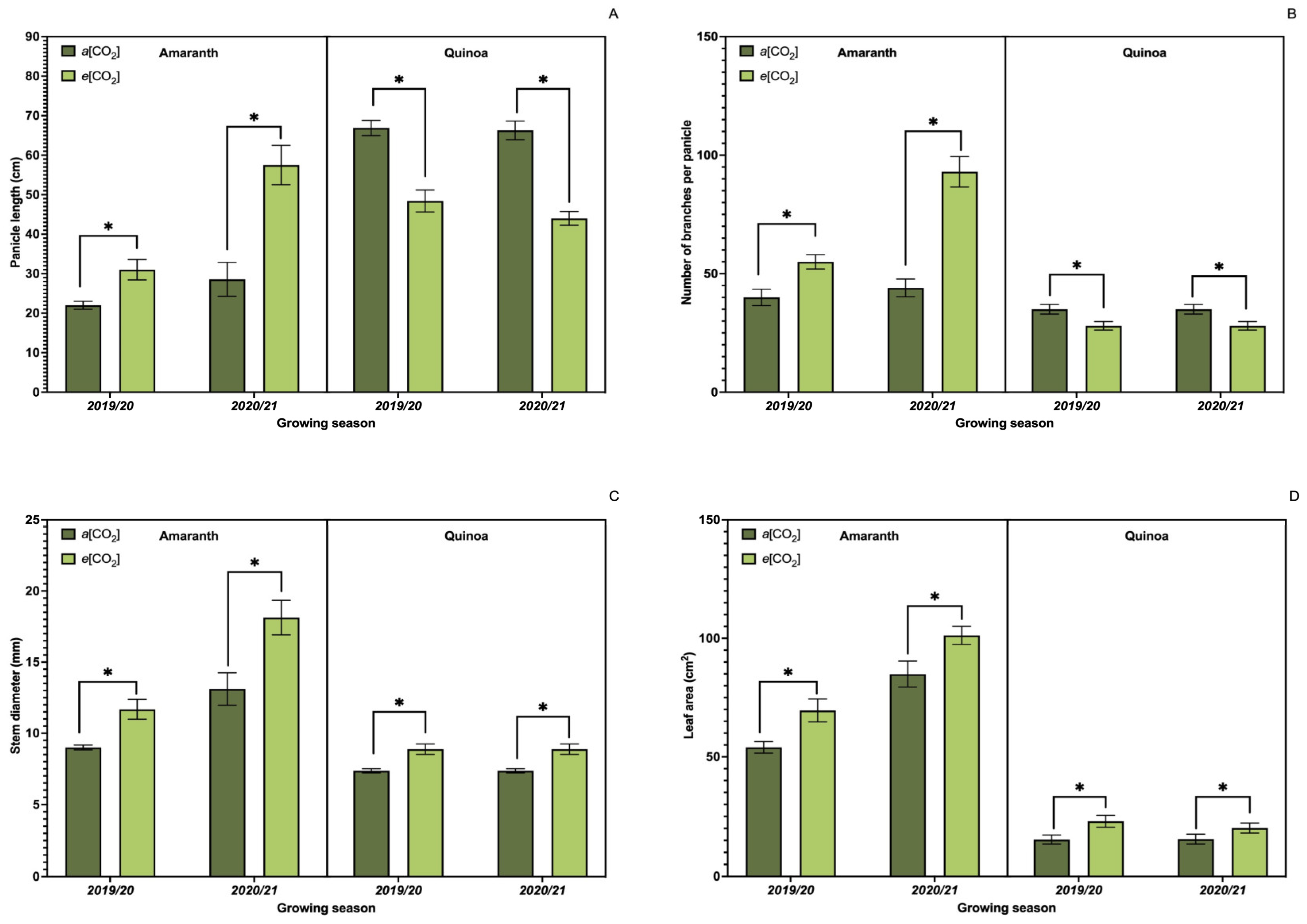
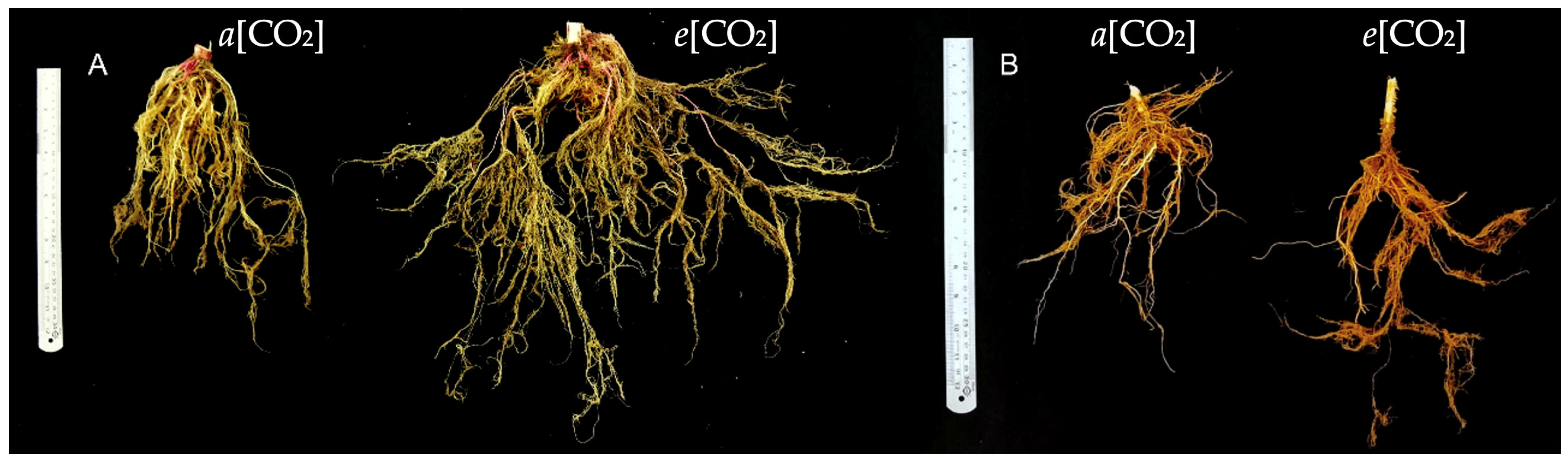
3.1. Growth Parameters
3.2. Photosynthetic Pigments
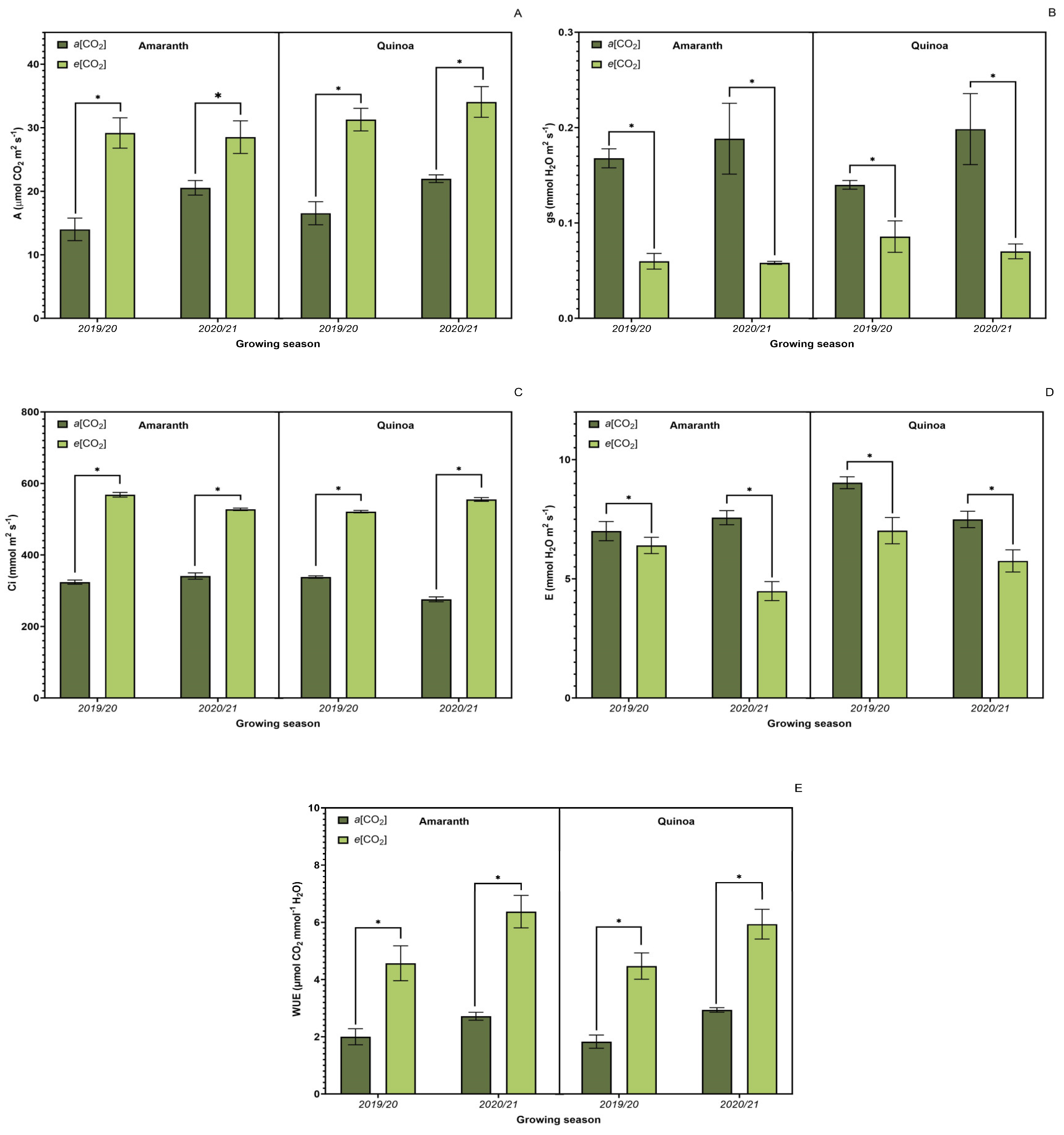
3.3. Leaf Gas Exchange
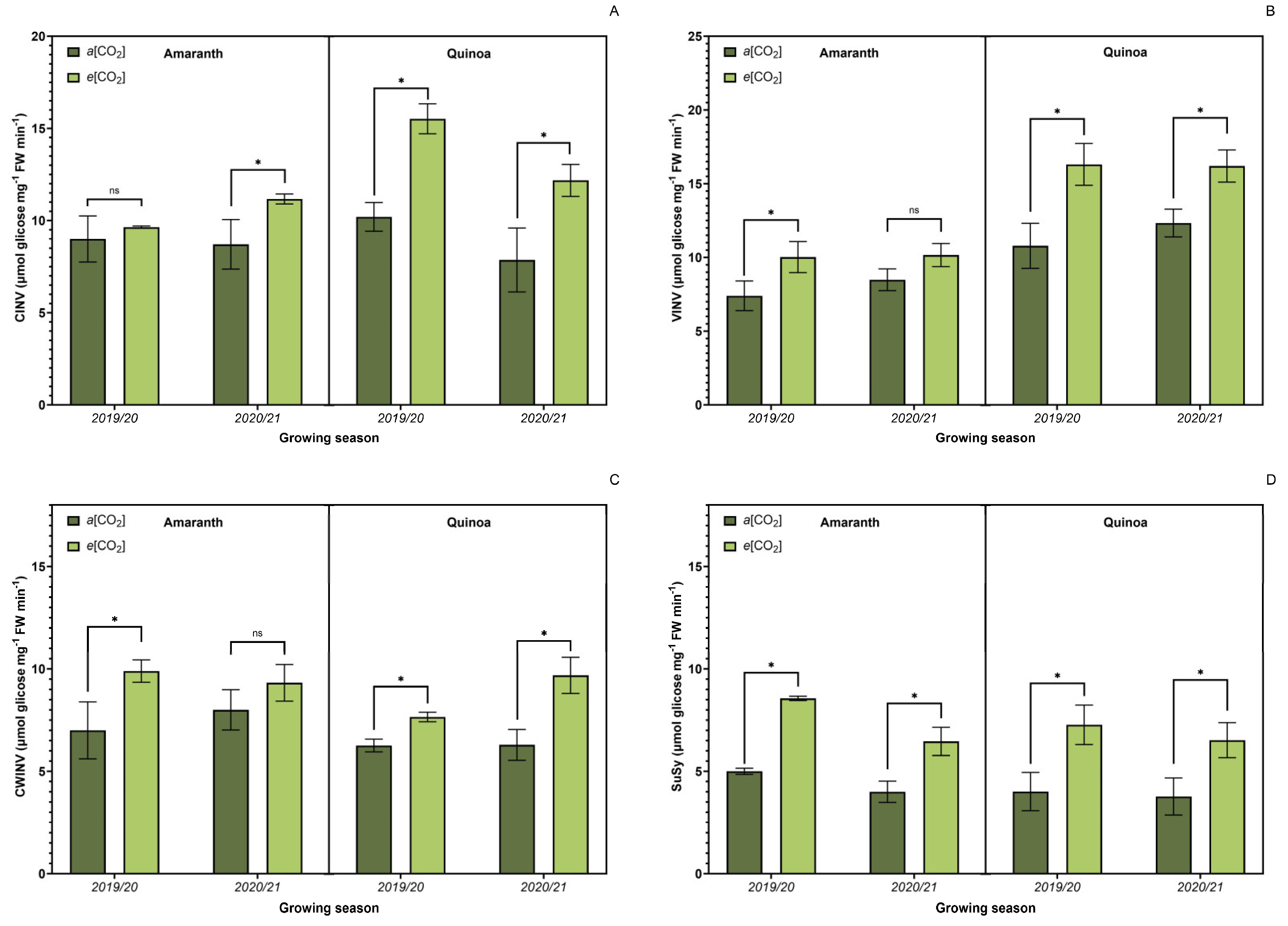
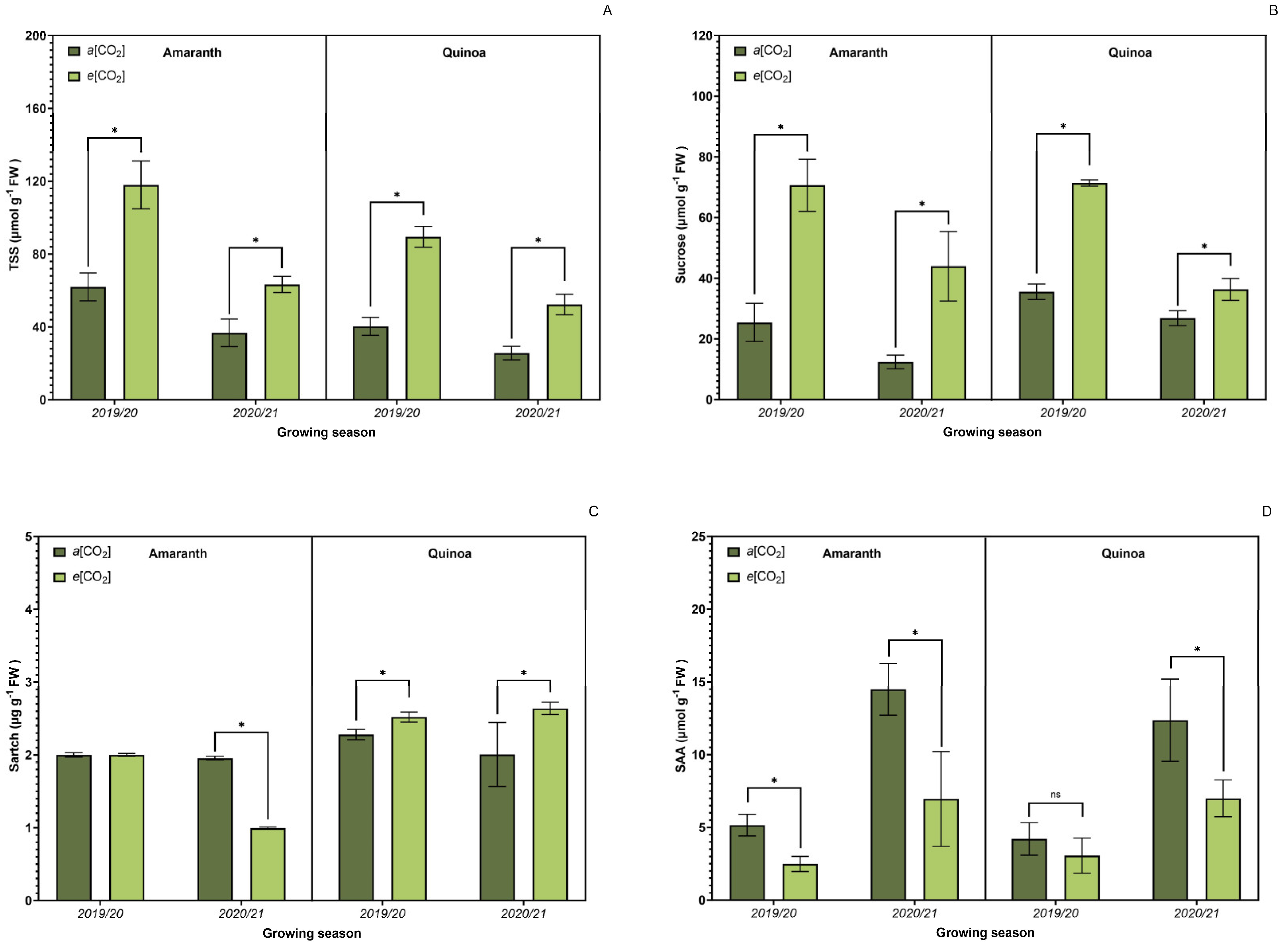
3.4. Sucrose Metabolism-Related Enzyme Activity
3.5. Carbohydrate Metabolism
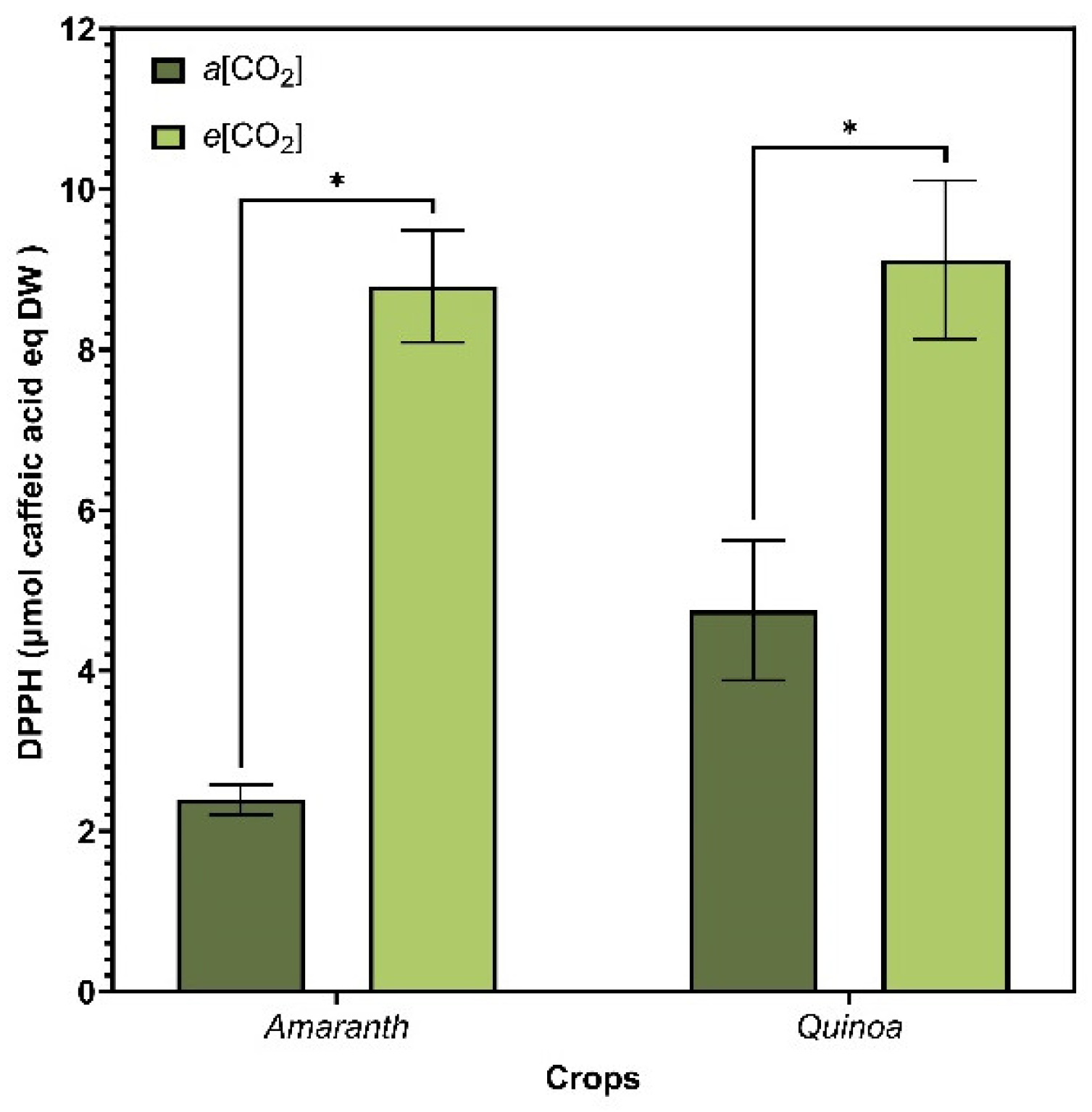
3.6. Antioxidant Activity
3.7. Nutrient Contents in Leaves
3.8. Yield Components
3.9. Crude Protein
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hannah, L. Climate Change Biology, 3rd ed.; Academic Press: London, UK, 2021. [Google Scholar]
- IPCC. Climate Change: Impacts, Adaptation, and Vulnerability; IPCC: Geneva, Switzerland, 2022. [Google Scholar]
- Letcher, T.M. Global warming-a complex situation. Clim. Change Obs. Impacts Planet Earth Third Ed. 2021, 3–17. [Google Scholar] [CrossRef]
- Leakey, A.D.B.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef] [PubMed]
- Kimball, B.A. Crop responses to elevated CO2 and interactions with H2O, N, and temperature. Curr. Opin. Plant Biol. 2016, 31, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Escribano, J.; Cabanes, J.; Jiménez-Atiénzar, M.; Ibañez-Tremolada, M.; Gómez-Pando, L.R.; García-Carmona, F.; Gandía-Herrero, F. Characterization of betalains, saponins and antioxidant power in differently colored quinoa (Chenopodium quinoa) varieties. Food Chem. 2017, 234, 285–294. [Google Scholar] [CrossRef]
- Antolin, L.A.; Heinemann, A.B.; Marin, F.R. Impact assessment of common bean availability in Brazil under climate change scenarios. Agric. Syst. 2021, 191, 103174. [Google Scholar] [CrossRef]
- Omar, M.E.D.M.; Moussa, A.M.A.; Hinkelmann, R. Impacts of climate change on water quantity, water salinity, food security, and socioeconomy in Egypt. Water Sci. Eng. 2021, 14, 17–27. [Google Scholar] [CrossRef]
- Manners, R.; Varela-Ortega, C.; van Etten, J. Protein-rich legume and pseudo-cereal crop suitability under present and future European climates. Eur. J. Agron. 2019, 113, 125974. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Nutritional constituents of pseudo cereals and their potential use in food systems: A review. Trends Food Sci. Technol. 2018, 75, 170–180. [Google Scholar] [CrossRef]
- Schoenlechner, R. Properties of pseudocereals, selected specialty cereals and legumes for food processing with special attention to gluten-free products/Verarbeitungseigenschaften von Pseudogetreide, ausgewählten Spezialitätengetreide und Leguminosen mit speziellem Fokuss auf glutenfreie Produkte. Die Bodenkultur J. Land Manag. Food Environ. 2016, 67, 239–248. [Google Scholar] [CrossRef]
- Chandra, A.K.; Chandora, R.; Sood, S.; Malhotra, N. Global production, demand, and supply. In Millets and Pseudo Cereals: Genetic Resources and Breeding Advancements; Woodhead Publishing: Cambridge, UK, 2020. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Walthall, C.L. Climate Change: Cropping System Changes and Adaptations. Encycl. Agric. Food Syst. 2014, 256–265. [Google Scholar] [CrossRef]
- Hofstra, G.; Hesketh, J.D. The Effects of Temperature and CO2 Enrichment on Photosynthesis in Soybean. In Environmental and Biological Control of Photosynthesis; Springer: Dordrecht, The Netherlands, 1975; pp. 71–80. [Google Scholar] [CrossRef]
- Sionit, N.; Hellmers, H.; Strain, B.R. Interaction of Atmospheric CO2 Enrichment and Irradiance on Plant Growth. Agron. J. 1982, 74, 721–725. [Google Scholar] [CrossRef]
- Long, S.P.; Zhu, X.; Naidu, S.L.; Ort, D.R. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 2006, 29, 315–330. [Google Scholar] [CrossRef]
- Zong, Y.-Z.; Shangguan, Z.-P. Increased sink capacity enhances C and N assimilation under drought and elevated CO2 conditions in maize. J. Integr. Agric. 2016, 15, 2775–2785. [Google Scholar] [CrossRef]
- Münch, E. Movement of Solutes within the Plant. Nature 1931, 127, 550–551. [Google Scholar] [CrossRef]
- Herbers, K.; Sonnewald, U. Molecular determinants of sink strength. Curr. Opin. Plant Biol. 1998, 1, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.C. Metabolism and Compartmentation of Imported Sugars in Sink Organs in Relation to Sink Strength. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 355–378. [Google Scholar] [CrossRef]
- Liu, J.; Hu, T.; Fang, L.; Peng, X.; Liu, F. CO2 elevation modulates the response of leaf gas exchange to progressive soil drying in tomato plants. Agric. For. Meteorol. 2019, 268, 181–188. [Google Scholar] [CrossRef]
- Khanam, U.K.S.; Oba, S. Bioactive substances in leaves of two amaranth species, Amaranthus tricolor and A. hypochondriacus. Can. J. Plant Sci. 2013, 93, 47–58. [Google Scholar] [CrossRef]
- Hariadi, Y.; Marandon, K.; Tian, Y.; Jacobsen, S.-E.; Shabala, S. Ionic and osmotic relations in quinoa (Chenopodium quinoa Willd.) plants grown at various salinity levels. J. Exp. Bot. 2010, 62, 185–193. [Google Scholar] [CrossRef]
- James, L.E.A. Chapter 1 Quinoa (Chenopodium quinoa Willd.): Composition, chemistry, nutritional, and functional properties. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2009; Volume 58, pp. 1–31. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Biondi, S.; Oses, R.; Acuña-Rodríguez, I.S.; Antognoni, F.; Martinez-Mosqueira, E.A.; Coulibaly, A.; Canahua-Murillo, A.; Pinto, M.; Zurita-Silva, A.; et al. Quinoa biodiversity and sustainability for food security under climate change. A review. Agron. Sustain. Dev. 2013, 34, 349–359. [Google Scholar] [CrossRef]
- Monteiro, J.E.B.A.; Sentelhas, P.C.; Chiavegato, E.J.; Guiselini, C.; Santiago, A.V.; Prela, A. Estimação da área foliar do algodoeiro por meio de dimensões e massa das folhas. Bragantia 2005, 64, 15–24. [Google Scholar] [CrossRef]
- Benincasa, M.M. Análise de Crescimento de Plantas: Noções Básicas, 2nd ed.; Jaboticabal Funep: São Paulo, Brazil, 2003. [Google Scholar]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Graham, D.; Smydzuk, J. Use of anthrone in the quantitative determination of hexose phosphates. Anal. Biochem. 1965, 11, 246–255. [Google Scholar] [CrossRef] [PubMed]
- van Handel, E. Direct microdetermination of sucrose. Anal. Biochem. 1968, 22, 280–283. [Google Scholar] [CrossRef]
- Cocking, E.C.; Yemm, E.W. Estimation of amino acids by ninhydrin. Process Biochem. 1954, 58. [Google Scholar]
- Zeng, Y.; Wu, Y.; Avigne, W.T.; Koch, K.E. Rapid Repression of Maize Invertases by Low Oxygen. Invertase/Sucrose Synthase Balance, Sugar Signaling Potential, and Seedling Survival. Plant Physiol. 1999, 121, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Lowell, C.A.; Tomlinson, P.T.; Koch, K.E. Sucrose-Metabolizing Enzymes in Transport Tissues and Adjacent Sink Structures in Developing Citrus Fruit. Plant Physiol. 1989, 90, 1394–1402. [Google Scholar] [CrossRef]
- Pérez-Tortosa, V.; López-Orenes, A.; Martínez-Pérez, A.; Ferrer, M.A.; Calderón, A.A. Antioxidant activity and rosmarinic acid changes in salicylic acid-treated Thymus membranaceus shoots. Food Chem. 2012, 130, 362–369. [Google Scholar] [CrossRef]
- Tedesco, M.; Gianello, C.; Bissiani, C.A.; Bohnem, H.; Volkweiss, S.J. Análise de Solo, Plantas e Outros Materiais; Universidade Federal do Rio Grande do Sul: Porto Alegre, Brazil, 1995. [Google Scholar]
- Kirk, P.L. Kjeldahl Method for Total Nitrogen. Anal. Chem. 1950, 22, 354–358. [Google Scholar] [CrossRef]
- Reich, P.B.; Hobbie, S.E.; Lee, T.D.; Pastore, M.A. Unexpected reversal of C3 versus C4 grass response to elevated CO2 during a 20-year field experiment. Science 2018, 360, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.D.M. Características da Planta (Árvore do Conhecimento-Feijão Cauipi); Agência Embrapa de Informação Tecnologica (Ageitec): Agência, Brazil, 2019. [Google Scholar]
- Xu, C.; McDowell, N.G.; Sevanto, S.; Fisher, R.A. Our limited ability to predict vegetation dynamics under water stress. New Phytol. 2013, 200, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Song, N.; Zhang, X.; Wang, F.; Zhang, C.; Tang, S. Elevated CO2 increases Cs uptake and alters microbial communities and biomass in the rhizosphere of Phytolacca americana Linn (pokeweed) and Amaranthus cruentus L. (purple amaranth) grown on soils spiked with various levels of Cs. J. Environ. Radioact. 2012, 112, 29–37. [Google Scholar] [CrossRef]
- Bunce, J.A. Variation in yield responses to elevated CO2 and a brief high temperature treatment in quinoa. Plants 2017, 6, 26. [Google Scholar] [CrossRef]
- Li, T.; Di, Z.; Han, X.; Yang, X. Elevated CO2 improves root growth and cadmium accumulation in the hyperaccumulator Sedum alfredii. Plant Soil 2011, 354, 325–334. [Google Scholar] [CrossRef]
- Pritchard, S.G.; Rogers, H.H. Spatial and temporal deployment of crop roots in CO2-enriched environments. New Phytol. 2000, 147, 55–71. [Google Scholar] [CrossRef]
- Tingey, D.T.; Phillips, D.L.; Johnson, M.G. Elevated CO2 and conifer roots: Effects on growth, life span and turnover. New Phytol. 2000, 147, 87–103. [Google Scholar] [CrossRef]
- Nie, M.; Lu, M.; Bell, J.; Raut, S.; Pendall, E. Altered root traits due to elevated CO2: A meta-analysis. Glob. Ecol. Biogeogr. 2013, 22, 1095–1105. [Google Scholar] [CrossRef]
- Jayme-Oliveira, A.; Júnior, W.Q.R.; Ramos, M.L.G.; Ziviani, A.C.; Jakelaitis, A. Amaranth, quinoa, and millet growth and development under different water regimes in the Brazilian Cerrado. Pesqui. Agropecu. Bras. 2017, 52, 561–571. [Google Scholar] [CrossRef]
- Xie, H.; Liu, K.; Sun, D.; Wang, Z.; Lu, X.; He, K. A field experiment with elevated atmospheric CO2-mediated changes to C4 crop-herbivore interactions. Sci. Rep. 2015, 5, 13923. [Google Scholar] [CrossRef] [PubMed][Green Version]
- DE Souza, A.P.; Gaspar, M.; DA Silva, E.A.; Ulian, E.C.; Waclawovsky, A.J.; Nishiyama, M.Y., Jr.; DOS Santos, R.V.; Teixeira, M.M.; Souza, G.M.; Buckeridge, M.S. Elevated CO2 increases photosynthesis, biomass and productivity, and modifies gene expression in sugarcane. Plant Cell Environ. 2008, 31, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, B.; Seneweera, S.; Zong, Y.; Li, F.Y.; Han, Y.; Hao, X. Photosynthesis and yield response to elevated CO2, C4 plant foxtail millet behaves similarly to C3 species. Plant Sci. 2019, 285, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Filho, A.F.D. Produção e Qualidade de Sementes de Quinoa em Função do Arranjo Espacial. Ph.D. Thesis, Universidade Federal do Ceará, Fortaleza, Brazil, 2017. [Google Scholar]
- Tozzi, F.R.O.; Ghini, R. Impacto do aumento da concentração atmosférica de dióxido de carbono sobre a ferrugem e o crescimento do cafeeiro. Pesqui. Agropecu. Bras. 2016, 51, 933–941. [Google Scholar] [CrossRef][Green Version]
- Gonzalez-Meler, M.A.; Silva, L.B.C.; Dias-De-Oliveira, E.; Flower, C.E.; Martinez, C.A. Experimental air warming of a Stylosanthes capitata, vogel dominated tropical pasture affects soil respiration and nitrogen dynamics. Front. Plant Sci. 2017, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Dorneles, K.; Posso, D.A.; Rebhahn, I.; Deuner, S.; Pazdiora, P.; Avila, L.; Dallagnol, L.J. Respostas morfofisiológicas e rendimento de grãos do trigo mediados pelo aumento da concentração de CO2 atmosférico. Rev. Bras. De Cienc. Agrar. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Busch, F.A.; Sage, R.F. The sensitivity of photosynthesis to O2 and CO2 concentration identifies strong Rubisco control above the thermal optimum. New Phytol. 2016, 213, 1036–1051. [Google Scholar] [CrossRef]
- Siefermann-Harms, D. The light-harvesting and protective functions of carotenoids in photosynthetic membranes. Physiol. Plant. 1987, 69, 561–568. [Google Scholar] [CrossRef]
- Conroy, J.; Hocking, P. Nitrogen nutrition of C3 plants at elevated atmospheric CO2 concentrations. Physiol. Plant. 1993, 89, 570–576. [Google Scholar] [CrossRef]
- Wang, L.; Deng, F.; Ren, W.-J. Shading tolerance in rice is related to better light harvesting and use efficiency and grain filling rate during grain filling period. Field Crop. Res. 2015, 180, 54–62. [Google Scholar] [CrossRef]
- Bloom, A.J.; Asensio, J.S.R.; Randall, L.; Rachmilevitch, S.; Cousins, A.B.; Carlisle, E.A. CO2 enrichment inhibits shoot nitrate assimilation in C3 but not C4 plants and slows growth under nitrate in C3 plants. Ecology 2012, 93, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, S.; Mitra, S. Elevated atmospheric CO2 and the future of crop plants. Plant Breed. 2020, 140, 1–11. [Google Scholar] [CrossRef]
- Krämer, K.; Kepp, G.; Brock, J.; Stutz, S.; Heyer, A.G. Acclimation to elevated CO2 affects the C/N balance by reducing de novo N-assimilation. Physiol. Plant. 2022, 174, e13615. [Google Scholar] [CrossRef] [PubMed]
- White, A.C.; Rogers, A.; Rees, M.; Osborne, C.P. How can we make plants grow faster? A source–sink perspective on growth rate. J. Exp. Bot. 2015, 67, 31–45. [Google Scholar] [CrossRef]
- Kuai, B.; Chen, J.; Hörtensteiner, S. The biochemistry and molecular biology of chlorophyll breakdown. J. Exp. Bot. 2017, 69, 751–767. [Google Scholar] [CrossRef]
- Makino, A.; Mae, T. Photosynthesis and plant growth at elevated levels of CO2. Plant Cell Physiol. 1999, 40, 999–1006. [Google Scholar] [CrossRef]
- Turkan, I.; Uzilday, B.; Dietz, K.-J.; Bräutigam, A.; Ozgur, R. Reactive oxygen species and redox regulation in mesophyll and bundle sheath cells of C4 plants. J. Exp. Bot. 2018, 69, 3321–3331. [Google Scholar] [CrossRef]
- Taub, D. Effects of rising atmospheric concentrations of carbon dioxide on plants. Nat. Educ. Knowl. 2010, 3, 21. [Google Scholar]
- Xu, Y.; Ibrahim, I.M.; Harvey, P.J. The influence of photoperiod and light intensity on the growth and photosynthesis of Dunaliella salina (chlorophyta) CCAP 19/30. Plant Physiol. Biochem. 2016, 106, 305–315. [Google Scholar] [CrossRef]
- Wang, T.; Wei, Q.; Wang, Z.; Liu, W.; Zhao, X.; Ma, C.; Gao, J.; Xu, Y.; Hong, B. CmNF-YB8 affects drought resistance in chrysanthemum by altering stomatal status and leaf cuticle thickness. J. Integr. Plant Biol. 2021, 64, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, A.; Lam, S.K.; Li, P.; Zong, Y.; Gao, Z.; Hao, X. Increased carbon uptake under elevated CO2 concentration enhances water-use efficiency of C4 broomcorn millet under drought. Agric. Water Manag. 2020, 245, 106631. [Google Scholar] [CrossRef]
- Davis, A.S.; Ainsworth, E.A. Weed interference with field-grown soyabean decreases under elevated [CO2] in a FACE experiment. Weed Res. 2012, 52, 277–285. [Google Scholar] [CrossRef]
- Dos Santos, L.A.; Pinto, C.C.; Szareski, V.J.; Carvalho, I.R.; Koch, F.; Pimentel, J.R.; Troyjack, C.; Dubal, T.P.; Reolon, F.; Da Rosa, T.C.; et al. Respiratory activity and physiological performance of maize seeds classified according to their shapes and sizes. Aust. J. Crop Sci. 2018, 12, 1882–1889. [Google Scholar] [CrossRef]
- Leakey, A.D.; Uribelarrea, M.; Ainsworth, E.A.; Naidu, S.L.; Rogers, A.; Ort, D.R.; Long, S.P. Photosynthesis, productivity, and yield of maize are not affected by open-air elevation of CO2 concentration in the absence of drought. Plant Physiol. 2006, 140, 779–790. [Google Scholar] [CrossRef]
- Monson, R.K. The Origins of C4 Genes and Evolutionary Pattern in the C4 Metabolic Phenotype. C4 Plant Biol. 1999, 377–410. [Google Scholar] [CrossRef]
- Sage, R.F. The evolution of C4 photosynthesis. New Phytol. 2003, 161, 341–370. [Google Scholar] [CrossRef]
- Stutz, S.S.; Hanson, D.T. Contribution and consequences of xylem-transported CO2 assimilation for C3 plants. New Phytol. 2019, 223, 1230–1240. [Google Scholar] [CrossRef]
- de Souza Osório, C.R.W.; Teixeira, G.C.M.; Barreto, R.F.; Campos, C.N.S.; Leal, A.J.F.; Teodoro, P.E.; Prado, R.D.M. Macronutrient deficiency in snap bean considering physiological, nutritional, and growth aspects. PLoS ONE 2020, 15, e0234512. [Google Scholar] [CrossRef]
- Wand, S.J.E.; Midgley, G.F.; Jones, M.H.; Curtis, P.S. Responses of wild C4 and C3 grass (Poaceae) species to elevated atmospheric CO2 concentration: A meta-analytic test of current theories and perceptions. Glob. Chang. Biol. 1999, 5, 723–741. [Google Scholar] [CrossRef]
- da Silva, R.G.; Alves, R.d.C.; Zingaretti, S.M. Increased [CO2] causes changes in physiological and genetic responses in C4 crops: A brief review. Plants 2020, 9, 1567. [Google Scholar] [CrossRef] [PubMed]
- Leegood, R.C. C4 photosynthesis: Principles of CO2 concentration and prospects for its introduction into C3 plants. J. Exp. Bot. 2002, 53, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Roitsch, T.; González, M.-C. Function and regulation of plant invertases: Sweet sensations. Trends Plant Sci. 2004, 9, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, X.; Manning, W.J. Effects of Elevated CO2 on Leaf Senescence, Leaf Nitrogen Resorption, and Late-Season Photosynthesis in Tilia americana L. Front. Plant Sci. 2019, 10, 1217. [Google Scholar] [CrossRef]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L.; et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef]
- Ma, N.; Ma, C.; Liu, Y.; Shahid, M.O.; Wang, C.; Gao, J. Petal senescence: A hormone view. J. Exp. Bot. 2018, 69, 719–732. [Google Scholar] [CrossRef]
- Ruan, Y.-L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Lal, M.K.; Sharma, N.; Adavi, S.B.; Sharma, E.; Altaf, M.A.; Tiwari, R.K.; Kumar, R.; Kumar, A.; Dey, A.; Paul, V.; et al. From source to sink: Mechanistic insight of photoassimilates synthesis and partitioning under high temperature and elevated [CO2]. Plant Mol. Biol. 2022, 110, 305–324. [Google Scholar] [CrossRef]
- Stein, O.; Granot, D. An overview of sucrose synthases in plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef]
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef]
- Thompson, M.; Gamage, D.; Hirotsu, N.; Martin, A.; Seneweera, S. Effects of elevated carbon dioxide on photosynthesis and carbon partitioning: A perspective on root sugar sensing and hormonal crosstalk. Front. Physiol. 2017, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Bühler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Gruda, N.; Lam, S.K.; Li, X.; Duan, Z. Effects of elevated CO2 on nutritional quality of vegetables: A review. Front. Plant Sci. 2018, 9, 924. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed: Porto Alegre, Brazil, 2017. [Google Scholar]
- Pazzagli, P.T.; Weiner, J.; Liu, F. Effects of CO2 elevation and irrigation regimes on leaf gas exchange, plant water relations, and water use efficiency of two tomato cultivars. Agric. Water Manag. 2016, 169, 26–33. [Google Scholar] [CrossRef]
- Escudero, A.; del Arco, J.M.; Sanz, I.C.; Ayala, J. Effects of leaf longevity and retranslocation efficiency on the retention time of nutrients in the leaf biomass of different woody species. Oecologia 1992, 90, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R. Nutrient Resorption from Senescing Leaves of Perennials: Are there General Patterns? J. Ecol. 1996, 84, 597. [Google Scholar] [CrossRef]
- Killingbeck, K.T. Nutrients in senesced leaves: Keys to the search for potential resorption and resorption proficiency. Ecology 1996, 77, 1716–1727. [Google Scholar] [CrossRef]
- Reed, S.C.; Townsend, A.R.; Davidson, E.A.; Cleveland, C.C. Stoichiometric patterns in foliar nutrient resorption across multiple scales. New Phytol. 2012, 196, 173–180. [Google Scholar] [CrossRef]
- Vergutz, L.; Manzoni, S.; Porporato, A.; Novais, R.F.; Jackson, R.B. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol. Monogr. 2012, 82, 205–220. [Google Scholar] [CrossRef]
- de Campos, M.C.R.; Pearse, S.J.; Oliveira, R.S.; Lambers, H. Downregulation of net phosphorus-uptake capacity is inversely related to leaf phosphorus-resorption proficiency in four species from a phosphorus-impoverished environment. Ann. Bot. 2013, 111, 445–454. [Google Scholar] [CrossRef]
- Pang, J.; Zhu, J.-G.; Xie, Z.-B.; Liu, G.; Zhang, Y.-L.; Chen, G.-P.; Zeng, Q.; Cheng, L. A new explanation of the N concentration decrease in tissues of rice (Oryza sativa L.) exposed to elevated atmospheric pCO2. Environ. Exp. Bot. 2005, 57, 98–105. [Google Scholar] [CrossRef]
- Crous, K.Y.; Wujeska-Klause, A.; Jiang, M.; Medlyn, B.E.; Ellsworth, D.S. Nitrogen and phosphorus retranslocation of leaves and stemwood in a mature eucalyptus forest exposed to 5 years of elevated CO2. Front. Plant Sci. 2019, 10, 664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.M.; Zhang, C.C.; Pan, G.X.; Zhang, X.H. Availability of soil nitrogen and phosphorus under elevated [CO2] and temperature in the Taihu Lake region, China. J. Plant Nutr. Soil Sci. 2014, 177, 343–348. [Google Scholar] [CrossRef]
- Xu, H.; Xie, H.; Wu, S.; Wang, Z.; He, K. Effects of elevated CO2 and increased n fertilization on plant secondary metabolites and chewing insect fitness. Front. Plant Sci. 2019, 10, 739. [Google Scholar] [CrossRef]
- Sudderth, E.A.; Stinson, K.A.; Bazzaz, F.A. Host-specific aphid population responses to elevated CO2 and increased N availability. Glob. Chang. Biol. 2005, 11, 1997–2008. [Google Scholar] [CrossRef]
- Müntz, K.; Belozersky, M.; Dunaevsky, Y.; Schlereth, A.; Tiedemann, J. Stored proteinases and the initiation of storage protein mobilization in seeds during germination and seedling growth. J. Exp. Bot. 2001, 52, 1741–1752. [Google Scholar] [CrossRef]
- Goyoaga, C.; Burbano, C.; Cuadrado, C.; Romero, C.; Guillamón, E.; Varela, A.; Pedrosa, M.M.; Muzquiz, M. Content and distribution of protein, sugars and inositol phosphates during the germination and seedling growth of two cultivars of Vicia faba. J. Food Compos. Anal. 2011, 24, 391–397. [Google Scholar] [CrossRef]
- Erbaş, S.; Tonguç, M.; Şanli, A. Mobilization of seed reserves during germination and early seedling growth of two sunflower cultivars. J. Appl. Bot. Food Qual. 2016, 89. [Google Scholar] [CrossRef]
- Gu, J.; Chao, H.; Gan, L.; Guo, L.; Zhang, K.; Li, Y.; Wang, H.; Raboanatahiry, N.; Li, M. Proteomic dissection of seed germination and seedling establishment in Brassica napus. Front. Plant Sci. 2016, 7, 1482. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, Q.; Ziska, L.H.; Zhu, J.; Liu, G.; Zhang, J.; Ni, K.; Seneweera, S.; Zhu, C. Seed vigor of contrasting rice cultivars in response to elevated carbon dioxide. Field Crop. Res. 2015, 178, 63–68. [Google Scholar] [CrossRef]
- Högy, P.; Wieser, H.; Köhler, P.; Schwadorf, K.; Breuer, J.; Franzaring, J.; Muntifering, R.; Fangmeier, A. Effects of elevated CO2 on grain yield and quality of wheat: Results from a 3-year free-air CO2 enrichment experiment. Plant Biol. 2009, 11, 60–69. [Google Scholar] [CrossRef] [PubMed]

| Treatment [CO2] | Amaranth (First Year) | Amaranth (Second Year) | Quinoa (First Year) | Quinoa (Second Year) |
|---|---|---|---|---|
| N (g kg−1) | ||||
| a[CO2] | 52.0 ± (1.79) * | 53.0 ± (1.66) * | 54.9 ± (4.57) * | 53.2 ± (0.54) * |
| e[CO2] | 49.2 ± (0.98) | 36.0 ± (1.79) | 48.4 ± (0.27) | 38.5 ± (1.71) |
| CV% | 1.15 | 1.57 | 2.52 | 1.12 |
| P (g kg−1) | ||||
| a[CO2] | 3.4 ± (0.13) * | 3.1 ± (0.08) * | 5.1 ± (0.42) * | 2.7 ± (0.08) * |
| e[CO2] | 4.3 ± (0.17) | 3.7 ± (0.17) | 4.0 ± (0.37) | 3.0 ± (0.18) |
| CV% | 1.65 | 1.56 | 3.54 | 2.03 |
| K (g kg−1) | ||||
| a[CO2] | 46.5 ± (2.33) * | 46.5 ± (3.87) * | 50.8 ± (2.07) * | 50.9 ± (1.97) * |
| e[CO2] | 30.7 ± (4.20) | 33.9 ± (1.62) | 46.3 ± (1.86) | 45.7 ± (0.64) |
| CV% | 3.54 | 2.97 | 1.63 | 1.22 |
| Ca (g kg−1) | ||||
| a[CO2] | 16.9 ± (1.88) * | 27.3 ± (2.75) * | 11.3 ± (0.46) ns | 21.7 ± (1.63) * |
| e[CO2] | 12.9 ± (1.63) | 16.9 ± (1.88) | 11.2 ± (2.58) | 15.3 ± (2.97) |
| CV% | 4.74 | 4.29 | 6.66 | 5.23 |
| Mg (g kg−1) | ||||
| a[CO2] | 8.1 ± (0.88) * | 8.8 ± (1.07) ns | 6.3 ± (1.13) ns | 8.9 ± (0.78) ns |
| e[CO2] | 9.9 ± (0.21) | 7.8 ± (1.34) | 6.0 ± (1.03) | 8.8 ± (0.71) |
| CV% | 2.99 | 5.87 | 7.06 | 3.38 |
| Treatment [CO2] | Amaranth (First Year) | Amaranth (Second Year) | Quinoa (First Year) | Quinoa (Second Year) |
|---|---|---|---|---|
| Fe (mg kg−1) | ||||
| a[CO2] | 164.4 ± (4.50) * | 174.5 ± (1.33) * | 80.1 ± (7.83) * | 180.0 ± (3.63) * |
| e[CO2] | 185.4 ± (2.37) | 207.8 ± (3.60) | 135.1 ± (8.64) | 235.5 ± (2.66) |
| CV% | 0.89 | 2.04 | 3.09 | |
| Zn (mg kg−1) | ||||
| a[CO2] | 22.2 ± (2.10) * | 36.1 ± (2.99) * | 11.3 ± (2.31) * | 49.0 ± (1.03) * |
| e[CO2] | 15.5 ± (4.45) | 26.0 ± (2.92) | 6.4 ± (4.55) | 20.8 ± (0.88) |
| CV% | 7.41 | 3.82 | 16.37 | 1.11 |
| Mn (mg kg−1) | ||||
| a[CO2] | 554.7 ± (9.19) * | 254.2 ± (4.18) * | 348.4(5.42) * | 755.0 ± (8.93) * |
| e[CO2] | 424.8 ± (2.60) | 193.7 ± (8.29) | 284.0 ± (3.86) | 585.1 ± (9.43) |
| CV% | 0.56 | 1.18 | 0.6 | |
| Cu (mg kg−1) | ||||
| a[CO2] | 17.6 ± (1.72) * | 19.6 ± (1.72) * | 12.4 ± (1.72) * | 14.0 ± (1.72) * |
| e[CO2] | 15.2 ± (1.72) | 17.6 ± (1.72) | 31.4 ± (1.72) | 29.2 ± (1.72) |
| CV% | 4.21 | 3.73 | 3.15 | 3.2 |
| Treatment [CO2] | Amaranth (First Year) | Amaranth (Second Year) | Quinoa (First Year) | Quinoa (Second Year) |
|---|---|---|---|---|
| Weight of 1000 grains (g) | ||||
| a[CO2] | 1.11 ± (0.01) * | 1.10 ± (0.00) * | 2.41 ± (0.07) * | 2.48 ± (0.01) * |
| e[CO2] | 0.86 ± (0.03) | 0.81 ± (0.01) | 1.71 ± (0.04) | 1.70 ± (0.00) |
| CV% | 1.87 | 0.74 | 1.89 | 0.28 |
| Number of grains per panicle | ||||
| a[CO2] | 10.921 ± (11.28) ns | 9.879 ± (7.5) ns | 2.598 ± (2.66) * | 2.772 ± (18.45) * |
| e[CO2] | 9.876 ± (10.52) | 10.922 ± (9.14) ns | 2.174 ± (3.48) | 1.830 ± (14.0) |
| CV% | 13.12 | 11.44 | 18.2 | 9.99 |
| Grain yield per pot (g) | ||||
| a[CO2] | 9.37 ± (0.96) ns | 8.40 ± (0.66) ns | 5.72 ± (0.69) * | 5.67 ± (0.78) * |
| e[CO2] | 9.30 ± (0.73) | 8.41 ± (0.70) | 2.84 ± (0.62) | 2.17 ± (0.47) |
| CV% | 12.83 | 11.38 | 21.58 | 23.08 |
| Treatment [CO2] | Crude Protein (%) | |||
|---|---|---|---|---|
| Amaranth (First Year) | Amaranth (Second Year) | Quinoa (First Year) | Quinoa (Second Year) | |
| a[CO2] | 18.28 ± (1.35) * | 17.99 ± (0.37) * | 15.97 ± (0.97) * | 15.94 ± (0.92) * |
| e[CO2] | 12.74 ± (0.62) | 11.87 ± (0.30) | 7.97 ± (0.89) | 7.79 ± (0.41) |
| CV% | 4.29 | 1.45 | 4.92 | 3.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, B.E.P.; Pires, S.N.; Teixeira, S.B.; Lucho, S.R.; Fagundes, N.d.S.; Centeno, L.H.; Carlos, F.S.; de Souza, F.R.; Avila, L.A.d.; Deuner, S. Physiological and Biochemical Responses of Pseudocereals with C3 and C4 Photosynthetic Metabolism in an Environment with Elevated CO2. Plants 2024, 13, 3453. https://doi.org/10.3390/plants13233453
Silva BEP, Pires SN, Teixeira SB, Lucho SR, Fagundes NdS, Centeno LH, Carlos FS, de Souza FR, Avila LAd, Deuner S. Physiological and Biochemical Responses of Pseudocereals with C3 and C4 Photosynthetic Metabolism in an Environment with Elevated CO2. Plants. 2024; 13(23):3453. https://doi.org/10.3390/plants13233453
Chicago/Turabian StyleSilva, Bruna Evelyn Paschoal, Stefânia Nunes Pires, Sheila Bigolin Teixeira, Simone Ribeiro Lucho, Natan da Silva Fagundes, Larissa Herter Centeno, Filipe Selau Carlos, Fernanda Reolon de Souza, Luis Antonio de Avila, and Sidnei Deuner. 2024. "Physiological and Biochemical Responses of Pseudocereals with C3 and C4 Photosynthetic Metabolism in an Environment with Elevated CO2" Plants 13, no. 23: 3453. https://doi.org/10.3390/plants13233453
APA StyleSilva, B. E. P., Pires, S. N., Teixeira, S. B., Lucho, S. R., Fagundes, N. d. S., Centeno, L. H., Carlos, F. S., de Souza, F. R., Avila, L. A. d., & Deuner, S. (2024). Physiological and Biochemical Responses of Pseudocereals with C3 and C4 Photosynthetic Metabolism in an Environment with Elevated CO2. Plants, 13(23), 3453. https://doi.org/10.3390/plants13233453








