A Bifunctional Nuclease Promotes the Infection of Zucchini Yellow Mosaic Virus in Watermelon by Targeting P3
Abstract
1. Introduction
2. Results
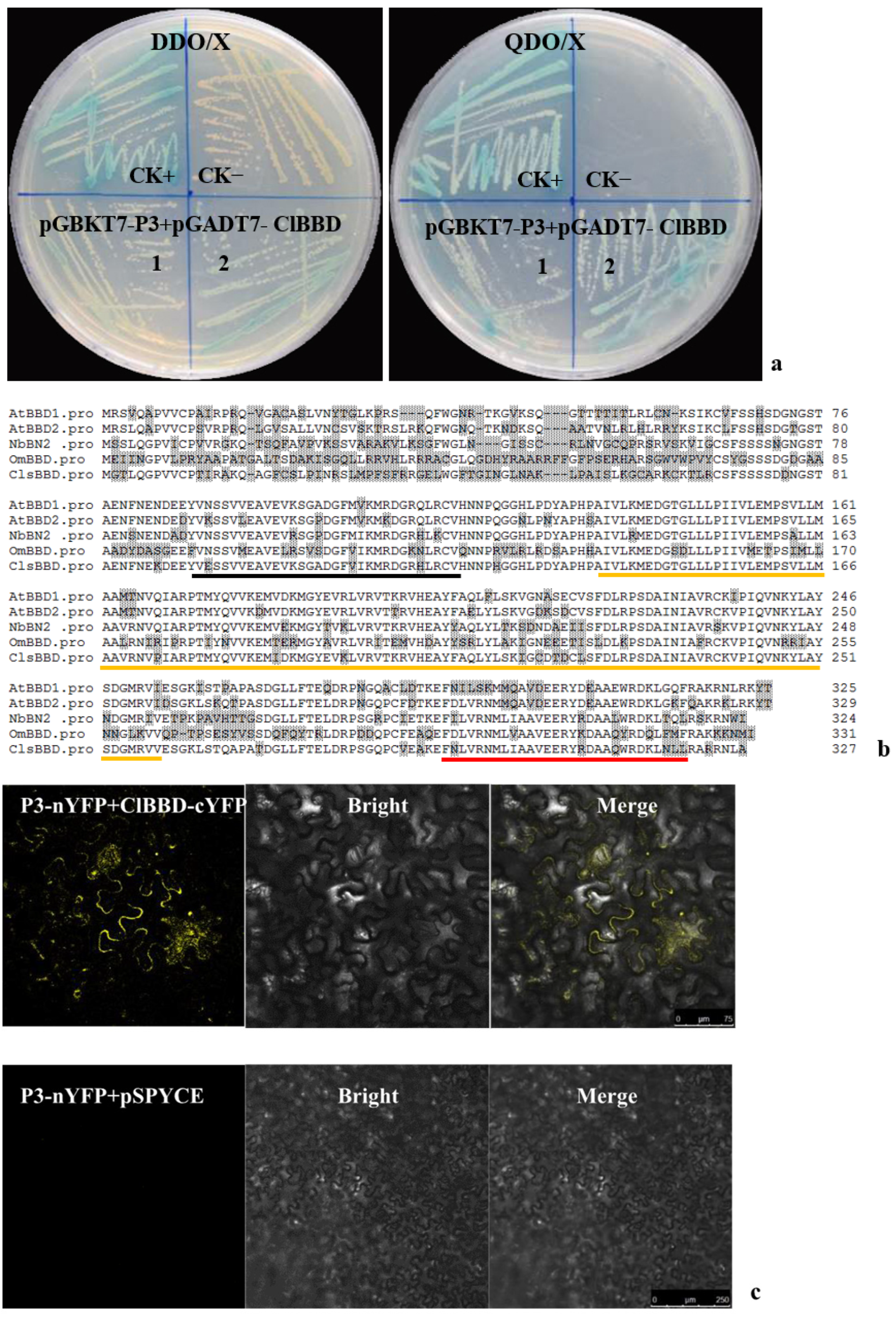
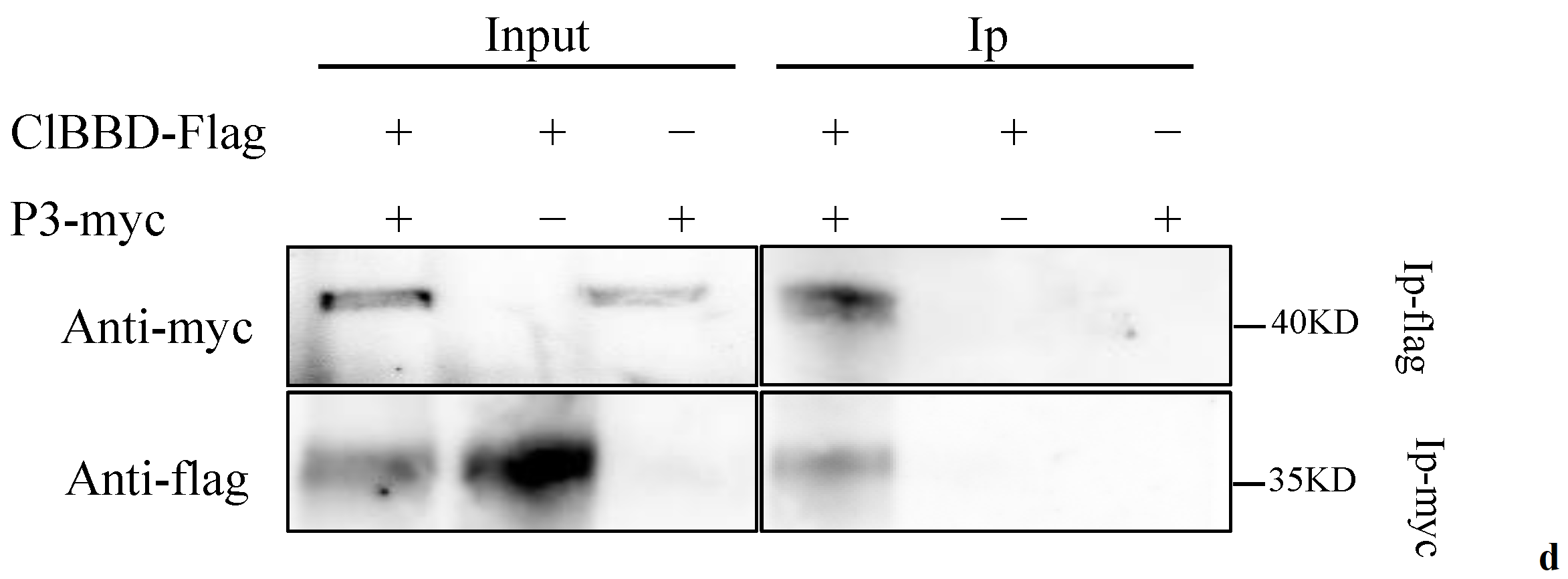
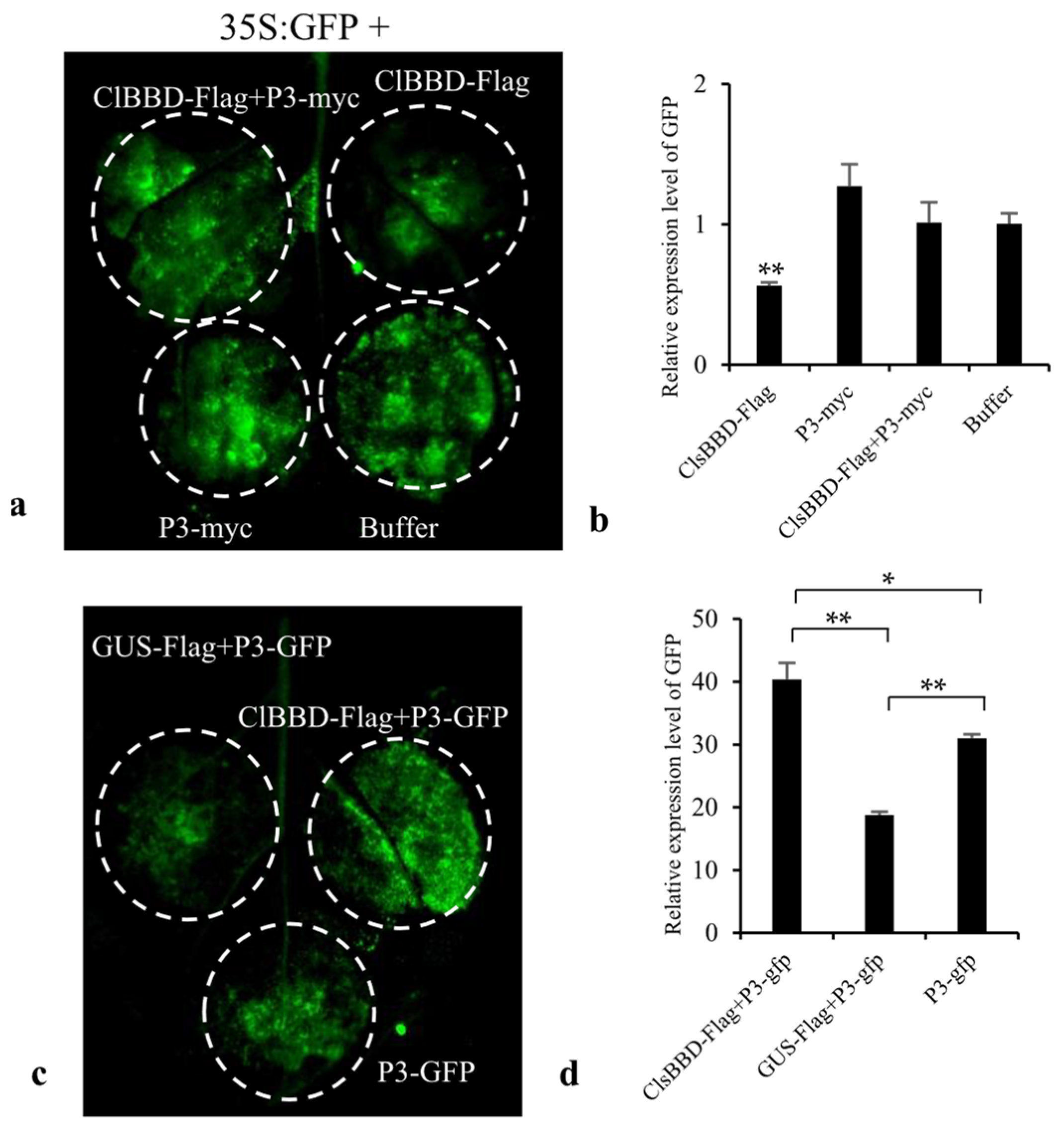
2.1. ZYMV P3 Protein Interacts with the Host Factor ClBBD
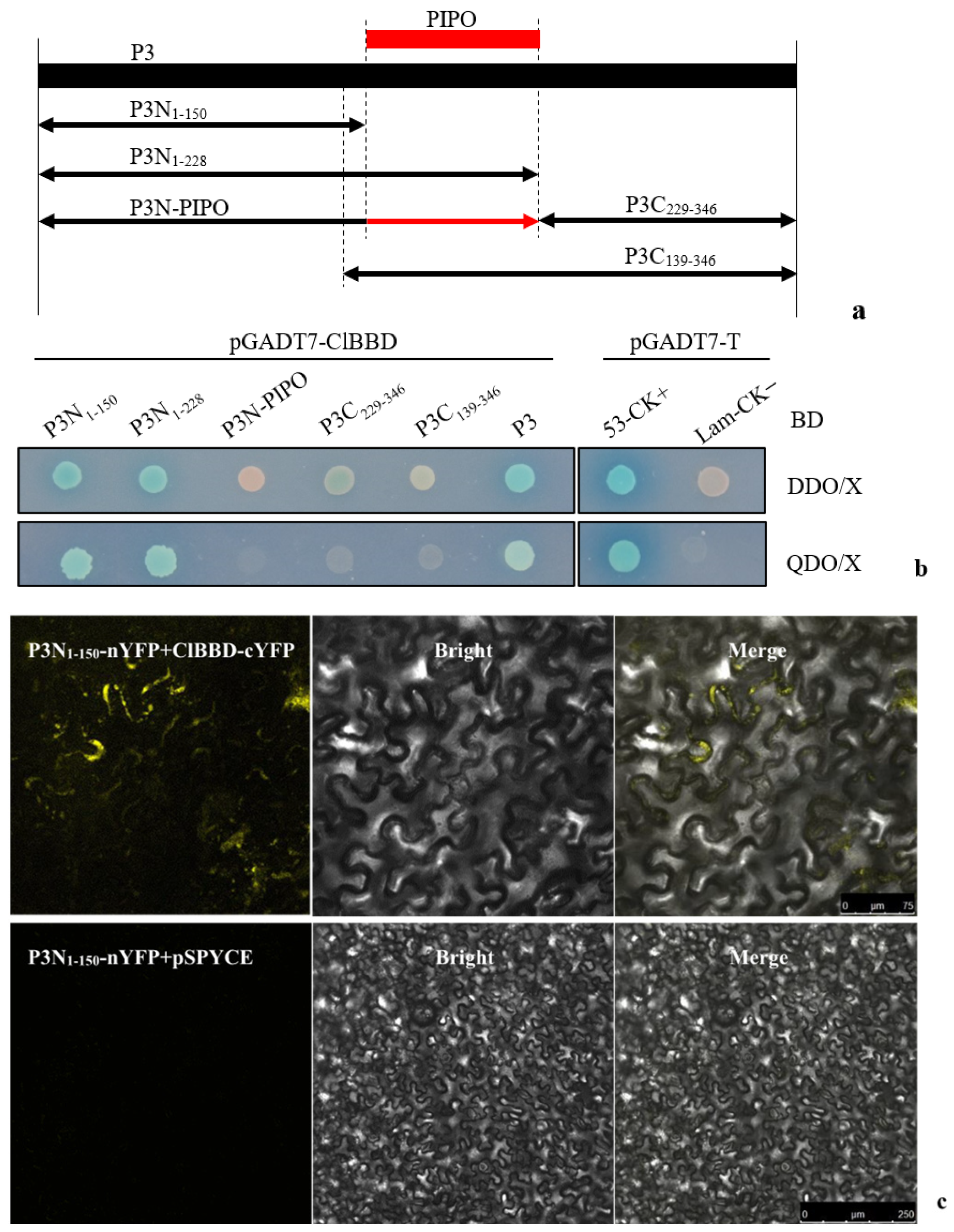
2.2. The Interacting Region of P3 with ClBBD Is at the P3 N-Terminus but Is Not P3N-PIPO
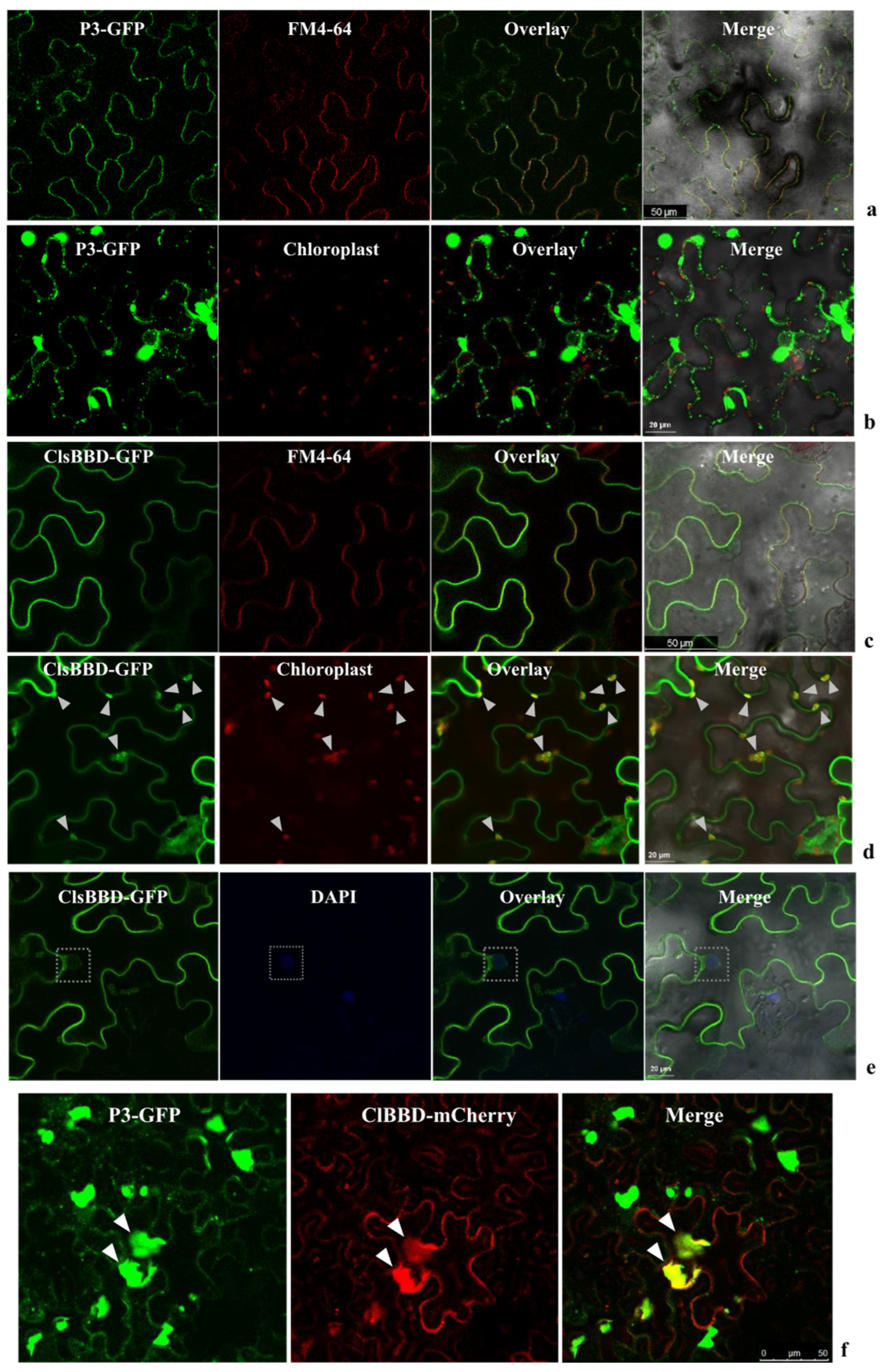
2.3. P3 Recruits ClBBD in the Cytoplasm
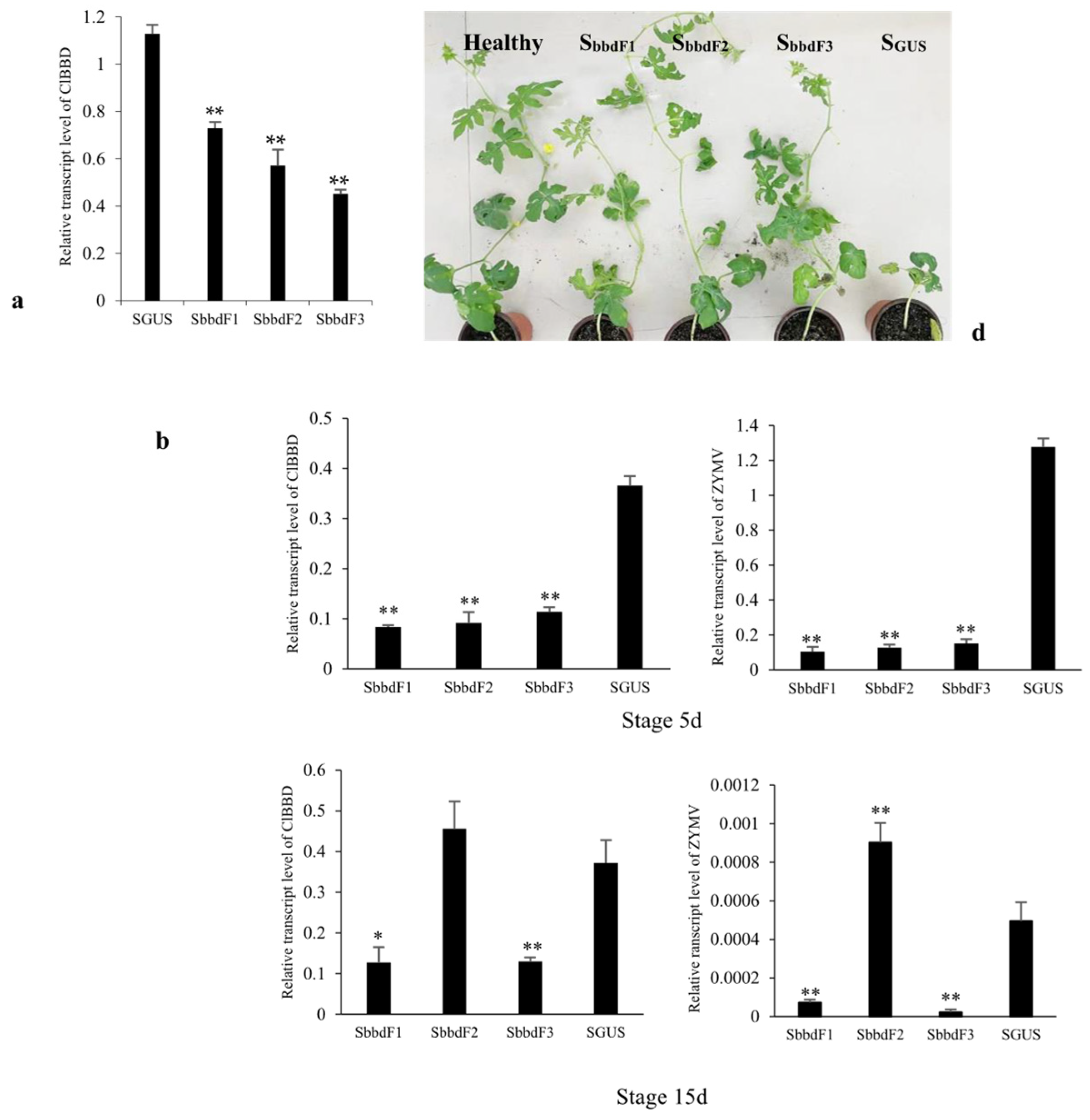
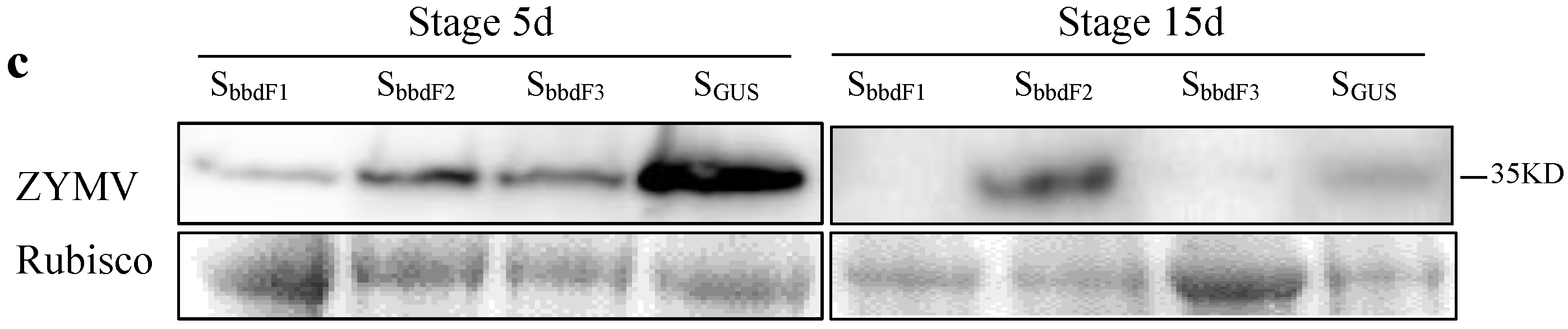
2.4. ClBBD Protein Positively Regulates the Viral Accumulation
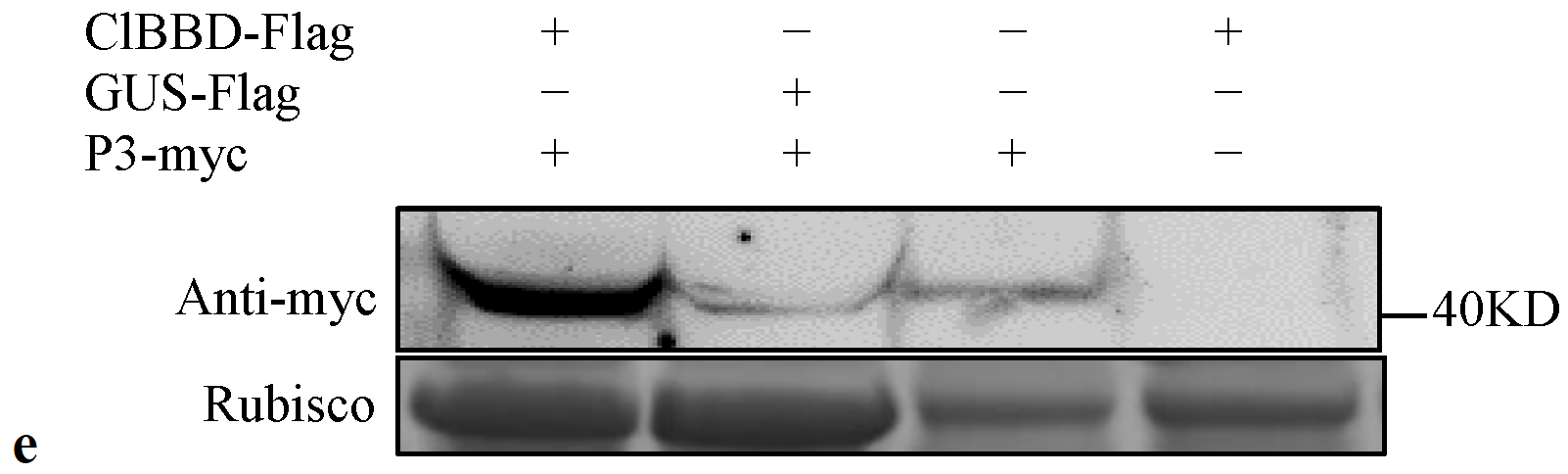
2.5. ClBBD Is Involved in Regulating the Expression of P3
3. Discussion
4. Conclusions
5. Experimental Procedures
5.1. Plant Material and Virus
5.2. Constructs
5.3. Yeast Two-Hybrid Assay
5.4. Agroinfiltration in N. benthamiana and Watermelon
5.5. Co-Immunoprecipitation Assays
5.6. Confocal Microscopy
5.7. Virus-Induced Gene Silencing (VIGS) Assay and Viral Infections
5.8. RNA Extraction and Quantitative Real-Time PCR Analysis
5.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desbiez, C.; Lecoq, H. Zucchini yellow mosaic virus. Plant Pathol. 1997, 46, 809–829. [Google Scholar] [CrossRef]
- Urcuqui-Inchima, S.; Haenni, A.L.; Bernardi, F. Potyvirus proteins: A wealth of functions. Virus Res. 2001, 74, 157–175. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.Y.; Miller, W.A.; Atkins, J.F.; Firth, A.E. An overlapping essential gene in the Potyviridae. Proc. Natl. Acad. Sci. USA 2008, 105, 5897–5902. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wei, T.; Chowda-Reddy, R.V.; Sun, G.; Wang, A. The Tobacco etch virus P3 protein forms mobile inclusions via the early secretory pathway and traffics along actin microfilaments. Virology 2010, 397, 56–63. [Google Scholar] [CrossRef]
- Johansen, I.E.; Lund, O.S.; Hjulsager, C.K.; Laursen, J. Recessive resistance in Pisum sativum and potyvirus pathotype resolved in a gene-for-cistron correspondence between host and virus. J. Virol. 2001, 75, 6609–6614. [Google Scholar] [CrossRef]
- Merits, A.; Guo, D.; Jarvekulg, L.; Saarma, M. Biochemical and genetic evidence for interactions between potato A potyvirus-encoded proteins P1 and P3 and proteins of the putative replication complex. Virology 1999, 263, 15–22. [Google Scholar] [CrossRef]
- Jenner, C.E.; Wang, X.; Tomimura, K.; Ohshima, K.; Ponz, F.; Walsh, J.A. The dual role of the potyvirus P3 protein of Turnip mosaic virus as a symptom and avirulence determinant in brassicas. Mol. Plant-Microbe Interact. MPMI 2003, 16, 777–784. [Google Scholar] [CrossRef]
- Choi, S.H.; Hagiwara-Komoda, Y.; Nakahara, K.S.; Atsumi, G.; Shimada, R.; Hisa, Y.; Naito, S.; Uyeda, I. Quantitative and qualitative involvement of P3N-PIPO in overcoming recessive resistance against Clover yellow vein virus in pea carrying the cyv1 gene. J. Virol. 2013, 87, 7326–7337. [Google Scholar] [CrossRef]
- Hajimorad, M.R.; Eggenberger, A.L.; Hill, J.H. Adaptation of Soybean mosaic virus avirulent chimeras containing P3 sequences from virulent strains to Rsv1-genotype soybeans is mediated by mutations in HC-Pro. Mol. Plant-Microbe Interact. MPMI 2008, 21, 937–946. [Google Scholar] [CrossRef]
- Chowda-Reddy, R.V.; Sun, H.; Chen, H.; Poysa, V.; Ling, H.; Gijzen, M.; Wang, A. Mutations in the P3 protein of Soybean mosaic virus G2 isolates determine virulence on Rsv4-genotype soybean. Mol. Plant-Microbe Interact. MPMI 2011, 24, 37–43. [Google Scholar] [CrossRef]
- Wang, Y.; Khatabi, B.; Hajimorad, M.R. Amino acid substitution in P3 of Soybean mosaic virus to convert avirulence to virulence on Rsv4-genotype soybean is influenced by the genetic composition of P3. Mol. Plant Pathol. 2015, 16, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.H.; Maroof, M.A.; Hajimorad, M.R. Amino acid changes in P3, and not the overlapping pipo-encoded protein, determine virulence of soybean mosaic virus on functionally immune Rsv1-genotype soybean. Mol. Plant Pathol. 2011, 12, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Desbiez, C.; Gal-On, A.; Girard, M.; Wipf-Scheibel, C.; Lecoq, H. Increase in Zucchini yellow mosaic virus Symptom Severity in Tolerant Zucchini Cultivars Is Related to a Point Mutation in P3 Protein and Is Associated with a Loss of Relative Fitness on Susceptible Plants. Phytopathology 2003, 93, 1478–1484. [Google Scholar] [CrossRef]
- Eiamtanasate, S.; Juricek, M.; Yap, Y.K. C-terminal hydrophobic region leads PRSV P3 protein to endoplasmic reticulum. Virus Genes. 2007, 35, 875–876. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Shine, M.B.; Cui, X.; Chen, X.; Ma, N.; Kachroo, P.; Zhi, H.; Kachroo, A. The Potyviral P3 Protein Targets Eukaryotic Elongation Factor 1A to Promote the Unfolded Protein Response and Viral Pathogenesis. Plant Physiol. 2016, 172, 221–234. [Google Scholar] [CrossRef]
- Luan, H.; Liao, W.; Niu, H.; Cui, X.; Chen, X.; Zhi, H. Comprehensive Analysis of Soybean Mosaic Virus P3 Protein Interactors and Hypersensitive Response-Like Lesion-Inducing Protein Function. Int. J. Mol. Sci. 2019, 20, 388. [Google Scholar] [CrossRef]
- Shi, F.; Wang, Y.; Zhang, F.; Yuan, X.; Chen, H.; Chen, X.; Chen, X.; Cui, X. Soybean Endo-1,3-Beta-Glucanase (GmGLU) Interaction with Soybean mosaic virus-Encoded P3 Protein May Contribute to the Intercelluar Movement. Front. Genet. 2020, 11, 536771. [Google Scholar] [CrossRef]
- Lin, L.; Luo, Z.; Yan, F.; Lu, Y.; Zheng, H.; Chen, J. Interaction between potyvirus P3 and ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO) of host plants. Virus Genes. 2011, 43, 90–92. [Google Scholar] [CrossRef]
- Lu, L.; Wu, G.; Xu, X.; Luan, H.; Zhi, H.; Cui, J.; Cui, X.; Chen, X. Soybean actin-depolymerizing factor 2 interacts with Soybean mosaic virus-encoded P3 protein. Virus Genes. 2015, 50, 333–339. [Google Scholar] [CrossRef]
- Chai, M.; Wu, X.; Liu, J.; Fang, Y.; Luan, Y.; Cui, X.; Zhou, X.; Wang, A.; Cheng, X. P3N-PIPO Interacts with P3 via the Shared N-Terminal Domain To Recruit Viral Replication Vesicles for Cell-to-Cell Movement. J. Virol. 2020, 94, e01898-19. [Google Scholar] [CrossRef]
- Cui, X.; Yaghmaiean, H.; Wu, G.; Wu, X.; Chen, X.; Thorn, G.; Wang, A. The C-terminal region of the Turnip mosaic virus P3 protein is essential for viral infection via targeting P3 to the viral replication complex. Virology 2017, 510, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Saruta, M.; Shimizu, T.; Shu, M.; Anai, T.; Komatsu, K.; Yamada, N.; Katayose, Y.; Ishikawa, M.; Ishimoto, M.; et al. Soybean antiviral immunity conferred by dsRNase targets the viral replication complex. Nat. Commun. 2019, 10, 4033. [Google Scholar] [CrossRef] [PubMed]
- Huque, A.; So, W.M.; You, M.K.; Shin, J.S. Phylogenetic Analysis and In Vitro Bifunctional Nuclease Assay of Arabidopsis BBD1 and BBD2. Molecules 2020, 25, 2169. [Google Scholar] [CrossRef] [PubMed]
- LeBrasseur, N.D.; MacIntosh, G.C.; Perez-Amador, M.A.; Saitoh, M.; Green, P.J. Local and systemic wound-induction of RNase and nuclease activities in Arabidopsis: RNS1 as a marker for a JA-independent systemic signaling pathway. Plant J. 2002, 29, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Perez-Amador, M.A.; Abler, M.L.; De Rocher, E.J.; Thompson, D.M.; van Hoof, A.; LeBrasseur, N.D.; Lers, A.; Green, P.J. Identification of BFN1, a bifunctional nuclease induced during leaf and stem senescence in Arabidopsis. Plant Physiol. 2000, 122, 169–180. [Google Scholar] [CrossRef]
- You, M.K.; Shin, H.Y.; Kim, Y.J.; Ok, S.H.; Cho, S.K.; Jeung, J.U.; Yoo, S.D.; Kim, J.K.; Shin, J.S. Novel bifunctional nucleases, OmBBD and AtBBD1, are involved in abscisic acid-mediated callose deposition in Arabidopsis. Plant Physiol. 2010, 152, 1015–1029. [Google Scholar] [CrossRef]
- Huque, A.; So, W.; Noh, M.; You, M.K.; Shin, J.S. Overexpression of AtBBD1, Arabidopsis Bifunctional Nuclease, Confers Drought Tolerance by Enhancing the Expression of Regulatory Genes in ABA-Mediated Drought Stress Signaling. Int. J. Mol. Sci. 2021, 22, 2936. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, Q.; Wu, Y.; Huang, F.; Ismayil, A.; Zhang, D.; Li, H.; Gu, H.; Ludman, M.; Fatyol, K.; et al. A calmodulin-binding transcription factor links calcium signaling to antiviral RNAi defense in plants. Cell Host Microbe 2021, 29, 1393–1406.e7. [Google Scholar] [CrossRef]
- Meiss, G.; Gast, F.U.; Pingoud, A.M. The DNA/RNA non-specific Serratia nuclease prefers double-stranded A-form nucleic acids as substrates. J. Mol. Biol. 1999, 288, 377–390. [Google Scholar] [CrossRef]
- Guan, R.B.; Li, H.C.; Fan, Y.J.; Hu, S.R.; Christiaens, O.; Smagghe, G.; Miao, X.X. A nuclease specific to lepidopteran insects suppresses RNAi. J. Biol. Chem. 2018, 293, 6011–6021. [Google Scholar] [CrossRef]
- Prentice, K.; Smagghe, G.; Gheysen, G.; Christiaens, O. Nuclease activity decreases the RNAi response in the sweetpotato weevil Cylas puncticollis. Insect Biochem. Mol. Biol. 2019, 110, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Du, J.; Li, S.; Wang, X. Identification and Characterization of a Double-Stranded RNA Degrading Nuclease Influencing RNAi Efficiency in the Rice Leaf Folder Cnaphalocrocis medinalis. Int. J. Mol. Sci. 2022, 23, 3961. [Google Scholar] [CrossRef] [PubMed]
- Megel, C.; Hummel, G.; Lalande, S.; Ubrig, E.; Cognat, V.; Morelle, G.; Salinas-Giege, T.; Duchene, A.M.; Marechal-Drouard, L. Plant RNases T2, but not Dicer-like proteins, are major players of tRNA-derived fragments biogenesis. Nucleic Acids Res. 2019, 47, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Lian, B.; Yuan, Y.; Kong, C.; Li, Y.; Liu, C.; Qi, Y. A 5’ tRNA-Ala-derived small RNA regulates anti-fungal defense in plants. Sci. China Life Sci. 2022, 65, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cognat, V.; Morelle, G.; Megel, C.; Lalande, S.; Molinier, J.; Vincent, T.; Small, I.; Duchene, A.M.; Marechal-Drouard, L. The nuclear and organellar tRNA-derived RNA fragment population in Arabidopsis thaliana is highly dynamic. Nucleic Acids Res. 2017, 45, 3460–3472. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, C.; Hou, X.; Sanfacon, H.; Wang, A. The SNARE protein Syp71 is essential for turnip mosaic virus infection by mediating fusion of virus-induced vesicles with chloroplasts. PLoS Pathog. 2013, 9, e1003378. [Google Scholar] [CrossRef]
- Liu, L.M.; Kang, B.S.; Peng, B.; Wu, H.J.; Su, Y.D.; Gu, Q.S. Construction of ZYMV infectious clone carrying eGFP and its infectivity. Acta Phytopathol. Sin. 2021, 51, 734–740. [Google Scholar]
- Walter, M.; Chaban, C.; Schutze, K.; Batistic, O.; Weckermann, K.; Nake, C.; Blazevic, D.; Grefen, C.; Schumacher, K.; Oecking, C.; et al. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 2004, 40, 428–438. [Google Scholar] [CrossRef]
- Lough, T.J.; Balmori, E.; Beck, D.L.; Forster, R.L. Western analysis of transgenic plants. Methods Mol. Biol. 1998, 81, 447–451. [Google Scholar]
- Fischer-Parton, S.; Parton, R.M.; Hickey, P.C.; Dijksterhuis, J.; Atkinson, H.A.; Read, N.D. Confocal microscopy of FM4-64 as a tool for analysing endocytosis and vesicle trafficking in living fungal hyphae. J. Microsc. 2000, 198 Pt 3, 246–259. [Google Scholar] [CrossRef]
- Liu, M.; Liang, Z.L.; Aranda, M.A.; Hong, N.; Liu, L.M.; Kang, B.S.; Gu, Q.S. A cucumber green mottle mosaic virus vector for virus-induced gene silencing in cucurbit plants. Plant Methods 2020, 16, 9. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, B.; Liu, L.; Liu, L.; Liu, M.; Wu, H.; Peng, B.; Liang, Z.; Liu, F.; Zang, Y.; Gu, Q. A Bifunctional Nuclease Promotes the Infection of Zucchini Yellow Mosaic Virus in Watermelon by Targeting P3. Plants 2024, 13, 3431. https://doi.org/10.3390/plants13233431
Kang B, Liu L, Liu L, Liu M, Wu H, Peng B, Liang Z, Liu F, Zang Y, Gu Q. A Bifunctional Nuclease Promotes the Infection of Zucchini Yellow Mosaic Virus in Watermelon by Targeting P3. Plants. 2024; 13(23):3431. https://doi.org/10.3390/plants13233431
Chicago/Turabian StyleKang, Baoshan, Lifeng Liu, Liming Liu, Mei Liu, Huijie Wu, Bin Peng, Zhiling Liang, Fengnan Liu, Yaoxing Zang, and Qinsheng Gu. 2024. "A Bifunctional Nuclease Promotes the Infection of Zucchini Yellow Mosaic Virus in Watermelon by Targeting P3" Plants 13, no. 23: 3431. https://doi.org/10.3390/plants13233431
APA StyleKang, B., Liu, L., Liu, L., Liu, M., Wu, H., Peng, B., Liang, Z., Liu, F., Zang, Y., & Gu, Q. (2024). A Bifunctional Nuclease Promotes the Infection of Zucchini Yellow Mosaic Virus in Watermelon by Targeting P3. Plants, 13(23), 3431. https://doi.org/10.3390/plants13233431




