In Vitro and In Silico Biological Activities Investigation of Ethyl Acetate Extract of Rubus ulmifolius Schott Leaves Collected in Algeria
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Screening
2.2. Determination of Total Phenolic, Total Flavonoid, and Flavonol Contents
2.3. GC-MS Analysis
2.4. Antioxidant Ability
2.5. Antibacterial Activity
2.6. In Silico Study
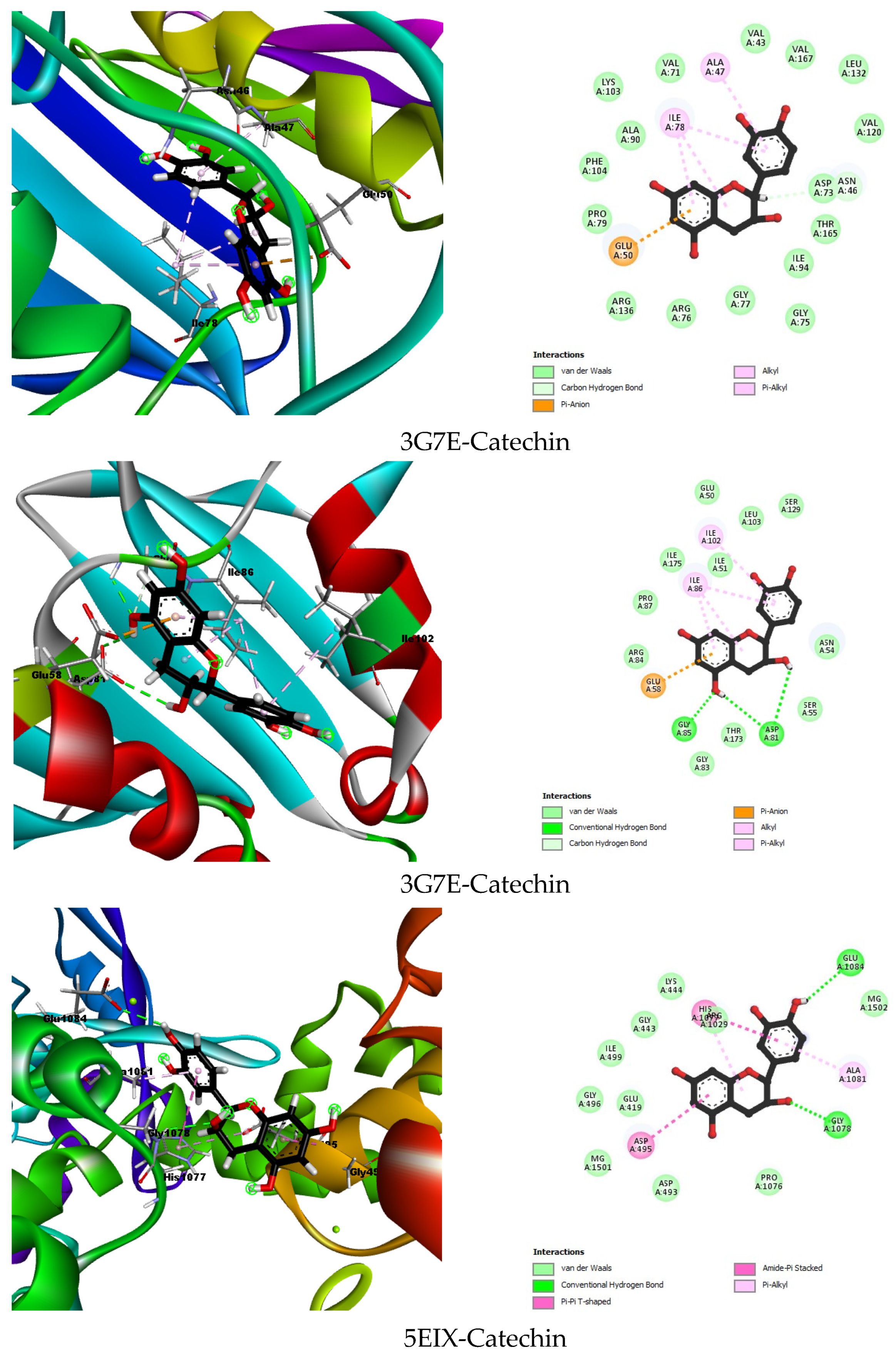
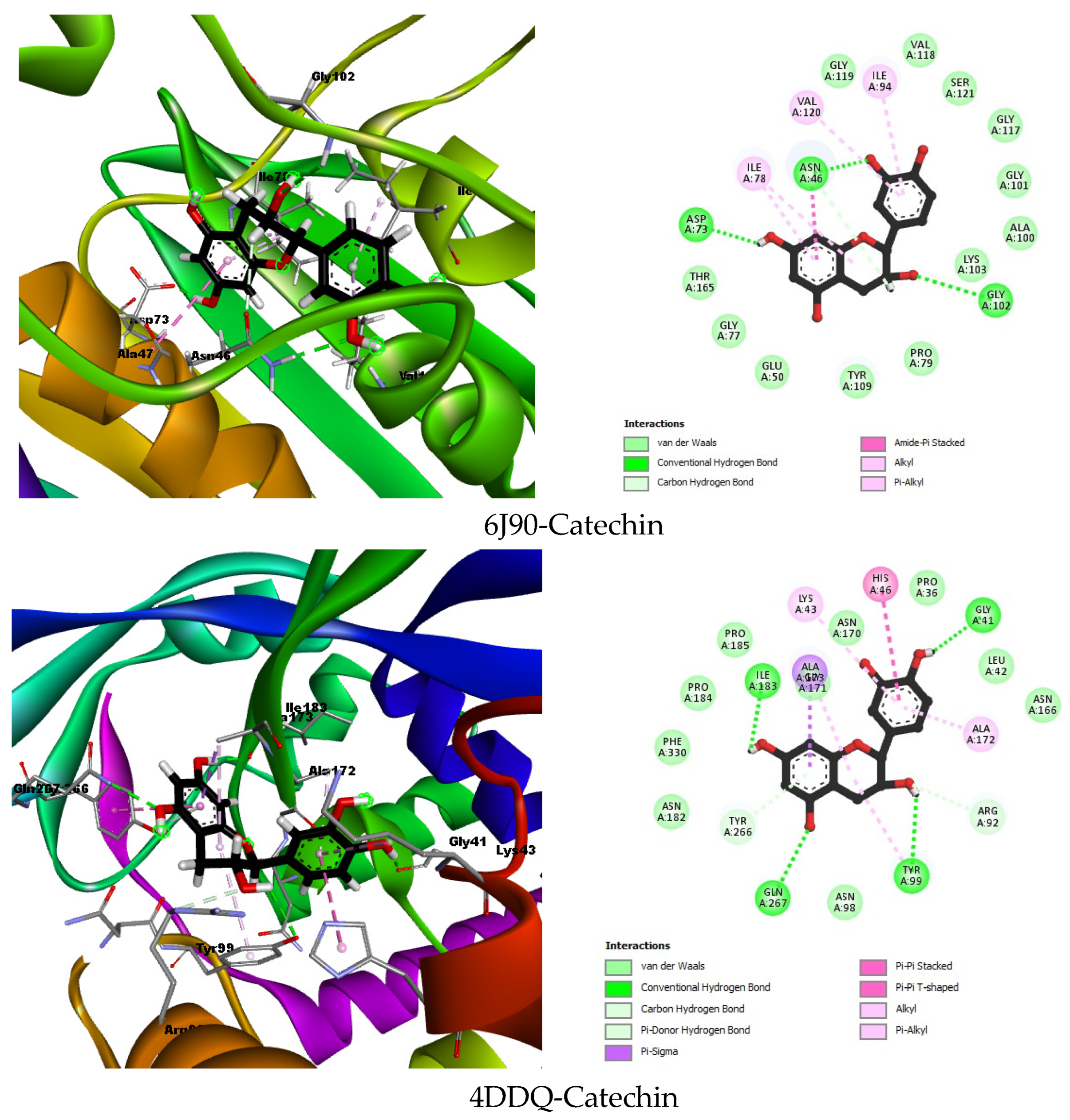
2.6.1. Molecular Docking
2.6.2. ADMET Properties
- -
- Polarity: d-(-)-fructofuranose was found to be the most polar.
- -
- Unsaturation: The high degree of unsaturation was present in gallic acid and caffeic acid followed by catechin and d-(-)-fructofuranose, respectively.
- -
- Flexibility: d-(-)-fructofuranose showed the most flexibility.
- -
- Lipophilicity and insolubility: The outcomes of all the examined compounds were comparable in these categories.
3. Materials and Methods
3.1. Collection and Identification of the Plant
3.2. Extraction of Secondary Metabolites
3.3. The Yield of Extraction
3.4. Phytochemical Screening
- Test for Flavonoids
- Test for Tannins
- Test for Coumarins
- Test for Alkaloids
- Test for Triterpenes, Sterols and Steroids
- Test for Saponins
- Test for Reducing Sugars
- Test for Anthocyanins
3.5. Determination of Total Phenolics, Total Flavonoids, and Flavonols
3.5.1. Determination of Total Phenolic Content (TPC)
3.5.2. Determination of Total Flavonoid Content
3.5.3. Determination of Total Flavonol Content
3.6. GC-MS Analysis
3.7. Biological Activities Evaluation In Vitro
3.7.1. Antioxidant Ability
3.7.2. Antibacterial Activity
3.8. Statistical Analysis
3.9. In Silico Study
3.9.1. Computational Analysis of Antibacterial Activity
3.9.2. ADMET Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Felidj, M.; Bouazza, M.; Ferouani, T. Short paper about the floristic community and the interest of Ammoides pussila (verticillata), a medicinal plant from Mounts of Tlemcen National Park (Western Algeria). Geo-Eco-Trop 2010, 34, 147–154. [Google Scholar]
- Da Silva, L.P.; Pereira, E.; Pires, T.C.S.P.; Alves, M.J.; Pereira, O.R.; Barros, L.; Ferreira, I.C.F.R. Rubus ulmifolius Schott fruits: A detailed study of its nutritional, chemical and bioactive properties. Food Res. Int. 2019, 119, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Sher, H. Ethnoecological evaluation of some medicinal and aromatic plants of Kot Malakand Agency, Pakistan. Sci. Res. Essays 2011, 6, 2164–2173. [Google Scholar] [CrossRef]
- Ahmad, N.; Anwar, S.; Fazal, H.; Abbasi, B.H. Medicinal plants used in indigenous therapy by people of Madyan Valley in district Swat, Pakistan. Int. J. Med. Arom. Plants 2013, 3, 47–54. [Google Scholar]
- Lemus, I.; Garcıa, R.; Delvillar, E.; Knop, G. Hypoglycaemic Activity of Four Plants Used in Chilean Popular Medicine. Phytother. Res. 1999, 13, 91–94. [Google Scholar] [CrossRef]
- Tabarki, S.; Aouadhi, C.; Mechergui, K.; Hammi, K.M.; Ksouri, R.; Raies, A.; Toumi, L. Comparison of Phytochemical Composition and Biological Activities of Rubus ulmifolius Extracts Originating from Four Regions of Tunisia. Chem. Biodivers. 2017, 14, e1600168. [Google Scholar] [CrossRef]
- Triggiani, D.; Franconi, R. Rubus ulmifolius: Traditional, Current and Future Pharmaceutical Uses. Curr. Tradit. Med. 2018, 4, 192–203. [Google Scholar] [CrossRef]
- Ali, N.; Shaoib, M.; Shah, S.W.; Shah, I.; Shuaib, M. Pharmacological profile of the aerial parts of Rubus ulmifolius Schott. BMC Complement. Altern. Med. 2017, 17, 59. [Google Scholar] [CrossRef]
- Kouamé, T.K.; Siaka, S.; Kassi, A.B.B.; Soro, Y. Détermination des teneurs en polyphénols totaux, flavonoïdes totaux et tanins de jeunes feuilles non encore ouvertes de Piliostigma thonningii (Caesalpiniaceae). Int. J. Biol. Chem. Sci. 2021, 15, 97–105. [Google Scholar] [CrossRef]
- Tlili-Ait Kaki, Y.; Bennadja, S.; Chefrour, A. Revalorisation d’une essence endémique: Le sapin de Numidie (Abies numidica). Fl. Medit. 2013, 23, 123–129. [Google Scholar] [CrossRef]
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 3. [Google Scholar] [CrossRef]
- Akhtar, K.; Shah, S.W.A.; Shah, A.A.; Shoaib, M.; Haleem, S.K.; Sultana, N. Pharmacological effect of Rubus ulmifolius Schott as antihyperglycemic and antihyperlipidemic on streptozotocin (STZ)-induced albino mice. Appl. Biol. Chem. 2017, 60, 411–418. [Google Scholar] [CrossRef]
- Martins, A.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Fernandes, I.P.; Barreiro, F.; Ferreira, I.C.F.R. Phenolic extracts of Rubus ulmifolius Schott flowers: Characterization, microencapsulation and incorporation into yogurts as nutraceutical sources. Food Funct. 2014, 5, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Akkari, H.; Hajaji, S.; B’chir, F.; Rekik, M.; Gharbi, M. Correlation of polyphenolic content with radical-scavenging capacity and anthelmintic effects of Rubus ulmifolius (Rosaceae) against Haemonchus contortus. Vet. Parasitol. 2016, 221, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Desmiaty, Y.; Elya, B.; Saputri, F.C.; Hanafi, M.; Prastiwi, R. Antioxidant Activity of Rubus fraxinifolius Poir. and Rubus rosifolius J. Sm. Leaves. J. Young Pharm. 2018, 10, 93–96. [Google Scholar] [CrossRef]
- Yousefbeyk, F.; Ghasemi, S.; Evazalipour, M.; Dabirian, S.; Schubert, C.; Hekmatnia, S.; Habibi, Y.; Eghbali Koohi, D.; Böhm, V. Phytochemical analysis, antioxidant, antibacterial, and cytotoxic activities of leaves and roots of Rubus hyrcanus Juz. Eur. Food Res. Technol. 2022, 248, 141–152. [Google Scholar] [CrossRef]
- Sehaki, C.; Jullian, N.; Ayati, F.; Fernane, F.; Gontier, E. A Review of Pistacia lentiscus Polyphenols: Chemical Diversity and Pharmacological Activities. Plants 2023, 12, 279. [Google Scholar] [CrossRef]
- Bhatt, S.C.; Naik, B.; Kumar, V.; Kumar Gupta, A.; Kumar, S.; Singh Preet, M.; Sharma, N.; Rustagi, S. Untapped potential of non-conventional Rubus species: Bioactivity, nutrition, and livelihood opportunities. Plant Methods 2023, 19, 114. [Google Scholar] [CrossRef]
- Mawazi, S.M.; Ann, T.J.; Widodo, R.T. Application of Niosomes in Cosmetics: A Systematic Review. Cosmetics 2022, 9, 127. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, B.M.; Sánchez-Moreno, C.; De Ancos, B.; Sánchez-Mata, M.D.C.; Fernández-Ruiz, V.; Cámara, M.; Tardío, J. Wild Arbutus unedo L. and Rubus ulmifolius Schott fruits are underutilized sources of valuable bioactive compounds with antioxidant capacity. Fruits 2014, 69, 435–448. [Google Scholar] [CrossRef]
- Schulz, M.; Tischer Seraglio, S.K.; Della Betta, F.; Nehring, P.; Camargo Valese, A.; Daguer, H.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Blackberry (Rubus ulmifolius Schott): Chemical composition, phenolic compounds and antioxidant capacity in two edible stages. Int. Food Res. 2019, 122, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; D’Addario, C.; Colacevich, A.; Focardi, S.; Borghini, F.; Santucci, A.; Figura, N.; Rossi, C. Antimicrobial activity against Helicobacter pylori strains and antioxidant properties of blackberry leaves (Rubus ulmifolius) and isolated compounds. Int. J. Antimicrob. Agents 2009, 34, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; Da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef]
- Sheng, Y.; Sun, Y.; Tang, Y.; Yu, Y.; Wang, J.; Zheng, F.; Li, Y.; Sun, Y. Catechins: Protective mechanism of antioxidant stress in atherosclerosis. Front. Pharmacol. 2023, 14, 1144878. [Google Scholar] [CrossRef]
- Blonk, B.; Cock, I.E. Interactive antimicrobial and toxicity profiles of Pittosporum angustifolium Lodd. Extracts with conventional antimicrobials. J. Integr. Med. 2019, 17, 261–272. [Google Scholar] [CrossRef]
- Panizzi, L.; Caponi, C.; Catalano, S.; Cioni, P.L.; Morelli, I. In vitro antimicrobial activity of extracts and isolated constituents of Rubus ulmifolius. J. Ethnopharmacol. 2002, 79, 165–168. [Google Scholar] [CrossRef]
- Quave, C.L.; Estévez-Carmona, M.; Compadre, C.M.; Hobby, G.; Hendrickson, H.; Beenken, K.E.; Smeltzer, M.S. Ellagic Acid Derivatives from Rubus ulmifolius Inhibit Staphylococcus aureus Biofilm Formation and Improve Response to Antibiotics. PLoS ONE 2012, 7, e28737. [Google Scholar] [CrossRef]
- Ibba, A.; Piras, A.; Rosa, A.; Maxia, A.; Fais, S.; Orrù, G.; Porcedda, S. Fatty Acid Profile and Antimicrobial Activity of Rubus ulmifolius Schott Extracts Against Cariogenic Bacterium Streptococcus mutans. Biointerface Res. Appl. Chem. 2022, 12, 25–30. [Google Scholar] [CrossRef]
- Da Silva, L.P.; Pereira, E.; Prieto, M.A.; Simal-Gandara, J.; Pires, T.C.S.P.; Alves, M.J.; Calhelha, R.; Barros, L.; Ferreira, I.C.F.R. Rubus ulmifolius Schott as a Novel Source of Food Colorant: Extraction Optimization of Coloring Pigments and Incorporation in a Bakery Product. Molecules 2019, 24, 2181. [Google Scholar] [CrossRef] [PubMed]
- Passos, M.R.; Almeida, R.S.; Lima, B.O.; Rodrigues, J.Z.S.; Macêdo Neres, N.S.; Pita, L.S.; Marinho, P.D.F.; Santos, I.A.; Da Silva, J.P.; Oliveira, M.C.; et al. Anticariogenic activities of Libidibia ferrea, gallic acid and ethyl gallate against Streptococcus mutans in biofilm model. J. Ethnopharmacol. 2021, 274, 114059. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Wei, S.; Su, H.; Zheng, S.; Xu, S.; Liu, M.; Bo, R.; Li, J. Bactericidal activity of gallic acid against multi-drug resistance Escherichia coli. Microb. Pathog. 2022, 173, 105824. [Google Scholar] [CrossRef] [PubMed]
- Kępa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wąsik, T.J. Antimicrobial Potential of Caffeic Acid against Staphylococcus aureus Clinical Strains. Biomed. Res. Int. 2018, 15, 7413504. [Google Scholar] [CrossRef]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs toward Microbial Pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef]
- Wu, M.; Brown, A.C. Applications of Catechins in the Treatment of Bacterial Infections. Pathogens 2021, 10, 546. [Google Scholar] [CrossRef]
- El Sayyed, H.; Le Chat, L.; Lebailly, E.; Vickridge, E.; Pages, C.; Cornet, F.; Lagomarsino, M.C.; Espéli, O. Mapping Topoisomerase IV Binding and Activity Sites on the E. coli Genome. PLoS Genet. 2016, 12, e1006025. [Google Scholar] [CrossRef]
- Stracy, M.; Wollman, A.J.M.; Kaja, E.; Gapinski, J.; Lee, J.E.; Leek, V.A.; McKie, S.J.; Mitchenall, L.A.; Maxwell, A.; Sherratt, D.J.; et al. Single-molecule imaging of DNA gyrase activity in living Escherichia coli. Nucleic Acids Res. 2019, 47, 210–220. [Google Scholar] [CrossRef]
- Shariati, A.; Noei, M.; Askarinia, M.; Khoshbayan, A.; Farahani, A.; Chegini, Z. Inhibitory Effect of Natural Compounds on Quorum Sensing System in Pseudomonas aeruginosa: A Helpful Promise for Managing Biofilm Community. Front. Pharmacol. 2024, 15, 1350391. [Google Scholar] [CrossRef]
- Kurnia, D.; Ramadhanty, Z.F.; Ardani, A.M.; Zainuddin, A.; Dharsono, H.D.A.; Satari, M.H. Bio-Mechanism of Catechin as Pheromone Signal Inhibitor: Prediction of Antibacterial Agent Action Mode by In Vitro and In Silico Study. Molecules 2021, 26, 6381. [Google Scholar] [CrossRef]
- Gradišar, H.; Pristovšek, P.; Plaper, A.; Jerala, R. Green Tea Catechins Inhibit Bacterial DNA Gyrase by Interaction with Its ATP Binding Site. J. Med. Chem. 2007, 50, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.B.; Segretti, M.C.F.; Polli, M.C.; Parise-Filho, R. Analysis of the Applicability and Use of Lipinski`s Rule for Central Nervous System Drugs. Lett. Drug. Des. Discov. 2016, 13, 999–1006. [Google Scholar] [CrossRef]
- Benouchenne, D.; Bellil, I.; Tachour, S.H.; Akkal, S.; Djeghim, H.; Kebaili, F.F.; Nieto, G.; Khelifi, D. Tyrosinase Inhibitory Ability and In Vitro, In Vivo Acute Oral and In Silico Toxicity Evaluation of Extracts Obtained from Algerian Fir (Abiesnumidica de Lannoy ex CARRIERE) Needles. Plants 2022, 11, 2389. [Google Scholar] [CrossRef] [PubMed]
- Bendjedid, S.; Benouchenne, D. In silico studies for assessing physicochemical, pharmacokinetic and cytotoxicity properties of bioactive molecules identified by LC-MS in Aloe vera leaves extracts. S. Afr. J. Bot. 2023, 157, 75–81. [Google Scholar] [CrossRef]
- Fan, J.; De Lannoy, I.A.M. Pharmacokinetics. Biochem. Pharmacol. 2014, 87, 93–120. [Google Scholar] [CrossRef]
- Shin, S.; Kim, N.S.; Kim, Y.A.; Oh, H.R.; Bang, O.S. Effect of the Phragmitis Rhizoma Aqueous Extract on the Pharmacokinetics of Docetaxel in Rats. Comb. Chem. High Throughput Screen. 2019, 22, 326–332. [Google Scholar] [CrossRef]
- Veber, D.F.; Stephen, R.J.; Hung, Y.C.; Smith, B.R.; Ward, K.W.; Kenneth, D.K. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef]
- Kleczka, A.; Dzik, R.; Kabała-Dzik, A. Caffeic Acid Phenethyl Ester (CAPE) Synergistically Enhances Paclitaxel Activity in Ovarian Cancer Cells. Molecules 2023, 28, 5813. [Google Scholar] [CrossRef]
- Yang, J.T.; Lee, I.N.; Chen, C.H.; Lu, F.J.; Chung, C.Y.; Lee, M.H.; Cheng, Y.C.; Chen, K.T.; Peng, J.Y.; Chen, C.H. Gallic Acid Enhances the Anti-Cancer Effect of Temozolomide in Human Glioma Cell Line via Inhibition of Akt and p38-MAPK Pathway. Processes 2022, 10, 448. [Google Scholar] [CrossRef]
- Paolini, A.; Curti, V.; Pasi, F.; Mazzini, G.; Nano, R.; Capelli, E. Gallic acid exerts a protective or an anti-proliferative effect on glioma T98G cells via dose-dependent epigenetic regulation mediated by miRNAs. Int. J. Oncol. 2015, 46, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Jafarinejad, S.; Martin, W.H.C.; Abu Ras, B.; Isreb, M.; Jacob, B.; Aziz, A.; Adoul, Z.; Lagnado, R.; Bowen, R.D.; Najafzadeh, M. The anticancer/cytotoxic effect of a novel gallic acid derivative in non-small cell lung carcinoma A549 cells and peripheral blood mononuclear cells from healthy individuals and lung cancer patients. BioFactors 2024, 50, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Varela-Rodríguez, L.; Talamás-Rohana, P.; Sánchez-Ramírez, B.; Hernández-Ramírez, V.I.; Varela-Rodríguez, H. Cytotoxic Activity of Gallic Acid and Myricetin against Ovarian Cancer Cells by Production of Reactive Oxygen Species. Biol. Life Sci. Forum 2021, 7, 7. [Google Scholar] [CrossRef]
- Moghtaderi, H.; Sepehri, H.; Delphi, L.; Attari, F. Gallic acid and curcumin induce cytotoxicity and apoptosis in human breast cancer cell MDA-MB-231. BioImpacts 2018, 8, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Meneses-Gutiérrez, C.L.; Hernández-Damián, J.; Pedraza-Chaverri, J.; Guerrero-Legarreta, I.; Téllez, D.I.; Jaramillo-Flores, M.E. Antioxidant Capacity and Cytotoxic Effects of Catechins and Resveratrol Oligomers Produced by Enzymatic Oxidation against T24 Human Urinary Bladder Cancer Cells. Antioxidants 2019, 8, 214. [Google Scholar] [CrossRef]
- Zatout, R.; Kacem Chaouche, N. Antibacterial activity screening of an edible mushroom Agaricus litoralis. Int. J. Botany Stud. 2023, 8, 49–52. [Google Scholar]
- Yakoubi, R.; Benouchenne, D.; Bendjedid, S.; Megateli, S.; Hadj Sadok, T.; Gali, L.; Azri, O. Chemical constituents and in vitro biological activities of Mentha rotundifolia’s essential oils extracted by ultrasound-assisted hydrodistillation compared to conventional hydrodistillation. Biomass Conv. Bioref. 2024, 1–13. [Google Scholar] [CrossRef]
- Benouchenne, D.; Bellil, I.; Akkal, S.; Khelifi, D. Investigation of Phytochemical and Evaluation of Antioxidant and Antibacterial Activities from Abies Extract. Sci. J. King Faisal Univ. 2021, 22, 26–32. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Topçu, G.; Ay, M.; Bilici, A.; Sarıkürkcü, C.; Öztürk, M.; Ulubelen, A. A new flavone from antioxidant extracts of Pistacia terebinthus. Food Chem. 2007, 103, 816–822. [Google Scholar] [CrossRef]
- Kumaran, A.; Joel Karunakaran, R. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT-Food Sci. Technol. 2007; 40, 344–352. [Google Scholar] [CrossRef]
- Guida, M.; Salvatore, M.M.; Salvatore, F.A. strategy for GC/MS quantification of polar compounds via their silylated surrogates: Silylation and quantification of biological amino acids. J. Anal. Bioanal. Tech. 2015, 6, 1–16. [Google Scholar] [CrossRef]
- NIST 20. Available online: https://www.nist.gov/srd/nist-standard-reference-database-1a (accessed on 15 October 2024).
- Tel, G.; Apaydın, M.; Duru, M.E.; Öztürk, M. Antioxidant and Cholinesterase Inhibition Activities of Three Tricholoma Species with Total Phenolic and Flavonoid Contents: The Edible Mushrooms from Anatolia. Food Anal. Methods 2012, 5, 495–504. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Szydłowska-Czerniak, A.; Dianoczki, C.; Recseg, K.; Karlovits, G.; Szłyk, E. Determination of antioxidant capacities of vegetable oils by ferric-ion spectrophotometric methods. Talanta 2008, 76, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on Products of Browning Reactions: Antioxidative Activities of Product of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Bramki, A.; Benslama, O.; Rahim, N.; Ghorri, S.; Bouchair, M.; Mameri, B. Antimicrobial potential of Aspergillus fumigatiaffinis and A. sclerotiorum: Insights from in vitro and molecular docking investigations. Not. Sci. Biol. 2024, 16, 11862. Available online: https://www.notulaebiologicae.ro/index.php/nsb/index (accessed on 20 May 2024). [CrossRef]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi. J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef]
- Dassamiour, S.; Bensaad, M.S.; Hambaba, L.; Melakhessou, M.A.; Sami, R.; Al-Mushin, A.A.M.; Aljahani, A.H.; Masoudi, M.A. In Silico Investigation of Some Compounds from the N-Butanol Extract of Centaurea tougourensis Boiss. & Reut. Crystal 2022, 12, 355. [Google Scholar] [CrossRef]
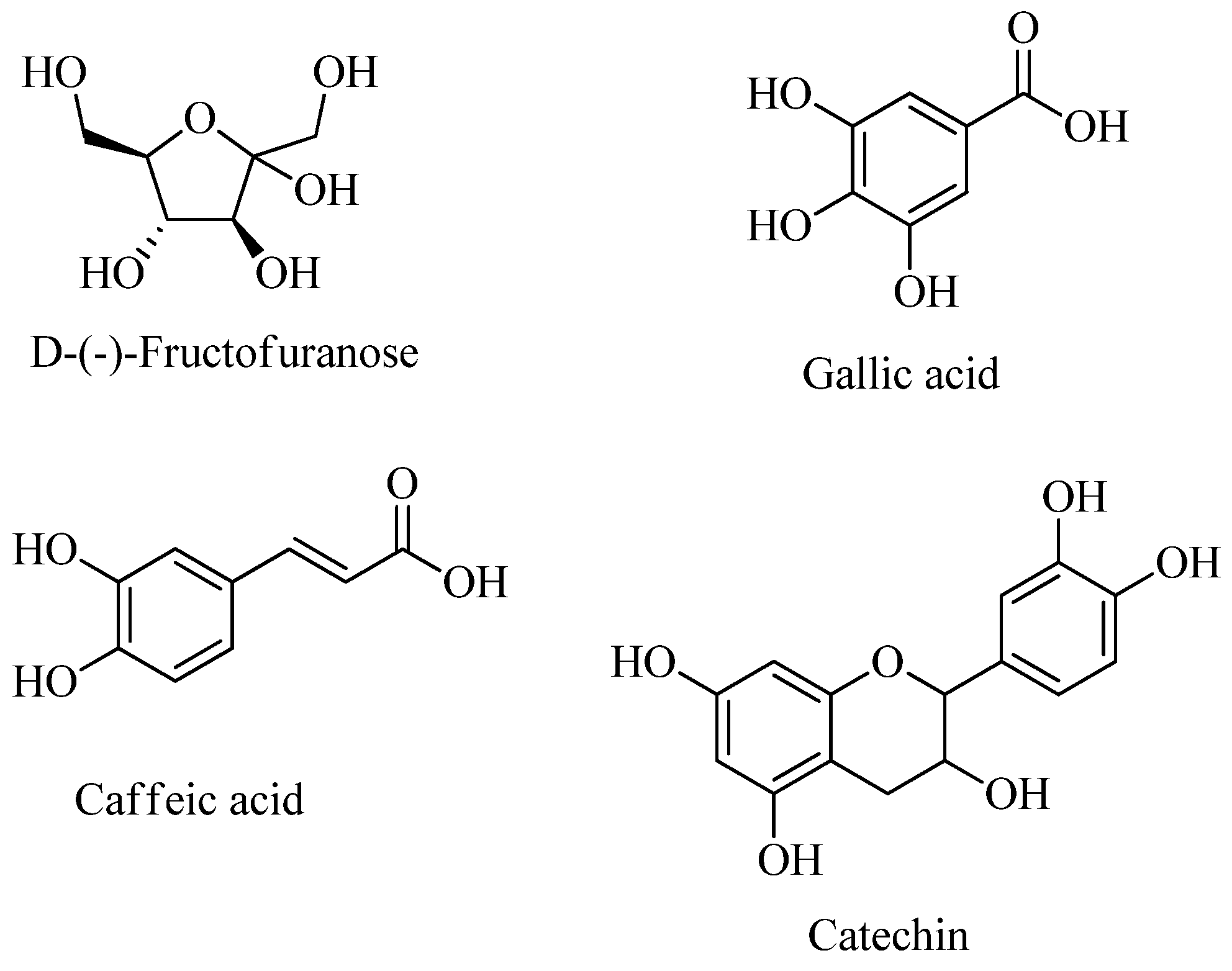
| Phytochemicals | Tannins | Flavonoids | Alkaloids | Coumarins | Sterols and Steroids | Triterpenes | Saponins | Reducing Sugars | Anthocyanins |
|---|---|---|---|---|---|---|---|---|---|
| Results | +++ | + | − | + | − | − | + | − | + |
| Total Phenolic Content (µg GAE/mg) | Flavonoid Content (µg QE/mg) | Flavonol Content (µg QE/mg) |
|---|---|---|
| 523.25 ± 3.53 | 20.41 ± 1.80 | 9.62 ± 0.51 |
| Compound | RT | RI |
|---|---|---|
| d-(-)-Fructofuranose, 5TMS | 12.669 | 1831 |
| Gallic acid, 4TMS | 13.28 | 1974 |
| Caffeic acid, 3TMS | 13.916 | 2148 |
| Catechin, 5TMS | 18.28 | 2900 |
| IC50 (µg/mL) | A0.5 (µg/mL) | |||
|---|---|---|---|---|
| DPPH• | ABTS•+ | Phenantroline | FRAP | |
| EtOAc | 98.82 ± 1.01 b | 4.20 ± 0.13 b | 21.22 ± 0.59 c | 94.09 ± 1.40 b |
| Trolox | 5.14 ± 0.09 a | 3.27 ± 0.17 a | 5.21 ± 0.07 b | 5.43 ± 0.31 a |
| Ascorbic acid | 4.40 ± 0.10 a | 3.07 ± 0.05 a | 3.08 ± 0.05 a | 3.76 ± 0.23 a |
| Strains Tested | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | K. pneumoniae | S. typhimurium | ||||||
| Inhibition Zone | MIC | Inhibition Zone | MIC | Inhibition Zone | MIC | Inhibition Zone | MIC | Inhibition Zone | MIC | |
| EtOAc | 22 ± 0.6 b | 0.78 | 24.5 ± 0.3 a | 0.78 | 21 ± 0.2 c | 0.78 | 20 ± 0.5 d | 3.12 | 20.2 ± 0.4 b | 0.78 |
| Gentamicin | 18 ± 0.5 f | − | 19.1 ± 0.8 e | − | 16.2 ± 0.9 g | − | 10.0 ± 0.2 j | − | 15.5 ± 0.7 h | − |
| Binding Energy (Kcal/mol) | Hydrogen Interactions (Distance Å) | Hydrophobic Interactions | |||
|---|---|---|---|---|---|
| 3G7E | Co-crystallized ligand | B46 | −10.7579 | Asp73 (1.69), Asn56 (1.88), Arg136 (2.46), Gly102 (2.82), Gly77 (2.60), Arg76 (3.05), Asp49 (2.52) | Val43, Val120, Leu130, Val167, Met95, Ile94, His116, Ala47, Ile78, Pro79 |
| Best docked compound | Catechin | −5.6915 | Asn46 (2.71) | Ala47, Ile78 | |
| 3G75 | Co-crystallized ligand | B48 | −6.1928 | Asp81 (1.82), Ser55 (3.39) | Ile175, Val79, Ile51, Ile86, Gly85, Asn54 |
| Best docked compound | Catechin | −6.1220 | Gly85 (2.21), Asp81 (2.80), Asp81 (2.95) | Ale102, Ile86 | |
| 5EIX | Co-crystallized ligand | LFX | −6.3059 | Ser1080 (2.90), Lys444 (2.73), Lys442 (1.83) | - |
| Best docked compound | Catechin | −3.6767 | Glu1084 (2.63), Gly1078 (2.54) | Asp495, Ala1081, His1077 | |
| 6J90 | Co-crystallized ligand | ATP | −10.3302 | Asp73 (1.84), Gly102 (1.90), Ser121 (2.76), Val118 (1.66), His116 (2.40), Leu115 (1.73), Gly119 (2.88), Gly117 (2.64), val120 (1.77), Asn46 (1.80) | Ile78 |
| Best docked compound | Catechin | −7.0767 | Ans46 (2.37), Asp73 (1.96), Gly102 (2.95) | Ile78, Ile94, Val120 | |
| 4DDQ | Best docked compound | Catechin | −6.3718 | Ile183 (1.96), Gnl267 (2.72), Tyr99 (2.87), Arg92 (3.43), Gly41 (1.80) | Ala171, Lys43, His46, Ala172 |
| Compound | Pa | Pi | Cell-Line (CL) | CL Full Name | Tissue | Tumor Type |
|---|---|---|---|---|---|---|
| d-(-)-Fructofuranose | 0.551 | 0.005 | CFPAC-1 | Pancreatic carcinoma | Pancreas | Carcinoma |
| 0.520 | 0.004 | SH-SY5Y | Bone marrow neuroblastoma | Brain | Neuroblastoma | |
| Gallic acid | 0.591 | 0.028 | Hs 683 | Oligodendroglioma | Glioma | |
| Caffeic acid | 0.607 | 0.010 | IGROV-1 | Ovarian adenocarcinoma | Ovarium | Adenocarcinoma |
| 0.538 | 0.021 | K562 | Erythroleukemia | Hematopoietic and lymphoid tissue | Leukemia | |
| Catechin | 0.556 | 0.006 | NCI-H187 | Small cell lung carcinoma | Lung | Carcinoma |
| 0.527 | 0.048 | Hs 683 | Oligodendroglioma | Brain | Glioma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bramki, A.; Benouchenne, D.; Salvatore, M.M.; Benslama, O.; Andolfi, A.; Rahim, N.; Moussaoui, M.; Ramoul, S.; Nessah, S.; Barboucha, G.; et al. In Vitro and In Silico Biological Activities Investigation of Ethyl Acetate Extract of Rubus ulmifolius Schott Leaves Collected in Algeria. Plants 2024, 13, 3425. https://doi.org/10.3390/plants13233425
Bramki A, Benouchenne D, Salvatore MM, Benslama O, Andolfi A, Rahim N, Moussaoui M, Ramoul S, Nessah S, Barboucha G, et al. In Vitro and In Silico Biological Activities Investigation of Ethyl Acetate Extract of Rubus ulmifolius Schott Leaves Collected in Algeria. Plants. 2024; 13(23):3425. https://doi.org/10.3390/plants13233425
Chicago/Turabian StyleBramki, Amina, Djamila Benouchenne, Maria Michela Salvatore, Ouided Benslama, Anna Andolfi, Noureddine Rahim, Mohamed Moussaoui, Sourore Ramoul, Sirine Nessah, Ghozlane Barboucha, and et al. 2024. "In Vitro and In Silico Biological Activities Investigation of Ethyl Acetate Extract of Rubus ulmifolius Schott Leaves Collected in Algeria" Plants 13, no. 23: 3425. https://doi.org/10.3390/plants13233425
APA StyleBramki, A., Benouchenne, D., Salvatore, M. M., Benslama, O., Andolfi, A., Rahim, N., Moussaoui, M., Ramoul, S., Nessah, S., Barboucha, G., Bensouici, C., Cimmino, A., Zorrilla, J. G., & Masi, M. (2024). In Vitro and In Silico Biological Activities Investigation of Ethyl Acetate Extract of Rubus ulmifolius Schott Leaves Collected in Algeria. Plants, 13(23), 3425. https://doi.org/10.3390/plants13233425











