Inhibitory Effects and Composition Analysis of Romanian Propolis: Applications in Organic and Sustainable Agriculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Propolis Samples
2.2. Cereal Samples
2.3. Physico-Chemical Analysis
2.3.1. Grain Analysis
2.3.2. Propolis Analysis
2.3.3. Obtaining the Aqueous Extract of Propolis
2.3.4. HPLC Analysis
2.4. The Phyto-Inhibitory Activity of Propolis
2.5. Antifungal Activity of Propolis
2.5.1. Fungal Cultures
2.5.2. Antifungal Properties of the Aqueous Propolis Extracts—Agar Disk-Diffusion Method
2.5.3. Minimal Inhibitory Concentration (MIC)
2.5.4. Minimal Fungicidal Concentration (MFC)
2.6. Statistical Analysis
3. Results
3.1. Physico-Chemical Analysis of the Propolis Samples
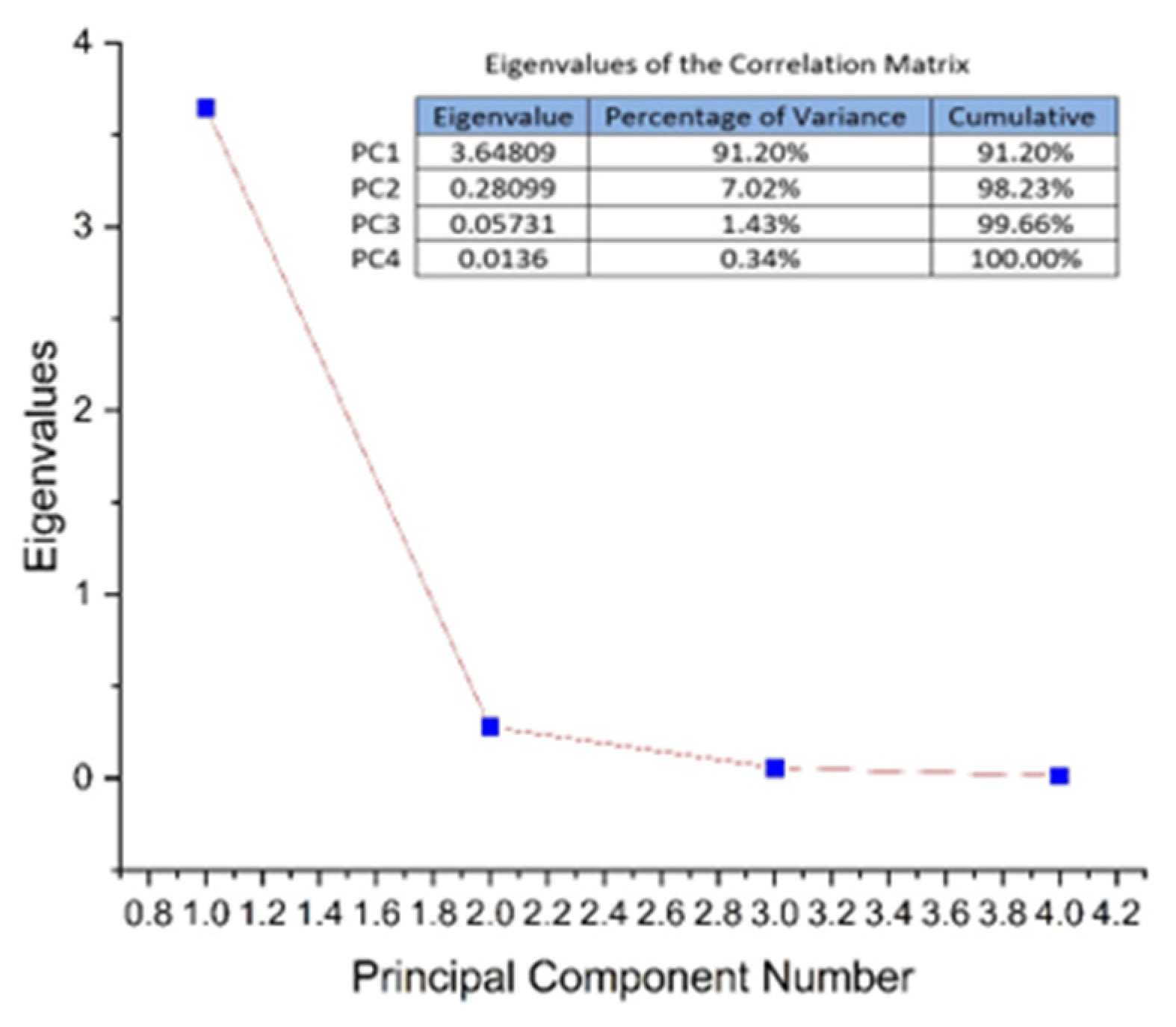
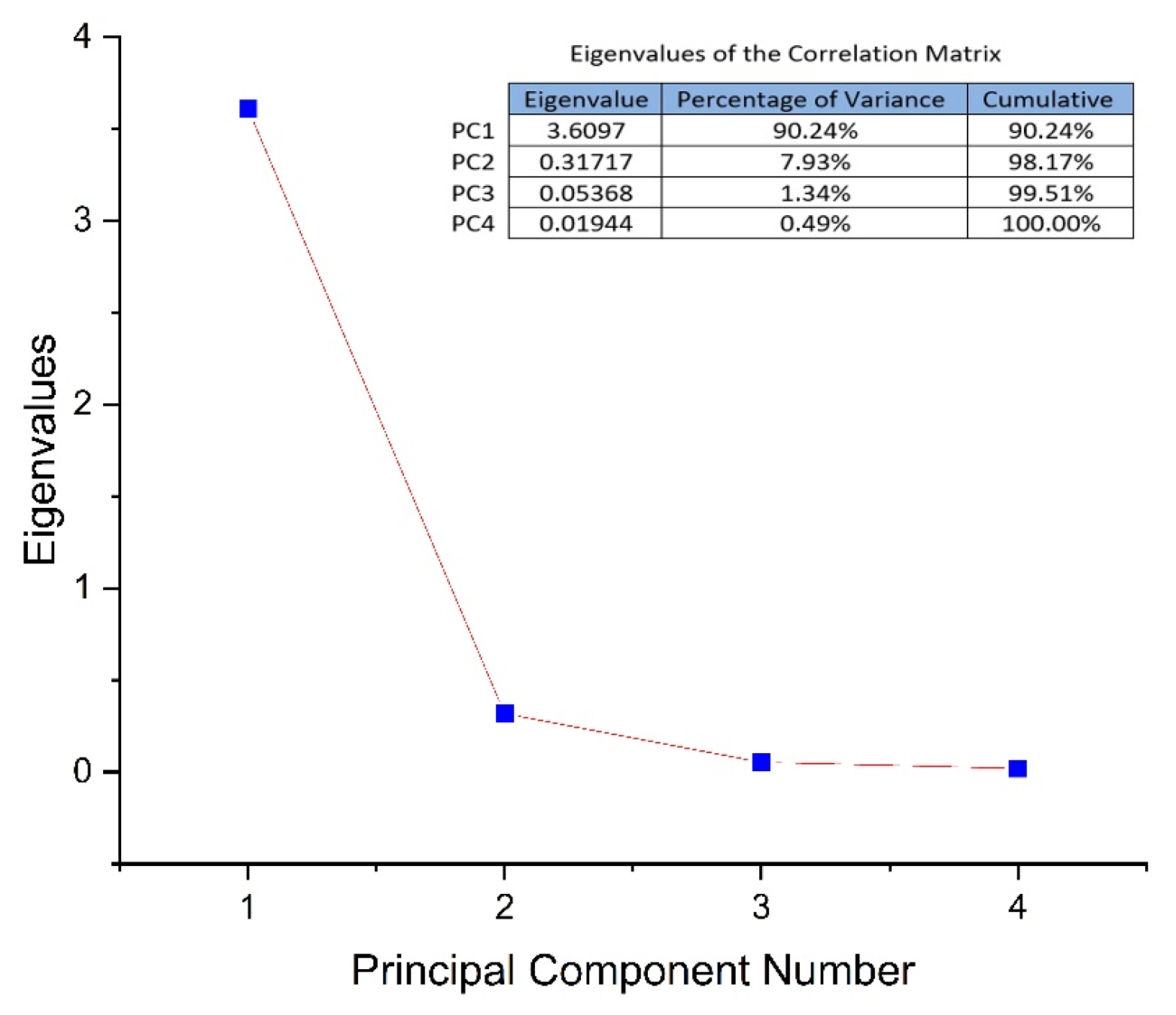
3.2. HPLC Analysis
3.3. Physico-Chemical Analysis of the Cereals
3.4. The Phyto-Inhibitory Activity of Propolis
3.5. Antifungal Activity of Propolis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malekahmadi, M.; Pahlavani, N.; Heshmati, J.; Clayton, Z.S.; Beigmohammadi, M.T.; Navashenaq, J.G.; Alavi-Naeini, A. Effect of Propolis Supplementation on Oxidative Stress Markers: A Systematic Review of Randomized Controlled Trials. J. Herb. Med. 2023, 40, 100679. [Google Scholar] [CrossRef]
- Wagh, V.D. Propolis: A Wonder Bees Product and Its Pharmacological Potentials. Adv. Pharmacol. Sci. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tsagkarakis, A.E.; Katsikogianni, T.; Gardikis, K.; Katsenios, I.; Spanidi, E.; Balotis, G.N. Comparison of Traps Collecting Propolis by Honey Bees. Adv. Entomol. 2017, 5, 68–74. [Google Scholar] [CrossRef][Green Version]
- Dezmirean, D.S.; Paşca, C.; Moise, A.R.; Bobiş, O. Plant Sources Responsible for the Chemical Composition and Main Bioactive Properties of Poplar-Type Propolis. Plants 2020, 10, 22. [Google Scholar] [CrossRef]
- Rivera-Yañez, N.; Rivera-Yañez, C.R.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Reyes-Reali, J.; Mendoza-Ramos, M.I.; Méndez-Cruz, A.R.; Nieto-Yañez, O. Effects of Propolis on Infectious Diseases of Medical Relevance. Biology 2021, 10, 428. [Google Scholar] [CrossRef]
- Almuhayawi, M.S. Propolis as a novel antibacterial agent. Saudi J. Biol. Sci. 2020, 27, 3079–3086. [Google Scholar] [CrossRef]
- Kasote, D.M.; Sharbidre, A.A.; Kalyani, D.C.; Nandre, V.S.; Lee, J.H.J.; Ahmad, A.; Telke, A.A. Propolis: A Natural Antibiotic to Combat Multidrug-Resistant Bacteria. In Non-traditional Approaches to Combat Antimicrobial Drug Resistance; Springer Nature: Singapore, 2023; pp. 281–296. [Google Scholar]
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical Aspects of Propolis Research in Modern Times. Evid. -Based Complement. Altern. Med. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D.; et al. Propolis: An update on its chemistry and pharmacological applications. Chin. Med. 2022, 17, 1–60. [Google Scholar] [CrossRef]
- Chuttong, B.; Lim, K.; Praphawilai, P.; Danmek, K.; Maitip, J.; Vit, P.; Wu, M.-C.; Ghosh, S.; Jung, C.; Burgett, M.; et al. Explor-639 ing the Functional Properties of Propolis, Geopropolis, and Cerumen, with a Special Emphasis on Their Antimicrobial Ef-640 fects. Foods 2023, 12, 3909. [Google Scholar] [CrossRef]
- Li, Y.-J.; Xuan, H.-Z.; Shou, Q.-Y.; Zhan, Z.-G.; Lu, X.; Hu, F.-L. Therapeutic effects of propolis essential oil on anxiety of restraint-stressed mice. Hum. Exp. Toxicol. 2011, 31, 157–165. [Google Scholar] [CrossRef]
- Woźniak, M.; Sip, A.; Mrówczyńska, L.; Broniarczyk, J.; Waśkiewicz, A.; Ratajczak, I. Biological Activity and Chemical Composition of Propolis from Various Regions of Poland. Molecules 2022, 28, 141. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Sagi, S.; Masoodi, M.H.; Bae, J.Y.; Wali, A.F.; Khan, I.A. Quantification and Characterization of Phenolic Compounds from Northern Indian Propolis Extracts and Dietary Supplements. J. AOAC Int. 2020, 103, 1378–1393. [Google Scholar] [CrossRef] [PubMed]
- Altuntaş, Ü.; Güzel, İ.; Özçelik, B. Phenolic Constituents, Antioxidant and Antimicrobial Activity and Clustering Analysis of Propolis Samples Based on PCA from Different Regions of Anatolia. Molecules 2023, 28, 1121. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.; Popova, M.; Trusheva, B. Propolis volatile compounds: Chemical diversity and biological activity: A review. Chem. Cent. J. 2014, 8, 28. [Google Scholar] [CrossRef]
- Li, B.; Wei, K.; Yang, S.; Yang, Y.; Zhang, Y.; Zhu, F.; Wang, D.; Zhu, R. Immunomodulatory effects of Taishan Pinus massoniana pollen polysaccharide and propolis on immunosuppressed chickens. Microb. Pathog. 2015, 78, 7–13. [Google Scholar] [CrossRef]
- Baltas, N.; Yildiz, O.; Kolayli, S. Inhibition properties of propolis extracts to some clinically important enzymes. J. Enzym. Inhib. Med. Chem. 2016, 31 (Suppl. 1), 52–55. [Google Scholar] [CrossRef]
- Tyszka-Czochara, M.; Paśko, P.; Reczyński, W.; Szlósarczyk, M.; Bystrowska, B.; Opoka, W. Zinc and Propolis Reduces Cytotoxicity and Proliferation in Skin Fibroblast Cell Culture: Total Polyphenol Content and Antioxidant Capacity of Propolis. Biol. Trace Elem. Res. 2014, 160, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Roman, A.; Madras-Majewska, B.; Popiela, E. Comparative study of selected toxic elements in propolis and honey. J. Apic. Sci. 2011, 55, 97–106. [Google Scholar]
- Zullkiflee, N.; Taha, H.; Usman, A. Propolis: Its Role and Efficacy in Human Health and Diseases. Molecules 2022, 27, 6120. [Google Scholar] [CrossRef] [PubMed]
- EUGreenDeal. Farm to Fork Strategy. For a Fair, Healthy and Environmentally-Friendly Food System; European Union: Brussels, Belgium, 2020. [Google Scholar]
- Carvalho, G.J.L.D.; Sodré, G.d.S. Application of propolis in agriculture. Arq. Inst. Biol. 2021, 88, e0632019. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M. Propolis: Harnessing Nature’s Hidden Treasure for Sustainable Agriculture. Agrochemicals 2023, 2, 581–597. [Google Scholar] [CrossRef]
- Moraes, W.B.; Jesus Junior, W.C.d.; Belan, L.L.; Peixoto, L.d.A.; Pereira, A.J. Aplicação foliar de fungicidas e produtos alternativos reduz a severidade do oídio do tomateiro. Nucleus 2011, 8, 57–68. [Google Scholar] [CrossRef]
- Pereira, C.S.; Souza, F.L.F.; Godoy, C.A. Extrato etanólico de própolis no controle da cercosporiose e no desenvolvimento de mudas de cafeeiro. Rev. Bras. De Agroecol. 2013, 8, 170–178. [Google Scholar]
- Pereira, C.S.; Matte, W.D.; Venâncio, P.H.B. Aplicação de extrato de própolis na agricultura. R. Cienc. Agro-Ambient. 2016, 14, 143–156. [Google Scholar]
- Cassiano, S.P.; Rodrigo, P.d.A.; Idelfonsa, B.Z.; Camila, B.R.; Everton, V.Z. Application of ethanolic extract of propolis typified in nutrition and vegetative growth of beans. Afr. J. Agric. Res. 2018, 13, 21–26. [Google Scholar] [CrossRef]
- Pereira, C.S.; Maia, L.F.P.; Paula, F.S.d. Aplicação de extrato etanólico de própolis no crescimento e produtividade do feijoeiro comum. Rev. Ceres 2014, 61, 98–104. [Google Scholar] [CrossRef]
- Machado, P.P.; Vieira, G.H.d.C.; Machado, R.A. Uso da Própolis e Óleo de Nim no Controle dos Fungos Lasiodiplodia Theobromae e Colletotrichum Gloesporioides: Principais Patógenos Que Acometem Os Frutos da Manga. J. Neotrop. Agric. 2015, 2, 31–37. [Google Scholar] [CrossRef][Green Version]
- Marini, D.; Mensch, R.; Freiberger, M.B.; Dartora, J.; Franzener, G.; Garcia, R.C.; Stangarlin, J.R. Efeito antifúngico de extratos alcoólicos de própolis sobre patógenos da videira. Arq. Inst. Biol. 2012, 79, 305–308. [Google Scholar] [CrossRef]
- Rosana Wuaden, C.; Gaio, I.; Sperhacke, T.; Paulo Barro, J.; Mendes Milanesi, P. Atividade Antifúngica do Extrato Alcoólico de Própolis, Álcool de Cereais e do Óleo Essencial de Manjericão Sobre Botrytis Cinerea. Colloq. Agrar. 2018, 14, 48–55. [Google Scholar] [CrossRef][Green Version]
- Irigoiti, Y.; Navarro, A.; Yamul, D.; Libonatti, C.; Tabera, A.; Basualdo, M. The use of propolis as a functional food ingredient: A review. Trends Food Sci. Technol. 2021, 115, 297–306. [Google Scholar] [CrossRef]
- Mateescu, C.; Dumitru, I.F. Propolisul şi Extractele de Propolis. Available online: https://apiardeal.ro/biblioteca/carti/Romanesti/Propolisul_si_extractele_de_propolis_-_52_pag.pdf (accessed on 31 March 2024).
- Farooqui, T.; Farooqui, A. Molecular Mechanism Underlying the Therapeutic Activities of Propolis: A Critical Review. Curr. Nutr. Food Sci. 2010, 6, 186–199. [Google Scholar] [CrossRef]
- Veiga, R.S.; De Mendonça, S.; Mendes, P.B.; Paulino, N.; Mimica, M.J.; Lagareiro Netto, A.A.; Lira, I.S.; Lopez, B.G.; Negrao, V.; Marcucci, M.C. Artepillin C and phenolic compounds responsible for antimicrobial and antioxidant activity of green propolis and Baccharis dracunculifolia DC. J. Appl. Microbiol. 2017, 122, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Lopez, B.G.-C.; de Lourenco, C.C.; Alves, D.A.; Machado, D.; Lancellotti, M.; Sawaya, A.C.H.F. Antimicrobial and cytotoxic activity of red propolis: An alert for its safe use. J. Appl. Microbiol 2015, 119, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, propolis, and royal jelly: A comprehensive review of their biological actions and health benefits. Oxid. Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef]
- Haddadin, M.S.Y.; Nazer, I.; Raddad, S.J.A.; Robinson, R.K. Effect of propolis on two bacterial species with probiotic potential. Pak. J. Nutr. 2008, 7, 391–394. [Google Scholar] [CrossRef]
- Rahman, M.M.; Richardson, A.; Sofian-Azirun, M. Antibacterial activity of propolis and honey against Staphylococcus aureus and Escherichia coli. Afr. J. Microbiol. Res. 2010, 4, 1872–1878. [Google Scholar]
- Hassanien, A.A.; Shaker, E.M.; El-Sharkawy, E.E.; Elsherif, W.M. Antifungal and antitoxin effects of propolis and its nanoemulsion formulation against Aspergillus flavus isolated from human sputum and milk powder samples. Vet. World 2021, 14, 2306–2312. [Google Scholar] [CrossRef] [PubMed]
- Gniewosz, M.; Pobiega, K.; Kraśniewska, K.; Synowiec, A.; Chaberek, M.; Galus, S. Characterization and Antifungal Activity of Pullulan Edible Films Enriched with Propolis Extract for Active Packaging. Foods 2022, 11, 2319. [Google Scholar] [CrossRef]
- Marghitas, L.A.; Mihai, C.M.; Chirila, F.; Dezmirean, D.S.; Fit, N.I. The Study of the Antimicrobial Activity of Transylvanian (Romanian) Propolis. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 40–44. [Google Scholar]
- Stan, T.; Teodor, E.D.; Gatea, F.; Chifiriuc, C.M.; Lazăr, V. Antioxidant and antifungal activity of Romanian propolis. Rom. Biotechnol. Lett. 2017, 22, 13116–13124. [Google Scholar]
- de W. Blackburn, C.; McClure, P.J. Foodborne Pathogens. Hazards, Risk Analysis and Control, 2nd ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2009. [Google Scholar]
- Romanian Standards Association. SR (Romanian Standard); Cereals and Legumes. Determination of the Mass of 1000 Grains; National Organization for Standardization—ASRO: Bucharest, Romania, 2002; 520/2002. [Google Scholar]
- Romanian Standards Association. SR (Romanian Standard); Agricultural Seeds. Wheat. Determination of Vitreousity; National Organization for Standardization—ASRO: Bucharest, Romania, 1984; 6283–2/84. [Google Scholar]
- Popescu, N.; Meica, S. Bee Products and Their CHEMICAL Analysis (Honey, Wax, Royal Jelly, Pollen, Bee Bread, Propolis, and Venom); Diacon Coresi: București, Romania, 1997. (In Romanian) [Google Scholar]
- Mărghitaş, L.A. Bees and Their Products (In Romanian); Ceres Publishing: Bucharest, Romania, 2008. [Google Scholar]
- AOAC International. Official Methods of Analysis Program. Available online: https://www.aoac.org/scientific-solutions/standards-and-official-methods/ (accessed on 19 February 2024).
- Norma Argentina. IRAM-INTA. Productos del NOA. Propóleos en Bruto; Instituto Argentino de Normalización y Certificación: Buenos Aires, Argentina, 2008; 15935-1. [Google Scholar]
- Bogdanov, S. Harmonised Methods of the International Honey Commission; Swiss Bee Research Centre, FAM: Liebefeld, Switzeland, 2009; p. 63. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Oxid. Antioxid. Part A 1999, 299, 152–178. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2020, 10, 178–182. [Google Scholar] [CrossRef]
- Mărghitas, L.A.; Dezmirean, D.; Moise, A.; Mihai, C.; Laslo, L. DPPH method for evaluation of propolis antioxidant activity. Bull. Univ. Agric. Sci. 2009, 66, 253–258. [Google Scholar]
- Popova, M.; Bankova, V.; Butovska, D.; Petkov, V.; Nikolova-Damyanova, B.; Sabatini, A.G.; Marcazzan, G.L.; Bogdanov, S. Validated methods for the quantification of biologically active constituents of poplar type propolis. Phytochem. Anal. 2004, 15, 235–240. [Google Scholar] [CrossRef]
- Biron, R.C.; Heghedűş-Mîndru, G.; Hădărugă, N.G.; Jianu, C.; Ştef, D.; Jianu, I. Activitatea fitoinhibitorie a propolisului recoltat din zona de vest a României—partea I, Universitatea de Ştiinţe Agricole şi Medicină Veterinară Iaşi. Lucrări Ştiinţifice Ser. Agron. 2007, 50, 462–467. (In Romanian) [Google Scholar]
- Vica, M.L.; Glevitzky, I.; Glevitzky, M.; Siserman, C.V.; Matei, H.V.; Teodoru, C.A. Antibacterial Activity of Propolis Extracts from the Central Region of Romania against Neisseria gonorrhoeae. Antibiotics 2021, 10, 689. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standard Institute. Performance Standards for Antimicrobial Susceptibsility Testing. 2020. Available online: https://clsi.org/media/3481/m100ed30_sample.pdf (accessed on 7 March 2024).
- The European Committee on Antimicrobial Susceptibility Testing—EUCAST. EUCAST Guidance Documents. Available online: https://www.eucast.org/eucastguidancedocuments (accessed on 7 March 2024).
- Vică, M.L.; Glevitzky, M.; Heghedűş-Mîndru, R.C.; Glevitzky, I.; Matei, H.V.; Balici, S.; Popa, M.; Teodoru, C.A. Potential Effects of Romanian Propolis Extracts against Pathogen Strains. Int. J. Environ. Res. Public Health 2022, 19, 2640. [Google Scholar] [CrossRef]
- Okińczyc, P.; Szumny, A.; Szperlik, J.; Kulma, A.; Franiczek, R.; Żbikowska, B.; Krzyżanowska, B.; Sroka, Z. Profile of Polyphenolic and Essential Oil Composition of Polish Propolis, Black Poplar and Aspens Buds. Molecules 2018, 23, 1262. [Google Scholar] [CrossRef] [PubMed]
- Imdorf, A.; Bogdanov, S.; Ochoa, R.I.; Calderone, N.W. Use of essential oils for the control of Varroa jacobsoni Oud. in honey bee colonies. Apidologie 1999, 30, 209–228. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Lyoussi, B.; Miguel, M.G.; Figueiredo, A.C. Characterization of volatiles from Moroccan propolis samples. J. Essent. Oil Res. 2018, 31, 27–33. [Google Scholar] [CrossRef]
- Grassi, G.; Capasso, G.; Gambacorta, E.; Perna, A.M. Chemical and Functional Characterization of Propolis Collected from Different Areas of South Italy. Foods 2023, 12, 3481. [Google Scholar] [CrossRef] [PubMed]
- Zarate, M.; Juárez, M.; García, A.; Ozuna, C.; Gutierrez-Chavez, A.; Segoviano-Garfias, J.; Ramos, F. Flavonoids, phenolic content, and antioxidant activity of propolis from various areas of Guanajuato, Mexico. Food Sci. Technol. Campinas 2018, 38, 210–215. [Google Scholar] [CrossRef]
- Indradi, B.; Fidrianny, I.; Wirasutisna, K. DPPH Scavenging Activities and Phytochemical Content of Four Asteraceae Plants. Int. J. Pharmacogn. Pharm. 2017, 9, 755–759. [Google Scholar] [CrossRef]
- Sorkun, K.; Bozcuk, S.; Gömürgen, A.N.; Tekin, F. An Inhibitory Effect of Propolis on Germination and Cell Division in the Root Tips of Wheat Seedlings. In Bee Products; Springer US: Boston, MA, USA, 1997; pp. 129–135. [Google Scholar]
- Sorkun, K.; Bozcuk, S. Investigation of the effect of propolis on seed germination of some culture plants. In Proceedings of the XIIth National Biology Conference, Edirne, Turkey, 6–8 July 1994; pp. 54–59. [Google Scholar]
- Dadgostar, S.; Nozari, J. Evaluation of propolis extract in preventing weed seed germination. Proc. Int. Acad. Ecol. Environ. Sci. 2020, 10, 125–130. [Google Scholar]
- King-Díaz, B.; Granados-Pineda, J.; Bah, M.; Rivero-Cruz, J.F.; Lotina-Hennsen, B. Mexican propolis flavonoids affect photosynthesis and seedling growth. J. Photochem. Photobiol. B 2015, 151, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Silva, C.C.; Lima, C.A.; Negri, G.; Salatino, M.L.F.; Salatino, A.; Mayworm, M.A.S. Composition of the volatile fraction of a sample of Brazilian green propolic and its phytotoxic activity. J. Sci. Food Agric. 2015, 95, 3091–3095. [Google Scholar] [CrossRef]
- Pusceddu, M.; Annoscia, D.; Floris, I.; Frizzera, D.; Zanni, V.; Angioni, A.; Satta, A.; Nazzi, F. Honeybees use propolis as a natural pesticide against their major ectoparasite. Proc. R. Soc. B 2021, 288, 20212101. [Google Scholar] [CrossRef]
- Ararso, Z.; Legesse, G. Insecticidal action of honeybees propolis extract against larvae of lesser wax moth. Agric. Biol. J. N. Am. 2016, 7, 302–306. [Google Scholar]
- Ożarowski, M.; Karpiński, T.M.; Alam, R.; Łochyńska, M. Antifungal Properties of Chemically Defined Propolis from Various Geographical Regions. Microorganisms 2022, 10, 364. [Google Scholar] [CrossRef]
- Franchin, M.; Saliba, A.S.M.C.; Giovanini de Oliveira Sartori, A.; Orestes Pereira Neto, S.; Benso, B.; Ikegaki, M.; Wang, K.; Matias de Alencar, S.; Granato, D. Food-grade delivery systems of Brazilian propolis from Apis mellifera: From chemical composition to bioactivities in vivo. Food Chem. 2024, 432, 137175. [Google Scholar] [CrossRef]
- Almas, K.; Dahlan, A.; Mahmoud, A. Propolis as a Natural Remedy: An Update. Saudi Dent. Soc. 2001, 13, 45–49. [Google Scholar]
- Yang, S.; Peng, L.; Cheng, Y.; Chen, F.; Pan, S. Control of citrus green and blue molds by Chinese propolis. Food Sci. Biotechnol. 2010, 19, 1303–1308. [Google Scholar] [CrossRef]
- Balderas-Cordero, D.; Canales-Alvarez, O.; Sánchez-Sánchez, R.; Cabrera-Wrooman, A.; Canales-Martinez, M.M.; Rodriguez-Monroy, M.A. Anti-Inflammatory and Histological Analysis of Skin Wound Healing through Topical Application of Mexican Propolis. Int. J. Mol. Sci. 2023, 24, 11831. [Google Scholar] [CrossRef]
- Vică, M.L.; Glevitzky, M.; Dumitrel, G.-A.; Bostan, R.; Matei, H.V.; Kartalska, Y.; Popa, M. Qualitative Characterization and Antifungal Activity of Romanian Honey and Propolis. Antibiotics 2022, 11, 1552. [Google Scholar] [CrossRef]
- Heghedűş-Mîndru, R.C.; Glevitzky, M.; Heghedűş-Mîndru, G.; Dumitrel, G.-A.; Popa, M.; Popa, D.M.; Radulov, I.; Vică, M.L. Applications of Romanian Propolis in Phyto-Inhibitory Activity and Antimicrobial Protection: A Comparative Study. Antibiotics 2023, 12, 1682. [Google Scholar] [CrossRef]
- Lagouri, V.; Prasianaki, D.; Krysta, F. Antioxidant Properties and Phenolic Composition of Greek Propolis Extracts. Int. J. Food Prop. 2013, 17, 511–522. [Google Scholar] [CrossRef]
- Vică, M.L.; Glevitzky, M.; Heghedűş-Mîndru, R.C.; Dumitrel, G.-A.; Heghedűş-Mîndru, G.; Popa, M.; Faur, D.M.; Bâlici, Ș.; Teodoru, C.A. Phyto-Inhibitory and Antimicrobial Activity of Brown Propolis from Romania. Antibiotics 2023, 12, 1015. [Google Scholar] [CrossRef]
- Al Aboody, M.S.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, G.; Brasili, E.; Pasqua, G. Antifungal Activity of Phenolic and Polyphenolic Compounds from Different Matrices of Vitis vinifera L. against Human Pathogens. Molecules 2020, 25, 3748. [Google Scholar] [CrossRef] [PubMed]
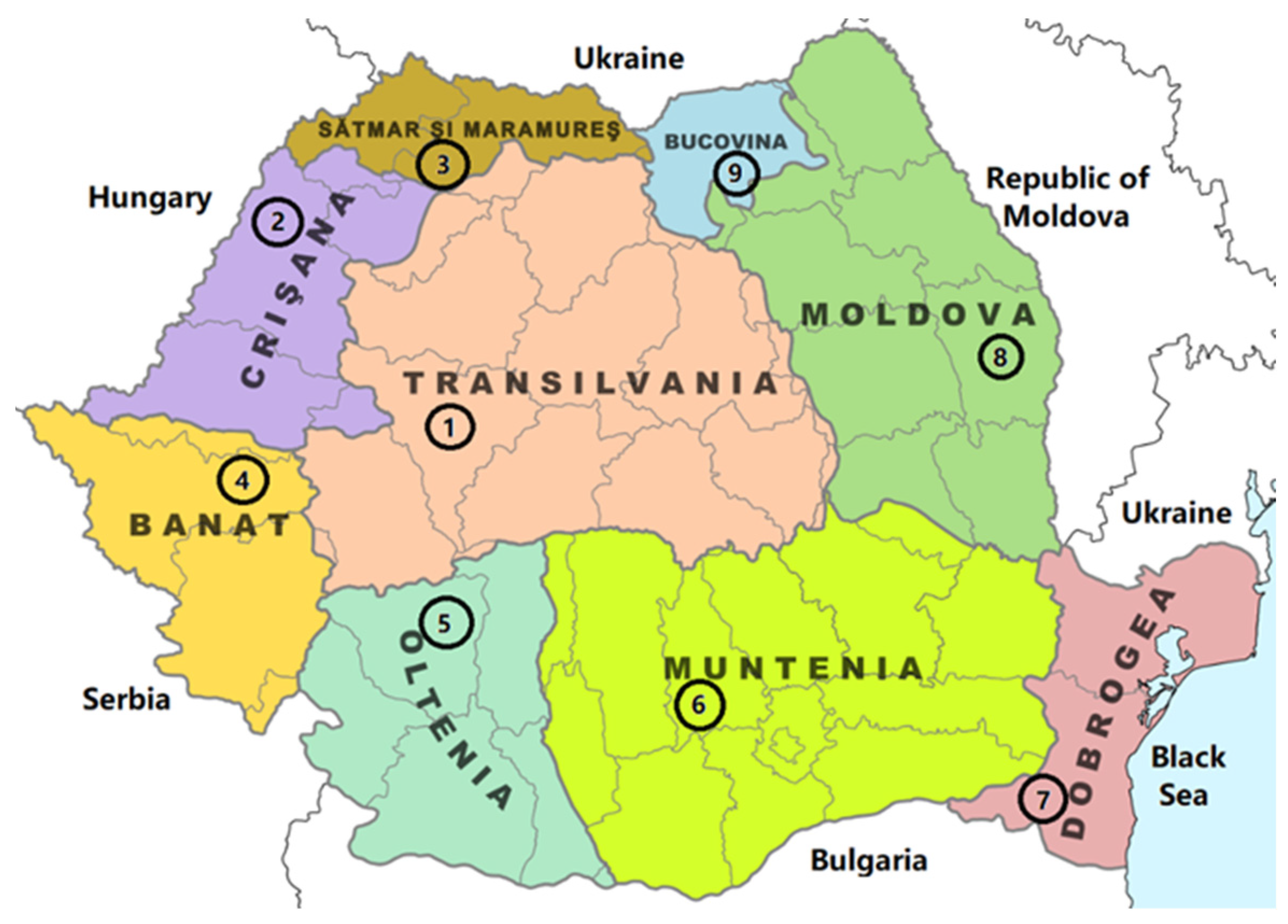
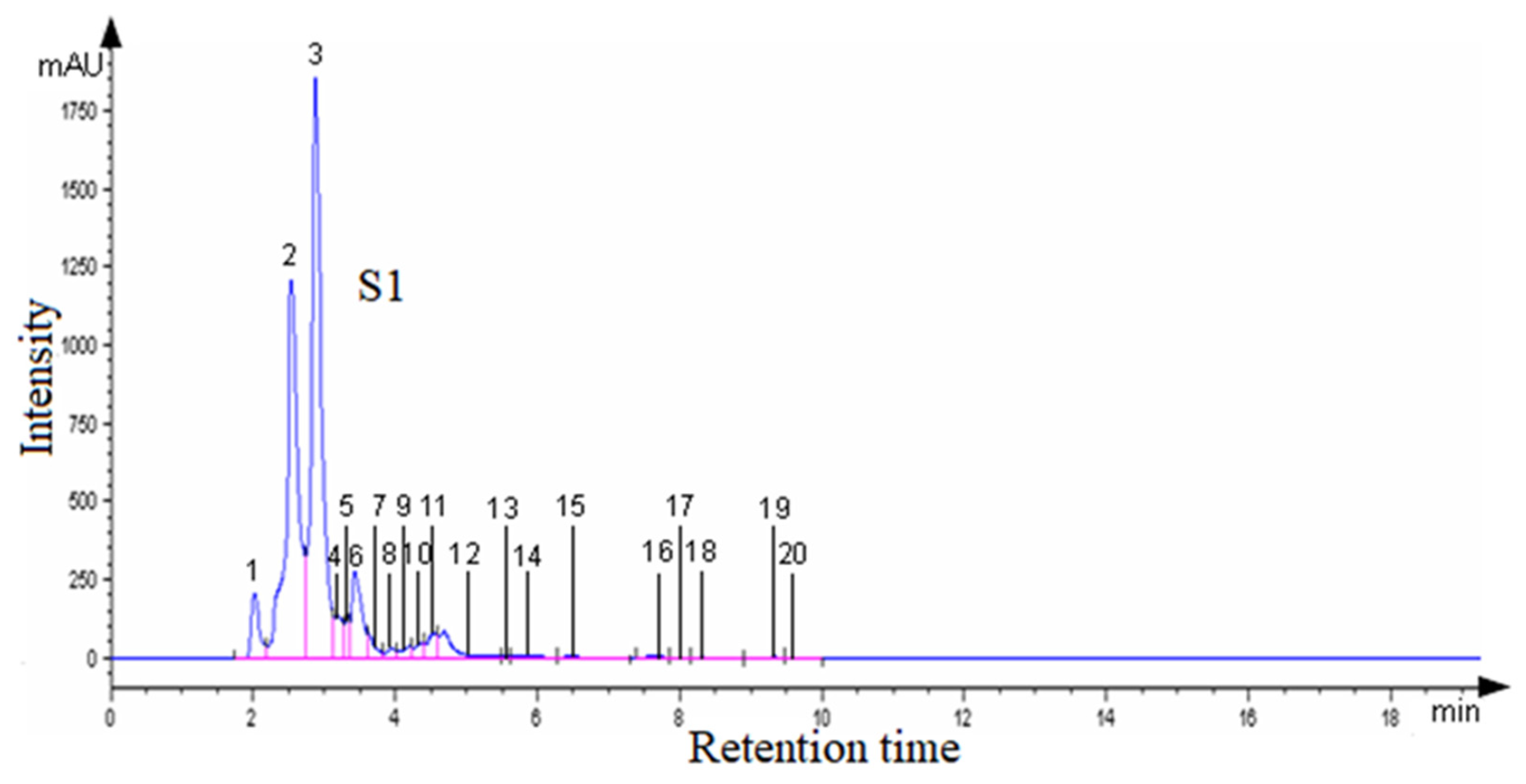
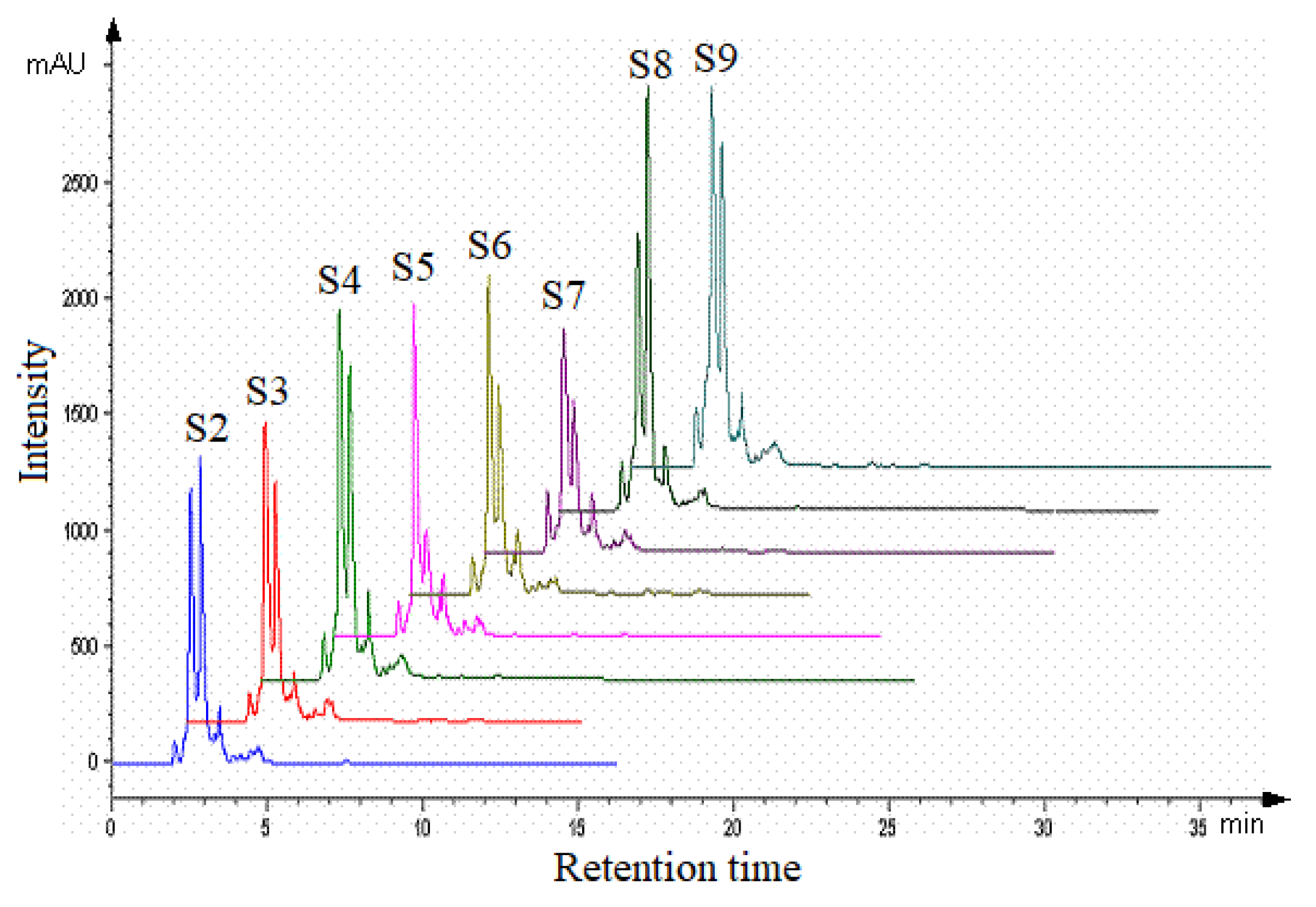
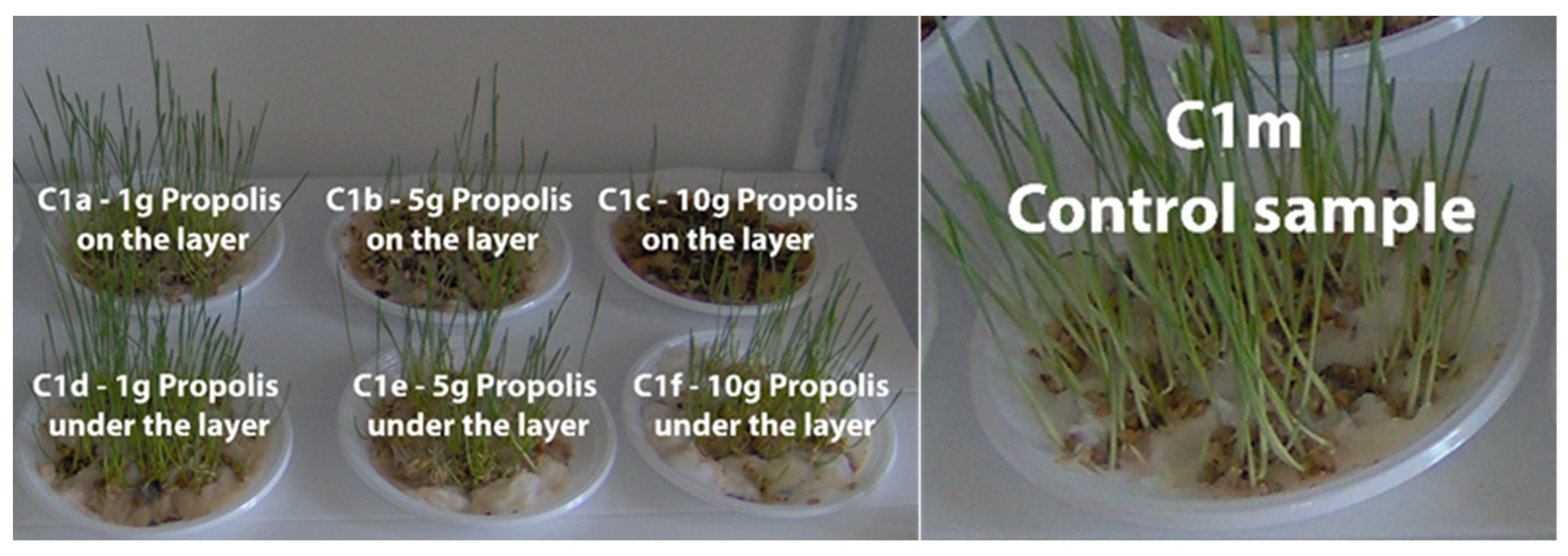

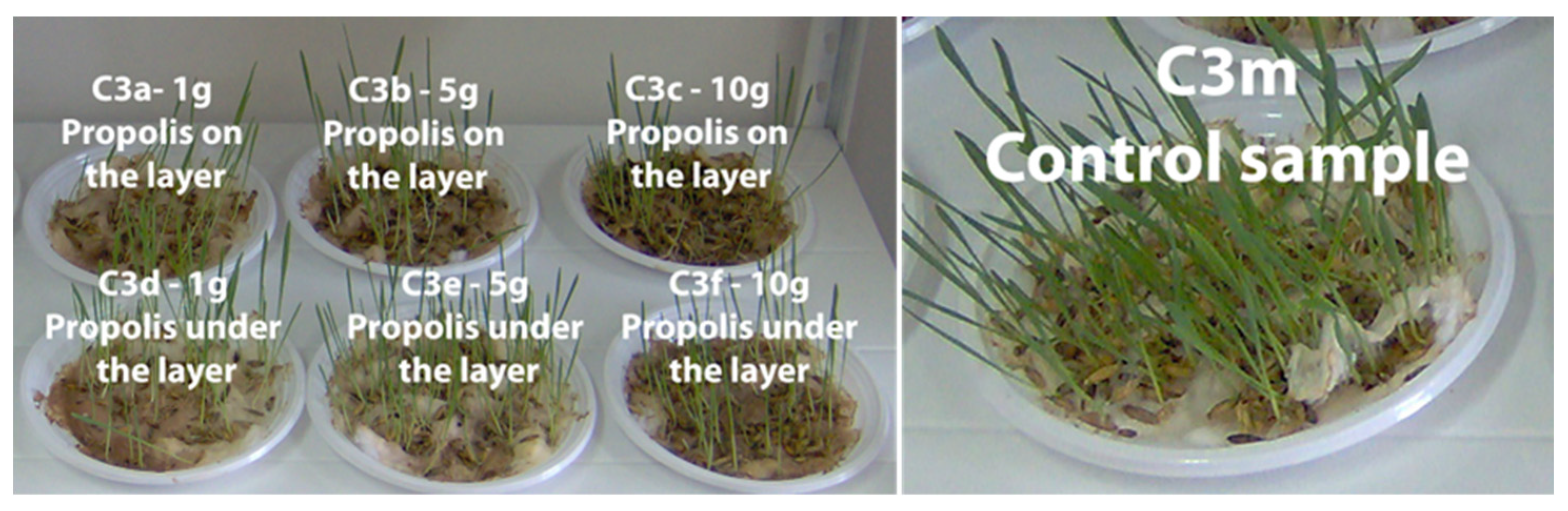
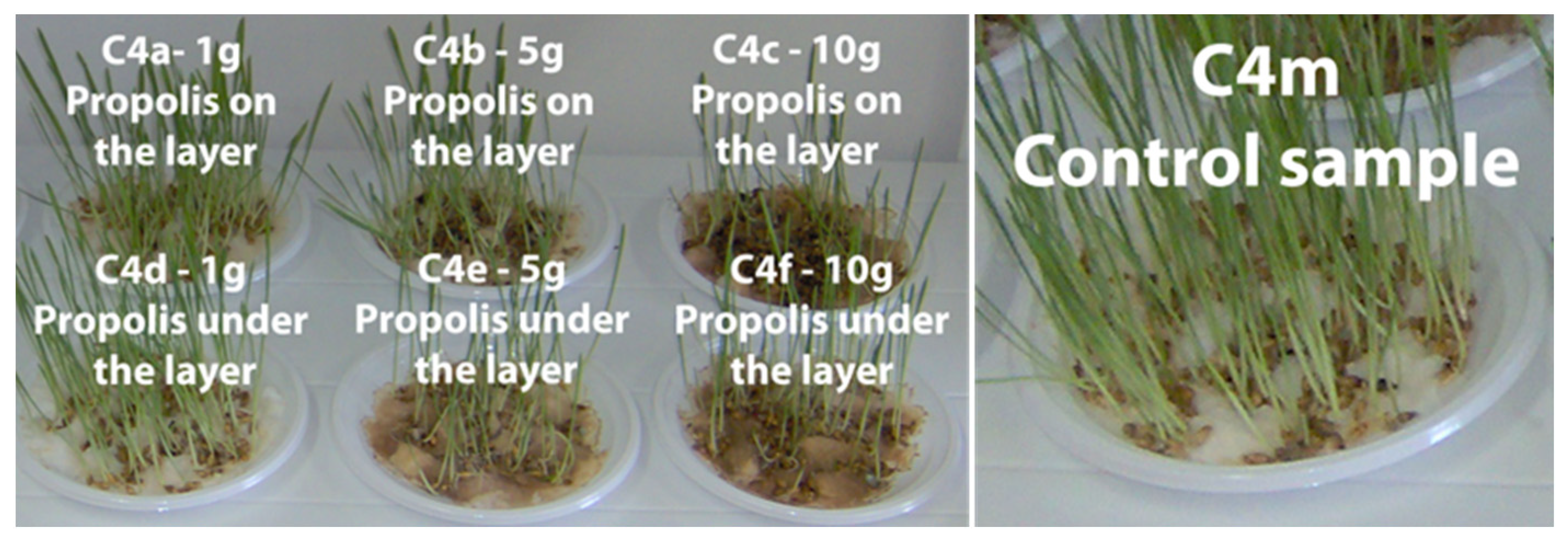
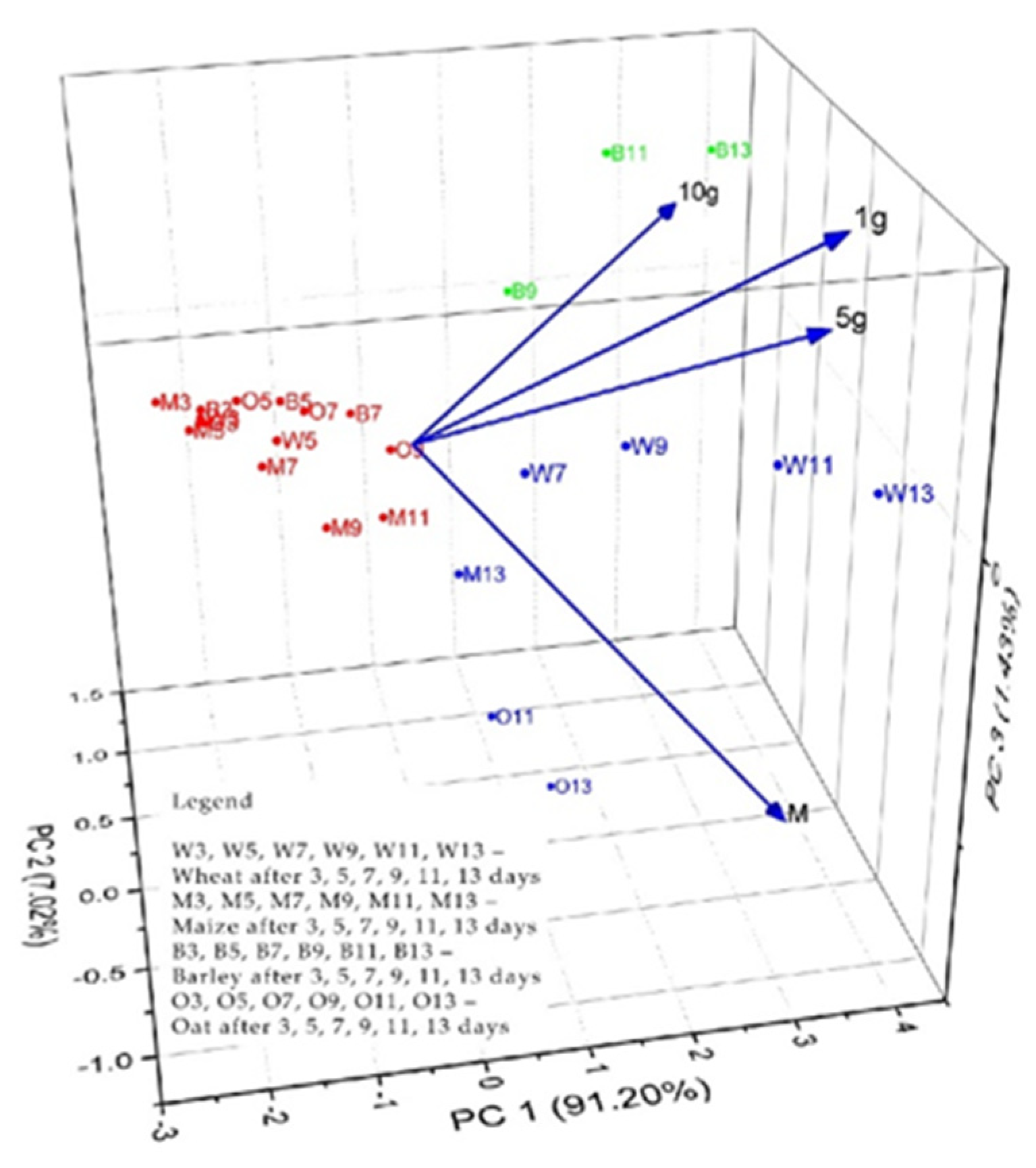
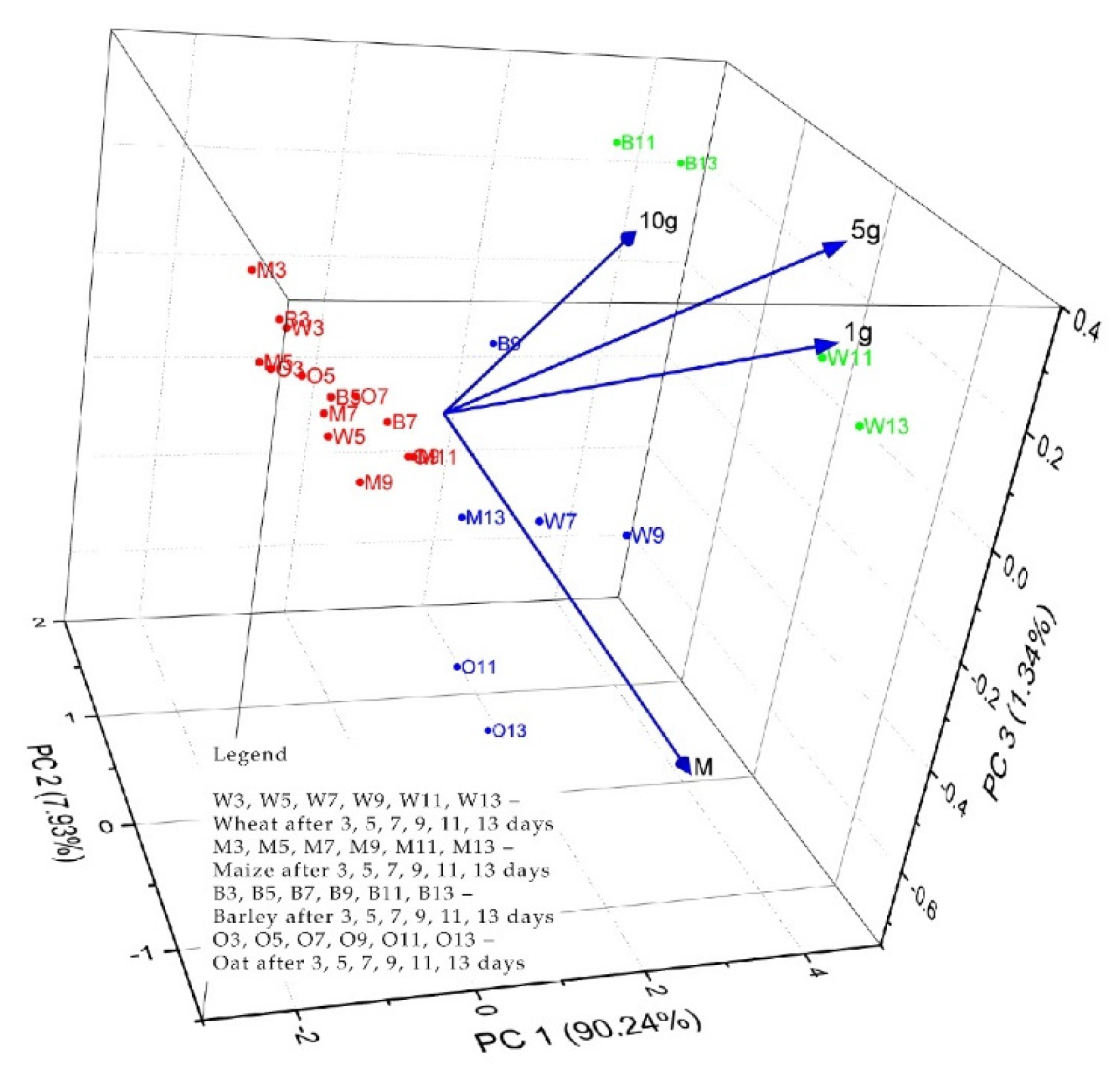
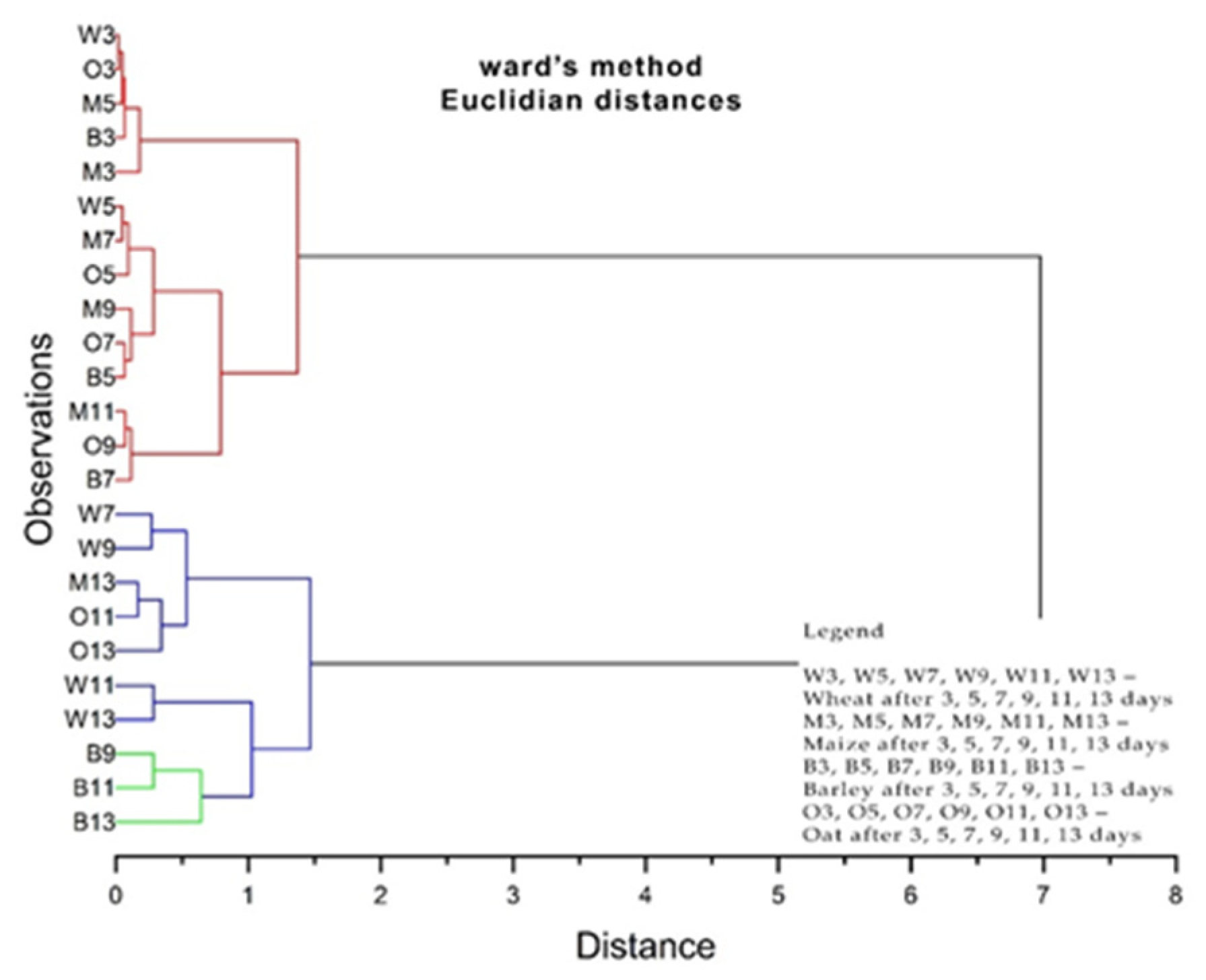
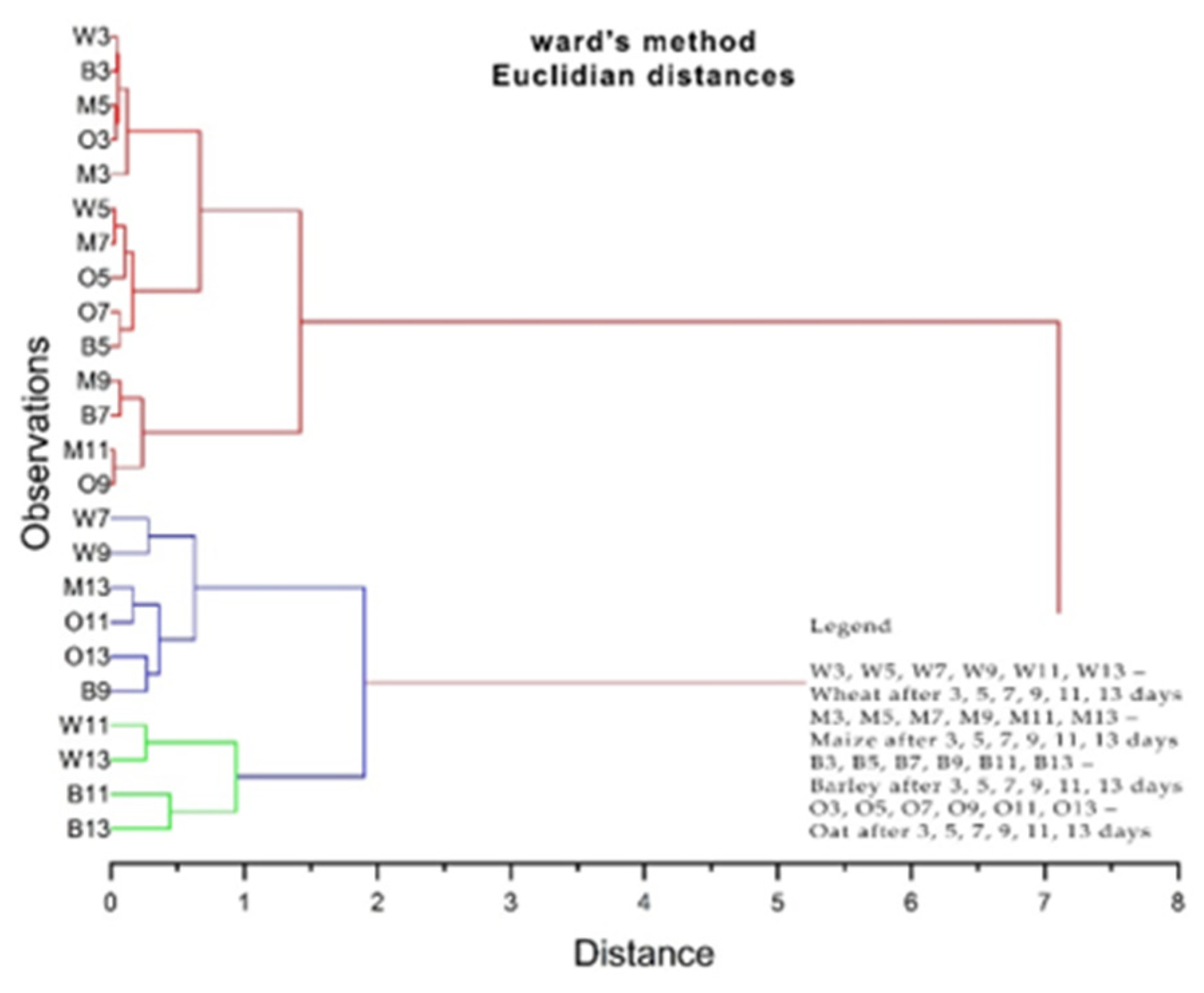
| Sample | Region | County of Origin | Latitude | Longitude | Landforms |
|---|---|---|---|---|---|
| S1 | Transilvania | Alba | 46°23′39.8″ N | 22°58′06.2″ E | Mountainous |
| S2 | Crișana | Bihor | 46°41′56.0″ N | 22°37′23.2″ E | Hilly |
| S3 | Sătmar and Maramureș | Maramureș | 47°32′35.2″ N | 23°55′31.8″ E | Mountainous |
| S4 | Banat | Timiș | 45°42′06.5″ N | 21°14′04.6″ E | Plain |
| S5 | Oltenia | Gorj | 45°10′09.3″ N | 23°07′53.9″ E | Sub-mountainous |
| S6 | Muntenia | Dâmbovița | 44°58′19.6″ N | 25°26′13.7″ E | Hilly |
| S7 | Dobrogea | Constanța | 43°48′25.1″ N | 28°31′27.3″ E | Plain |
| S8 | Moldova | Vaslui | 46°38′01.9″ N | 27°21′31.3″ E | Hilly |
| S9 | Bucovina | Suceava | 47°34′16.1″ N | 25°07′01.3″ E | Mountainous |
| County | Cultivated Plants | Surface, ha * | Essentially Flora * | Floral Melliferous Potential (t) * |
|---|---|---|---|---|
| Alba | Sunflower | 7636 | Spruce, fir tree, beech, mountain ash, birch, acacia, blueberry, juniper, raspberry, poppies, orchards, pastures, meadow | 6758 |
| Rapeseed and other oilseeds | 3410 | |||
| Vegetables and legumes | 4686 | |||
| Bihor | Sunflower | 31,750 | Spruce, fir tree, mountain buckthorn, sedge, goat willow, cherry, tart cherry, apple, dandelion, Tatar maple, white mustard, sorrel, raspberry, mountain alder, juniper, blueberry and cranberry, beech, sessile oak, ash, hazelnut oak, garneau, tatar maple, and linden. | 6503 |
| Rapeseed and other oilseeds | 24,835 | |||
| Vegetables and legumes | 4774 | |||
| Maramureș | Sunflower | 758 | Spruce, plane tree, mountain ash, mountain alder, fir tree, larch, pine tree, yew, beech, hornbeam, elm, juniper, blueberry, cranberry, orchards, raspberry | 8246.4 |
| Rapeseed and other oilseeds | 8 | |||
| Vegetables and legumes | 1591 | |||
| Timiș | Sunflower | 46,000 | Fir tree, spruce, beech, oak, linden, sycamore, poplar, willow, rush, reed, water lily | 4817.7 |
| Rapeseed and other oilseeds | 10,000 | |||
| Vegetables and legumes | 318,700 | |||
| Gorj | Sunflower | 596 | Acacia, linden, dwarf willow, gorse, blueberry, cranberry, mountain carnation, lamb’s grass, conifers, beech, oak, chestnut | 7048.7 |
| Rapeseed and other oilseeds | 155 | |||
| Vegetables and legumes | 7150 | |||
| Dâmbovița | Sunflower | 16,134 | Spruce, fir tree, beech, hornbeam, sessile, turkey oak, dogwood, acacia, linden, black anise, poplar and willow | 3577.4 |
| Rapeseed and other oilseeds | 5220 | |||
| Vegetables and legumes | 11,636 | |||
| Constanța | Sunflower | 74,005 | Acacia, linden, oak, dwarf almonds, doves, hawthorn bushes, mint, sage, white clover, cinquefoil, sweet peas, bellflower, carnation | 3002 |
| Rapeseed and other oilseeds | 38,307 | |||
| Vegetables and legumes | 1958 | |||
| Vaslui | Sunflower | 51,202 | Sessile oak, pedunculated oak, hornbeam, beech, linden, field elm, boxwood, maple, ash, acacia | 3395 |
| Rapeseed and other oilseeds | 13,204 | |||
| Vegetables | 5215 | |||
| Rapeseed and other oilseeds | 4990 | |||
| Suceava | Sunflower | 10,018 | Spruce, fir tree, pine, aspen, linden, birch, boxwood, yew, sorrel, bluebells, foxglove, water lily, raspberry, blackberry, elder, honeysuckle, hazel bushes, strawberries, blueberry | 11,256.6 |
| Rapeseed and other oilseeds | 1600 | |||
| Vegetables and legumes | 9452 |
| Sample | Cereal Type | Scientific Name |
|---|---|---|
| C1 | Hexaploid bread wheat | Triticum aestivum |
| C2 | Maize | Zea mays L. |
| C3 | Oats | Avena sativa L. |
| C4 | Barley | Hordeum vulgare L. |
| Sample | VO, % | Wax, % | OI, s | MP, °C | Dry Matter, % | Ash, % | Resin, % |
|---|---|---|---|---|---|---|---|
| S1 | 0.2 (0.02) b | 28.11 (1.51) c,d | 14.8 (3.5) c | 63.4 (0.2) c,d | 3.46 (0.22) a,b | 0.90 (0.05) b | 40.16 (1.37) g |
| S2 | 0.3 (0.01) c | 26.55 (1.28) a,b,c | 13.1 (4.0) b | 64.1 (0.1) d | 3.51 (0.19) a,b | 0.92 (0.03) b | 29.93 (1.76) b |
| S3 | 0.1 (0.01) a | 30.02 (1.54) d | 12.9 (3.3) b | 63.0 (0.2) b,c | 3.73 (0.27) a,b | 1.48 (0.07) d | 34.31 (1.52) e |
| S4 | 0.4 (0.03) d | 24.54 (1.37) a | 13.6 (2.1) b | 62.2 (0.2) a,b | 3.27 (0.31) a | 2.01 (0.14) f | 27.05 (1.32) a |
| S5 | 0.2 (0.05) b | 28.40 (1.16) c,d | 12.8 (3.3) b | 66.7 (0.3) f | 3.80 (0.26) b | 0.80 (0.05) a | 31.44 (1.15) c,d |
| S6 | 0.2 (0.01) b | 29.08 (1.41) d | 10.7 (1.9) a | 63.5 (0.3) c,d | 3.74 (0.15) a,b | 1.06 (0.04) c | 30.72 (1.64) b,c |
| S7 | 0.5 (0.08) e | 25.90 (1.09) a,b | 14.3 (3.6) c | 65.3 (0.2) e | 3.32 (0.20) a,b | 1.77 (0.09) e | 38.13 (2.03) f |
| S8 | 0.3 (0.02) c | 27.77 (1.62) b,c | 11.0 (2.2) a | 62.0 (0.1) a | 3.66 (0.28) a,b | 0.79 (0.05) a | 29.84 (1.10) b |
| S9 | 0.2 (0.04) b | 29.43 (1.93) d | 13.2 (2.7) b | 63.4 (0.2) c,d | 3.58 (0.25) a,b | 1.23 (0.08) c | 32.20 (1.01) d |
| Sample | TPC, mg GAE/g | TFC, mg QE/g | IC50, µg/mL |
|---|---|---|---|
| S1 | 189.4 (5.82) f | 84.31 (0.09) e | 0.333 (0.002) b |
| S2 | 172.9 (3.25) e | 78.55 (0.08) c | 0.514 (0.016) c |
| S3 | 152.2 (6.80) d | 70.10 (0.16) b | 0.725 (0.003) e |
| S4 | 144.0 (2.09) c,d | 81.09 (0.98) d,e | 0.669 (0.010) d |
| S5 | 138.2 (3.06) c | 77.62 (0.20) c | 0.884 (0.028) e |
| S6 | 102.7 (2.43) a | 65.30 (0.11) a | 0.964 (0.031) f |
| S7 | 189.0 (4.55) f | 85.19 (0.07) e | 0.086 (0.001) a |
| S8 | 126.3 (3.14) b | 82.36 (0.09) d,e | 0.517 (0.004) c |
| S9 | 150.1 (4.37) d | 79.54 (0.13) c,d | 0.615 (0.005) d |
| Parameter | VO | Wax | OI | MP | Dry Matter | Ash | Resin | TPC | TFC |
|---|---|---|---|---|---|---|---|---|---|
| Wax | −0.884 | - | - | - | - | - | - | - | - |
| OI | 0.279 | −0.358 | - | - | - | - | - | - | - |
| MP | 0.102 | 0.011 | 0.261 | - | - | - | - | - | - |
| Dry matter | −0.781 | 0.825 | −0.660 | 0.183 | - | - | - | - | - |
| Ash | 0.519 | −0.505 | 0.390 | −0.171 | −0.662 | - | - | - | - |
| Resin | −0.046 | 0.224 | 0.595 | 0.291 | −0.150 | −0.037 | - | - | - |
| TPC | 0.329 | −0.324 | 0.903 | 0.290 | −0.606 | 0.202 | 0.683 | - | - |
| TFC | 0.608 | −0.575 | 0.624 | 0.077 | −0.660 | 0.107 | 0.303 | 0.667 | - |
| IC50 | −0.619 | 0.445 | −0.637 | −0.072 | 0.693 | −0.237 | −0.616 | −0.827 | −0.792 |
| Sample | Quercetin (mg/mL); RSD% | Rutin (mg/mL); RSD% |
|---|---|---|
| S1 | 0.57; 1.66 a | 0.0143; 1.47 |
| S2 | 0.62; 2.80 a,b | 0.0127; 1.30 |
| S3 | 0.65; 2.83 a | 0.0093; 2.03 |
| S4 | 0.74; 2.94 | 0,0102; 2.24 |
| S5 | 0.71; 2.79 | 0.0134; 1.31 |
| S6 | 0.78; 2.85 | 0.0119; 1.19 |
| S7 | 0.66; 2.44 | 0.0185; 1.02 |
| S8 | 0.81; 1.91 | 0.0168; 1.55 |
| S9 | 0,59; 1.72 a | 0.0150; 1.96 |
| Sample | Relative Weight of 1000 Seeds, g | Absolute Mass of 1000 Seeds, g | Moisture, % | Hectoliter Mass, kg/hL | Glassiness, % |
|---|---|---|---|---|---|
| C1 | 40 | 35 | 13.8 | 77.1 | 91 |
| C2 | 169 | 287 | 14.4 | 73.8 | 88 |
| C3 | 25 | 23 | 12.9 | 41.1 | - |
| C4 | 41 | 60 | 14.2 | 63.7 | - |
| Sample No. | Strain | ||||
|---|---|---|---|---|---|
| A. niger | A. flavus | P. chrysogenum | F. oxysporum | R. stolonifer | |
| S1 | 23.83 ± 0.29 | 26.33 ± 0.29 | 21.67 ± 0.58 | 27.77 ± 1.14 * | 25.5 ± 0.50 |
| S2 | 18.67 ± 0.58 | 17.00 ± 0.00 | 17.16 ± 0.29 | 23.67 ± 0.58 | 20.00 ± 0.00 |
| S3 | 16.17 ± 0.29 | 20.17 ± 0.29 | 18.67 ± 0.58 * | 26.50 ± 1.73 * | 22.17 ± 0.76 |
| S4 | 15.67 ± 0.57 | 18.00 ± 0.87 | 16.83 ± 0.29 | 22.00 ± 0.00 | 24.33 ± 0.58 |
| S5 | 16.67 ± 1.04 | 21.67 ± 1.44 * | 18.00 ± 0.00 | 25.00 ± 0.00 | 19.17 ± 0.29 |
| S6 | 18.17 ± 0.29 | 19.00 ± 0.00 | 16.17 ± 0.29 | 22.67 ± 0.58 | 23.00 ± 4.33 * |
| S7 | 17.50 ± 0.50 | 17.00 ± 0.87 | 18.67 ± 0.58 * | 26.33 ± 0.58 | 20.00 ± 0.00 |
| S8 | 18.00 ± 0.00 | 21.33 ± 0.58 | 17.00 ± 0.00 | 24.00 ± 0.00 | 25.00 ± 0.00 |
| S9 | 16.50 ± 0.50 | 18.00 ± 0.00 | 17.67 ± 0.58 * | 21.67 ± 0.58 | 21.00 ± 3.50 * |
| Voriconazole 1 μg | 45.00 ± 0.00 | 43.00 ± 0.00 | 18.00 ± 0.00 | 29.00 ± 0.00 | 16.00 ± 0.00 |
| Tested Microorganism (MIC) | Pearson Correlation With: | |||
|---|---|---|---|---|
| TPC | p-Value | TFC | p-Value | |
| A. niger | 0.438 | 0.238 | 0.667 | 0.050 |
| A. flavus | 0.109 | 0.781 | 0.155 | 0.691 |
| P. chrysogenum | 0.731 | 0.025 | 0.435 | 0.242 |
| F. oxysporum | 0.600 | 0.088 | 0.667 | 0.050 |
| R. stolonifer | −0.164 | 0.674 | 0.096 | 0.050 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heghedűş-Mîndru, G.; Glevitzky, M.; Heghedűş-Mîndru, R.C.; Dumitrel, G.-A.; Popa, M.; Glevitzky, I.; Obiștioiu, D.; Cocan, I.; Vică, M.L. Inhibitory Effects and Composition Analysis of Romanian Propolis: Applications in Organic and Sustainable Agriculture. Plants 2024, 13, 3355. https://doi.org/10.3390/plants13233355
Heghedűş-Mîndru G, Glevitzky M, Heghedűş-Mîndru RC, Dumitrel G-A, Popa M, Glevitzky I, Obiștioiu D, Cocan I, Vică ML. Inhibitory Effects and Composition Analysis of Romanian Propolis: Applications in Organic and Sustainable Agriculture. Plants. 2024; 13(23):3355. https://doi.org/10.3390/plants13233355
Chicago/Turabian StyleHeghedűş-Mîndru, Gabriel, Mirel Glevitzky, Ramona Cristina Heghedűş-Mîndru, Gabriela-Alina Dumitrel, Maria Popa, Ioana Glevitzky, Diana Obiștioiu, Ileana Cocan, and Mihaela Laura Vică. 2024. "Inhibitory Effects and Composition Analysis of Romanian Propolis: Applications in Organic and Sustainable Agriculture" Plants 13, no. 23: 3355. https://doi.org/10.3390/plants13233355
APA StyleHeghedűş-Mîndru, G., Glevitzky, M., Heghedűş-Mîndru, R. C., Dumitrel, G.-A., Popa, M., Glevitzky, I., Obiștioiu, D., Cocan, I., & Vică, M. L. (2024). Inhibitory Effects and Composition Analysis of Romanian Propolis: Applications in Organic and Sustainable Agriculture. Plants, 13(23), 3355. https://doi.org/10.3390/plants13233355






