The Application of Stress Modifiers as an Eco-Friendly Approach to Alleviate the Water Scarcity in Ajwain (Carum copticum L.) Plants
Abstract
1. Introduction
2. Results
2.1. Two-Year Effect of Water Treatments and Stress Modifier Applications on Biological and Seed Yield and Nutritional Traits of Ajwain Plants
2.1.1. Biological and Seed Yield
2.1.2. Fixed Oil and Fixed Oil Yield, Essential Oil and Essential Oil Yields
2.1.3. Nitrogen, Phosphorus, and Potassium Leaf Concentration
2.2. Two-Year Effect of Water Treatments and Stress Modifier Applications on Eco-Physiological Traits and Antioxidant Defenses of Ajwain Plants
2.2.1. Leaf Relative Water Content, Chlorophyll a and b Concentrations
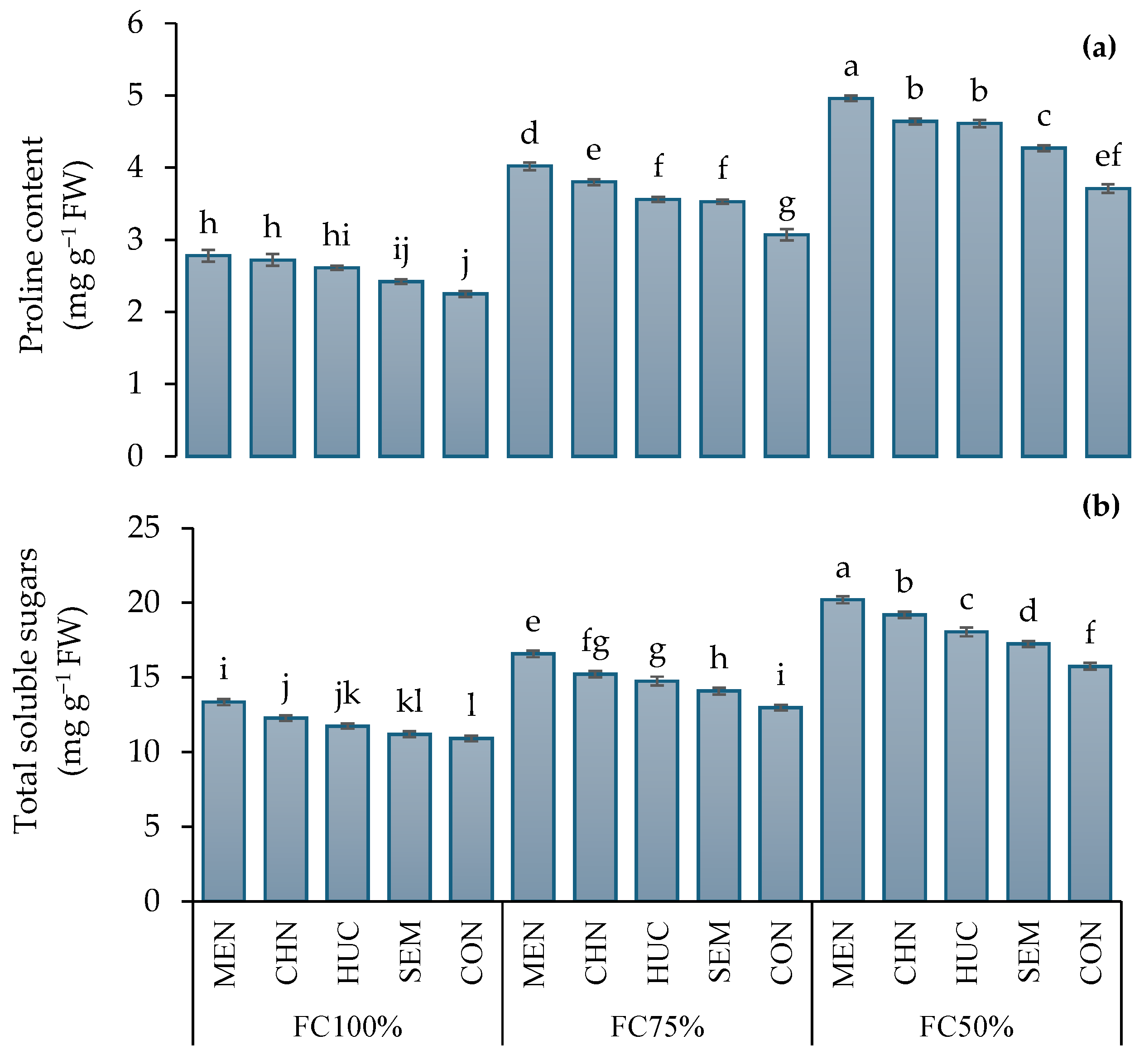
2.2.2. Osmotic Concentration
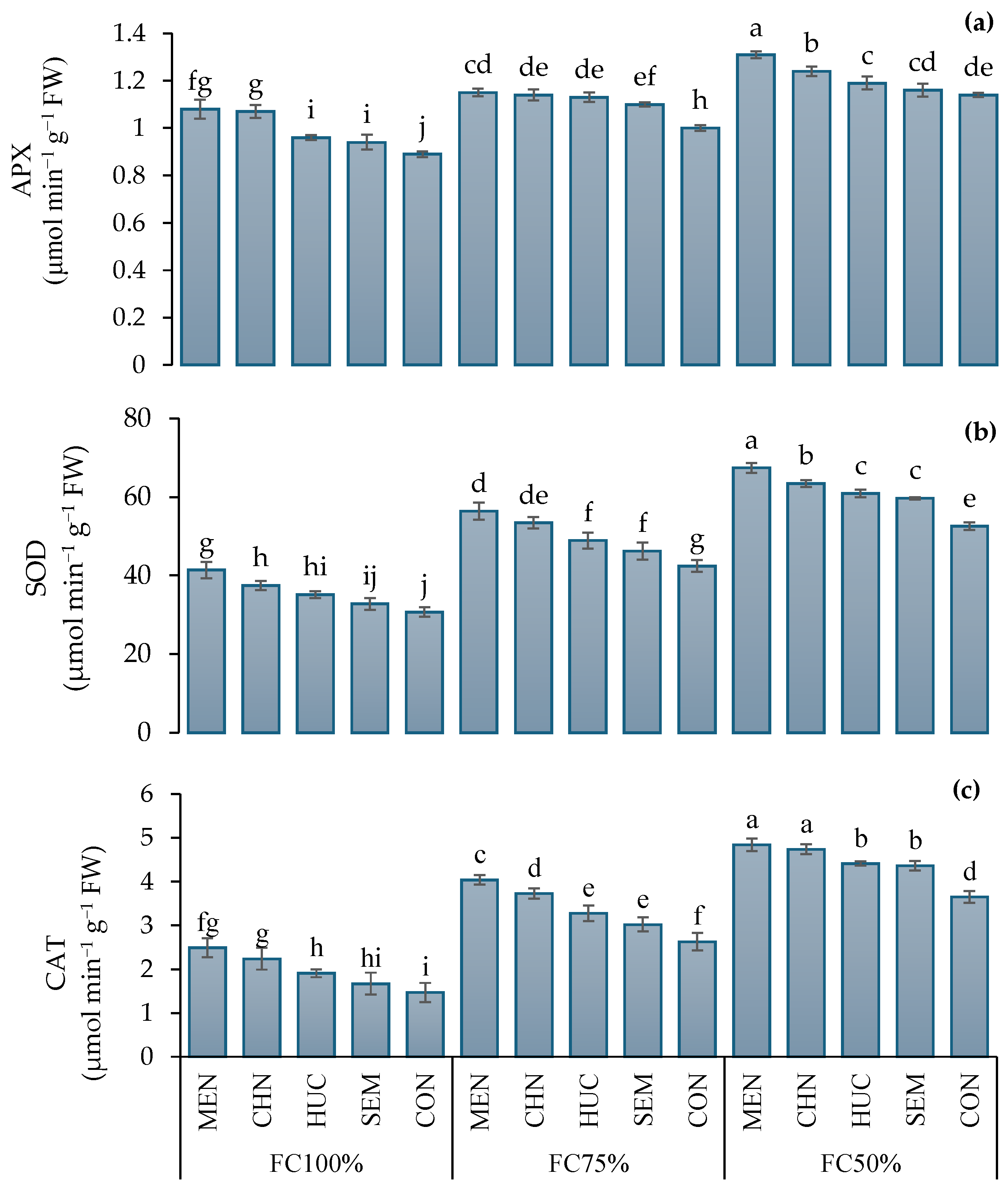
2.2.3. Antioxidant Enzyme Activity
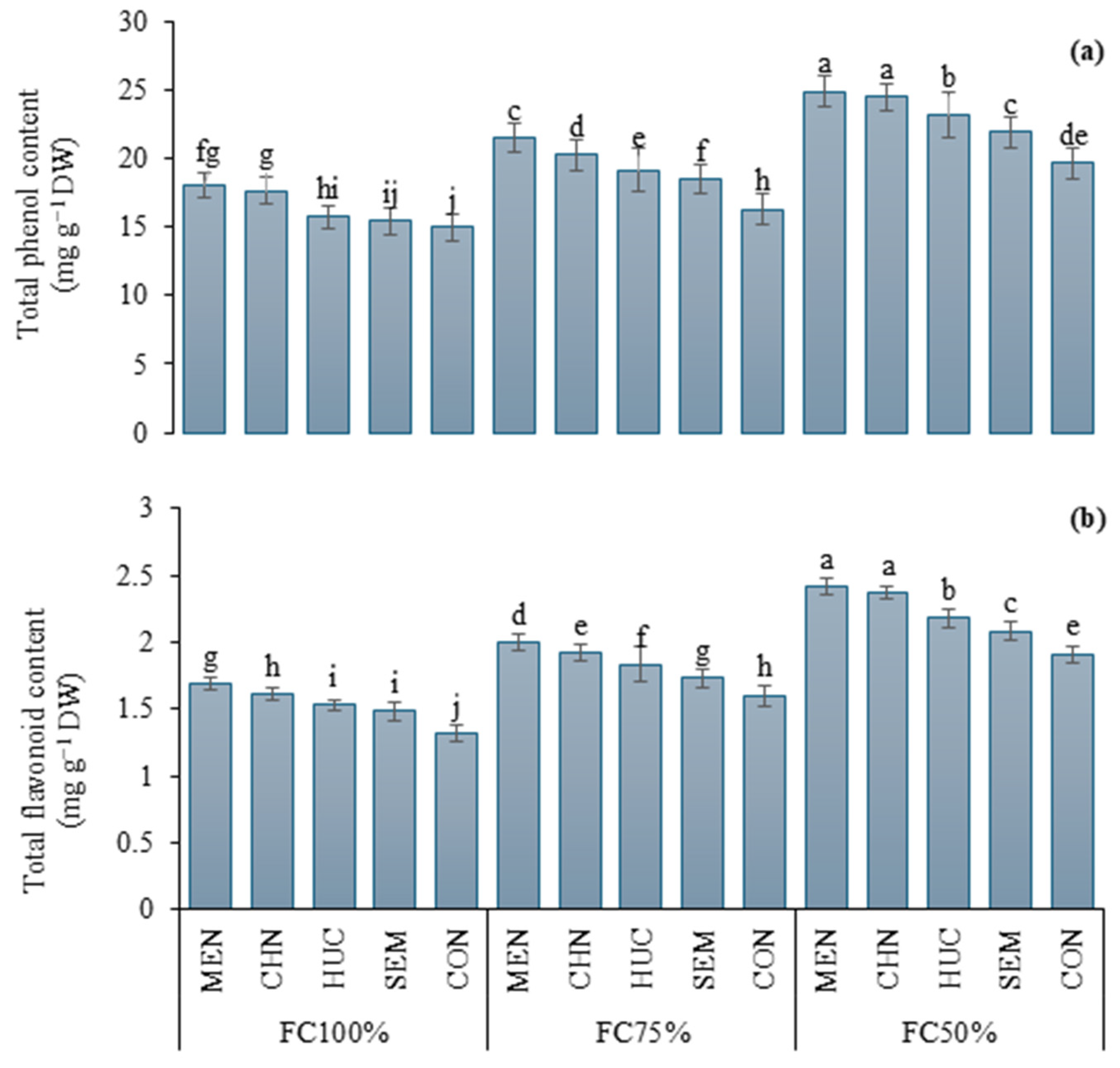
2.2.4. Total Phenol and Flavonoid Content
3. Discussion
3.1. Effect of Stress Modifier Application on Essential Oil Attributes, Leaf Nutritional Status, and Water Relationships
3.2. Effect of Stress Modifier Application on Leaf Photosynthetic Pigments, Osmotic Concentration, and Antioxidant System
4. Materials and Methods
4.1. Experimental Setup
4.2. Soil Classification, Plant Material, and Stress Modulators Application
4.3. Oil Content and Yield Determination
4.4. Leaf Traits and Biochemical Analyses
4.4.1. Determination of Nitrogen, Phosphorus, and Potassium Concentration
4.4.2. Relative Water Content (RWC)
4.4.3. Chlorophyll a and b Content
4.4.4. Total Soluble Sugars
4.4.5. Proline Content
4.4.6. Enzyme Extractions and Assays
4.4.7. Total Polyphenol and Flavonoid Leaf Content
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rezaei-Chiyaneh, E.; Mahdavikia, H.; Subramanian, S.; Alipour, H.; Siddique, K.H.; Smith, D.L. Co-inoculation of phosphate-solubilizing bacteria and mycorrhizal fungi: Effect on seed yield, physiological variables, and fixed oil and essential oil productivity of ajwain (Carum copticum L.) under water deficit. J. Soil Sci. Plant Nutr. 2021, 21, 3159–3179. [Google Scholar] [CrossRef]
- Razavizadeh, R.; Mosayebi, Z.; Forghani, A.H. The role of chitosan in improvisation the drought stress in carum copticum through biochemical and essential oil components. Russ. J. Plant Physiol. 2022, 69, 156. [Google Scholar]
- Ziaee, M.; Sheikhzadeh Takabi, A.; Ebadollahi, A. Fabrication of Carum copticum essential oil-loaded chitosan nanoparticles and evaluation its insecticidal activity for controlling Rhyzopertha dominica and Tribolium confusum. Front. Plant Sci. 2023, 14, 1187616. [Google Scholar] [CrossRef] [PubMed]
- Heydarzadeh, S.; Arena, C.; Vitale, E.; Rahimi, A.; Mirzapour, M.; Nasar, J.; Kisaka, O.; Sow, S.; Ranjan, S.; Gitari, H. Impact of different fertilizer sources under supplemental irrigation and rainfed conditions on eco-physiological responses and yield characteristics of dragon’s head (Lallemantia iberica). Plants 2023, 12, 1693. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Zarepour Moshizi, M.; Yousefi, A.; Amini, A.M. Rural vulnerability to water scarcity in Iran: An integrative methodology for evaluating exposure, sensitivity and adaptive capacity. Geo J. 2023, 88, 2121–2136. [Google Scholar]
- Amirnia, R.; Ghiyasi, M.; Siavash Moghaddam, S.; Rahimi, A.; Damalas, C.A.; Heydarzadeh, S. Nitrogen-fixing soil bacteria plus mycorrhizal fungi improve seed yield and quality traits of lentil (Lens culinaris Medik). J. Soil Sci. Plant Nutr. 2019, 19, 592–602. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Heydarzadeh, S.; Jalilian, J.; Pirzad, A.; Jamei, R.; Petrussa, E. Fodder value and physiological aspects of rainfed smooth vetch affected by biofertilizers and supplementary irrigation in an agri-silviculture system. Agrofor. Syst. 2022, 95, 221–232. [Google Scholar] [CrossRef]
- Das, D.; Bisht, K.; Chauhan, A.; Gautam, S.; Jaiswal, J.P.; Salvi, P.; Lohani, P. Morpho-physiological and Biochemical responses in wheat foliar sprayed with zinc-chitosan-salicylic acid nanoparticles during drought stress. Plant Nano Biol. 2023, 4, 100034. [Google Scholar] [CrossRef]
- Farouk, S.; Al-Amri, S.M. Ameliorative roles of melatonin and/or zeolite on chromium-induced leaf senescence in marjoram plants by activating antioxidant defense, osmolyte accumulation, and ultrastructural modification. Ind. Crops Prod. 2019, 142, 111823. [Google Scholar] [CrossRef]
- Mustafa, G.; Shehzad, M.A.; Tahir, M.H.N.; Nawaz, F.; Akhtar, G.; Bashir, M.A.; Ghaffar, A. Pretreatment with chitosan arbitrates physiological processes and antioxidant defense system to increase drought tolerance in alfalfa (Medicago sativa L.). J. Soil Sci. Plant Nutr. 2022, 22, 2169–2186. [Google Scholar] [CrossRef]
- Labulo, A.H.; David, O.A.; Terna, A.D.; Omotosho, T.P.; Tanko, N.S.; Hassan, I.; Oluwole, B.R.; Odebode, A. Modulation of physiological and biochemical activities of Eugenia uniflora by green-synthesized silver nanoparticle and melatonin under drought stress. Plant Biotechnol. Rep. 2024, 18, 289–299. [Google Scholar] [CrossRef]
- Soltani, R.; Pazoki, A.; Monem, R. Co-application of chitosan and ascorbic acid alleviated drought stress in common chicory (Cichorium intybus L.) by improving physiological and biochemical changes. J. Plant Growth Regul. 2024, 43, 1184–1194. [Google Scholar] [CrossRef]
- Tadele, K.T.; Zerssa, G.W. Biostimulants and phytohormones improve productivity and quality of medicinal plants under abiotic stress. In Medicinal Plants: Their Response to Abiotic Stress; Springer Nature: Singapore, 2023; pp. 335–362. [Google Scholar]
- Zafar, F.; Noreen, Z.; Shah, A.A.; Usman, S. Co-application of humic acid, potassium dihydrogen phosphate, and melatonin to ameliorate the effects of drought stress on barley (Hordeum vulgare L.). J. Soil Sci. Plant Nutr. 2024, 24, 618–634. [Google Scholar] [CrossRef]
- Rahimi, A.; Mohammadi, M.M.; Siavash Moghaddam, S.; Heydarzadeh, S.; Gitari, H. Effects of stress modifier biostimulants on vegetative growth, nutrients, and antioxidants contents of garden thyme (Thymus vulgaris L.) under water deficit conditions. J. Plant Growth Regul. 2022, 41, 2059–2072. [Google Scholar] [CrossRef]
- Gohari, G.; Farhadi, H.; Panahirad, S.; Zareei, E.; Labib, P.; Jafari, H.; Mahdavinia, G.; Hassanpouraghdam, M.B.; Ioannou, A.; Kulak, M.; et al. Mitigation of salinity impact in spearmint plants through the application of engineered chitosan-melatonin nanoparticles. Int. J. Biol. Macromol. 2023, 224, 893–907. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Liu, J.; Zhang, S.; Guo, J. Exogenous melatonin and salicylic acid enhance the drought tolerance of hibiscus (Hibiscus syriacus L.) by regulating photosynthesis and antioxidant system. J. Soil Sci. Plant Nutr. 2024, 24, 497–511. [Google Scholar] [CrossRef]
- Zohra, E.; Ikram, M.; Omar, A.A.; Hussain, M.; Satti, S.H.; Raja, N.I.; Mashwani, Z.U.R.; Ehsan, M. Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives. Green Process. Synth. 2021, 10, 456–475. [Google Scholar] [CrossRef]
- Soliman, M.H.; Alnusairi, G.S.; Khan, A.A.; Alnusaire, T.S.; Fakhr, M.A.; Abdulmajeed, A.M.; Aldesuquy, H.S.; Yahya, M.; Najeeb, U. Biochar and selenium nanoparticles induce water transporter genes for sustaining carbon assimilation and grain production in salt-stressed wheat. J. Plant Growth Regul. 2023, 42, 1522–1543. [Google Scholar] [CrossRef]
- Sheikhalipour, M.; Gohari, G.; Esmaielpour, B.; Panahirad, S.; Milani, M.H.; Kulak, M.; Janda, T. Melatonin and tio2 nps application-induced changes in growth, photosynthesis, antioxidant enzymes activities and secondary metabolites in stevia (Stevia rebaudiana bertoni) under drought stress conditions. J. Plant Growth Regul. 2023, 42, 2023–2040. [Google Scholar] [CrossRef]
- Liu, H.; Xiao, C.; Qiu, T.; Deng, J.; Cheng, H.; Cong, X.; Cheng, S.; Rao, S.; Zhang, Y. Selenium regulates antioxidant, photosynthesis, and cell permeability in plants under various abiotic stresses: A review. Plants 2022, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Peng, Y.; Zheng, L.; He, C.; Peng, S.; Huang, Y.; Wang, L.; Liu, H.; Feng, G. Chitosan–Se Engineered Nanomaterial Mitigates Salt Stress in Plants by Scavenging Reactive Oxygen Species. J. Agric. Food Chem. 2023, 72, 176–188. [Google Scholar] [CrossRef] [PubMed]
- García-García, A.L.; Matos, A.R.; Feijão, E.; Cruz de Carvalho, R.; Boto, A.; Marques da Silva, J.; Jiménez-Arias, D. The use of chitosan oligosaccharide to improve artemisinin yield in well-watered and drought-stressed plants. Front. Plant Sci. 2023, 14, 1200898. [Google Scholar] [CrossRef] [PubMed]
- Basit, F.; Abbas, S.; Zhu, M.; Tanwir, K.; El-Keblawy, A.; Sheteiwy, M.S.; Raza, A.; Hu, J.; Hu, W.; Guan, Y. Ascorbic acid and selenium nanoparticles synergistically interplay in chromium stress mitigation in rice seedlings by regulating oxidative stress indicators and antioxidant defense mechanism. Environ. Sci. Pollut. Res. 2023, 30, 120044–120062. [Google Scholar] [CrossRef] [PubMed]
- Bijanzadeh, E.; Moosavi, S.M.; Bahadori, F. Quantifying water stress of safflower (Carthamus tinctorius L.) cultivars by crop water stress index under different irrigation regimes. Heliyon 2022, 8, e09010. [Google Scholar] [CrossRef]
- Abdali, R.; Rahimi, A.; Siavash Moghaddam, S.; Heydarzadeh, S.; Arena, C.; Vitale, E.; Zamanian, M. The Role of Stress Modifier Biostimulants on Adaptive Strategy of Oregano Plant for Increasing Productivity under Water Shortage. Plants 2023, 12, 4117. [Google Scholar] [CrossRef] [PubMed]
- Mohammadreza, S.; Madani, H.; Pourdad, S.S.; Nour-Mohammadi, G.; Changizi, M. Impact of deficit irrigation on the physiological and agronomic traits of 24 safflower (Carthamus tinctorius L.) genotypes grown in Iran. OCL 2023, 30, 27. [Google Scholar]
- Barati, A.A.; Pour, M.D.; Sardooei, M.A. Water crisis in Iran: A system dynamics approach on water, energy, food, land and climate (WEFLC) nexus. Sci. Total Environ. 2023, 882, 163549. [Google Scholar] [CrossRef]
- Hao, T.; Yang, Z.; Liang, J.; Yu, J.; Liu, J. Foliar application of carnosine and chitosan improving drought tolerance in bermudagrass. Agronomy 2023, 13, 442. [Google Scholar] [CrossRef]
- Mehralian, M.; Bidabadi, S.S.; Azad, M.; Ebrahimi, S.N.; Mirjalili, M.H. Melatonin-mediated alleviation of drought stress by modulation of physio-biochemical and metabolic status in Dracocephalum kotschyi Boiss (Lamiaceae). Ind. Crops Prod. 2023, 204, 117321. [Google Scholar] [CrossRef]
- Naghizadeh, M.; Reiter, R.J.; Kabiri, R.; Moradi, R. Melatonin improves antioxidant defense mechanism of basil under drought stress. Hortic. Environ. Biotechnol. 2024, 65, 83–94. [Google Scholar] [CrossRef]
- Rafique, N.; Ilyas, N.; Aqeel, M.; Raja, N.I.; Shabbir, G.; Ajaib, M.; Sayyed, R.Z.; Alharbi, S.A.; Ansari, M.J. Interactive effects of melatonin and salicylic acid on Brassica napus under drought condition. Plant Soil 2023, 493, 1–20. [Google Scholar] [CrossRef]
- Khan, M.N.; Khan, Z.; Luo, T.; Liu, J.; Rizwan, M.; Zhang, J.; Xu, Z.; Wu, H.; Hu, L. Seed priming with gibberellic acid and melatonin in rapeseed: Consequences for improving yield and seed quality under drought and non-stress conditions. Ind. Crops Prod. 2020, 156, 112850. [Google Scholar] [CrossRef]
- Parsa Motlagh, B.; Shahdadi, F.; Salehi Sardoei, A.; Parviz, L.; Ghorbanpour, M. Foliar-applied melatonin alters grain yield and the fatty acid profile of sesame (Sesamum indicum L.) under drought stress. J. Crop Health 2024, 76, 725–737. [Google Scholar] [CrossRef]
- Sheikhalipour, M.; Mohammadi, S.A.; Esmaielpour, B.; Spanos, A.; Mahmoudi, R.; Mahdavinia, G.R.; Milani, M.H.; Kahnamoei, A.; Nouraein, M.; Antoniou, C.; et al. Seedling nanopriming with selenium-chitosan nanoparticles mitigates the adverse effects of salt stress by inducing multiple defence pathways in bitter melon plants. Int. J. Biol. Macromol. 2023, 242, 124923. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.A.; Gul, F.Z.; Zaman, G.; Iqbal, J.; Drouet, S.; Tungmunnithum, D.; Hano, C.; Abbasi, B.H. The influence of exogenous melatonin and chitosan on secondary metabolites’ production and biological activities of tissue extracts in agitated micro-shoot cultures of Ajuga integrifolia Buch. Ham. ex D. Don. Acta Physiol. Plant. 2023, 45, 106. [Google Scholar] [CrossRef]
- Tabassum, M.; Noreen, Z.; Aslam, M.; Shah, A.N.; Usman, S.; Waqas, A.; Alsherif, E.A.; Korany, S.M.; Nazim, M. Chitosan modulated antioxidant activity, inorganic ions homeostasis and endogenous melatonin to improve yield of Pisum sativum L. accessions under salt stress. Sci. Hortic. 2024, 323, 112509. [Google Scholar] [CrossRef]
- Mohtashami, R.; Dehnavi, M.M.; Balouchi, H.; Faraji, H. Improving yield, oil content and water productivity of dryland canola by supplementary irrigation and selenium spraying. Agric. Water. Manag. 2020, 232, 106046. [Google Scholar] [CrossRef]
- Samany, S.M.A.; Pirbalouti, A.G.; Malekpoor, F. Phytochemical and morpho-physiological changes of hyssop in response to chitosan-spraying under different levels of irrigation. Ind. Crops Prod. 2022, 176, 114330. [Google Scholar] [CrossRef]
- Gholami, R.; Hoveizeh, N.F.; Zahedi, S.M.; Gholami, H.; Carillo, P. Melatonin alleviates the adverse effects of water stress in adult olive cultivars (Olea europea cv. Sevillana & Roughani) in field condition. Agric. Water Manag. 2022, 269, 107681. [Google Scholar]
- Ahmad, I.; Younas, Z.; Mashwani, Z.U.R.; Raja, N.I.; Akram, A. Phytomediated selenium nanoparticles improved physio-morphological, antioxidant, and oil bioactive compounds of sesame under induced biotic stress. ACS Omega 2023, 8, 3354–3366. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Anjum, S.; Skalicky, M.; Waraich, E.A.; Muhammad Sabir Tariq, R.; Ayub, M.A.; Hossain, A.; Hassan, M.M.; Brestic, M.; Sohidul Islam, M.; et al. Selenium alleviates the adverse effect of drought in oilseed crops camelina (Camelina sativa L.) and canola (Brassica napus L.). Molecules 2021, 26, 1699. [Google Scholar] [CrossRef]
- Rahbari, A.; Sinaki, J.M.; Damavandi, A.; Rezvan, S. Castor bean (Ricinus communis L.) responses to drought stress and foliar application of Zn-nano fertilizer and humic acid: Grain yield, oil content, antioxidant activity, and photosynthetic pigments. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12003. [Google Scholar] [CrossRef]
- Nazim, M.; Ali, M.; Li, X.; Anjum, S.; Ahmad, F.; Zulfiqar, U.; Shahzad, K.; Soufan, W. Unraveling the synergistic effects of microbes and selenium in alleviating drought stress in Camelina sativa L. Plant Stress 2023, 9, 100193. [Google Scholar] [CrossRef]
- Khazaie, H.R.; Nadjafi, F.; Bannayan, M. Effect of irrigation frequency and planting density on herbage biomass and oil production of thyme (Thymus vulgaris) and hyssop (Hyssopus officinalis). Ind. Crops Prod. 2008, 27, 315–321. [Google Scholar] [CrossRef]
- Alizadeh, S.; Roozbahani, A.; Rad, A.H.S.; Seyedhadi, M.H. Foliar application of humic acids improves seed yield and oil quality of rapeseed (Brassica napus L.) genotypes at well-time and late planting dates. J. Soil Sci. Plant Nutr. 2022, 22, 549–559. [Google Scholar] [CrossRef]
- Jafari, M.; Shahsavar, A. The effect of foliar application of melatonin on changes in secondary metabolite contents in two citrus species under drought stress conditions. Front. Plant Sci. 2021, 12, 692735. [Google Scholar] [CrossRef]
- Attaran Dowom, S.; Karimian, Z.; Mostafaei Dehnavi, M.; Samiei, L. Chitosan nanoparticles improve physiological and biochemical responses of Salvia abrotanoides (Kar.) under drought stress. BMC Plant Biol. 2022, 22, 364. [Google Scholar] [CrossRef]
- Mohammadzadeh, S.; Pirzad, A. Biochemical responses of mycorrhizal-inoculated Lamiaceae (Lavender, Rosemary and Thyme) plants to drought: A field study. J. Soil Sci. Plant Nutr. 2021, 67, 41–49. [Google Scholar] [CrossRef]
- Caser, M.; Chitarra, W.; D’Angiolillo, F.; Perrone, I.; Demasi, S.; Lovisolo, C.; Pistelli, L.; Pistelli, L.; Scariot, V. Drought stress adaptation modulates plant secondary metabolite production in Salvia dolomitica Codd. Ind. Crops Prod. 2019, 129, 85–96. [Google Scholar] [CrossRef]
- Sattar, A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Hussain, S.; Rasheed, U.; Hussain, J.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Mahmoud, S.F.; et al. Modulation of antioxidant defense mechanisms and morpho-physiological attributes of wheat through exogenous application of silicon and melatonin under water deficit conditions. Sustainability 2023, 15, 7426. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Naz, S.; Altaf, M.M.; Khan, L.U.; Tiwari, R.K.; Lal, M.K.; Shahid, M.A.; Kumar, R.; et al. Melatonin improves drought stress tolerance of tomato by modulating plant growth, root architecture, photosynthesis, and antioxidant defense system. Antioxidants 2022, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Mazrou, R.M.; Hassan, F.A.; Mansour, M.M.F.; Moussa, M.M. Melatonin enhanced drought stress tolerance and productivity of Pelargonium graveolens L. (Herit) by regulating physiological and biochemical responses. Horticulturae 2023, 9, 1222. [Google Scholar] [CrossRef]
- Awan, S.A.; Khan, I.; Wang, Q.; Gao, J.; Tan, X.; Yang, F. Pre-treatment of melatonin enhances the seed germination responses and physiological mechanisms of soybean (Glycine max L.) under abiotic stresses. Front. Plant Sci. 2023, 14, 1149873. [Google Scholar] [CrossRef]
- Ahmad, S.; Su, W.; Kamran, M.; Ahmad, I.; Meng, X.; Wu, X.; Javed, T.; Han, Q. Foliar application of melatonin delay leaf senescence in maize by improving the antioxidant defense system and enhancing photosynthetic capacity under semi-arid regions. Protoplasma 2020, 257, 1079–1092. [Google Scholar] [CrossRef]
- Hasnain, Z.; Zafar, S.; Usman, S.; Zhang, L.; Elansary, H.O. Elucidating role of melatonin foliar spray in ameliorating adverse effects of drought stress on growth and physio-biochemical attributes of Brassica rapa plants. Sci. Hortic. 2023, 321, 112336. [Google Scholar] [CrossRef]
- Vitale, L.; Vitale, E.; Costanzo, G.; De Maio, A.; Arena, C. Photo-Protective Mechanisms and the Role of Poly (ADP-Ribose) Polymerase Activity in a Facultative CAM Plant Exposed to Long-Term Water Deprivation. Plants 2020, 9, 1192. [Google Scholar] [CrossRef]
- Samadi, M.; Kazemeini, S.A.; Razzaghi, F.; Edalat, M.; Andersen, M.N.; Jacobsen, S.E.; Mastinu, A. Melatonin priming manipulates antioxidant regulation and secondary metabolites production in favor of drought tolerance in Chenopodium quinoa Willd. S. Afr. J. Bot. 2024, 166, 272–286. [Google Scholar] [CrossRef]
- Mokhtassi-Bidgoli, A.; AghaAlikhani, M.; Nassiri-Mahallati, M.; Zand, E.; GonzalezAzndujar, J.L.; Azari, A. Agronomic performance, seed quality and nitrogen uptake of Descurainia sophia in response to different nitrogen rates and water regimes. Ind. Crops Prod. 2013, 44, 583–592. [Google Scholar] [CrossRef]
- Okalebo, J.R.; Gathua, K.W.; Woomer, P.L. Laboratory methods of soil and plant analysis: A working manual second edition. Sacred Afr. Nairobi 2002, 21, 25–26. [Google Scholar]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Rowell, D.L. Soil Science: Methods and Applications; Routledge: London, UK, 2014. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate (Issue 939); US Department of Agriculture: Washington, DC, USA, 1954.
- Zahedi, S.M.; Hosseini, M.S.; Abadía, J.; Marjani, M. Melatonin foliar sprays elicit salinity stress tolerance and enhance fruit yield and quality in strawberry (Fragaria× ananassa Duch.). Plant Physiol. Biochem. 2020, 149, 313–323. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen? total. In Methods of Soil Analysis: Part 3 Chemical Methods; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1085–1122. [Google Scholar]
- Li, B.; Wang, X.; Chen, R.; Huangfu, W.; Xie, G. Antibacterial activity of chitosan solution against Xanthomonas pathogenic bacteria isolated from Euphorbia pulcherrima. Carbohydr. Polym. 2008, 72, 287–292. [Google Scholar] [CrossRef]
- Piñero, M.C.; Otálora, G.; Collado-González, J.; López-Marín, J.; del Amor, F.M. Effects of selenium on the chlorophylls, gas exchange, antioxidant activity and amino acid composition of lettuce grown under an aquaponics system. Horticulturae 2021, 8, 30. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices; The American Oil Society Champaign: Champaign, IL, USA, 1993. [Google Scholar]
- Zamani, F.; Amirnia, R.; Rezaei-Chiyaneh, E.; Gheshlaghi, M.; Von Cossel, M.; Siddique, K.H.M. Optimizing essential oil, fatty acid profiles, and phenolic compounds of dragon’s head (Lallemantia iberica) intercropped with chickpea (Cicer arietinum L.) with biofertilizer inoculation under rainfed conditions in a semi-arid region. Arch. Agron. Soil Sci. 2022, 69, 1687–1704. [Google Scholar] [CrossRef]
- Taghizadeh, Y.; Amirnia, R.; Rezaei-Chiyaneh, E.; Ghiyasi, M.; Razavi, B.S.; Siddique, K.H.M. Co-inoculation of mycorrhizal fungi with bacterial fertilizer along with intercropping scenarios improves seed yield and oil constituents of sesame. J. Soil Sci. Plant Nutr. 2023, 23, 2258–2272. [Google Scholar] [CrossRef]
- Clevenger, J.F. Apparatus for the determination of volatile oil. J. Am. Pharm. Assoc. 1928, 17, 345–349. [Google Scholar] [CrossRef]
- Faridvand, S.; Rezaei-Chiyaneh, E.; Battaglia, M.L.; Gitari, H.I.; Raza, M.A.; Siddique, K.H. Application of bio and chemical fertilizers improves yield, and essential oil quantity and quality of Moldavian balm (Dracocephalum moldavica L.) intercropped with mung bean (Vigna radiata L.). Food. Energy Secur. 2022, 11, 319. [Google Scholar] [CrossRef]
- Perkin, E. Analytical Methods for Atomic Absorbtion Spectrophotometry; Perkin-Elmer Inc.: Waltham, MA, USA, 1982. [Google Scholar]
- Waling, I.; Van Vark, W.; Houba, V.J.G.; Van der Lee, J.J. Soil and Plant Analysis, a Series of Syllabi: Plant Analysis Procedures; Wageningen Agriculture University Press: Wageningen, The Netherlands, 1989. [Google Scholar]
- Schuman, G.E.; Stanley, M.A.; Knudsen, D. Automated total nitrogen analysis of soil and plant samples. Soil Sci. Soc. Am. J. 1973, 37, 480–481. [Google Scholar] [CrossRef]
- Saadat, B.; Pirzad, A.; Jalilian, J. Yield-related biochemical response of understory mycorrhizal yellow sweet clover (Melilotus officinalis L.) to drought in agrisilviculture. Arch. Agron. Soil Sci. 2021, 67, 603–1620. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Singh, R.; Saffeullah, P.; Umar, S.; Abass, S.; Ahmad, S.; Iqbal, N. Comparing the individual and combined effects of nano zinc and conventional zinc fertilization on growth, yield, phytochemical properties, antioxidant activity, and secoisolariciresinol diglucoside content in linseed. Plant Nano Biol. 2024, 10, 100098. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Tejera, G.N.A.; Olivera, M.; Iribarne, C.; Lluch, C. Partial purification and characterization of a non-specific acid phosphatase in leaves and root nodules of Phaseolus vulgaris. Plant Physiol. Biochem. 2004, 42, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in bulgaria fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 89, 569–575. [Google Scholar] [CrossRef]
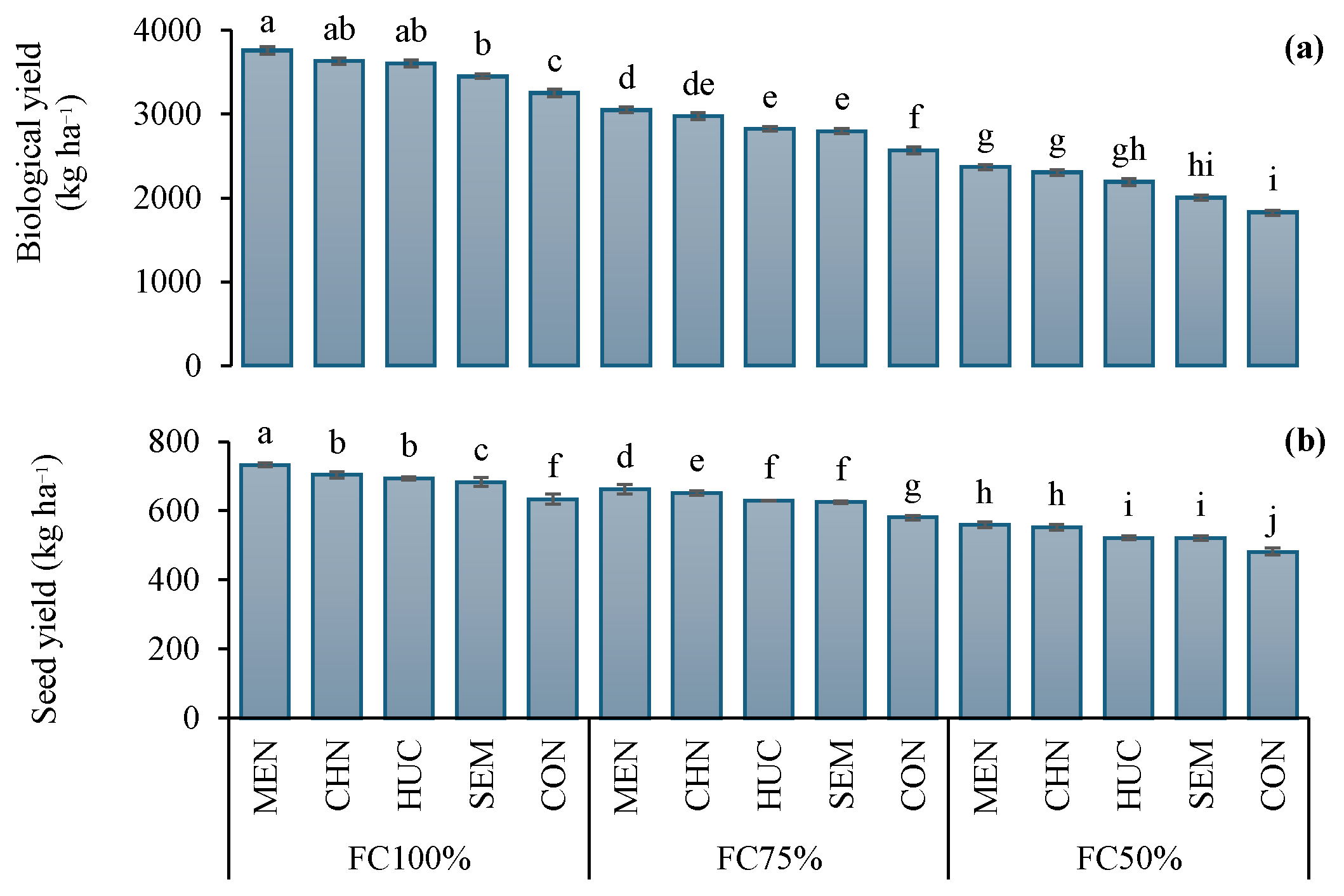
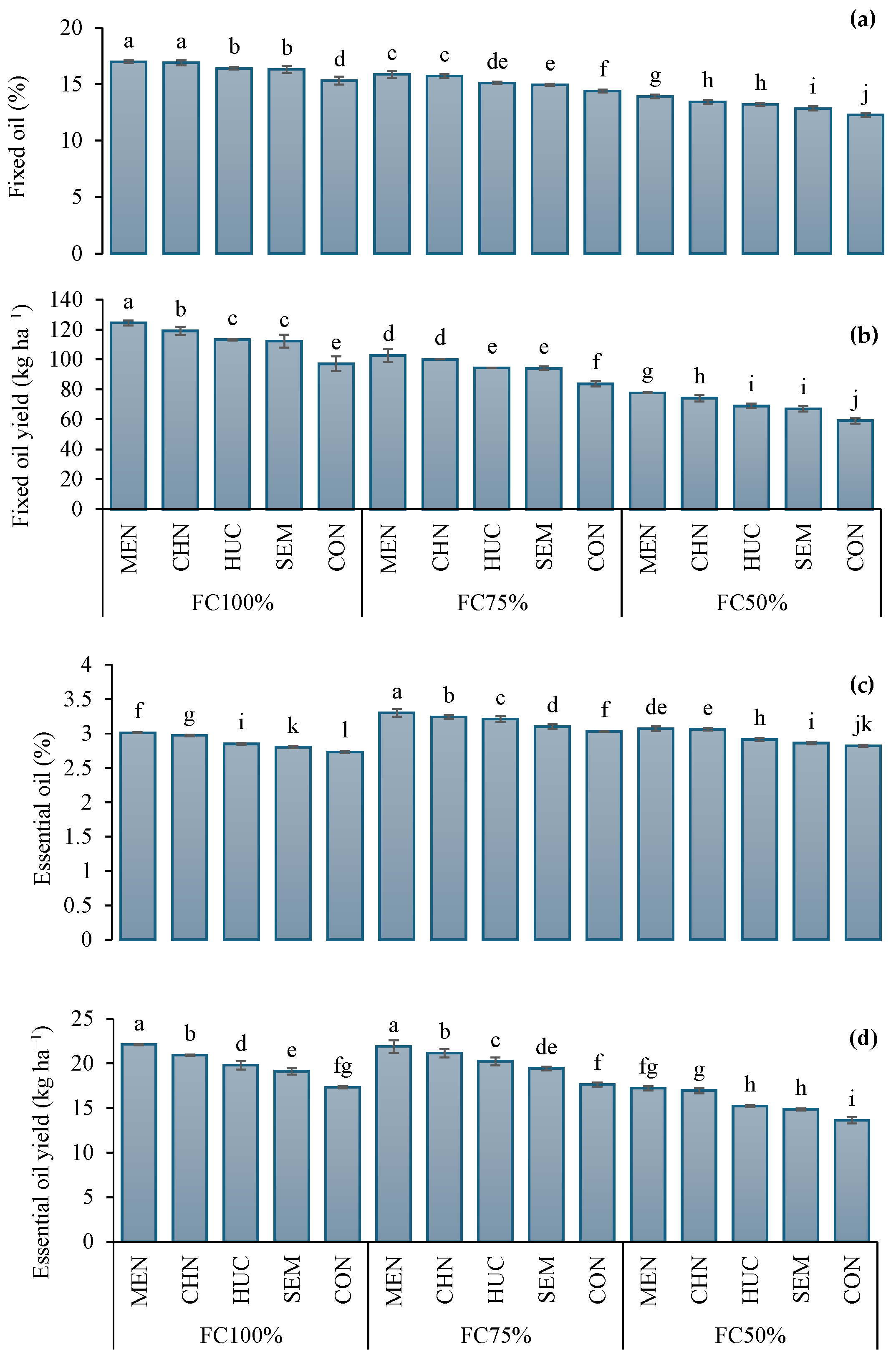
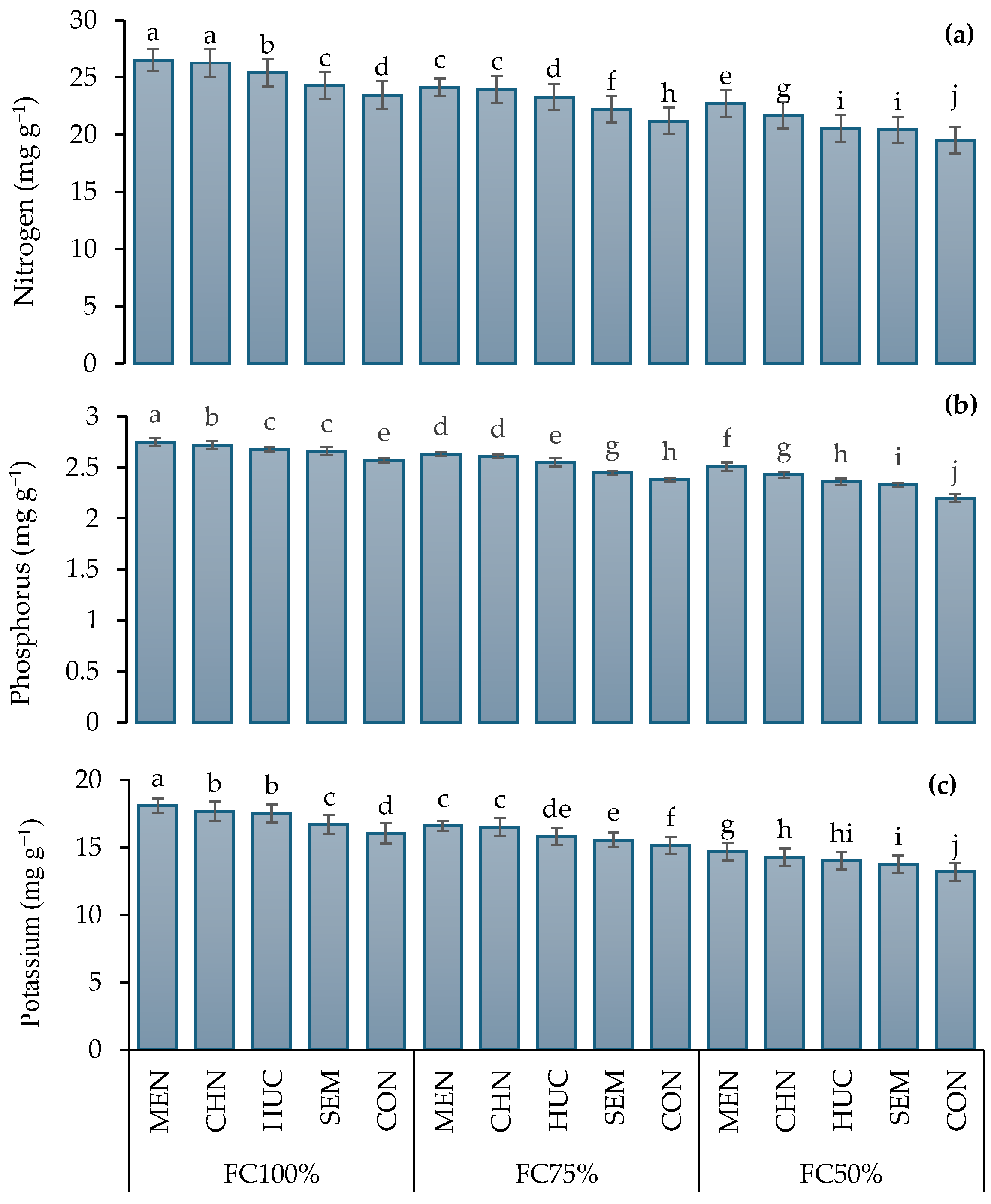
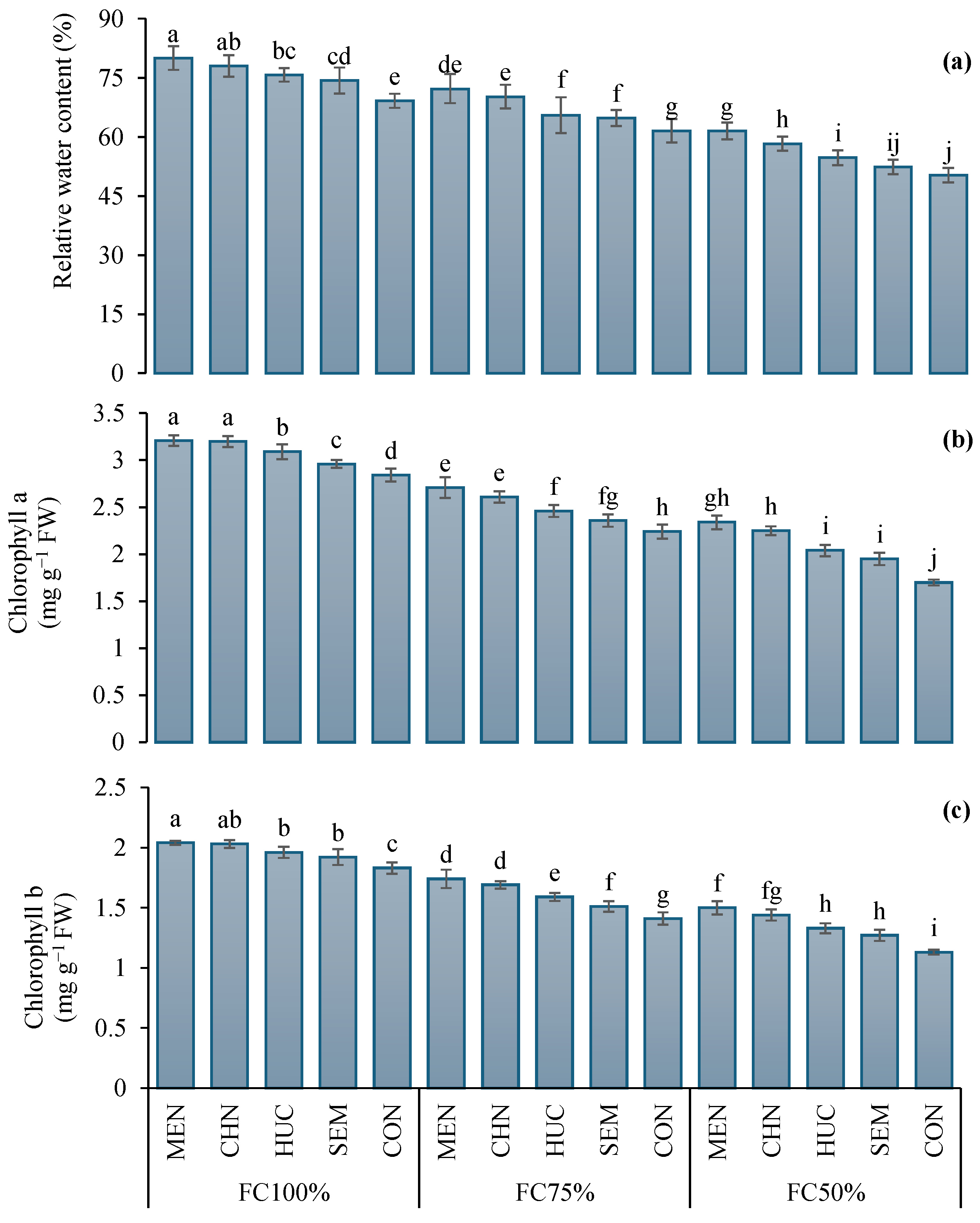

| Source of Variation | df | BY | SY | FO | FOY | EO | EOY | N | P | K |
|---|---|---|---|---|---|---|---|---|---|---|
| Year (Y) | 1 | 632,488 ** | 18,208 ** | 0.01 NS | 370.43 ** | 0.0008 NS | 14.99 ** | 90.70 ** | 0.42 ** | 26.35 ** |
| Rep (Y) | 4 | 14,060 | 69.35 | 0.03 | 6.99 NS | 0.001 NS | 0.17 | 0.05 | 0.0003 | 0.02 |
| (Ir) | 2 | 13,509,080 ** | 194,134 ** | 78.87 ** | 14,290 ** | 0.76 ** | 193.22 ** | 137.04 ** | 0.88 ** | 72.13 ** |
| Y × Ir | 2 | 8122 NS | 41.81 NS | 0.02 NS | 6.68 NS | 0.001 NS | 0.03 NS | 0.04 NS | 0.0003 NS | 0.04 NS |
| (M) | 4 | 1,618,004 ** | 6941 ** | 5.43 ** | 722.3 ** | 0.22 ** | 48.95 ** | 18.92 ** | 0.06 ** | 8.14 ** |
| Y × M | 4 | 454 NS | 51.33 NS | 0.02 NS | 2.97 NS | 0.0002 NS | 0.09 NS | 0.12 NS | 0.0002 NS | 0.06 NS |
| Ir × M | 8 | 1,280,608 ** | 8599 ** | 1.42 ** | 386.3 ** | 0.003 ** | 0.45 ** | 3.84 ** | 0.01 ** | 1.83 ** |
| Y × Ir × M | 8 | 718 NS | 77.59 NS | 0.03 NS | 6.14 NS | 0.0002 NS | 0.05 NS | 0.04 NS | 0.0002 NS | 0.02 NS |
| Error | 56 | 27,838 | 79.77 | 0.04 | 7.00 | 0.0006 | 0.12 | 0.10 | 0.0004 | 0.06 |
| Year | ||||||||||
| 2022 | 2758 ± 52.92 a | 601.6 ± 10.23 b | 14.9 ± 1.49 a | 90.8 ± 4.80 b | 3.00 ± 0.17 a | 18.1 ± 2.58 b | 22.0 ± 2.12 b | 2.45 ± 0.18 b | 15.2 ± 1.51 b | |
| 2023 | 2690 ± 72.03 a | 630.0 ± 9.300 a | 14.9 ± 1.47 b | 94.9 ± 4.21 a | 2.98 ± 0.16 a | 18.9 ± 2.62 a | 24.1 ± 2.07 a | 2.59 ± 0.15 a | 16.2 ± 1.47 a | |
| Irrigation Regime | ||||||||||
| FC100% | 3405 ± 93.51 a | 693.5 ± 12.55 a | 16.5 ± 0.51 a | 114 ± 4.50 a | 2.87 ± 0.11 c | 19.8 ± 1.73 b | 25.3 ± 1.51 a | 2.68 ± 0.09 a | 17.3 ± 0.87 a | |
| FC75% | 2979 ± 39.26 b | 620.9 ± 10.10 b | 15.0 ± 0.67 b | 93.5 ± 5.74 b | 3.18 ± 0.10 a | 20.1 ± 1.61 a | 22.8 ± 1.64 b | 2.54 ± 0.10 b | 15.6 ± 1.03 b | |
| FC50% | 1790 ± 19.53 c | 532.9 ± 8.540 c | 13.2 ± 0.73 c | 70.7 ± 4.30 c | 2.95 ± 0.11 b | 15.6 ± 1.44 c | 21.0 ± 1.44 c | 2.34 ± 0.11 c | 14.2 ± 0.93 c | |
| Modifiers | ||||||||||
| MEN | 3056 ± 16.05 a | 642 ± 5.68 a | 15.7 ± 1.08 a | 101 ± 6.68 a | 3.13 ± 0.13 a | 20.4 ± 2.37 a | 24.2 ± 1.94 a | 2.60 ± 0.14 a | 16.5 ± 0.18 a | |
| CHN | 2960 ± 58.24 a | 630 ± 4.59 b | 15.2 ± 1.03 b | 96.8 ± 4.59 b | 3.09 ± 0.12 b | 19.7 ± 2.07 b | 23.9 ± 1.92 b | 2.56 ± 0.12 b | 16.3 ± 0.32 b | |
| HUC | 2756 ± 44.84 b | 610 ± 9.30 c | 14.8 ± 1.59 c | 91.2 ± 7.50 c | 2.99 ± 0.16 c | 18.4 ± 2.41 c | 23.1 ± 2.32 c | 2.52 ± 0.18 c | 15.7 ± 1.56 c | |
| SEM | 2510 ± 32.10 c | 602 ± 7.33 d | 14.5 ± 1.53 d | 88.8 ± 6.45 d | 2.92 ± 0.14 d | 17.8 ± 2.20 d | 22.2 ± 2.30 d | 2.47 ± 0.17 d | 15.2 ± 1.70 d | |
| CON | 2341 ± 58.99 d | 595 ± 9.24 e | 14.3 ± 1.74 e | 85.8 ± 7.39 e | 2.86 ± 0.13 e | 16.2 ± 1.93 e | 21.9 ± 2.33 e | 2.46 ± 0.21 e | 14.9 ± 1.64 e | |
| CV (%) | 5.77 | 1.45 | 1.37 | 2.85 | 0.87 | 1.88 | 1.41 | 0.82 | 1.58 |
| Source of Variation | df | RWC | Chl a | Chl b | TSS | Pro | APX | SOD | CAT | TFC | TPC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year (Y) | 1 | 248.93 ** | 0.23 ** | 0.09 ** | 46.32 ** | 0.09 NS | 0.02 ** | 4.34 NS | 0.05 NS | 0.29 ** | 59.17 ** |
| Rep (Y) | 4 | 0.32 | 0.009 | 0.004 | 0.59 | 0.01 | 0.001 | 0.32 NS | 0.003 | 0.004 | 0.76 |
| (Ir) | 2 | 1946.39 ** | 7.06 ** | 2.32 ** | 260.05 ** | 24.53 ** | 0.27 ** | 2941.11 ** | 27.87 ** | 2.97 ** | 315.44 ** |
| Y × Ir | 2 | 0.23 NS | 0.0004 NS | 0.004 NS | 0.08 NS | 0.007 NS | 0.0004 NS | 7.75 NS | 0.09 NS | 0.001 NS | 0.10 NS |
| (M) | 4 | 387.06 ** | 0.93 ** | 0.23 ** | 28.05 ** | 0.2.80 ** | 0.05 ** | 612.36 ** | 6.16 ** | 0.42 ** | 37.85 ** |
| Y × M | 4 | 0.20 NS | 0.003 NS | 0.001 NS | 0.007 NS | 0.0004 NS | 0.0001 NS | 2.48 NS | 0.02 NS | 0.0009 NS | 0.01 NS |
| Ir × M | 8 | 244.13 ** | 0.32 ** | 0.18 ** | 10.01 ** | 0.31 ** | 0.03 ** | 382.36 ** | 3.29 ** | 0.13 ** | 11.24 ** |
| Y × Ir × M | 8 | 0.51 NS | 0.0002 NS | 0.001 NS | 0.004 NS | 0.0006 NS | 0.0001 NS | 3.37 NS | 0.03 NS | 0.0003 NS | 0.005 NS |
| Error | 56 | 6.91 | 0.008 | 0.004 | 0.25 | 0.02 | 0.0008 | 6.91 | 0.06 | 0.002 | 0.32 |
| Year | |||||||||||
| 2022 | 64.3 ± 9.50 b | 2.46 ± 0.50 b | 1.59 ± 0.28 b | 15.6 ± 2.92 a | 3.56 ± 0.85 a | 1.12 ± 0.12 a | 48.7 ± 12.2 a | 3.26 ± 1.19 a | 1.90 ± 0.32 a | 20.2 ± 3.23 a | |
| 2023 | 67.6 ± 9.29 a | 2.57 ± 0.47 a | 1.66 ± 0.30 a | 14.2 ± 2.84 b | 3.50 ± 0.86 a | 1.08 ± 0.10 a | 48.3 ± 10.9 a | 3.21 ± 1.06 a | 1.79 ± 0.29 b | 18.6 ± 3.14 b | |
| Irrigation Regime | |||||||||||
| FC100% | 74.2 ± 6.15 a | 2.99 ± 0.31 a | 1.90 ± 0.15 a | 11.8 ± 1.95 c | 2.55 ± 0.55 c | 1.02 ± 0.09 c | 38.9 ± 7.41 c | 2.28 ± 0.67 c | 1.52 ± 0.21 c | 16.0 ± 2.13 c | |
| FC75% | 65.5 ± 8.49 b | 2.54 ± 0.35 b | 1.63 ± 0.23 b | 15.2 ± 1.89 b | 3.69 ± 0.43 b | 1.07 ± 0.09 b | 47.9 ± 10.2 b | 3.21 ± 1.00 b | 1.85 ± 0.22 b | 19.8 ± 2.14 b | |
| FC50% | 58.1 ± 5.71 c | 2.02 ± 0.17 c | 1.35 ± 0.14 c | 17.7 ± 1.20 a | 4.34 ± 0.21 a | 1.20 ± 0.07 a | 58.7 ± 6.74 a | 4.21 ± 0.68 a | 2.15 ± 0.14 a | 22.5 ± 1.40 a | |
| Stress Modifiers | |||||||||||
| MEN | 71.2 ± 6.99 a | 2.80 ± 0.59 a | 1.75 ± 0.35 a | 16.7 ± 2.88 a | 3.98 ± 0.80 a | 1.16 ± 0.11 a | 55.2 ± 7.05 a | 3.92 ± 0.72 a | 2.08 ± 0.30 a | 21.7 ± 3.15 a | |
| CHN | 68.3 ± 11.06 b | 2.73 ± 0.50 b | 1.72 ± 0.30 a | 15.6 ± 1.78 b | 3.80 ± 0.86 b | 1.13 ± 0.16 b | 51.2 ± 15.1 b | 3.55 ± 1.39 b | 1.91 ± 0.19 b | 19.9 ± 2.01 b | |
| HUC | 66.5 ± 6.02 b | 2.41 ± 0.39 c | 1.59 ± 0.22 b | 14.4 ± 2.76 c | 3.59 ± 0.99 c | 1.11 ± 0.07 bc | 49.3 ± 6.68 c | 3.32 ± 0.67 c | 1.78 ± 0.70 c | 17.7 ± 4.08 c | |
| SEM | 64.7 ± 7.67 c | 2.37 ± 0.35 c | 1.55 ± 0.24 b | 14.2 ± 2.52 c | 3.26 ± 0.62 d | 1.09 ± 0.05 c | 47.5 ± 10.3 c | 3.00 ± 0.98 d | 1.75 ± 0.24 d | 18.6 ± 2.87 c | |
| CON | 58.9 ± 9.57 d | 2.29 ± 0.38 d | 1.50 ± 0.25 c | 13.6 ± 2.67 d | 3.01 ± 0.62 e | 1.01 ± 0.10 d | 39.4 ± 11.1 d | 2.37 ± 1.11 e | 1.70 ± 0.33 e | 18.2 ± 2.88 d | |
| CV (%) | 3.98 | 3.68 | 3.92 | 3.36 | 4.56 | 2.57 | 5.41 | 8.13 | 2.63 | 2.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heydarzadeh, S.; Tobeh, A.; Jahanbakhsh, S.; Farzaneh, S.; Vitale, E.; Arena, C. The Application of Stress Modifiers as an Eco-Friendly Approach to Alleviate the Water Scarcity in Ajwain (Carum copticum L.) Plants. Plants 2024, 13, 3354. https://doi.org/10.3390/plants13233354
Heydarzadeh S, Tobeh A, Jahanbakhsh S, Farzaneh S, Vitale E, Arena C. The Application of Stress Modifiers as an Eco-Friendly Approach to Alleviate the Water Scarcity in Ajwain (Carum copticum L.) Plants. Plants. 2024; 13(23):3354. https://doi.org/10.3390/plants13233354
Chicago/Turabian StyleHeydarzadeh, Saeid, Ahmad Tobeh, Sodabeh Jahanbakhsh, Salim Farzaneh, Ermenegilda Vitale, and Carmen Arena. 2024. "The Application of Stress Modifiers as an Eco-Friendly Approach to Alleviate the Water Scarcity in Ajwain (Carum copticum L.) Plants" Plants 13, no. 23: 3354. https://doi.org/10.3390/plants13233354
APA StyleHeydarzadeh, S., Tobeh, A., Jahanbakhsh, S., Farzaneh, S., Vitale, E., & Arena, C. (2024). The Application of Stress Modifiers as an Eco-Friendly Approach to Alleviate the Water Scarcity in Ajwain (Carum copticum L.) Plants. Plants, 13(23), 3354. https://doi.org/10.3390/plants13233354







